Abstract
Methacrylated hyaluronic acid (HA) hydrogels provide a backbone polymer with which mesenchymal stem cells (MSCs) can interact through several cell surface receptors that are expressed by MSCs, including CD44 and CD168. Previous studies showed that this 3D hydrogel environment supports the chondrogenesis of MSCs, and here we demonstrate through functional blockade that these specific cell–material interactions play a role in this process. Beyond matrix interactions, cadherin molecules, a family of transmembrane glycoproteins, play a critical role in tissue development during embryogenesis, and N-cadherin is a key factor in mediating cell–cell interactions during mesenchymal condensation and chondrogenesis. In this study, we functionalized HA hydrogels with N-cadherin mimetic peptides and evaluated their role in regulating chondrogenesis and cartilage matrix deposition by encapsulated MSCs. Our results show that conjugation of cadherin peptides onto HA hydrogels promotes both early chondrogenesis of MSCs and cartilage-specific matrix production with culture, compared with unmodified controls or those with inclusion of a scrambled peptide domain. This enhanced chondrogenesis was abolished via treatment with N-cadherin–specific antibodies, confirming the contribution of these N-cadherin peptides to chondrogenesis. Subcutaneous implantation of MSC-seeded constructs also showed superior neocartilage formation in implants functionalized with N-cadherin mimetic peptides compared with controls. This study demonstrates the inherent biologic activity of HA-based hydrogels, as well as the promise of biofunctionalizing HA hydrogels to emulate the complexity of the natural cell microenvironment during embryogenesis, particularly in stem cell-based cartilage regeneration.
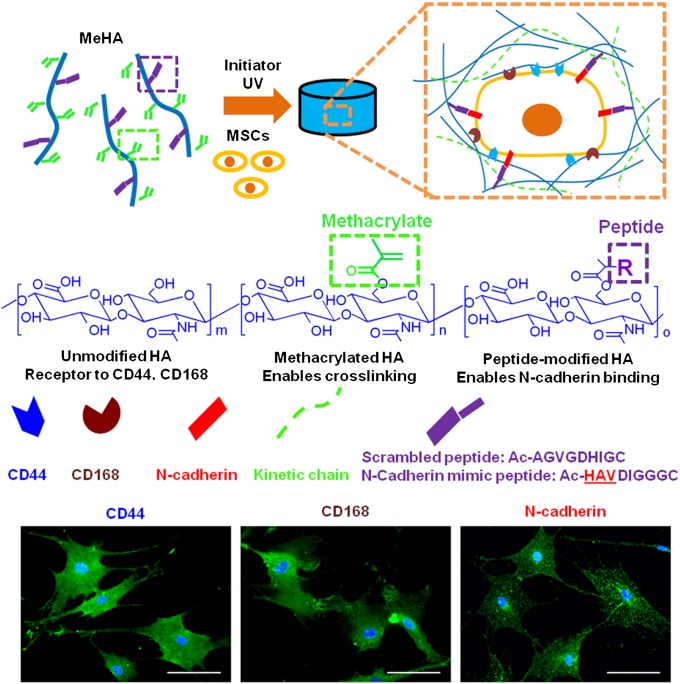
Mesenchymal stem cells (MSCs) have emerged as a clinically relevant cell source for regenerative medicine due to their potential to differentiate into several mesenchymal lineages including cartilage, bone, and fat (1, 2). The multipotent differentiation of MSCs is tightly regulated by both soluble and physical cues present in the pericellular microenvironment, including cell–cell and cell–matrix interactions, cues that can be engineered into a variety of natural and synthetic biomaterial scaffolds (3). These materials may be either permissive to chondrogenesis (inert materials including agarose and PEG) or inductive to chondrogenesis by mimicking components of the natural pericellular microenvironment (4, 5). For example, photopolymerizable hydrogels composed of methacrylated (Me) hyaluronic acid (HA) may provide biological cues such as CD44 and CD168 interactions based on the role of HA in cellular signaling (6–8) (Fig. 1). Coincident with the onset of condensation and the first appearance of cartilage in the embryo is the appearance of specific binding sites for HA on bud limb mesenchymal cells (9). Large HA molecules are involved in the aggregation of these cells during condensation via multivalent cross-bridging (10), and HA has already been shown to enhance chondrogenesis in hydrogels compared with inert hydrogels (11).
Fig. 1.
Hydrogel design to harness cell–matrix and cell–cell interactions. hMSCs were photoencapsulated within hydrogels that present epitopes for interaction with CD44 and CD168 receptors, as well as N-cadherin. Macromers were designed from hyaluronic acid (to bind to receptors), modified with methacrylates for photo cross-linking, and modified with peptides that either mediate N-cadherin binding or act as scrambled sequence controls. Fluorescent images of hMSCs immunostained for CD44, CD168, or N-cadherin (green) surface receptors and nuclei (blue). (Scale bars, 100 μm.)
Biomaterials are evolving to increase their complexity for a variety of applications, yet the field is still limited in many aspects of cellular signaling. As one example, many hydrogels inherently limit the direct cell–cell interactions that are essential for early mesenchymal condensation events, including rapid proliferation of cells within the developing limb bud. The temporal expression patterns of matrix molecules (e.g., fibronectin fragments) and those that mediate cell–cell adhesion (e.g., N-cadherin) regulate this process. Many studies have attempted to improve the biological functionality of biomaterial scaffolds by tethering ECM molecules, growth factors, or other bioactive groups (5, 12–15). A recent study showed that modification of PEG hydrogels with fusion proteins that support cellular communication promotes the viability and functionality of encapsulated pancreatic cells (16). However, little attention has been focused on mimicking early cell–cell interactions in hydrogels for stem cell-based cartilage regeneration.
