Abstract
Rotaviruses cause life-threatening gastroenteritis in children worldwide; the enormous disease burden has focused efforts to develop vaccines and led to the discovery of novel mechanisms of gastrointestinal virus pathogenesis and host responses to infection. Two live-attenuated vaccines for gastroenteritis (Rotateq and Rotarix) have been licensed in many countries. This review summarizes the latest data on these vaccines, their effectiveness and challenges to global vaccination. Recent insights into rotavirus pathogenesis are also discussed, including information on extra-intestinal infection, viral antagonists of the interferon response and the first described viral enterotoxin. Rotavirus-induced diarrhea is now considered to be a disease that can be prevented through vaccination, although there are many challenges to achieving global effectiveness. Molecular biology studies of rotavirus replication and pathogenesis have identified unique viral targets that might be useful in developing therapies for immunocompromised children with chronic infections.
Introduction
Rotavirus infects every child in the world and is the leading cause of life-threatening diarrheal disease among infants and young children in many countries. The global disease burden stimulated efforts to develop vaccines, some of which are licensed and now being used. This article summarizes information about rotavirus that led to recent vaccines, challenges to global vaccination, and mechanisms of gastrointestinal (GI) virus pathogenesis and mucosal immunity.
Virology
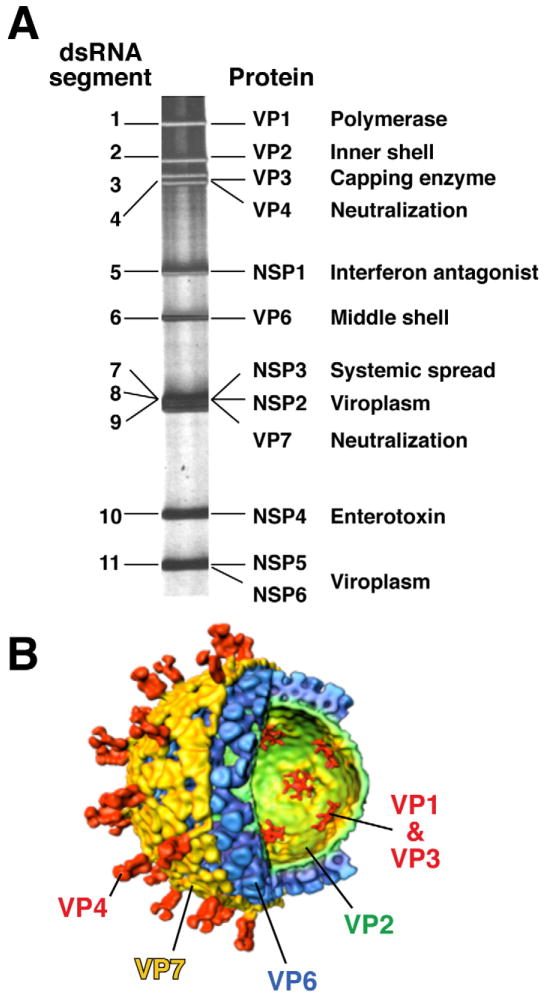
Rotaviruses are members of the Rotavirus genus of the Reoviridae family, which contains viruses with segmented, double-stranded RNA genomes. Rotavirus particles are large (1000 Å) and complex, with 3 concentric protein layers that surround the viral genome of 11 segments of double-stranded RNA (Figure 1) 1. The rotavirus genome segments encode 6 structural proteins that make up virus particles (viral protein or VP) and 6 nonstructural proteins (NSP). The NSP are synthesized in infected cells and function in some aspect of the viral replication cycle or interact with host proteins to influence pathogenesis or the immune response to infection. The rotavirus protein VP7 makes up the outer capsid protein shell and VP4 forms spikes that emanate through the outer capsid shell; these induce neutralizing antibody responses and are the basis of a binary classification system for viral serotypes. Thus, VP7 (a glycoprotein or G-type antigen) and VP4 (a protease sensitive protein or P-type antigen) are used to classify rotaviruses. VP7 types are classified as serotypes by neutralization assays or as genotypes by sequencing; these 2 assays yield concordant results, so viruses are referred to by their G serotype alone (e.g., G1, G2, G3, etc). VP4 serotypes are also classified by neutralization and sequencing assays, but the results do not always agree, so there is a dual system for P typing. P serotypes are referred to by their serotype numbers (e.g., P1, P2) and P genotypes are denoted in brackets (e.g., P[8], P[4]). P genotyping is the most widely used method for classification because of difficulties in standardizing VP4 serotype assays. Currently, 19G and 28[P] types are known.
Figure 1.
Structure and proteins of rotavirus. A). The viral genome of 11 segments of double-stranded RNA is analyzed by polyacrylamide gel electrophoresis. Each gene codes for at least one protein as shown with at least one major function of the protein indicated. B). A cut-away of the viral structure as determined by image reconstruction after electron cryo-microscopy is shown with the proteins designated that make up each concentric protein layer. Adapted from Estes, 2001. 1
In addition to being clinically significant pathogens, rotaviruses are fascinating in that they exhibit unusual aspects of structural complexity and have unique replication features. There have several key properties that are relevant to their success as GI pathogens. First, the triple layer capsid is very stable, which facilitates fecal–oral transmission and delivery of virus into the small intestine, where it infects nondividing differentiated enterocytes near the tips of the villus (Figures 2 and 3). It is thought that mature enterocytes express factors required for efficient infection and/or replication. The 60 spikes that protrude from the surface of the outer viral capsid are composed of a complex of molecules that act as an initial viral attachment protein to bind host receptors. The spike protein is susceptible to proteolytic cleavage, a common feature of attachment proteins of many viruses that infect mucosal surfaces. Proteolytic cleavage by trypsin induces a remarkable conformational change in the structure of the spike that exposes additional attachment sites on the surface glycoprotein for interaction with a series of co-receptors. The multistep attachment and entry process, which remains incompletely understood, is complex, with activation of infectivity resulting in virus delivery across the plasma membrane and into the cell. The virus is never completely uncoated, but instead the outer capsid shell is removed and double-layered particles are delivered into the cell cytoplasm. These particles function as molecular machines, producing capped viral mRNAs that are extruded from transcribing particles into the cytoplasm. There, they are translated into proteins and replicated to produce new genomic RNA. The proteins in the core of the incoming particles possess all the enzymatic activities required to produce the viral transcripts from the viral genome dsRNA, because eukaryotic cells do not express RNA polymerases that transcribe mRNA from dsRNA templates.
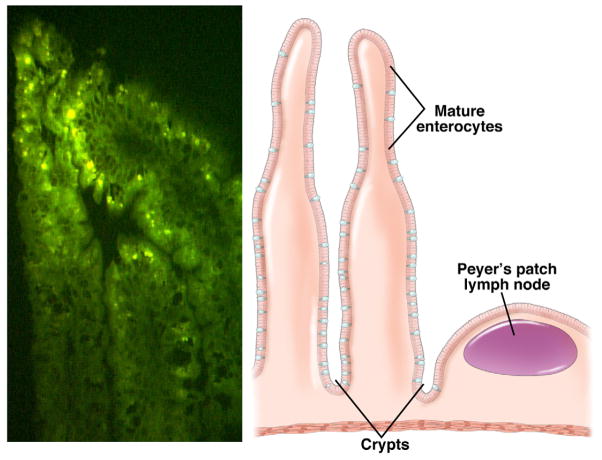
Figure 2.
