Abstract
Aberrant signaling by oncogenic mutant rat sarcoma (Ras) proteins occurs in ∼15% of all human tumors, yet direct inhibition of Ras by small molecules has remained elusive. Recently, several small-molecule ligands have been discovered that directly bind Ras and inhibit its function by interfering with exchange factor binding. However, it is unclear whether, or how, these ligands could lead to drugs that act against constitutively active oncogenic mutant Ras. Using a dynamics-based pocket identification scheme, ensemble docking, and innovative cell-based assays, here we show that andrographolide (AGP)—a bicyclic diterpenoid lactone isolated from Andrographis paniculata—and its benzylidene derivatives bind to transient pockets on Kirsten-Ras (K-Ras) and inhibit GDP–GTP exchange. As expected for inhibitors of exchange factor binding, AGP derivatives reduced GTP loading of wild-type K-Ras in response to acute EGF stimulation with a concomitant reduction in MAPK activation. Remarkably, however, prolonged treatment with AGP derivatives also reduced GTP loading of, and signal transmission by, oncogenic mutant K-RasG12V. In sum, the combined analysis of our computational and cell biology results show that AGP derivatives directly bind Ras, block GDP–GTP exchange, and inhibit both wild-type and oncogenic K-Ras signaling. Importantly, our findings not only show that nucleotide exchange factors are required for oncogenic Ras signaling but also demonstrate that inhibiting nucleotide exchange is a valid approach to abrogating the function of oncogenic mutant Ras.
Keywords: cancer, molecular dynamics, allosteric site, drug design
Monomeric rat sarcoma (Ras) proteins are molecular switches that cycle between inactive GDP-bound and active GTP-bound conformational states and regulate multiple cell signaling pathways that include the MAPK cascade (1). Activation of Ras is facilitated by guanine nucleotide exchange factors (GEFs) and inactivation by GTPase-activating proteins (GAPs) (2, 3). About 15% of all human cancers are associated with somatic Ras mutations at amino acid positions 12, 13, or 61 that impair GAP-catalyzed GTP hydrolysis. Of the three most common human Ras isoforms N-, H-, and Kirsten-Ras4B (K-Ras4B), mutations on K-Ras4B (hereafter K-Ras) are most prevalent in cancers, including in up to 90% of cases of pancreatic cancer (4). However, decades of efforts to inhibit oncogenic Ras by small molecules have to date been unsuccessful (5). Attempts to abrogate the plasma membrane binding of Ras, which is required for biological activity, by inhibiting farnesyl transferase (6, 7) have failed because N-Ras and K-Ras are also good substrates for geranyl-geranyl transferase 1 in cells treated with farnesyl transferase inhibitors (8, 9). Other efforts along this line include the development of farnesyl analogs, currently in clinical trials (10), and other compounds that dislodge Ras from the plasma membrane (11, 12). Although the potential therapeutic value and mechanism of action of these compounds are still under investigation, it is clear that they do not directly bind to Ras. Recent efforts by us (13) and others (14–16) toward direct inhibition of Ras have yielded promising initial results. For instance, using fragment screening, crystallography, and other methods, two groups reported ligands that directly bind Ras and inhibit GEF-dependent nucleotide exchange (15, 16). However, it is unclear whether, or how, these ligands could lead to drugs that act against constitutively active, GTP-loaded mutant Ras.
GTP-Ras can exist in at least two conformational states (17, 18). When in state 1, Ras has reduced affinity for effectors and harbors open pockets (19, 20), whereas in state 2, Ras is able to effectively bind effectors (21, 22). In principle, small-molecule inhibitors that can selectively stabilize the state 1 conformation have the potential to inhibit Ras signaling by interfering with either effector or exchange factor binding. Along this line, a recent study found a compound that binds to an open switch 1 conformation of state 1 Ras (23). In this work, we use ensembles of K-Ras obtained from molecular dynamics (MD) simulations that sample state 1 and other intermediate structures (24) to address two major questions: first, to evaluate whether the reported anticancer activity (25) of andrographolide (AGP), a diterpene from the medicinal plant Andrographis paniculata (26), and its benzylidene derivatives involves direct inhibition of Ras; and second, to use these compounds to test a unique hypothesis that prolonged inhibition of nucleotide exchange can abrogate the function of oncogenic mutant Ras. Combining data from ensemble docking, simulations, and experiments in intact cells, we show that AGP and its derivatives inhibit Ras function by preventing GEF-induced nucleotide exchange. We further show that prolonged treatment with AGP derivatives significantly impairs oncogenic K-RasG12V signaling, and highlight how inhibiting nucleotide exchange can be a valid approach to abrogating the function of oncogenic mutant Ras.