N-cadherin is widely considered to be the key factor in directing cell–cell interactions during mesenchymal condensation, a process mediated by surface contacts that results in aggregation of progenitor cells (17–20). Studies have shown that the expression of the deletion mutant form of N-cadherin, which lacks either the extracellular homotypic interaction domains or the intracellular β-catenin binding site, results in decreased cellular condensation and impaired chondrogenesis (21, 22). These findings suggest that both extracellular homotypic interaction and intracellular interaction with the catenin complex are essential for proper N-cadherin signaling (23). The evolutionarily conserved His-Ala-Val (HAV) motif in the first extracellular domain (ECD1) of N-cadherin is critical to the homotypic protein interaction that mediates cell–cell adhesion (24). This sequence is present at the interface of the ECD1 adhesion dimer crystal, where side chains from this sequence account for 14% of the adhesion interface (25). Optimized synthetic peptides containing the HAV domain have been shown to possess N-cadherin–like binding activity (26).
With this in mind, the design of a hydrogel that incorporates cues of both matrix and cell–cell interactions may enhance the chondrogenesis of MSCs. The incorporation of these developmentally relevant cues is possible in HA hydrogels, where provision for receptor interactions and inclusion of peptides that mimic the extracellular domain of N-cadherin can be engineered into the synthetic microenvironment. Here, these interactions are investigated and blocked to understand their role in human MSC (hMSC) chondrogenesis and in neocartilage production, including both in vitro and in vivo studies.
Results
HA macromers were modified with methacrylates (29%) to enable cross-linking via radical polymerization of methacrylates to form MeHA hydrogels (1.5%, wt/vol) (Fig. 1). Unmodified portions of the macromers provided a base to investigate the contribution of cellular signaling of hMSCs via CD44 and CD168 surface receptors to chondrogenesis. To investigate the effect of cell–cell signaling on chondrogenesis, methacrylates were used to covalently bind cadherin mimetic (Cadherin) or scrambled (Scrambled) peptides through addition reaction. For in vitro studies, hMSCs were encapsulated in MeHA hydrogels and cultured in differentiation media, containing TGF-β3 added directly to the media for in vitro studies or TGF-β3 delivery with microspheres for in vivo studies (Fig. S1). Immunostaining of the hMSCs cultured on tissue culture plastic revealed the expression of CD44, CD168, and N-cadherin surface receptors by the majority of the starting hMSC population used in this study (CD44, ∼99%; CD168, ∼95%; and N-cadherin, ∼99%; Fig. 1 and Fig. S2). Although expression decreased to almost half of the initial levels for calcium binding receptors CD168 and N-cadherin during the cell detachment process, the expression was back to initial levels with 4 h of culture (Fig. S2).
Interactions Between hMSCs and HA via CD44 and CD168 Enhance Chondrogenesis and Neocartilage Formation.
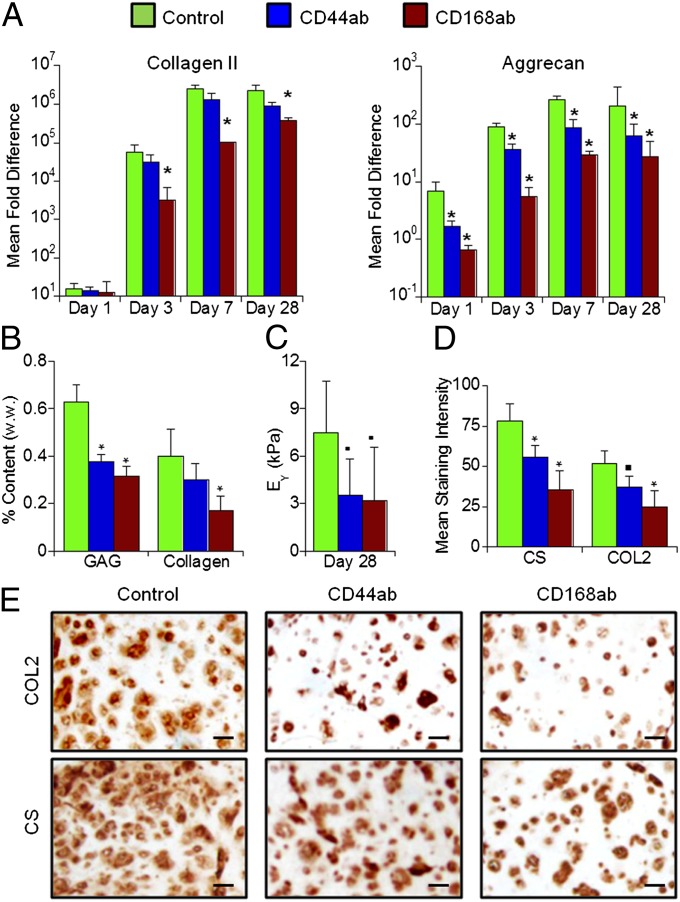
In differentiation media, but not in the growth media, hMSCs underwent chondrogenesis in MeHA hydrogels (Fig. 2 and Fig. S3). However, treatment of hMSCs before encapsulation with an antibody to CD168 (CD168ab) significantly reduced the expression of chondrogenic markers including type II collagen (COL2), aggrecan (AGG), and sex determining region Y-box 9 (Sox 9) through day 28 of the culture compared with the untreated control (Fig. 2A and Fig. S4). Treatment with CD44 antibody (CD44ab) significantly lowered the expression of AGG. Both antibody-treated construct groups developed lower glycosaminoglycan (GAG), collagen content, and equilibrium modulus compared with the control constructs after 28 d of culture (Fig. 2 B and C). Immunohistochemical staining also showed more intense and distributed chondroitin sulfate (CS) and COL2 staining in the control constructs compared with the antibody treatment groups such that the mean staining intensity (CS and COL2, respectively) was significantly higher for control (∼78 and 52) compared with CD44ab (∼56 and 37) and CD168ab (∼36 and 25) (Fig. 2 D and E). Finally, no differences in cell survival for encapsulated hMSCs were observed when cells were treated with CD44 or CD168, compared with untreated cells (Fig. S5). Little staining was observed for COL1 for all groups (Fig S6).