Rotavirus infection of small intestinal enterocytes. Left panel. Immunofluorescence analysis detects rotavirus replication in the ileum of a 5-day-old neonatal rat pup infected with rhesus rotavirus. Right panel. Schematic of villus showing the site of rotavirus replication in the mature enterocytes. Adapted from Ciarlet et al., 2002.96
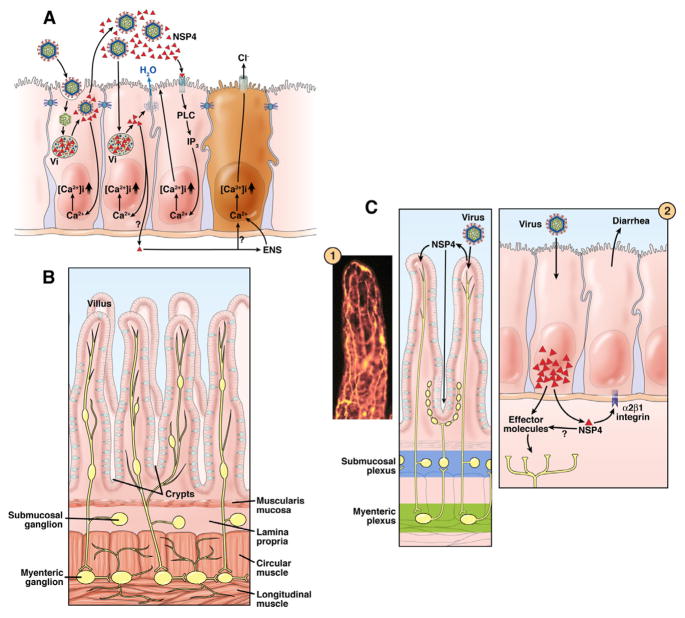
Figure 3.
Mechanisms by which rotaviruses cause diarrhea. A). Events that occur following rotavirus infection of enterocytes are shown in order from left to right. Not all events are shown in each cell. 1) Infection of the initial cell by luminal virus leads to virus entry, uncoating, transcription, translation of viral proteins, formation of viroplasms (Vi), and apical release of virus and viral protein. Nonstructural protein 4 (NSP4, red triangle) and virus particles are released by a nonclassical secretory pathway. Intracellular NSP4 also induces the release of Ca2+, from internal stores, primarily the endoplasmic reticulum, leading to increasing intracellular calcium [Ca2+]i. 2) Another outcome can result from a cell being infected with virus. NSP4 produced by the infection disrupts tight junctions, allowing paracellular flow of water and electrolytes (blue arrow). 3) NSP4 released from previously infected cells binds to a specific receptor and triggers a signaling cascade through phospholipase C (PLC) and inositol phosphatase (IP)3 that results in release of Ca2+ and an increase in [Ca2+]i. Intracellular expression of NSP4 increases [Ca2+]i through a PLC-independent mechanism. The increase in [Ca2+]i also disrupts the microvillar cytoskeleton. 4) A crypt cell (brown) can be acted on directly by NSP4 or NSP4 can stimulate the enteric nervous system (ENS), which in turn signals an increase in [Ca2+]i that induces Cl− secretion. Panel B shows the normal architecture of the small intestine, without the circulatory system shown. This panel shows the ENS and its ganglia in the different submucosal levels. Panel C shows a reflex arc in the ENS that can receive signals from the villus epithelium and activate the crypt epithelium. Inset 1 shows a whole-mount of an adult mouse small intestinal villus, stained with antibody to the gene product 9.5 neuroendocrine marker to reveal the rich innervation (yellow). Inset 2 shows that infected villus enterocytes can stimulate the ENS by the basolateral release of NSP4 or other effector molecules. The integrin α2β1 can bind NSP4 and elicit diarrhea in neonatal mice. Adapted with permission from Ramig, 2004. 97
Several aspects of the rotavirus replication process are unique. Viral replication is restricted to the cell cytoplasm and occurs within specialized electron-dense structures called viroplasms, which are localized adjacent to the cell nucleus and near the endoplasmic reticulum (Figure 3). Viroplasms are composed of nascent viral proteins and their size and shape change during the replication cycle. Newly made double-layered particles containing newly replicated dsRNA bud from viroplasms into the endoplasmic reticulum (ER) in a unique process during which particles become transiently enveloped. The budding of particles into the ER is initiated by the binding of newly made double-layered particles to an intracellular viral receptor. This receptor consists of the cytoplasmic tail of a rotavirus nonstructural protein (NSP4) that is a transmembrane ER glycoprotein. The outer capsid proteins are incorporated into new particles during the budding process through protein rearrangements that occur as the transient envelope is lost. Mature virus particles are released from cells either by cell lysis or by delivery of particles to the apical plasma membrane of polarized cells by a nonclassical trafficking pathway.
Rotavirus are important models for studying non-enveloped virus penetration of intracellular membranes and viral modulation of cell Ca 2+ homeostasis. In spite of the synthesis of rotavirus glycoproteins and intracellular viral protein and particle trafficking, regulation of rotavirus replication and morphogenesis does not involve protein trafficking to the Golgi. Instead, levels of intracellular calcium ([Ca2+]i)regulate rotavirus replication. Rotaviruses affect and exploit calcium (Ca2+) signaling to control replication, morphogenesis and pathogenesis. Thus, rotavirus infections result in at least 3-fold increases of [Ca2+]i, and up to 10-fold increases in uptake of 45Ca2+ into cells 2, 3. Ca2+ also has an important role in virion assembly and disassembly processes. Ca2+ maintains the integrity of the rotavirus outer capsid layer; VP7 is a Ca2+ binding protein and Ca2+ chelation is one way to activate the endogenous RNA polymerase. NSP5 also is a Ca2+ binding protein and viroplasm formation requires Ca2+ 4. Rotavirus morphogenesis depends on the presence of adequate Ca2+ levels in cells. Without Ca2+, virus morphogenesis is stopped at the double-layered particle (DLP) step and VP7 is excluded from hetero-oligomeric complexes made of NSP4 and VP4 that participate in the budding of DLPs into the ER 5. Furthermore, Ca2+ depletion of the ER by the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pump inhibitor thapsigargin inhibits VP7 and NSP4 glycosylation and virus maturation 6. NSP4 is the only rotavirus protein that mobilizes [Ca2+]i in cells7, 8. Release of [Ca2+]i from the ER alters plasma membrane permeability and compensatory entry of extracellular Ca2+ into cells. It is not clear how intracellular NSP4 releases Ca2+ from the ER, but this is a phospholipase C (PLC)-independent mechanism7–9. NSP4 itself might function as, or regulate, a Ca2+ channel10, 11. Alternatively, NSP4 might simply cause Ca2+ to leak from the ER by co-translational insertion into the ER membrane or by activity of its membrane destabilization domain(s). Understanding this process could identify new therapeutic targets that could be use to treat immunocompromised patients with chronic rotavirus infections.
Epidemiology and Transmission
Human rotaviruses were discovered 36 years ago—a decade after the first animal rotaviruses were visualized12–14. Because large amounts of human rotaviruses are shed in the stool, the development of specific and sensitive solid-phase immunoassay systems for detection was straightforward; within 10 years of discovery, it was clear that rotaviruses were ubiquitous and associated with approximately 20%–30% of severe diarrheal diseases that required hospitalization in children under the age of 5, worldwide15. Virtually every study into the role of rotavirus as an etiologic cause of gastroenteritis has found that rotavirus-associated illness tends to be more severe than gastroenteritis caused by other enteric pathogens 16–18. In the early 1980’s, it was estimated that rotaviruses were responsible for approximately 870,000 deaths per year in young children15. Under the auspices of the Centers for Disease Control (CDC), the World Health Organization (WHO) and other regional surveillance agencies, techniques of rotavirus detection have become more sensitive, widespread and uniform over the past several years; as a result, the global burden of diarrheal disease has fallen sharply—from over 4 million to under 2 million per year. It has been estimated that there are over 114 million rotavirus diarrheal episodes per year that result in approximately 24 million clinic visits, 2.4 million hospitalizations and over 500,000 deaths in children under 5 years of age19, 20. By 5 years of age, 1 in 50 children will be hospitalized and 1 in 205 will die from rotavirus-associated causes. Virtually all these deaths occur in children living in developing countries21. Recent worldwide surveillance data from the CDC revealed that of the 62,584 hospitalizations for diarrhea, 40% were due to rotavirus infection 19. The reason for the apparent increase in the proportion of severe diseases associated with rotavirus worldwide is not entirely clear; it might result from more standardized methodology of defining and selecting cases and improved diagnostic testing. Perhaps more importantly, however, it could also reflect a relative decrease over the last 20 years in the absolute number of severe diarrheal cases caused by bacterial pathogens. This decrease has been associated with general improvements in water supply and hygiene without a concomitant decrease in number of rotavirus disease cases. The global mortality burden associated with rotavirus infection continues to be great—it represents one of a handful of vaccine preventable causes of significant infant mortality worldwide.