Results and Discussion
AGP and Benzylidene Derivatives Target the Switch Regions of K-Ras.
AGP has oxidative, antiviral, and anticancer properties, and its benzylidene derivatives (Fig. 1) exhibit an enhanced ability to induce apoptosis and G1 cell-cycle arrest in breast and colon cancer cells (25, 27). Other studies have shown that AGP interferes with MAPK activation, increases sensitivity of Ras-transformed cells to radiation treatment in vitro and in vivo (27–30), and is not toxic (31). The drug-like (32) AGP has three hydrogen-bond donors and five acceptors and a LogP of 2.6. Its slightly larger SRJ series of derivatives each has one donor, five acceptors, and an estimated LogP of 5.6.
Fig. 1.
Chemical structures of AGP and its benzylidene derivatives SRJ09, SRJ10, and SRJ23.
We docked these ligands onto a diverse set of 75 K-Ras conformers and ranked them by their preference for a given site and receptor conformation as described in Materials and Methods and SI Materials and Methods, with appropriate controls (Fig. S1). We found that the ligands preferentially target three distinct pockets: p1, p2, and p3. The residues defining these pockets are listed in Table 1 and their location in the 3D structure is shown in Fig. 2. Pocket 1 comprises the effector binding loop (residues 30–40), β2 (residues 55 and 57), and several residues in α-helix 1. Pocket 2 involves the core β-strands 1 and 2, part of the effector loop, and switch 2. The C-terminal pocket 3 is bounded by α-helix 5 plus the N-terminal and preceding loop residues of β5 and β6. Remarkably, each of these pockets is very similar to those we have previously characterized using a different approach and a different set of probes (13). Mapping the energetic preference of small-molecule fragments by FTMap (33) identified the same binding hotspots, with binding preference for each site modulated by protein motion (Fig. S2). Opening of pocket 1 is primarily a function of the displacement of Y40 that occurs during the simulations (Figs. S3 and S4). Relative to the reference X-ray structure Protein Data Bank (PDB) ID code 3GFT, the Cα of Y40 is displaced by 4 Å and the side chain is oriented away from D38 (Fig. S3). Similarly, expansion of a narrow surface groove between switches 1 and 2 (Fig. S4 B and D) opens p2, a pocket surrounded by hydrophobic (I36 and M67) and polar residues (Y64 and E37) with V7 at the bottom (Fig. 3B). To check whether structures within each cluster have reasonably similar binding-site topologies, we calculated the SD of the mean solvent-accessible surface area of p1 residues, which was found to be 33% smaller for structures within clusters (averaged over all 75 clusters) than the corresponding value across clusters.
Table 1.
Pockets on K-RasQ61H targeted by AGP and derivatives SRJ09, SRJ10, and SRJ23
| Residues | Locations | |
| 1 | 17, 20, 21, 24, 25, 29, 30–40, 55, and 57 | α1, switch 1, β2 |
| 2 | 4–7, 35–39, 54–59, 61–78 | β1, β2, switch 2 |
| 3 | 97, 101, 107–111, 137–140, 162, 163, 166 | α5, β5, β6, loops α3/β5 and α4/β6 |
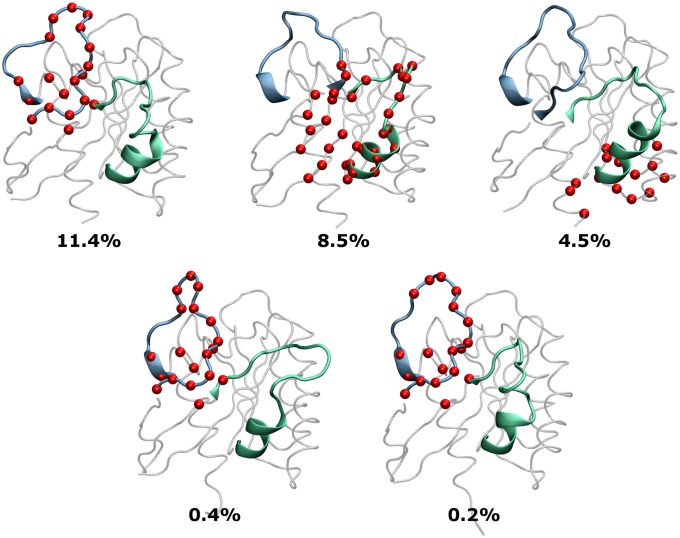
Fig. 2.