Fig. 2.
hMSC interactions with CD44 and CD168 influence chondrogenesis. (A) Mean fold change in gene expression (normalized to GAPDH and monolayer cells before encapsulation) of selected chondrogenic markers (COL2 and AGG) of MSCs cultured in HA hydrogels (1.5% MeHA, wt/vol) after 1, 3, 7, or 28 d of in vitro culture in differentiation media with no treatment (control) or after treatment with CD44 (CD44ab) or CD168 (CD168ab) antibodies before encapsulation. (B) GAG and total collagen content normalized by sample wet weight, and (C) equilibrium modulus (EY). (D and E) Quantification and images of immunohistochemical staining for CS and COL2 of MSC-laden HA hydrogel constructs after 28 d of in vitro culture. *P < 0.05 vs. control group at the same culture time (n = 4); ▪P < 0.1 vs. control group (n = 5). (Scale bars, 50 µm.)
Cadherin Mimetic Peptides Enhance Early Chondrogenesis.
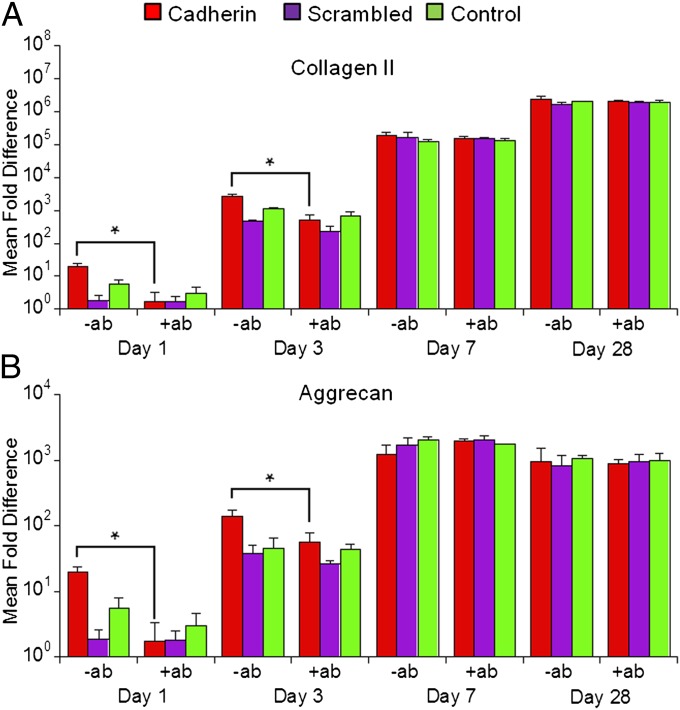
MeHA macromers were functionalized with Cadherin or Scrambled peptides (10 mol% of the methacrylates), and a control group with no peptide conjugation was used for comparison. Peptide binding efficiency was quantified to be 88 ± 11% and 85 ± 6% of the theoretical amount for Cadherin and Scrambled groups, respectively (Fig S7). The expression of chondrogenic markers including COL2, AGG, and Sox 9 on days 1 and 3 of the culture for the Cadherin group were significantly higher than for the Scrambled and control groups. No significant differences were observed between the Scrambled and control groups. Specifically, the expression of COL2, AGG, and Sox 9 in Cadherin hydrogels increased by 6.2-, 3.3-, and 6.5-fold on day 3, respectively, compared with the Scrambled group. By day 7, the chondrogenic effect of N-cadherin peptides had diminished, such that no significant differences in chondrogenic gene expression were found among the groups. Mechanical testing showed that modification of acellular hydrogels with peptides (at 10% level) resulted in no change in the baseline Young’s modulus compared with unmodified hydrogels.
To verify that the enhanced chondrogenesis was indeed attributable to cadherin peptides, hMSCs were treated with a monoclonal N-cadherin antibody (+ab) before encapsulation. The antibody treatment abrogated the chondrogenic effect of N-cadherin peptides and reduced the expression of chondrogenic genes to levels similar to those of the control group (Fig. 3 and Fig. S3). For example, antibody treatment reduced the expression of COL2 and AGG in the Cadherin group by ∼91% and ∼79%, respectively, on day 1. In contrast, the antibody treatment had no significant impact on gene expression in the Scrambled and control groups. Immunohistochemical staining against β-catenin revealed expression of β-catenin by cells encapsulated in the cadherin peptide modified hydrogels (Cadherin) on day 7 of the culture (Fig. S8). In contrast, very little staining was observed in the Scrambled and control hydrogels. Furthermore, N-cadherin antibody treatment significantly reduced β-catenin staining in the Cadherin modified hydrogels (Fig. S8).
Fig. 3.
HA-based presentation of N-cadherin mimetic peptides enhances early hMSC chondrogenesis. Mean fold change in gene expression (normalized to GAPDH and monolayer cells before encapsulation) of selected chondrogenic markers (A) COL2 and (B) AGG of MSCs cultured in HA hydrogels (1.5% MeHA wt/vol, 10% methacrylate consumption with Cadherin or Scrambled peptides) after 1, 3, 7, or 28 d of in vitro culture in differentiation media either untreated (−ab) or with treatment with the N-cadherin antibody before encapsulation (+ab). *P < 0.05 vs. +ab group of the same scaffold at the same culture time (n = 4).