The burden of disease from rotavirus infection is not limited, however, to the less-developed world. Studies from Western Europe found that 50% of cases of gastroenteritis in children less than 5 years of age that were treated in emergency departments was caused by rotavirus and that the infection resulted in 230 deaths per year 22–24. In recent studies from the US, 50% of children hospitalized or treated in the emergency department for gastroenteritis had rotavirus infection25, leading to estimates that in children under the age of 3, one of every 150 would be hospitalized and 1 of 11 would be seen as an outpatient in an emergency department for rotavirus disease. In the US rotavirus is estimated to cause 20–60 deaths, 55000–70,000 hospitalizations and 410,000 physician visits annually26, 27. Rotavirus also appears to be a common cause of nosocomial infection. One review study from the US indicated that over 20% of patients admitted to hospitals develop concurrent rotavirus infections. Other studies estimated that every 4 children admitted to the hospital for rotavirus illness results in 1 case of nosocomial rotavirus illness in the hospital28. The overall health and societal costs of rotavirus disease in the US have been estimated to exceed $1 billion per year.
Because rotavirus has a segmented dsRNA genome, the genes that encode VP4 and VP7 can, in theory, segregate independently. Of the 28 P types and 15 G types thus far identified, 11 VP4 P types and 10 VP7 G types have been isolated from viruses from humans. So, the potential serotypic diversity for human rotaviruses seems to be vast. In fact, over 40 G/P combinations have been observed at least once in people29. However, in nature it does not appear that all VP4 and VP7 proteins are equally efficient in competing for a niche in the human GI tract; only a relatively small number of P- and G-type combinations have been encountered with any significant frequency and just 4 combinations (P(8)G 1, G2, or G3 and P(4)G2) account for over 90% of isolates. This finding has remained relatively unchanged for many years. In Europe, for example, P(8)G1 strains account for almost 70% of all human isolates. This is not to say that serotypic diversity does not change over time or on a geographical basis. In fact, in the last decade, P(8)G9 viruses have been more frequently encountered than in past decades 29 and in some regions, such as India, much greater serotypic diversity is routinely encountered. It has generally been assumed that interspecies transmission of rotaviruses between animals and humans is primarily responsible for the generation of serotypic diversity; there are numerous case reports in the literature of infections of humans with animal strains30. In addition, a variety of human isolates have been shown to be recombinants of human and animal rotavirus strains31. The critical relationship between serotypic diversity and protective immunity remains to be fully understood and is one of the important unanswered questions in rotavirology. The emergence of new serotypes such as the G9 strains would seem to indicate that immune selection can drive serotypic diversification. On the other hand, the efficacy of single serotype vaccines, the general restriction of severe illness to the first infection and the general persistence and dominance of G1 strains in the environment of Western Europe and the US for many years indicate that other factors could have a more critical role in determining rotavirus serotypic distribution than immune selection.
Unlike many bacterial enteric pathogens, rotaviruses subsist in temperate and tropical climates, as well as in developed and less-developed social settings. Rotaviruses are shed in the feces in amounts up to 1010 particles per gram of stool; limited infectivity studies indicating that 10 or less particles are likely infectious32, 33. The large amount of virus shed is probably the reason that improvements in hygiene in the developed world have not greatly reduced the incidence of rotavirus disease, although respiratory transmission might also have a role in dissemination34. In temperate climates, rotavirus disease is seasonal, occurring in the cooler dryer months of the year35. In a study from Australia, performed over a 10 year period, higher weekly temperatures and humidity in the previous week correlated with decreased rotavirus admissions for diarrhea in the following week during rotavirus seasons36. However, there are regional variations in the rotavirus season. For example, in the US, it tends to start in the Southwest in the fall and end in the Northeast in the spring; in Europe it tends to spread from south to north over generally the same timeframe37, 38. The mechanism responsible for this seasonality (relative humidity, average temperature, indoor population density) is not clear. Although rotavirus infections fluctuate far less in tropical climates, rates are not flat in these areas. In fact, a recent systematic review of 26 studies concluded that the highest number of infections also occurred in the coolest and driest months of the year in the tropics 38.
Pathogenesis
Intestinal infection
Rotavirus infection can result in asymptomatic or symptomatic infection. The outcome of infection is affected by both viral and host factors. The most prominent host factor that affects the clinical outcome of infection is age. Thus, neonates infected with rotavirus rarely have symptomatic disease; this protection is thought to be mediated primarily by transplacental transfer of maternal antibodies 39. Reductions in these antibodies coincide with the age of maximum susceptibility of infants to severe rotavirus-induced disease (3 mos to 2 years). Rotavirus can infect adults, but severe symptomatic disease is relatively uncommon and can result from infections with an unusual virus strain or extremely high doses of virus.
Virus virulence is related to properties of the proteins encoded by a subset of the 11 viral genes. Virus virulence is multigenic and has been associated with genes 3, 4, 5, 9, and 10. The basis for the involvement of these genes is only partially understood. Gene 3 encodes the capping enzyme that affects levels of viral RNA replication; genes 4 and 9 produce the outer capsid proteins required to initiate infection. Gene 5 codes for a protein (NSP1) that functions as an interferon antagonist (discussed below in the immunity section). Gene 10 codes for the nonstructural protein NSP4, which functions to regulate calcium homeostasis, virus replication and as an enterotoxin.
Diarrhea is the main clinical manifestation of rotavirus infection in infants and young children. A hallmark of viral-induced diarrhea that distinguishes it from bacterial-induced diarrhea is that little inflammation is seen in infected intestines. Rotavirus primarily infects intestinal villus enterocytes and crypt cells are spared (Figures. 2 and 3). Our understanding of disease pathogenesis is based primarily on studies in a variety of animal models. Disease pathogenesis is multifactorial and malabsorptive diarrhea occurs due to virus-mediated destruction of absorptive enterocytes, virus-induced downregulation of the expression of absorptive enzymes, and functional changes in tight junctions between enterocytes that lead to paracellular leakage. There is a secretory component of rotavirus diarrhea that is thought to be mediated by activation of the enteric nervous system and the effects of NSP4—the first described virus-encoded enterotoxin (Figure. 3). Studies of the virus and the effects of NSP4 alone, in cultured cells and animal models, indicate that rotavirus- induced diarrhea results, in part, from activation of cellular Cl− channels, which increases secretion of Cl− and consequently water (Fig 2). This Cl− secretion does not occur through the cystic fibrosis transmembrane regulator—rotavirus and NSP4 induce diarrhea in mouse pups that lack this channel as well as in children with cystic fibrosis10, 40. Villus ischemia and alterations in intestinal motility have also been reported in some animal models but their role in disease in children remains poorly documented.
Systemic infection
Rotavirus infection is not limited to the intestine—its extra-intestinal spread was documented over 45 years ago in mice, when virus was detected in multiple organs12, 41. These studies were largely forgotten until sensitive techniques re-evaluated systemic infection in a variety of animal models and in children42–45. It is clear that all infected individuals and animals undergo at least a short period of viremia and virus can be detected in the several other tissues of immunocompetent hosts46. The clinical consequences of such systemic infection remain unclear. Although there are many case reports associating rotavirus with many systemic illnesses, there is no proof of causation from extraintestinal spread of rotavirus; this would be difficult to prove if this form of the disease is rare. However, it is important for clinicians to consider the possibility of systemic infections and to be attuned to possible cases in which causation can be shown. It is not known if the most recently developed, live attenuated vaccines result in viremia, but unexpected systemic infections have not been associated with these vaccines.