Overview of the K-RasQ61H structures derived from MD simulations for ensemble docking. From among the 75 unique K-Ras conformers used for docking (Materials and Methods), 5 representative cluster centroids are shown along with the percentage of the total conformers they represent. Pockets most frequently targeted by SRJ23 are highlighted in red van der Waals spheres. Notice the major conformational changes in switch 1 (cyan) and switch 2 (green).
Fig. 3.
Overview of transiently opening pockets 1 and 2 on K-RasQ61H with relevant residues colored according to their electrostatic potential. (A) Docking pose of SRJ23 at pocket 1, where its phenyl group occupies the space previously occupied by Y40 and is stabilized by the residues I21 and T20. (B) Docking pose of SRJ23 at pocket 2 opened by the movement of Y71 and lined by hydrophobic residues I36 and M67. (C) MD-optimized complex of SRJ23 at pocket 1. (D) The conformation in C is modified to visualize the proximity of SRJ23 to Mg2+ and GTP (switch 1 is now shown as a transparent surface). The hydroxyl group on the lactone ring of SRJ23 forms a hydrogen bond with the α-phosphate of GTP as well as an electrostatic contact with Mg2+ similar to that made by the hydroxyl of T35 on Ras (20). Electrostatic potentials were calculated using the Adaptive Poisson-Boltzmann Solver (50).
Whereas the parent compound AGP displays some preference for p3 in addition to p1 and p2, the derivatives have much less preference for p3, as can be seen from the binding frequency histograms in Fig. S5 for the top five ligand clusters. In fact, the SRJ ligands consistently hit p3 only when docked onto the crystal structure. When the structure is relaxed but still somewhat close to the starting crystal structure PDB ID code 3GFT (Cα rmsd of 2.8 and 2.9 Å, respectively, for switches 1 and 2), the top-ranked ligand cluster targets p2 with a probability of 5.6%. When it adopts a more open switch conformation (3.6 and 4.3 Å), SRJ23 [3,19-(3-chloro-4-fluorobenzylidene)andrographolide] targets p1 with a probability of 3.1% (Table 2). To probe whether the ligand might prefer a region proximal to p2, such as the site occupied by the ligands benzimidazole (BZIM) and its halogenated derivative 4,6-dichloro-2-methyl-3-aminoethyl-indole (DCAI) (15), we docked SRJ23 onto the K-Ras–BZIM structure (PDB ID code 4DSU). We found that SRJ23 does not recognize this region, because four of the top five ligand clusters (predicted affinity 0.04–1.2 μM) targeted p3. We conclude that pockets near the highly dynamic canonical switches, which became accessible during the simulations, represent the most probable binding sites for AGP and its derivatives.
Table 2.
Ranking ligand–receptor pairs by the joint probability of occurrence of a given receptor conformation and ligand pose, followed by visual pocket identification
| Receptor |
Ligand |
Receptor–ligand pair |
|||
| Cluster | Fraction total conformers | Cluster | Fraction total poses | Ranking by joint probability, % | Pocket ID |
| 4 | 0.09 | 2 | 0.66 | 5.6 | 2 |
| 2 | 0.11 | 3 | 0.27 | 3.1 | 1 |
| 7 | 0.04 | 1 | 0.64 | 2.9 | 3 |
| 1 | 0.12 | 17 | 0.20 | 2.4 | 2 |
| 1 | 0.12 | 18 | 0.17 | 1.4 | 2 |
Shown here are data for a few preferred poses of SRJ23 on selected K-RasQ61H cluster representatives, highlighting the relative preference of the ligand for a specific binding-site and receptor conformation. The joint probability is simply the product of the fraction of individual ligand and receptor clusters (column 2 times column 4). Note that these are just samples and do not reflect the total probability of binding to a particular site; binding sites for the rest of the ligands and receptor conformations were determined in a similar way.