Cadherin Mimetic Peptides Promote in Vitro Neocartilage Formation over Long-Term Culture.
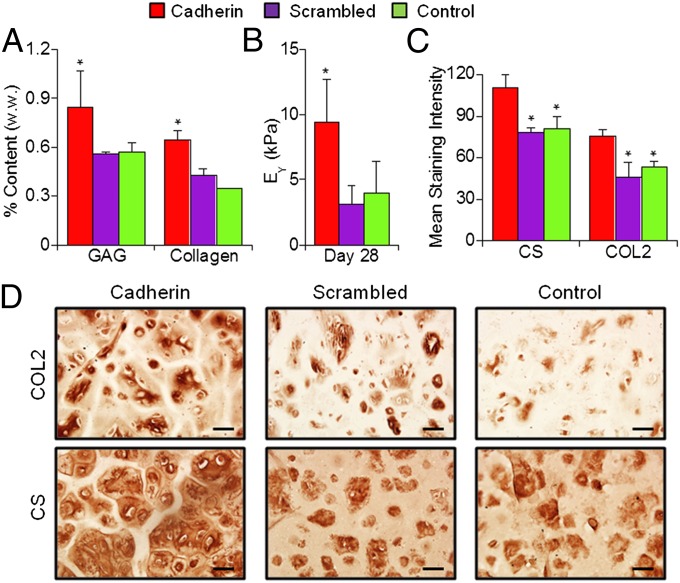
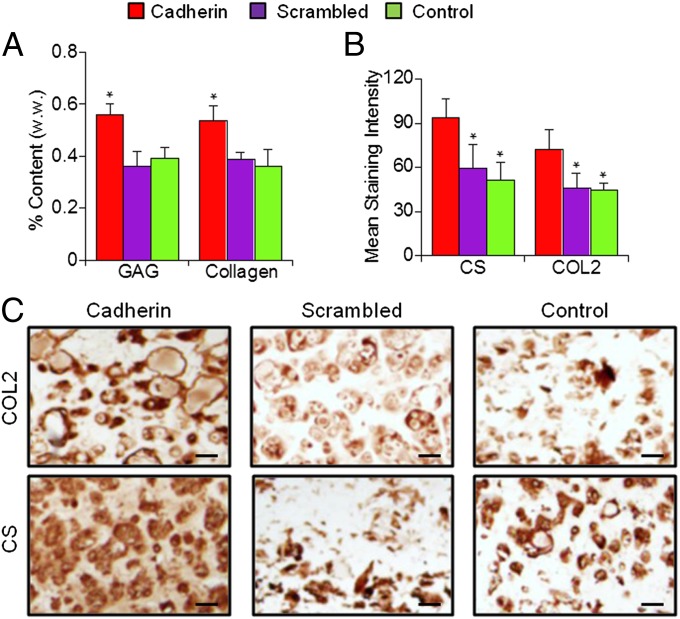
To evaluate the long-term outcome in neocartilage formation as a result of the enhanced early chondrogenesis in the presence of cadherin mimetic peptides, hMSC-laden constructs were cultured in differentiation media for 28 d (Fig. S1). Staining revealed that >90% of encapsulated hMSCs remained viable on day 28 in all groups (Fig. S5). Quantification of cartilage-specific matrix components showed that Cadherin hydrogels possessed higher GAG and collagen content compared with Scrambled or control groups (Fig. 4A). GAG and collagen content for the Cadherin group increased by ∼52% and ∼83%, respectively, compared with the control group after 28 d of culture. Mechanical testing indicated that Cadherin hydrogel constructs developed higher mechanical stiffness compared with the Scrambled or control hydrogels after 28 d of culture (Fig. 4B). Immunohistochemical staining for CS and COL2 revealed more intense and distributed staining in the pericellular and extracellular space, suggesting superior cartilage matrix elaboration in the Cadherin constructs compared with the Scrambled and control constructs (Fig. 4 C and D), which likely explains the enhanced mechanical properties. The mean staining intensity (CS and COL2, respectively) was significantly higher for the Cadherin group (∼110 and 75) compared with the Scrambled (∼79 and 46) and control (∼80 and 53) groups. Little staining was observed for COL1 for all groups (Fig. S6).
Fig. 4.
N-cadherin biomimetic peptides enhance neocartilage formation by hMSCs in vitro. (A) GAG and total collagen content normalized by sample wet weight, (B) equilibrium modulus (EY), and (C and D) immunohistochemical staining results for chondroitin sulfate (CS) and type II collagen (COL2) of MSC-laden HA hydrogel constructs after 28 d of in vitro culture in differentiation media. *P < 0.05 vs. Scrambled or control group (n = 4). (Scale bars, 50 µm.)
Cadherin Mimetic Peptides Enhance in Vivo Neocartilage Formation.
To evaluate the efficacy of the N-cadherin peptide in vivo, hMSC-laden MeHA hydrogel disks (Cadherin, Scrambled, or control groups) were implanted in s.c. pockets of nude mice for 28 d (Fig. S1). Alginate microspheres loaded with TGF-β3 were also encapsulated to provide chondrogenic induction in vivo (27). As observed in vitro, the implants containing N-cadherin peptides reached higher GAG and collagen content compared with those from the Scrambled and control groups (Fig. 5A). For example, GAG and collagen content in Cadherin hydrogels increased by ∼36% and ∼45%, respectively, compared with the control group after 28 d of implantation. Immunohistochemical staining also showed more intense and distributed COL2 and CS staining in the Cadherin implants compared with the Scrambled and control implants (Fig. 5 B and C). The mean staining intensity (CS and COL2, respectively) was significantly higher for the Cadherin group (∼94 and 73) compared with the Scrambled (∼58 and 46) and control (∼52 and 44) groups. Little staining was observed for COL1 for all groups (Fig. S6).