Immunity
Studies of natural rotavirus infection in humans and animals were the first to demonstrate the existence of acquired immunity both to recurrent disease, and to a lesser extent, re-infection following primary infection47. These observations were followed by a large number of experimental studies in a variety of small and large animal models, all of which demonstrated the presence of acquired immunity. Each of the animal models has advantages and disadvantages. The 2 most widely studied models are the neonatal gnotobiotic pig model and the mouse model. The pig remains susceptible to disease longer than the mouse, can be symptomatically infected with virulent human as well as porcine rotavirus strains and is therefore preferable for studies of protection from disease (as opposed to restriction of re-infection). Gnotobiotic pigs that recover from infection with virulent human rotavirus possess high numbers of intestinal immunoglobulin (Ig)A rotavirus-specific primary antibody-secreting cells, measured by ELISPOT; these correlate with complete protection against homotypic challenge48. Because it is not possible to disrupt genes in pigs (make knockout animals) and because of the lack of large numbers of T- and B-cell–depleting antibody reagents, the pig model has been less effective for addressing fundamental questions about the effector mechanisms responsible for antiviral immunity. On the other hand, it has been an excellent system to evaluate vaccine candidates—live rotavirus infection (symptomatic or asymptomatic) has been by far the most efficient inducer of protective immunity. This protection has generally been correlated with a number of markers of mucosal immunity, including levels of anti-rotavirus intestinal IgA, enteric rotavirus reactive antibody-secreting cells, and IgA memory cells.
The mouse model has been the other widely used system to study rotavirus immunity. Its advantages include the animal’s small size and general availability, the existence of several virulent mouse rotavirus strains, and the large number of immunologic reagents available to measure and manipulate the model. Unfortunately, mice become maturationally resistant to diarrheal disease by 14 days of age, so they cannot be used to study active immunization against disease. Fortunately, mice remain susceptible to infection throughout their life so they are useful to examine acquired resistance to infection. Passive transfer studies of monoclonal antibodies in the mouse model demonstrated that neutralizing antibodies to VP4 or VP7 transfered homotypic or heterotypic protection, depending on the antibody specificity in vitro 49; the other rotavirus proteins appear to be targets for viral neutralization. Interestingly, other studies have shown that non-neutralizing IgA against the antigenically conserved VP6 protein can also mediate protection, apparently via an intracellular antiviral effect occurring during transcytosis50. Viral-induced diarrhea is also significantly reduced in mouse pups born to dams immunized with a variety of forms of the enterotoxin NSP451, 52. The relevance of these protective mechanisms to human immunity is unknown.
Studies in mice demonstrated that B cells were the primary determinant of protection from reinfection after natural infection, whereas CD8+T cells were responsible for shortening the course of primary infection53. CD4+T cells were generally involved in supplying help to CD8+ T cells and B cells, but also appear to mediate active protection, via a interferon (IFN)-γ–dependent pathway, after immunization with recombinant VP654. Regulatory T cells do not appear to mediate or modulate rotavirus immunity during primary infection55. Lymphocyte homing also has a prominent role in regulating rotavirus immunity and B cell trafficking to the intestine seems to be critical for generating optimal protection in a chronically infected mouse model 56.
Recent studies have also drawn attention to the importance of the innate immune response and IFN in regulating rotavirus immunity. In the gnotobiotic porcine model, the probiotic lactobacillus acidophilus significantly increased both B and T cell responses to attenuated live virus infection57. It remains to be determined if these effects would occur in humans, because their GI tract is colonized by bacteria shortly after birth. The role of the innate immune response in rotavirus infection, and specifically in IFN-induced antiviral effects, has been examined both in vivo and in vitro. Levels of type I and II IFNs increase in rotavirus-infected children and animals58. Types I and II interferon are able to limit rotavirus infection in vitro and, in early studies, IFN-α administration reduced rotavirus-associated diarrhea in cattle and pigs59, 60. On the other hand, IFNs appear to have little if any effect on the course of diarrhea or virus shedding during acute rotavirus infection in suckling mice. In adult mice, STAT1 deficiency was associated with increased viral shedding61. Interferon regulatory factor (IRF)3 has been shown to interact with the rotavirus protein NSP1, linking rotavirus infection to innate immunity62. Additional in vitro studies indicated that the rotavirus non-structural protein NSP1 functions as a virally encoded E3 ligase, interacting with and promoting the degradation of IRF3 and IRF7 via a proteosome-mediated mechanism that involved ubiquitination63. NSP1 also inhibits activation of NF B by a novel mechanism that involves targeted degradation of an F-box protein of the E3 ligase complex64. Studies in vivo demonstrated that the systemic virulence of selected strains of rotavirus was increased and a lethal biliary and pancreatic disease was induced when interferon signaling was blocked during rotavirus infection65. Innate immunity has an important role in modulating rotavirus infection in vitro and in animal model systems, but the role in humans is unexplored.
Vaccines
Attempts to develop a vaccine against human rotavirus began in the early 1980s. Initial efforts used a “Jennerian” approach (in reference to Edward Jenner’s cowpox vaccine against smallpox) to vaccinate children against rotaviruses, which normally infect animals. It has been observed that injection of an attenuated bovine rotavirus strain protected gnotobiotic calves against subsequent challenge with human rotavirus 66–69. Several important findings emerged from the first vaccine studies. The RIT4237 bovine rotavirus vaccine candidate was safe and highly effective (greater than 80%) in preventing severe diarrhea in Finnish children, but significantly less effective in clinical trials in African and Latin American children. The RIT4237 vaccine was less effective (as have been all subsequent vaccine candidates) at preventing any diarrhea than at preventing severe illness. Finally, and most interestingly from the standpoint of the role of vaccine serotype in protection, the bovine RIT vaccine was effective despite the fact that it was antigenically mismatched with all circulating human rotavirus strains. Because of its failure in clinical trials in Africa, the RIT4237 candidate was not pursued.
These initial studies were followed by a more sustained effort from investigators at the National Institutes of Health (NIH) and Wyeth Pharmaceuticals to develop an improved animal rotavirus-based vaccine. A simian rotavirus (RRV) was initially evaluated as a monovalent candidate70 that appeared to be effective in preliminary trials, but subsequent studies revealed diminished efficacy. This failure was proposed to reflect differences between the serotype of the RRV vaccine (G3) and circulating human strains at the time of the trial. In addition, the monovalent RRV strain possessed a considerable amount of residual reactogenicity, primarily in the form of fever. To circumvent the possible serologic problems inherent in a monovalent vaccine, a modified strategy was employed, in which the gene encoding VP7 from RRV (which was a G3 strain) was replaced with genes encoding human G1, 2 and 4 VP7s and a tetravalent vaccine containing G1,2,3 (from the original RRV) and 4 was evaluated71. This vaccine, called RotaShield™ or RRV-TV, was evaluated in an extensive series of safety and efficacy studies in the US, Finland and Venezuela, which all indicated it was highly effective (80%–100%) in preventing severe diarrheal disease47, 72–74.
Although the tetravalent vaccine was highly effective, the immunologic basis for this efficacy was unclear. Of note, neutralization responses to the 4 G serotypes contained in the vaccine were much lower than the apparent efficacy rates of the vaccine. To explain this apparent contradiction, it has been postulated that the primary advantage of multivalent rotavirus vaccines is not their serotypic diversity but rather their increased ability, compared to monovalent constructs, to boost the immune response on the second or third administration70. In any case, the RotaShield™ vaccine was judged to be safe and effective in several pivotal phase 3 clinical trials and was licensed for general use in children 2 to 6 months of age in the US in August, 1998 with high expectations that the dangers of rotavirus infection would soon be eliminated.
Approximately 600,000 infants in the US received RRV-TV before its utilization came to a halt in July 1999, when it was reported that the first dose of RRV-TV was associated with a substantial increased relative risk (at least 25-fold) of intussusception within the first 10 days after administration16, 75–77. The mechanism that underlies the association between RRV-TV and intussusception is unknown, but has been postulated to be specific to the RRV strain, since wild-type rotaviruses and other live attenuated vaccines have not been reproducibly associated with an increased rate of intussusception. Based primarily on the increase in relative risk, Rotashield was judged to be unsafe for routine use and withdrawn from commercial manufacture. It took another 7 years before new rotavirus vaccine candidates were available; during this seven 7-year hiatus, rotavirus caused morbidity and mortality continued unabated. Many ethical questions concerning the appropriateness of eliminating the availability of RRV-TV vaccine remain, especially for children in less-developed parts of the world, where the risk:benefit ratio for utilization of RRV-TV was very different than in the US.