MD Simulations of K-Ras–SRJ23 Complexes Suggest Stable Binding at p1 but Not p2.
To further evaluate the viability of ligand binding at p1 or p2, we conducted multiple MD simulations of K-Ras in complex with SRJ23 with different initial velocity assignments. The ligand dissociated within 60 ns in four out of five of the runs with SRJ23 at p2 and after an additional 40 ns in the fifth run (Fig. S6A). By contrast, the K-Ras–SRJ23 complex remained stable (with 45–80% of the ligand surface area buried) during four out of five simulations with SRJ23 bound at p1 (Fig. S6 A and C). In the remaining run, switch 1 moved away from the GTP and the pose of SRJ23 was altered, but it did not dissociate. These results suggest that p1 is the preferred pocket for our ligands, despite the fact that p2 showed up repeatedly during docking (Fig. S6). We conclude that switch 1 is a viable target whose dynamics leads to the opening of a pocket that can accommodate even comparatively large ligands such as SRJ23.
During the simulations with p1-bound SRJ23, the ligand forms a hydrogen bond with the α-phosphate of GTP (Fig. 3D), but it targets the same pocket when docked onto a nucleotide-free–like conformer (Fig. 4B). This indicates that the nucleotide is not required by SRJ23 for binding but that it might stabilize the bound ligand. As noted earlier, SRJ ligands target an open switch 1 conformation in which, for instance, the distance between D33 and D38 Cα atoms is 14 Å instead of 11 Å, as in the classic (i.e., closed) GTP-bound structures (Fig. S3). Relaxing the K-Ras–SRJ23 complex by MD further expanded the pocket to better accommodate the halogenated phenyl ring (Fig. 3 A and C). This led us to the question of what might constitute a minimal scaffold to target this pocket. To address this question, we fragmented SRJ23 into 16 substructures and docked them onto the K-Ras conformer that served as the starting point for the K-Ras–SRJ23 simulations. A fragment common to all three SRJ ligands has the highest preference for this site (256/256 poses) (Fig. S7A). We predict that this three-membered ring represents the chemical signature of AGP derivatives to target p1, but the role of the halogenations for affinity, if any, is not obvious (SI Materials and Methods). To test whether compounds that share structural similarity with SRJ bind to p1, we carried out virtual screening of about 1,000 ligands from the ZINC database (http://zinc.docking.org) selected based on a Tanimoto similarity index of 0.6 (SI Materials and Methods). The results suggest that these ligands would bind to p1 with a micro- to nanomolar affinity (AutoDock Vina energy score of −6 to −10 kcal/mol) when switch 1 is open (Fig. S7B). Their affinity for closed switch 1 conformations was about three orders of magnitude weaker. This result not only reemphasizes the correlation between Ras dynamics and ligand binding but also suggests that it may be possible to find other small-molecule Ras binders that preserve the key features of SRJ but bind with better affinity (Fig. S7C).
Fig. 4.
Comparison of known Ras structures bound to SOS and small-molecule ligands. (A) Overlay of the switch 2 region of a K-Ras–SRJ23 snapshot (orange), the crystal structure of K-RasG12D–DCAI from PDB ID code 4DST (purple, with only residues 62–75 shown for clarity), K-Ras–0QX from PDB ID code 4EVP (yellow, residues 62–75), and two structures of H-Ras–SOS from PDB ID codes 1NVV (ice blue) and 1BKD (cyan). (B) Projection of simulated K-Ras conformers onto a PC space defined by crystallographic structures, with the cluster of GDP–H-Ras structures highlighted in blue and the cluster of loss-of-function mutants in orange. PDB ID code 4DST (purple) lies in the major GTP cluster, whereas PDB ID code 4EPV (yellow) is intermediate to the GTP and GDP clusters. Two K-Ras–ligand conformations from docking (MD-Lf, green dots) are shown to illustrate the ability of MD to capture putative excited-state structures with open p1 that are preferred by our ligands. An example of simulated K-Ras–SRJ23 (MD-Lb) lies between Ras–SOS (cyan/ice blue) and Ras–inhibitor (purple/yellow) conformations. The crystal structure of nucleotide-free H-Ras (PDB ID code 1BKD) is also shown, with SRJ23 docked at switch 1. SRJ23 is shown as a yellow surface and switches 1 and 2 as blue and green surfaces, respectively. The Bio3D package (http://thegrantlab.org/bio3d) was used to generate the figure in B.