Fig. 5.
N-cadherin biomimetic peptides enhance neocartilage formation by hMSCs in vivo. (A) GAG and total collagen content normalized by sample wet weight and (B and C) immunohistochemical staining results for CS and COL2 of MSC-laden HA hydrogel constructs 28 d after s.c. implantation in nude mice. *P < 0.05 vs. Scrambled or control group (n = 4). (Scale bars, 50 µm.)
Discussion
Recent in vitro studies have demonstrated successful chondrogenesis of hMSCs photoencapsulated in HA hydrogels formed through cross-linking of HA modified with methacrylate groups (27–29). However, it still remains a challenge to recapitulate the functional properties of the native articular cartilage using only hMSCs, particularly compared with donor-matched articular chondrocytes, as shown in a bovine model (30, 31). The selection and modification of scaffold materials is critical to the improvement of chondrogenesis and cartilage formation by hMSCs. This work demonstrates that the interactions between hMSCs and HA via cell receptors CD44 and CD168 significantly influence chondrogenesis and neocartilage formation. Furthermore, coupling N-cadherin mimetic peptides onto HA hydrogels enhances early chondrogenic differentiation of encapsulated hMSCs, leading to superior long-term neocartilage formation under both in vitro and in vivo conditions. In vitro studies also revealed the necessity of soluble factors present in the media supplemented with TGF-β3 for hMSC chondrogenesis in HA hydrogels. In the absence of soluble factors, chondrogenic differentiation of hMSCs was diminished even in the presence of N-cadherin mimetic peptides, indicating a synergistic response of hMSCs to cadherin and soluble factors. This finding highlights the importance of functionalizing hydrogels to emulate complex cell microenvironments during skeletogenesis to advance stem cell-based cartilage tissue engineering.
CD44- and CD168-mediated cell interactions with HA are involved in many physiological processes, including embryonic development, tissue regeneration, and cell migration and proliferation. Studies have shown that stem-cell interactions with HA promote expression of chondrogenic markers including COL2, AGG, and Sox 9 (32, 33). In this study, blocking the hMSC–HA interactions using antibodies specifically targeting CD44 or CD168 significantly down-regulated the expression of major chondrogenic markers, confirming the importance of receptor-mediated HA interactions in chondrogenesis. Although binding of HA via CD44 or CD168 is known to trigger various cell signaling events, such as altered kinase activities (34) and cytoskeletal rearrangement (35), the molecular mechanisms underlying their roles in chondrogenesis remain largely undefined.
Cadherin plays a critical role in mediating early chondrogenic events during cartilage formation. It is known that N-cadherin function is correlated with cytoplasmic-associated proteins including β-catenin (36, 37). The observed increase in the expression of β-catenin in the cadherin peptide-modified hydrogels demonstrates that peptide functionalization leads to hMSC signaling. Here, the beneficial effects of cadherin mimetic peptides on the expression of chondrogenic marker genes were most evident at early time points (days 1 and 3), but this effect had diminished to baseline levels by day 7 of culture. A previous study reported that N-cadherin expression by limb mesenchymal cells increased dramatically at the initiation of condensation, but that this effect decreased with cartilage formation and maturation (38). Therefore, the diminishing effect of the coupled cadherin peptides was potentially a concomitant consequence of decreasing N-cadherin density on hMSCs with chondrogenesis. In fact, persistent expression of N-cadherin via viral transfection in limb mesenchymal cell culture had an inhibitory effect on chondrogenesis, possibly due to prolonged maintenance of increased cell–cell adhesiveness, stressing the importance of precise temporal presentation of N-cadherin (21). Moreover, the encapsulated hMSCs elaborate and deposit increasing amounts of cartilage matrix with culture, which could block interactions with the peptide with time. Several previous studies showed that a transient but optimal exposure of soluble chondrogenic factors resulted in superior subsequent neocartilage formation by hMSCs (31, 39). In this study, it was demonstrated that a short-term effect of synthetic cues on cellular interactions during early stages of chondrogenesis leads to improved cartilage development in the long term.
The HAV motif, the evolutionary conserved sequence in the ECD1 of N-cadherin considered essential to cadherin activity, is critical to its homotypic binding (24). Monomeric versions of this motif generally function as antagonists by competing against natural binding sites in the N-cadherin ECD1 domain, whereas dimeric versions are capable of acting as agonists by promoting dimerization of N-cadherins in cells (40). In this study, because one end (C-terminal) of the peptide was conjugated to the HA hydrogel backbone, a monomeric linear peptide sequence containing the HAV motif was capable of mimicking N-cadherin agonistic activity. Studies have shown that the identity of the amino acids that flank the HAV motif determines both the activity and specificity of the mimetic peptides (26). Furthermore, the cyclic form of cadherin mimetic peptides exhibits significantly higher activity than linear peptides (40). Therefore, further optimization of the peptide sequence and structure could produce an even more robust chondrogenic effect than that observed here.