Fortunately, research on rotavirus vaccines continued after the unexpected problems with the RRV-TV and in 2006, 2 new rotavirus vaccines were licensed in the US, the European Union, as well as many countries in Central and South America70, 78–83 (Table 1). One of these new vaccines represents an alternative approach. In this case, a bovine rotavirus strain (WC3), isolated in the US, was used as a backbone to create a pentavalent vaccine that contained 5 separate viruses that expressed either human G1, 2, 3 or 4 VP7s and a human P(8) VP4 on the bovine WC3 backbone84–86. The WC3 strain was initially studied as a stand-alone monovalent candidate (much like the RIT vaccine). It was found to be appropriately attenuated but clinical trials yielded varying efficacy rates, which led to the modification and inclusion of the various human G and P types. The pentavalent WC3-based vaccine is manufactured by Merck and is marketed under the trade name RotaTeq™. Because of the safety issues with RRV-TV, registration trials required almost 70,000 infants. In these trials, which were primarily but not exclusively carried out in the US and other developed countries, the vaccine was highly efficacious with protection rates against any rotavirus diarrhea of 74%, against diarrhea requiring a physician visit of 87% and against severe rotavirus disease of as high as 100%. RotaTeq’s efficacy rates did not appear to be affected by breastfeeding and administration of this vaccine did not interfere with immune responses induced by other vaccines37, 84. Most importantly, the vaccine was safe and not associated with intussusception. In fact the rates of intussusception were somewhat lower in vaccine recipients. Recent follow-up, post-licensure studies from the CDC have not disclosed rate of intussusception that is greater than expected for vaccine recipients87.
Table 1.
Comparison of the Two Licensed Rotavirus Vaccines **
| Rotateq™ | Rotarix™ | |
|---|---|---|
| Manufacturer | Merck Vaccine Division | GlaxoSmithKline |
| Genetic Backbone | Bovine Rotavirus-WC3 | Human rotavirus-89-12 |
| Composition | 5 human; bovine reassortant | Single human rotavirus |
| Genotypes | G1,2,3,4 and [P8] | G1 [P8] |
| Dosage schedule | 3 doses @ 2, 4, & 6 months of age | 2 doses @ 2 & 4 months of age |
| Administration | Oral | Oral |
| Presentation | Liquid | Lyophilized-reconstituted |
| * Protection against severe disease | 85% (72–92) | 95% (91–97)* |
| Virus shedding | 9% | 50% or more |
| Intussusception | No | No |
The molecular basis for the attenuation of the WC3-based vaccine is not presently known. In fact, the basis for host range restriction of rotaviruses in general is poorly understood. It is assumed, but not proven, that a vaccine that is attenuated on the basis of host range restriction will be genetically stable. RotaTeq is given in a 3-dose schedule and preliminary data indicate that at least 2 doses are required to generate significant levels of protection70. As expected for a vaccine based on an animal rotavirus isolate, vaccine shedding has been reported as infrequent and at a low level. The vaccine appeared to be effective in preventing severe disease caused by a variety of rotavirus serotypes, including G9 strains, although a G type component is not present in the actual vaccine. Additional evidence supporting the notion that serotype specific immunity is not solely responsible for protection is the finding (as was also seen with the Rotashield vaccine) that type-specific neutralization response rates following vaccination were much lower than the protection rates observed in clinical trials. RotaTeq was licensed in the US in 2006 and by late 2008 its effect on reported diarrheal disease in children were assessed in a nationwide study 88. The CDC estimated that vaccination was associated with a substantial delay in the annual onset of the rotavirus season and a greater than 50% decrease in rotavirus activity. This substantial reduction was more significant because it took place during a period when only a minority of the susceptible children had been given the vaccine, so it might be able to reduce transmission and provide ‘herd-immunity’ (community-based) as well as individual immunity.
A live attenuated human rotavirus vaccine was licensed in 2006 under the trade name Rotarix™. This virulent G1P49 human rotavirus strain (89–12) was passaged for multiple rounds in monkey kidney cell cultures to achieve attenuation. The initial passaged material possessed residual virulence, but following subsequent additional passages and plaque purification, carried out by GlaxoSmithKline, a highly attenuated product was attained. As with the Merck vaccine (RotaTeq), the molecular basis for the attenuation of the Rotarix vaccine is unknown, although a sequence comparison with its wild-type parent strain could identify the genes changes that are associated with attenuation. Although there has been no direct comparison between RotaTeq and the GlaxoSmithKline vaccine, this human rotavirus vaccine is apparently shed in substantially greater amounts than RotaTeq, the bovine-derived vaccine89. This would likely indicate a higher likelihood of transmission from vaccinated to unvaccinated contacts. However, better understanding of the genetic basis of its attenuation and the degree of its genetic stability following transmission would aide development of future vaccines.
The rationale underlying the development of Rotarix was that a single natural rotavirus infection, either symptomatic or asymptomatic, provides protective immunity against subsequent severe disease, irrespective of serotype47. Therefore, it seemed logical to predict that an attenuated human rotavirus strain might do the same. Because of prior safety concerns with the RRV-TV, large scale (>60,000 children) safety and efficacy trials were required for licensure. Unlike RotaTeq, these were carried out primarily, but not exclusively in countries in Central and South America. Rotarix requires only 2 doses, probably because it is better adapted to replication in the human GI tract than the bovine-based vaccine and it can be administered at a dose approximately 100-fold lower than that of RotaTeq. The large-scale safety study conducted in Latin America showed no association between Rotarix and intussusception90. Efficacy trials in Latin America and Europe showed the vaccine to be highly effective90, 91. In a subset of the large registration safety study cohort, the vaccine was 85% effective against preventing severe diarrhea and 100% effective against the most severe cases. Interestingly, despite the monovalent nature of the vaccine, it was effective (92%) against homotypic G1 strains and 88% effective against heterotypic G3, 4 and 9 strains. In this study, efficacy against G2 strains (41%) was not significant but subsequent meta-analysis studies and other efficacy studies from Europe showed substantial (81%) efficacy against G2P(4) strains. Recent 2-year efficacy data for Rotarix have shown that Rotarix does not interfere with other routine childhood vaccinations91, 92. Because different disease symptom scoring systems were used by Merck and GlaxoSmithKlein during their clinical trial programs, it is virtually impossible to directly compare the efficacies of RotaTeq and Rotarix 93, although each vaccine is highly effective. However, there are lingering suspicions that Rotarix is less effective against G2 strains and that this relative deficiency might, under some circumstances, produce problems82.
Several 3rd-generation rotavirus vaccines are in development because of possible safety issues associated with the of RotaTeq and Rotarix; because of this several groups are pursuing inactivated virus or recombinant virus-like-particle approaches. Parenteral immunization with inactive virus has proven effective in animal models but no proof of principle for this approach exists for humans94. Similarly, parenteral or intranasal immunization with recombinant nonreplicating virus-like particle vaccines have been effective in all animal models tested, and these candidate vaccines are ready for phase 1 testing in humans95. Another rationale for the development of additional vaccine candidates is cost—rotavirus vaccine will never be fully affordable in the poorest countries until vaccine manufacturers in the developing world are able to compete with large pharmaceutical companies. At least 2 3rd-generation vaccine candidates are currently being evaluated in clinical trials. A neonatal G9P(10) rotavirus isolated from a newborn nursery in New Delhi (strain 116E) is about to undergo pivotal phase III efficacy trials in India. This vaccine is produced by an Indian biotech company and was shown to be safe and highly immunogenic in initial phase I and II studies. A series of bovine reassortants that utilize the UK bovine strain as a backbone have been licensed by the NIH to several companies in China, India and Brazil86. This vaccine is being formulated as a tetravalent G1, 2, 3, 4 vaccine on the UK backbone. Earlier efficacy trials with this vaccine in Finland showed it to be non-reactogenic, highly immunogenic and highly efficacious. If either of these vaccine candidatess proves to be safe and effective, they could be produced at substantial savings by the manufactures in developing nations for widespread use.