Stabilization of a Unique K-Ras Conformation.
The docking results indicate that our ligands favor state 1-like conformers with an open switch 1 (Fig. 4B). To test whether the ligands stabilize a particular Ras conformation, we used a principal component (PC)-based analysis developed previously (13, 34–36) to map conformers from K-Ras–SRJ23 trajectories onto a PC plane defined by crystal structures. Fig. 4B shows that p1-bound SRJ23 stabilizes conformations that are different from the canonical GTP/GDP or nucleotide-free states (see Fig. S8 for full PC data). Alignment of the simulated K-Ras structures with p1-bound SRJ23 onto those from a control ligand-free simulation further shows stabilization of D38 in an orientation that allows for an opening of a pore behind switch 1 (Fig. S3). Given their structural similarity, we expect SRJ09 and SRJ10 will have a similar effect.
Proposed Mechanism of Action.
Recent reports revealed that ligand binding at a pocket between the core β-sheet and helix 2 of K-Ras stabilizes alternate side-chain conformations at or around switch 2 and thereby affects exchange factor binding (15, 16). For instance, the side chains of both Y64 and Y71 were displaced in these ligand-bound structures relative to an SOS-bound H-Ras structure (15, 16). We therefore compared the orientation of these side chains in our K-Ras–SRJ23 conformers with those in K-Ras–DCAI (PDB ID code 4DST), H-Ras–SOS [PDB ID codes 1BKD and 1NVV (37, 38)], and other K-Ras–ligand complexes (PDB ID code 4EPV) (Fig. 4A). The orientation of Y71 in K-Ras–SRJ23 mimics that in K-Ras–DCAI and PDB ID code 4EPV. Moreover, Y64 is displaced by >5 Å (Cα atom) in K-Ras–SRJ23 relative to its position in the H-Ras–SOS complex (Movie S1). Although other modes of action cannot be ruled out, these observations suggest that our ligands may stabilize Ras in a conformation that is not conducive to GEF binding. AGP and its derivatives could thus inhibit GEF-induced nucleotide exchange in a similar manner as those reported by Maurer et al. (15) and Sun et al. (16).
In Vitro Assays Indicate That SRJ Compounds Inhibit Ras GTP Loading and Cancer Cell Growth.
To explore the effects of AGP and its derivatives on Ras function in intact cells, we first measured activation of the Ras/MAPK cascade in response to EGF stimulation. Serum-starved BHK cells were incubated with AGP or derivatives for 6 h and stimulated with EGF. Fig. 5A shows that SRJ09 and SRJ23 significantly reduced Ras GTP loading, as measured in Ras-binding domain (RBD) pull-down assays. The reduction in Ras activation correlated closely with a concomitant reduction in MAPK activation (Fig. 5B). The concentrations of AGP required to inhibit EGF-stimulated extracellular-signal–regulated kinase (ERK) activation were ∼10-fold higher than the active concentrations of SRJ09 and SRJ23, indicating that the SRJ compounds were considerably more potent than the parent compound (Fig. 5B compared with Fig. S9B). We then immunoblotted the RBD pull-down assays with isoform-specific antisera to determine whether all Ras isoforms were equally sensitive to SRJ09 and SRJ23. Fig. 5C shows that K-Ras GTP loading was significantly suppressed by SRJ09 and SRJ23, whereas H-Ras and N-Ras GTP loading were much less sensitive. For example, SRJ23 reduced K-Ras, H-Ras, and N-Ras GTP levels by 47%, 28% and 13%, respectively. The structural basis for K-Ras selectivity is not immediately clear, but it is consistent with previous suggestions (34, 36) that K-Ras might be more dynamic than H-Ras and samples open switch 1 conformations more frequently. This is supported by results from MD simulations of wild-type K- and H-Ras (Fig. S10). Importantly, none of the compounds suppressed activation of the EGF receptor, as measured by Y1068 phosphorylation. Furthermore, 5 μM SRJ09 did not inhibit CRaf-mediated MAPK activation (Fig. S11), showing that the andrographolides do not inhibit any of the kinases in the Raf/MEK/ERK signaling cascade. These results strongly suggest that AGP, SRJ09, and SRJ23 directly target Ras to block the exchange of GDP for GTP and thus prevent Ras activation. Consistent with this mechanism of action, a 6-h incubation in SRJ09 and SRJ23 (5 μM) had no measurable effect on the extent of GTP loading of oncogenic mutant K-, H-, and N-RasG12V, or on the extent of MAPK activation in Ras-transformed cell lines (Fig. 5D).