Beyond the peptide used, the dosing and mechanical loading environment may also need to be optimized. For example, we observed a robust cellular response with a peptide dosage that consumed ∼10% of the methacrylate groups, a consumption that did not lead to differences in initial mechanical properties. It was reported that moderate (twofold) overexpression of full-length N-cadherin in a transfected mesenchymal cell line augmented, whereas higher (fourfold) overexpression inhibited, chondrogenesis (41). Therefore, the response to the mimetic peptides seems to be biphasic, and there could be an optimal dosage, which will need to be elucidated in future studies. It has recently been shown that cells can exert traction forces through cadherin cell–cell contacts (42, 43), so hydrogel mechanics and the loading environment may need to be considered in the utility of such materials. It has also been shown that cluster presentation of certain mimetic peptides, such as those containing an integrin-binding Arg–Gly–Asp (RGD) domain, significantly increases their binding efficacy (44, 45). Therefore, the local presentation (e.g., clustering and tether length) may be optimized independent of hydrogel mechanics to further mimic and present the molecule for cellular interactions.
The biochemical content (GAG and collagen) and mechanical properties of healthy human articular cartilage generally differ between locations and with age. For femoral articular cartilage in adult human knees, the GAG, total collagen content, and mechanical stiffness was reported to be around 2–4% (by wet weight), 8–14% (by wet weight), and 400–600 kPa, respectively (46, 47). Although this study describes a significant advancement in cartilage tissue engineering with human MSCs and design of functional hydrogels, the values obtained are still well below those of the healthy cartilage reported above. There is definitely room for further enhancing the chondrogenesis of hMSCs to approach the functional properties of native cartilage tissue.
The findings from this study showed that hMSC interactions with HA hydrogels via CD44 and CD168 promote chondrogenesis. Furthermore, tethering of N-cadherin mimetic peptides further enhanced early expression of chondrogenic markers and promoted long-term cartilage matrix production under both in vitro and in vivo conditions. This finding highlights the importance of material selection and functionalization to guide stem cell differentiation by providing instructive microenvironmental biochemical signals. It also demonstrated the promising potential of biofunctionalizing HA hydrogels to emulate the complexity of the natural cell microenvironment during embryogenesis to further stem cell-based cartilage regeneration approaches.
Materials and Methods
Macromer Synthesis and Peptide Conjugation.
MeHA was synthesized as previously reported (48) through the reaction of methacrylic anhydride (94%, formula weight 154.17; Sigma) with 1% (wt/vol) HA (sodium hyaluronate powder, molecular weight ∼74 kDa; Lifecore) in deionized water at a pH of 8, purification via dialysis (molecular weight cutoff 6–8 kDa), followed by lyophilization. Methacrylation of the final macromer was confirmed by 1H NMR to be ∼29%. Scrambled (Ac-AGVGDHIGC) and N-cadherin mimic (Ac-HAVDIGGGC) peptides with a cysteine residue at the C-terminal end to permit Michael-type addition reaction with MeHA were obtained from GenScript. MeHA macromers and peptides were dissolved in triethanolamine-buffered saline (TEOA buffer, 0.2 M TEOA, 0.3 M total osmolarity, pH 8.0) and maintained at 37 °C overnight for peptide coupling. For determination of peptide binding efficiency refer to SI Materials and Methods.
Sample Preparation and in Vitro Culture.
Human MSCs (Lonza) expanded to passage 3 in growth media consisting of α-MEM with 16.7% (vol/vol) FBS and 1% (vol/vol) penicillin–streptomycin (supplemented with 5 ng/mL fibroblast growth factor 2) were used in all experiments. For blocking studies, hMSCs were incubated with anti-CD44 (3/1,000 mouse mAb CD44; Abcam), anti-CD168 (3/1,000 rabbit mAb Cd168; Epitomics), or anti–GC-4 (50 μg/mL; Sigma), which binds to the N-terminal half of the extracellular domain of human N-cadherin (49, 50), in a buffer [2% (vol/vol) FBS in PBS] for 45 min on ice, washed twice in buffer, and resuspended in growth media. Expression of surface markers (CD44, CD168, and N-cadherin) was determined via immunostaining of the hMSCs before cell detachment (SI Materials and Methods gives details). For hydrogel constructs, hMSCs (20 million/mL) were photoencapsulated (365 nm, 1.2 mW/cm2, 10 min) in 1.5% (wt/vol) MeHA [containing 0.05% 2-methyl-1-[4-(hydroxyethoxy) phenyl]-2-methyl-1-propanone (I2959; Ciba)] hydrogel disks (Ø5 mm, 2.6-mm thickness) conjugated with cadherin mimetic (Cadherin group) or scrambled (Scrambled group) peptides or no peptide controls (control group) (Ø5mm, 2.6-mm thickness; Fig. 1 and Fig. S1). The constructs were cultured in chondrogenic media [DMEM, 1% (vol/vol) ITS+ Premix, 50 µg/mL l-proline, 0.1 µM dexamethasone, 0.9 mM sodium pyruvate, 50 µg/mL ascorbate, and antibiotics] supplemented with 10 ng/mL TGF-β3 and changed three times per week (51).
Subcutaneous Implantation in Nude Mice.
TGF-β3–releasing alginate microspheres were fabricated as described previously (27). hMSCs and microspheres were coencapsulated in HA hydrogels (each construct contained microspheres loaded with 100 ng of TGF-β3, eight constructs for each group) as described above before being implanted on the following day in four s.c. pockets for 28 d in male nude mice (NCRNU, age 4 wk; Taconic) (Fig. S1). All animal procedures were approved and guided by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Construct Analysis.