There are a number of important basic and practical issues to be resolved concerning rotavirus vaccine development but perhaps the single most important one is to determine whether RotaTeq and/or Rotarix are effective in very poor counties in Asia or Africa. Other vaccines, especially orally administered vaccines, have been found to have greatly diminished efficacy in certain very poor regions of India and Africa. Currently, under the auspice of the Seattle-based nonprofit organization PATH (formerly called Program for Appropriate Technology in Health) and the support of the Gates foundation, the efficacy of Rotarix and RotaTeq is being studied in parts of Africa and or Asia. The results of these clinical trials are greatly anticipated. Another important issue is to determine whether the restricted timing of administration of the first dose of these vaccines will limit their usefulness in any country. Some children in the US are not benefitting from rotavirus vaccination because the first dose needs to be administered by a maximum of 2 months of age; it not clear if these timing restrictions are suitable for developing countries. Vaccine safety in children with immunodeficiencies also needs to monitored; cases of chronic infection occurred in babies with severe combined immunodeficiency (SCID) that received the vaccine before they were diagnosed with this disorder (M Estes-personal communication). This outcome is not unexpected as it has occurred with other live attenuated vaccines in SCID babies, but we need to establish ways to manage and prevent these situations.
Conclusions
Rotavirus diarrhea is considered to be a vaccine-preventable disease, based on recent successful outcomes in children in developed countries. Almost 50 years of basic and clinical research on rotavirus have culminated in this breakthrough and have also led to new knowledge about these fascinating pathogens and how they interact with the GI tract. Despite this impressive progress, much remains to be learned about rotavirus infection. We still do not know the mechanism by which current vaccines induce immunity to the broad array of serotypes encountered in nature. The molecular basis for host range restriction or cell tropism, which generally limits rotavirus infection to a single species and cell type, is unknown. Although we have learned much about the possible mechanisms by which rotavirus causes diarrhea, the exact contribution of each of these to the actual disease state remains unclear. Many of these questions can only be adequately addressed by the development of a tractable reverse genetics system, which has yet to occur. Given the rapid progress that has been made since the discovery of human rotavirus in 1973, it seems likely that in the next decade, many of these questions will be answered.
Acknowledgments
This work was supported in part by Public Health Service grants RO1 AI 021362 (HG), P30 DK 56339 (HG), RO1 DK 30144 (MKE), P30 DK56338 (MKE) from the NIH and a VA Merit Review Research Award (HG).
Biographies


Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Harry B. Greenberg, Email: harry.greenberg@stanford.edu, Senior Associate Dean for Research, Joseph D. Grant Professor of Medicine and Microbiology & Immunology, Stanford University School of Medicine, Alway Bldg, Rm M-121 | 300 Pasteur Dr, Stanford, CA 94305-5119, phone: 650-725-9722, fax: 650-725-7368
Mary K. Estes, Email: mestes@bcm.edu, Cullen Endowed Chair of Molecular and Human Virology, Departments of Molecular Virology and Microbiology and Medicine -GI, Baylor College of Medicine, One Baylor Plaza BCM-385, Houston, TX 77030-3498, 713-798-3585, 713-798-3586 fax
References
- 1.Estes MK. Rotaviruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1747–1785. [Google Scholar]
- 2.Brunet JP, Cotte-Laffitte J, Linxe C, et al. Rotavirus infection induces an increase in intracellular calcium concentration in human intestinal epithelial cells: role in microvillar actin alteration. J Virol. 2000;74:2323–32. doi: 10.1128/jvi.74.5.2323-2332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz MC, Cohen J, Michelangeli F. Role of Ca2+in the replication and pathogenesis of rotavirus and other viral infections. Cell Calcium. 2000;28:137–49. doi: 10.1054/ceca.2000.0142. [DOI] [PubMed] [Google Scholar]
- 4.Sen A, Sen N, Mackow ER. The formation of viroplasm-like structures by the rotavirus NSP5 protein is calcium regulated and directed by a C-terminal helical domain. J Virol. 2007;81:11758–67. doi: 10.1128/JVI.01124-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poruchynsky MS, Maass DR, Atkinson PH. Calcium depletion blocks the maturation of rotavirus by altering the oligomerization of virus-encoded proteins in the ER. J Cell Biol. 1991;114:651–6. doi: 10.1083/jcb.114.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michelangeli F, Liprandi F, Chemello ME, et al. Selective depletion of stored calcium by thapsigargin blocks rotavirus maturation but not the cytopathic effect. J Virol. 1995;69:3838–47. doi: 10.1128/jvi.69.6.3838-3847.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkova Z, Morris AP, Estes MK. Cytoplasmic calcium measurement in rotavirus enterotoxin-enhanced green fluorescent protein (NSP4-EGFP) expressing cells loaded with Fura-2. Cell Calcium. 2003;34:55–68. doi: 10.1016/s0143-4160(03)00022-8. [DOI] [PubMed] [Google Scholar]
- 8.Tian P, Hu Y, Schilling WP, et al. The nonstructural glycoprotein of rotavirus affects intracellular calcium levels. J Virol. 1994;68:251–7. doi: 10.1128/jvi.68.1.251-257.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian P, Estes MK, Hu Y, et al. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J Virol. 1995;69:5763–72. doi: 10.1128/jvi.69.9.5763-5772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris AP, Scott JK, Ball JM, et al. NSP4 elicits age-dependent diarrhea and Ca(2+)mediated I(−) influx into intestinal crypts of CF mice. Am J Physiol. 1999;277:G431–44. doi: 10.1152/ajpgi.1999.277.2.G431. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz MC, Diaz Y, Pena F, et al. Ca2+ permeability of the plasma membrane induced by rotavirus infection in cultured cells is inhibited by tunicamycin and brefeldin A. Virology. 2005;333:54–65. doi: 10.1016/j.virol.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Adams WR, Kraft LM. Epizootic diarrhea of infant mice: indentification of the etiologic agent. Science. 1963;141:359–60. doi: 10.1126/science.141.3578.359. [DOI] [PubMed] [Google Scholar]
- 13.Bishop RF, Davidson GP, Holmes IH, et al. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973;2:1281–3. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- 14.Malherbe H, Harwin R. The cytopathic effects of vervet monkey viruses. S Afr Med J. 1963;37:407–11. [PubMed] [Google Scholar]
- 15.Snyder JD, Merson MH. The magnitude of the global problem of acute diarrhoeal disease: a review of active surveillance data. Bull World Health Organ. 1982;60:605–13. [PMC free article] [PubMed] [Google Scholar]
- 16.Intussusception among recipients of rotavirus vaccine--United States, 1998–1999. MMWR Morb Mortal Wkly Rep. 1999;48:577–81. [PubMed] [Google Scholar]
- 17.Albano F, Bruzzese E, Bella A, et al. Rotavirus and not age determines gastroenteritis severity in children: a hospital-based study. Eur J Pediatr. 2007;166:241–7. doi: 10.1007/s00431-006-0237-6. [DOI] [PubMed] [Google Scholar]
- 18.Nelson EA, Bresee JS, Parashar UD, et al. Rotavirus epidemiology: the Asian Rotavirus Surveillance Network. Vaccine. 2008;26:3192–6. doi: 10.1016/j.vaccine.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 19.Rotavirus surveillance--worldwide, 2001–2008. MMWR Morb Mortal Wkly Rep. 2008;57:1255–7. [PubMed] [Google Scholar]
- 20.Parashar UD, Gibson CJ, Bresse JS, et al. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–6. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka G, Faruque AS, Luby SP, et al. Deaths from rotavirus disease in Bangladeshi children: estimates from hospital-based surveillance. Pediatr Infect Dis J. 2007;26:1014–8. doi: 10.1097/INF.0b013e318125721c. [DOI] [PubMed] [Google Scholar]