Fig. 5.
Mean fold increase in Ras-GTP (A) and ppERK (B) ± SEM from three independent experiments where wild-type BHK cells were treated with SRJ09 or SRJ23 for 6 h in the absence of serum, followed by 25 ng/mL EGF stimulation for 2 min. Ras-GTP levels were measured using an RBD pull-down assay, and ppERK levels were measured by quantitative immunoblotting. (C) Representative blots from three independent experiments in which wild-type BHK cells were treated with 5 μM SRJ23 for 6 h in the absence of serum, followed by 25 ng/mL EGF stimulation for 2 min. Levels of K-, H-, and N-Ras–GTP loadings were measured using an RBD pull-down assay, and phospho-EGF receptor (EGFR; Y1068) levels were measured by quantitative immunoblotting. (D) Mean ± SEM from three independent experiments in which BHK cells stably expressing oncogenic Ras isoforms were treated with 5 μM SRJ09 or SRJ23 for 6 h in the absence of growth serum. ppERK levels were measured by quantitative immunoblotting. Mean Ras-GTP (E) and ppERK (F) ± SEM from three independent experiments in which BHK cells stably expressing oncogenic K-Ras were treated with 5 μM SRJ09 or SRJ23 for 72 h. Growth media with the drug were replaced every 24 h. Ras-GTP levels were measured using an RBD pull-down assay, and ppERK levels were measured by quantitative immunoblotting. Differences between DMSO- and drug-treated cells were assessed using one-way ANOVA tests. Significant differences are indicated (*P < 0.05; **P < 0.01; ***P < 0.001).
Oncogenic mutant Ras is constitutively GTP-loaded because the oncogenic mutation blocks the ability of GAP to stimulate GTP hydrolysis and return Ras to the inactive GDP-bound ground state. We reasoned, however, that the intrinsic GTPase activity of oncogenic mutant Ras must eventually return Ras to the ground state even though the expected rate would be very slow (∼8–17 h, with kcat of 1–2 × 10−3 min−1) (39, 40). Once in the GDP ground state, oncogenic Ras would then need to interact with an exchange factor to be reloaded with GTP. If the SRJ compounds are present at this point and bind to RasG12V–GDP (or other substates with an open switch 1 conformation), GTP loading may be abrogated. We tested this hypothesis by incubating oncogenic mutant K-Ras–transformed cells in SRJ09 and SRJ23 (5 μM) for 3 d to give sufficient time for GTP hydrolysis and thus reveal a requirement for GDP–GTP exchange. Remarkably, after this prolonged incubation period, SRJ09 and SRJ23 reduced K-RasG12V GTP levels by ∼50% (Fig. 5E), which resulted in a concomitant reduction in phosphorylated ERK (ppERK) levels in K-RasG12V–transformed cells (Fig. 5F). Moreover, the ability of AGP, SRJ09, and SRJ23 to induce growth inhibition against PC-3 (prostate), HCT-116 (colon), and MDA-231 (breast) cancer cells was assessed as described previously (25). PC-3 cancer cells harbor wild-type Ras for all of the three different isoforms (H-, K-, and N-Ras), whereas HCT-116 and MDA-231 cancer cells express G13D mutant K-Ras and wild-type H- and N-Ras. Table 3 shows the 50% growth-inhibition (GI50) values of AGP, SRJ09, and SRJ23 in this panel of cancer cell lines. One can see that SRJ09 and SRJ23 are generally more active than the parent compound AGP and, more importantly, cancer cells with endogenous mutant K-Ras (HCT-116 and MDA-231) were found to be significantly more sensitive toward the compounds. The mean GI50 value in these cells was approximately half compared with the cancer cell harboring wild-type K-Ras (PC-3). We conclude that cancer cells with mutant Ras are more sensitive toward the compounds, which is consistent with our results with ectopically expressed mutant Ras.