For gene expression analysis, samples were homogenized in TRIzol reagent and RNA was extracted, quantified, and reverse-transcribed into cDNA and PCR was performed (primers listed in Table S1). The relative gene expression was calculated using the ΔΔCT method, where fold difference was calculated using the expression 2ΔΔCt, normalized to GAPDH and expression levels of hMSCs at encapsulation. At 28 d, samples were removed from the culture or from s.c. pockets and processed for bulk mechanics as described previously (52) and then digested in proteinase-K and assessed for DNA content with PicoGreen, GAG content with dimethylmethylene blue, and overall collagen via orthohydroxyproline via dimethylaminobenzaldehyde and chloramine T assay. Remaining constructs were fixed in 4% (wt/vol) formalin, processed with standard paraffin embedding, and immunostained for chondroitin sulfate and COL1 and COL2. Quantification of mean staining intensity is given in Fig S9. All data are presented as mean ± SD. Statistica (Statsoft) was used to perform statistical analyses using two-way ANOVA with Tukey’s honestly significant difference post hoc test of the means used to make comparisons between groups (n = 4 samples per group), with culture duration and experimental group as independent factors.
Supplementary Material
Acknowledgments
The authors thank Hoang Lu for assistance in peptide design, Elena Tous and Reena Rai for assistance with animal surgeries, and Emily Zhang for assistance in sample analyses. This work was supported by National Institutes of Health Grant R01EB008722.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214100110/-/DCSupplemental.
References
- 1.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 2.Rogers JJ, Young HE, Adkison LR, Lucas PA, Black AC., Jr Differentiation factors induce expression of muscle, fat, cartilage, and bone in a clone of mouse pluripotent mesenchymal stem cells. Am Surg. 1995;61(3):231–236. [PubMed] [Google Scholar]
- 3.Huang AH, Farrell MJ, Mauck RL. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. J Biomech. 2010;43(1):128–136. doi: 10.1016/j.jbiomech.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connelly JT, García AJ, Levenston ME. Inhibition of in vitro chondrogenesis in RGD-modified three-dimensional alginate gels. Biomaterials. 2007;28(6):1071–1083. doi: 10.1016/j.biomaterials.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Lee HJ, et al. Enhanced chondrogenesis of mesenchymal stem cells in collagen mimetic peptide-mediated microenvironment. Tissue Eng Part A. 2008;14(11):1843–1851. doi: 10.1089/ten.tea.2007.0204. [DOI] [PubMed] [Google Scholar]
- 6.Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7(2):79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 7.Lisignoli G, et al. Hyaluronan-based polymer scaffold modulates the expression of inflammatory and degradative factors in mesenchymal stem cells: Involvement of Cd44 and Cd54. J Cell Physiol. 2006;207(2):364–373. doi: 10.1002/jcp.20572. [DOI] [PubMed] [Google Scholar]
- 8.Zhu H, et al. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24(4):928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 9.Knudson CB, Toole BP. Hyaluronate-cell interactions during differentiation of chick embryo limb mesoderm. Dev Biol. 1987;124(1):82–90. doi: 10.1016/0012-1606(87)90462-3. [DOI] [PubMed] [Google Scholar]
- 10.Maleski MP, Knudson CB. Hyaluronan-mediated aggregation of limb bud mesenchyme and mesenchymal condensation during chondrogenesis. Exp Cell Res. 1996;225(1):55–66. doi: 10.1006/excr.1996.0156. [DOI] [PubMed] [Google Scholar]
- 11.Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15(2):243–254. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varghese S, et al. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27(1):12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 14.Seliktar D, Zisch AH, Lutolf MP, Wrana JL, Hubbell JA. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J Biomed Mater Res A. 2004;68(4):704–716. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- 15.Mann BK, Schmedlen RH, West JL. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22(5):439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 16.Lin CC, Anseth KS. Cell-cell communication mimicry with poly(ethylene glycol) hydrogels for enhancing beta-cell function. Proc Natl Acad Sci USA. 2011;108(16):6380–6385. doi: 10.1073/pnas.1014026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahrens PB, Solursh M, Reiter RS. Stage-related capacity for limb chondrogenesis in cell culture. Dev Biol. 1977;60(1):69–82. doi: 10.1016/0012-1606(77)90110-5. [DOI] [PubMed] [Google Scholar]
- 18.San Antonio JD, Tuan RS. Chondrogenesis of limb bud mesenchyme in vitro: stimulation by cations. Dev Biol. 1986;115(2):313–324. doi: 10.1016/0012-1606(86)90252-6. [DOI] [PubMed] [Google Scholar]
- 19.Oberlender SA, Tuan RS. Spatiotemporal profile of N-cadherin expression in the developing limb mesenchyme. Cell Adhes Commun. 1994;2(6):521–537. doi: 10.3109/15419069409014216. [DOI] [PubMed] [Google Scholar]
- 20.Tavella S, Raffo P, Tacchetti C, Cancedda R, Castagnola P. N-CAM and N-cadherin expression during in vitro chondrogenesis. Exp Cell Res. 1994;215(2):354–362. doi: 10.1006/excr.1994.1352. [DOI] [PubMed] [Google Scholar]
- 21.DeLise AM, Tuan RS. Alterations in the spatiotemporal expression pattern and function of N-cadherin inhibit cellular condensation and chondrogenesis of limb mesenchymal cells in vitro. J Cell Biochem. 2002;87(3):342–359. doi: 10.1002/jcb.10308. [DOI] [PubMed] [Google Scholar]
- 22.Delise AM, Tuan RS. Analysis of N-cadherin function in limb mesenchymal chondrogenesis in vitro. Dev Dyn. 2002;225(2):195–204. doi: 10.1002/dvdy.10151. [DOI] [PubMed] [Google Scholar]