- 22.The paediatric burden of rotavirus disease in Europe. Epidemiol Infect. 2006;134:908–16. doi: 10.1017/S0950268806006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soriano-Gabarro M, Mrukowicz J, Vesikari T, et al. Burden of rotavirus disease in European Union countries. Pediatr Infect Dis J. 2006;25:S7–S11. doi: 10.1097/01.inf.0000197622.98559.01. [DOI] [PubMed] [Google Scholar]
- 24.Van Damme P, Giaquinto C, Huet F, et al. Multicenter prospective study of the burden of rotavirus acute gastroenteritis in Europe, 2004–2005: the REVEAL study. J Infect Dis. 2007;195 (Suppl 1):S4–S16. doi: 10.1086/516714. [DOI] [PubMed] [Google Scholar]
- 25.Payne DC, Staat MA, Edwards KM, et al. Active, population-based surveillance for severe rotavirus gastroenteritis in children in the United States. Pediatrics. 2008;122:1235–43. doi: 10.1542/peds.2007-3378. [DOI] [PubMed] [Google Scholar]
- 26.Fischer TK, Viboud C, Parashar U, et al. Hospitalizations and deaths from diarrhea and rotavirus among children <5 years of age in the United States, 1993–2003. J Infect Dis. 2007;195:1117–25. doi: 10.1086/512863. [DOI] [PubMed] [Google Scholar]
- 27.Parashar UD, Burton A, Lanata C, et al. World Health Organization estimates of the global mortality from rotavirus in children in the year 2004. Journal of Infectious Diseases. 2004 In press. [Google Scholar]
- 28.Chandran A, Heinzen RR, Santosham M, et al. Nosocomial rotavirus infections: a systematic review. J Pediatr. 2006;149:441–7. doi: 10.1016/j.jpeds.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 29.Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol. 2005;15:29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- 30.Tsugawa T, Hoshino Y. Whole genome sequence and phylogenetic analyses reveal human rotavirus G3P[3] strains Ro1845 and HCR3A are examples of direct virion transmission of canine/feline rotaviruses to humans. Virology. 2008;380:344–53. doi: 10.1016/j.virol.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthijnssens J, Rahman M, Van Ranst M. Two out of the 11 genes of an unusual human G6P[6] rotavirus isolate are of bovine origin. J Gen Virol. 2008;89:2630–5. doi: 10.1099/vir.0.2008/003780-0. [DOI] [PubMed] [Google Scholar]
- 32.Glass RI, Bresee J, Jiang B, et al. Gastroenteritis viruses: an overview. Novartis Found Symp. 2001;238:5–19. doi: 10.1002/0470846534.ch2. discussion 19–25. [DOI] [PubMed] [Google Scholar]
- 33.Graham DY, Dufour GR, Estes MK. Minimal infective dose of rotavirus. Arch Virol. 1987;92:261–71. doi: 10.1007/BF01317483. [DOI] [PubMed] [Google Scholar]
- 34.Dennehy PH. Transmission of rotavirus and other enteric pathogens in the home. Pediatr Infect Dis J. 2000;19:S103–5. doi: 10.1097/00006454-200010001-00003. [DOI] [PubMed] [Google Scholar]
- 35.Turcios RM, Curns AT, Holman RC, et al. Temporal and geographic trends of rotavirus activity in the United States, 1997–2004. Pediatr Infect Dis J. 2006;25:451–4. doi: 10.1097/01.inf.0000214987.67522.78. [DOI] [PubMed] [Google Scholar]
- 36.D’Souza RM, Hall G, Becker NG. Climatic factors associated with hospitalizations for rotavirus diarrhoea in children under 5 years of age. Epidemiol Infect. 2008;136:56–64. doi: 10.1017/S0950268807008229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook SM, Glass RI, LeBaron CW, et al. Global seasonality of rotavirus infections. Bull World Health Organ. 1990;68:171–7. [PMC free article] [PubMed] [Google Scholar]
- 38.Levy K, Hubbard AE, Eisenberg JN. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol. 2008 doi: 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray PG, Kelkar SD, Walimbe AM, et al. Rotavirus immunoglobulin levels among Indian mothers of two socio-economic groups and occurrence of rotavirus infections among their infants up to six months. J Med Virol. 2007;79:341–9. doi: 10.1002/jmv.20804. [DOI] [PubMed] [Google Scholar]
- 40.Cukor G, Blacklow NR, Braverman LE. Antibodies to gastroenteritis viruses in cystic fibrosis patients. J Med Virol. 1982;9:161–4. doi: 10.1002/jmv.1890090302. [DOI] [PubMed] [Google Scholar]
- 41.Kraft RO, Fry WJ. Operative technic of selective gastric vagotomy. Am J Surg. 1963;105:423–35. doi: 10.1016/0002-9610(63)90357-x. [DOI] [PubMed] [Google Scholar]
- 42.Azevedo MS, Yuan L, Jeong KI, et al. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J Virol. 2005;79:5428–36. doi: 10.1128/JVI.79.9.5428-5436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blutt SE, Conner ME. Rotavirus: to the gut and beyond! Curr Opin Gastroenterol. 2007;23:39–43. doi: 10.1097/MOG.0b013e328011829d. [DOI] [PubMed] [Google Scholar]
- 44.Blutt SE, Kirkwood CD, Parreno V, et al. Rotavirus antigenaemia and viraemia: a common event? Lancet. 2003;362:1445–9. doi: 10.1016/S0140-6736(03)14687-9. [DOI] [PubMed] [Google Scholar]
- 45.Crawford SE, Patel DG, Cheng E, et al. Rotavirus viremia and extraintestinal viral infection in the neonatal rat model. J Virol. 2006;80:4820–32. doi: 10.1128/JVI.80.10.4820-4832.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramig RF. Systemic rotavirus infection. Expert Rev Anti Infect Ther. 2007;5:591–612. doi: 10.1586/14787210.5.4.591. [DOI] [PubMed] [Google Scholar]
- 47.Velazquez FR, Matson DO, Calva JJ, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–8. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 48.Yuan L, Saif LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunopathol. 2002;87:147–60. doi: 10.1016/S0165-2427(02)00046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Angel J, Franco MA, Greenberg HB. Rotavirus vaccines: recent developments and future considerations. Nat Rev Microbiol. 2007;5:529–39. doi: 10.1038/nrmicro1692. [DOI] [PubMed] [Google Scholar]
- 50.Corthesy B, Benureau Y, Perrier C, et al. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J Virol. 2006;80:10692–9. doi: 10.1128/JVI.00927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ball JM, Tian P, Zeng CQ, et al. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–4. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 52.Choi NW, Estes MK, Langridge WH. Oral immunization with a shiga toxin B subunit: rotavirus NSP4(90) fusion protein protects mice against gastroenteritis. Vaccine. 2005;23:5168–76. doi: 10.1016/j.vaccine.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 53.Franco MA, Greenberg HB. Challenges for rotavirus vaccines. Virology. 2001;281:153–5. doi: 10.1006/viro.2001.0830. [DOI] [PubMed] [Google Scholar]
- 54.McNeal MM, Stone SC, Basu M, et al. IFN-gamma is the only anti-rotavirus cytokine found after in vitro stimulation of memory CD4+ T cells from mice immunized with a chimeric VP6 protein. Viral Immunol. 2007;20:571–84. doi: 10.1089/vim.2007.0055. [DOI] [PubMed] [Google Scholar]
- 55.Kim B, Feng N, Narvaez CF, et al. The influence of CD4+ CD25+ Foxp3+ regulatory T cells on the immune response to rotavirus infection. Vaccine. 2008;26:5601–11. doi: 10.1016/j.vaccine.2008.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuklin NA, Rott L, Feng N, et al. Protective intestinal anti-rotavirus B cell immunity is dependent on alpha 4 beta 7 integrin expression but does not require IgA antibody production. J Immunol. 2001;166:1894–902. doi: 10.4049/jimmunol.166.3.1894. [DOI] [PubMed] [Google Scholar]
- 57.Zhang W, Azevedo MS, Wen K, et al. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008;26:3655–61. doi: 10.1016/j.vaccine.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Dennehy PH, Keyserling HL, et al. Rotavirus infection alters peripheral T-cell homeostasis in children with acute diarrhea. J Virol. 2007;81:3904–12. doi: 10.1128/JVI.01887-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lecce JG, Cummins JM, Richards AB. Treatment of rotavirus infection in neonate and weanling pigs using natural human interferon alpha. Mol Biother. 1990;2:211–6. [PubMed] [Google Scholar]