- 23.Tuan RS. Cellular signaling in developmental chondrogenesis: N-cadherin, Wnts, and BMP-2. J Bone Joint Surg Am. 2003;85-A(Suppl 2):137–141. doi: 10.2106/00004623-200300002-00019. [DOI] [PubMed] [Google Scholar]
- 24.Blaschuk OW, Sullivan R, David S, Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Dev Biol. 1990;139(1):227–229. doi: 10.1016/0012-1606(90)90290-y. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro L, et al. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374(6520):327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- 26.Williams E, Williams G, Gour BJ, Blaschuk OW, Doherty P. A novel family of cyclic peptide antagonists suggests that N-cadherin specificity is determined by amino acids that flank the HAV motif. J Biol Chem. 2000;275(6):4007–4012. doi: 10.1074/jbc.275.6.4007. [DOI] [PubMed] [Google Scholar]
- 27.Bian L, et al. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32(27):6425–6434. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung C, Beecham M, Mauck RL, Burdick JA. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009;30(26):4287–4296. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erickson IE, et al. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage. 2009;17(12):1639–1648. doi: 10.1016/j.joca.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14(2):179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Kim M, Erickson IE, Choudhury M, Pleshko N, Mauck RL. Transient exposure to TGF-β3 improves the functional chondrogenesis of MSC-laden hyaluronic acid hydrogels. J Mech Behav Biomed Mater. 2012;11:92–101. doi: 10.1016/j.jmbbm.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz Z, Griffon DJ, Fredericks LP, Lee HB, Weng HY. Hyaluronic acid and chondrogenesis of murine bone marrow mesenchymal stem cells in chitosan sponges. Am J Vet Res. 2011;72(1):42–50. doi: 10.2460/ajvr.72.1.42. [DOI] [PubMed] [Google Scholar]
- 33.Wu SC, Chang JK, Wang CK, Wang GJ, Ho ML. Enhancement of chondrogenesis of human adipose derived stem cells in a hyaluronan-enriched microenvironment. Biomaterials. 2010;31(4):631–640. doi: 10.1016/j.biomaterials.2009.09.089. [DOI] [PubMed] [Google Scholar]
- 34.Hall CL, Wang C, Lange LA, Turley EA. Hyaluronan and the hyaluronan receptor RHAMM promote focal adhesion turnover and transient tyrosine kinase activity. J Cell Biol. 1994;126(2):575–588. doi: 10.1083/jcb.126.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Assmann V, Jenkinson D, Marshall JF, Hart IR. The intracellular hyaluronan receptor RHAMM/IHABP interacts with microtubules and actin filaments. J Cell Sci. 1999;112(Pt 22):3943–3954. doi: 10.1242/jcs.112.22.3943. [DOI] [PubMed] [Google Scholar]
- 36.Balsamo J, et al. Regulated binding of PTP1B-like phosphatase to N-cadherin: Control of cadherin-mediated adhesion by dephosphorylation of beta-catenin. J Cell Biol. 1996;134(3):801–813. doi: 10.1083/jcb.134.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoschuetzky H, Aberle H, Kemler R. Beta-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol. 1994;127(5):1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oberlender SA, Tuan RS. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development. 1994;120(1):177–187. doi: 10.1242/dev.120.1.177. [DOI] [PubMed] [Google Scholar]
- 39.Huang AH, Stein A, Tuan RS, Mauck RL. Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng Part A. 2009;15(11):3461–3472. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams G, Williams EJ, Doherty P. Dimeric versions of two short N-cadherin binding motifs (HAVDI and INPISG) function as N-cadherin agonists. J Biol Chem. 2002;277(6):4361–4367. doi: 10.1074/jbc.M109185200. [DOI] [PubMed] [Google Scholar]
- 41.Haas AR, Tuan RS. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: II. Stimulation by bone morphogenetic protein-2 requires modulation of N-cadherin expression and function. Differentiation. 1999;64(2):77–89. doi: 10.1046/j.1432-0436.1999.6420077.x. [DOI] [PubMed] [Google Scholar]
- 42.Ganz A, et al. Traction forces exerted through N-cadherin contacts. Biol Cell. 2006;98(12):721–730. doi: 10.1042/BC20060039. [DOI] [PubMed] [Google Scholar]
- 43.Leckband DE, le Duc Q, Wang N, de Rooij J. Mechanotransduction at cadherin-mediated adhesions. Curr Opin Cell Biol. 2011;23(5):523–530. doi: 10.1016/j.ceb.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci. 2000;113(Pt 10):1677–1686. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- 45.Petrie TA, et al. Multivalent integrin-specific ligands enhance tissue healing and biomaterial integration. Sci Transl Med. 2010;2(45):45ra60. doi: 10.1126/scitranslmed.3001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Treppo S, et al. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J Orthop Res. 2000;18(5):739–748. doi: 10.1002/jor.1100180510. [DOI] [PubMed] [Google Scholar]
- 47.Jurvelin JS, Buschmann MD, Hunziker EB. Mechanical anisotropy of the human knee articular cartilage in compression. Proc Inst Mech Eng H. 2003;217(3):215–219. doi: 10.1243/095441103765212712. [DOI] [PubMed] [Google Scholar]
- 48.Smeds KA, et al. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res. 2001;54(1):115–121. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 49.Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001;61(9):3819–3825. [PubMed] [Google Scholar]
- 50.Puch S, et al. N-cadherin is developmentally regulated and functionally involved in early hematopoietic cell differentiation. J Cell Sci. 2001;114(Pt 8):1567–1577. doi: 10.1242/jcs.114.8.1567. [DOI] [PubMed] [Google Scholar]
- 51.Byers BA, Mauck RL, Chiang IE, Tuan RS. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;14(11):1821–1834. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mauck RL, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122(3):252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.