- 60.Schwers A, Vanden Broecke C, Maenhoudt M, et al. Experimental rotavirus diarrhoea in colostrum-deprived newborn calves: assay of treatment by administration of bacterially produced human interferon (Hu-IFN alpha 2) Ann Rech Vet. 1985;16:213–8. [PubMed] [Google Scholar]
- 61.Vancott JL, McNeal MM, Choi AH, et al. The role of interferons in rotavirus infections and protection. J Interferon Cytokine Res. 2003;23:163–70. doi: 10.1089/107999003321532501. [DOI] [PubMed] [Google Scholar]
- 62.Graff JW, Mitzel DN, Weisend CM, et al. Interferon regulatory factor 3 is a cellular partner of rotavirus NSP1. J Virol. 2002;76:9545–50. doi: 10.1128/JVI.76.18.9545-9550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barro M, Patton JT. Rotavirus NSP1 inhibits expression of type I interferon by antagonizing the function of interferon regulatory factors IRF3, IRF5, and IRF7. J Virol. 2007;81:4473–81. doi: 10.1128/JVI.02498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Graff JW, Ettayebi K, Hardy ME. Rotavirus NSP1 inhibits NFkB activation by inducing proteasome-dependent degradation of Beta-TrCP: a novel mechanism of IFN antagonism. PLoS Pathogens. 2009;5:1–12. doi: 10.1371/journal.ppat.1000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feng N, Kim B, Fenaux M, et al. Role of interferon in homologous and heterologous rotavirus infection in the intestines and extraintestinal organs of suckling mice. J Virol. 2008;82:7578–90. doi: 10.1128/JVI.00391-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruuska T, Vesikari T, Delem A, et al. Evaluation of RIT 4237 bovine rotavirus vaccine in newborn infants: correlation of vaccine efficacy to season of birth in relation to rotavirus epidemic period. Scand J Infect Dis. 1990;22:269–78. doi: 10.3109/00365549009027047. [DOI] [PubMed] [Google Scholar]
- 67.Salinas B, Perez Schael I, Linhares AC, et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: A randomized, placebo-controlled trial in Latin American infants. Pediatr Infect Dis J. 2005;24:807–16. doi: 10.1097/01.inf.0000178294.13954.a1. [DOI] [PubMed] [Google Scholar]
- 68.Vesikari T, Isolauri E, D’Hondt E, et al. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984;1:977–81. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- 69.Vesikari T, Karvonen A, Korhonen T, et al. Safety and immunogenicity of RIX4414 live attenuated human rotavirus vaccine in adults, toddlers and previously uninfected infants. Vaccine. 2004;22:2836–42. doi: 10.1016/j.vaccine.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 70.Vesikari T. Rotavirus vaccines. Scand J Infect Dis. 2008;40:691–5. doi: 10.1080/00365540802040570. [DOI] [PubMed] [Google Scholar]
- 71.Kapikian AZ, Hoshino Y, Chanock RM, et al. Jennerian and modified Jennerian approach to vaccination against rotavirus diarrhea using a quadrivalent rhesus rotavirus (RRV) and human-RRV reassortant vaccine. Arch Virol Suppl. 1996;12:163–75. doi: 10.1007/978-3-7091-6553-9_18. [DOI] [PubMed] [Google Scholar]
- 72.Joensuu J, Koskenniemi E, Pang XL, et al. Randomised placebo-controlled trial of rhesus-human reassortant rotavirus vaccine for prevention of severe rotavirus gastroenteritis. Lancet. 1997;350:1205–9. doi: 10.1016/S0140-6736(97)05118-0. [DOI] [PubMed] [Google Scholar]
- 73.Perez-Schael I, Guntinas MJ, Perez M, et al. Efficacy of the rhesus rotavirus-based quadrivalent vaccine in infants and young children in Venezuela. N Engl J Med. 1997;337:1181–7. doi: 10.1056/NEJM199710233371701. [DOI] [PubMed] [Google Scholar]
- 74.Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370:1757–63. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- 75.Bines J. Intussusception and rotavirus vaccines. Vaccine. 2006;24:3772–6. doi: 10.1016/j.vaccine.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 76.Peter G, Myers MG. Intussusception, rotavirus, and oral vaccines: summary of a workshop. Pediatrics. 2002;110:e67. doi: 10.1542/peds.110.6.e67. [DOI] [PubMed] [Google Scholar]
- 77.Simonsen L, Viboud C, Elixhauser A, et al. More on RotaShield and intussusception: the role of age at the time of vaccination. J Infect Dis. 2005;192 (Suppl 1):S36–43. doi: 10.1086/431512. [DOI] [PubMed] [Google Scholar]
- 78.Dennehy PH. Rotavirus vaccines: an overview. Clin Microbiol Rev. 2008;21:198–208. doi: 10.1128/CMR.00029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Glass RI, Bresee J, Jiang B, et al. Rotavirus and rotavirus vaccines. Adv Exp Med Biol. 2006;582:45–54. doi: 10.1007/0-387-33026-7_5. [DOI] [PubMed] [Google Scholar]
- 80.Glass RI, Parashar UD, Bresee JS, et al. Rotavirus vaccines: current prospects and future challenges. Lancet. 2006;368:323–32. doi: 10.1016/S0140-6736(06)68815-6. [DOI] [PubMed] [Google Scholar]
- 81.Grimwood K, Kirkwood CD. Human rotavirus vaccines: too early for the strain to tell. Lancet. 2008;371:1144–5. doi: 10.1016/S0140-6736(08)60501-2. [DOI] [PubMed] [Google Scholar]
- 82.Nakagomi O, Cunliffe NA. Rotavirus vaccines: entering a new stage of deployment. Curr Opin Infect Dis. 2007;20:501–7. doi: 10.1097/QCO.0b013e3282a56ba0. [DOI] [PubMed] [Google Scholar]
- 83.Parashar UD, Alexander JP, Glass RI. Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–13. [PubMed] [Google Scholar]
- 84.Heaton PM, Ciarlet M. Vaccines: the pentavalent rotavirus vaccine: discovery to licensure and beyond. Clin Infect Dis. 2007;45:1618–24. doi: 10.1086/522997. [DOI] [PubMed] [Google Scholar]
- 85.Vesikari T, Karvonen AV, Majuri J, et al. Safety, efficacy, and immunogenicity of 2 doses of bovine-human (UK) and rhesus-rhesus-human rotavirus reassortant tetravalent vaccines in Finnish children. J Infect Dis. 2006;194:370–6. doi: 10.1086/505151. [DOI] [PubMed] [Google Scholar]
- 86.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 87.Gentsch J, Wallace G, Bartlett D. Postmarketing monitoring of intussusception after RotaTeq vaccination--United States, February 1, 2006–February 15, 2007. MMWR Morb Mortal Wkly Rep. 2007;56:218–22. [PubMed] [Google Scholar]
- 88.Delayed onset and diminished magnitude of rotavirus activity--United States, November 2007–May 2008. MMWR Morb Mortal Wkly Rep. 2008;57:697–700. [PubMed] [Google Scholar]
- 89.Anderson EJ. Rotavirus vaccines: viral shedding and risk of transmission. Lancet Infect Dis. 2008;8:642–9. doi: 10.1016/S1473-3099(08)70231-7. [DOI] [PubMed] [Google Scholar]
- 90.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 91.Linhares AC, Velazquez FR, Perez-Schael I, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–9. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 92.Rodriguez ZM, Goveia MG, Stek JE, et al. Concomitant use of an oral live pentavalent human-bovine reassortant rotavirus vaccine with licensed parenteral pediatric vaccines in the United States. Pediatr Infect Dis J. 2007;26:221–7. doi: 10.1097/01.inf.0000254391.71103.e8. [DOI] [PubMed] [Google Scholar]
- 93.Givon-Lavi N, Greenberg D, Dagan R. Comparison between two severity scoring scales commonly used in the evaluation of rotavirus gastroenteritis in children. Vaccine. 2008;26:5798–801. doi: 10.1016/j.vaccine.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 94.Jiang B, Gentsch JR, Glass RI. Inactivated rotavirus vaccines: A priority for accelerated vaccine development. Vaccine. 2008;26:6754–8. doi: 10.1016/j.vaccine.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 95.Jansen K, Conner ME, Estes MK. Virus-like particles (VLP) as vaccines and vaccine delivery systems. Marcel Dekker, Inc; 2009. [Google Scholar]
- 96.Ciarlet M, Conner ME, Finegold MJ, et al. Group A rotavirus infection and age-dependent diarrheal disease in rats: a new animal model to study the pathophysiology of rotavirus infection. J Virol. 2002;76:41–57. doi: 10.1128/JVI.76.1.41-57.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramig RF. Pathogenesis of intestinal and systemic rotavirus infection. J Virol. 2004;78:10213–20. doi: 10.1128/JVI.78.19.10213-10220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]