Table 3.
Growth-inhibitory effect of AGP, SRJ09, and SRJ23 on prostate (PC-3), colon (HCT-116), and breast MDA-231 cancer cells
| GI50, µM |
|||
| Compounds | PC-3* | HCT-116† | MDA-231† |
| AGP | 9.7 ± 1.3 | 4.5 ± 1.8 | 5.9 ± 1.4 |
| SRJ09 | 12.4 ± 1.3 | 3.1 ± 0.7 | 5.0 ± 0.4 |
| SRJ23 | 6.6 ± 0.5 | 4.0 ± 1.2 | 5.8 ± 0.7 |
Ras status was obtained from the Catalog of Somatic Mutations in Cancer, Wellcome Trust Sanger Institute. Values represent mean ± SD of three independent experiments.
Wild-type for H-, K-, and N-Ras.
Mutant K-Ras (substitution, p.G13D) and wild-type for H- and N-Ras.
Taking the computational and cell biology results together, we conclude that SRJ09 and SRJ23 bind to Ras and prevent GTP loading, thus effectively blocking GDP–GTP exchange in live cells. Using these compounds that directly target Ras, we also show that exchange factors are required to maintain oncogenic mutant Ras in the active GTP-bound state, and that inhibiting nucleotide exchange is a valid approach to abrogating the function of oncogenic mutant Ras.
Conclusion
Using molecular docking and simulations, we show that AGP and its derivatives bind to the switch regions of Ras, preferentially targeting a transient pore behind switch 1 as well as a groove between switches 1 and 2. Binding of AGP derivatives to p1 stabilizes Ras in a unique conformation where key residues on the nucleotide-binding switches, such as Y64 and Y71, reorganize in a manner that would impair interaction with Ras modulators. Combining these findings with earlier reports (15, 16), we predict that AGP derivatives should inhibit Ras function by preventing GEF-assisted nucleotide exchange. We confirmed this prediction by experiments showing that SRJ treatment blocks acute GTP loading of wild-type Ras. Even more intriguing was the observation that extended incubation with SRJ compounds also reduced oncogenic mutant Ras-GTP levels and hence signal output. The likely explanation for this observation is that the SRJ ligands deplete the pool of GTP-Ras over time by allowing intrinsic hydrolysis of bound GTP while preventing reactivation via GEF-catalyzed GDP–GTP exchange. This mechanism can operate because oncogenic mutations prevent the ability of GAP to accelerate the intrinsic GTPase activity of Ras and only modestly reduce the actual intrinsic GTPase activity (41–44). In showing that GEF-mediated nucleotide exchange is critical for oncogenic Ras signaling, our findings represent an important proof of concept for anti-Ras drug discovery. Drug development need not be limited to ligands that abrogate effector interactions of GTP-loaded Ras or directly inhibit Ras effectors, but may be expanded to exploit the requirement of GEF-mediated GTP loading for a sustained signaling through oncogenic Ras. We therefore propose that future efforts toward Ras inhibition should additionally consider ligands that abrogate GEF binding and/or prevent GDP–GTP exchange; such ligands may potentially be more effective and selective given the fewer number of exchange factors than effectors and the possibility that Ras isoforms may differ in dynamics and response.
Materials and Methods
Ensemble Docking.
Expanding the concept of a relaxed complex scheme for structure-based drug discovery (45, 46), we built an ensemble of K-Ras conformers that contained infrequently sampled structures. Such structures could potentially harbor open binding sites that are invisible in crystal structures. To this end, we isolated an ensemble of 75 structures based on rmsd clustering of GTP-bound K-RasQ61H conformers derived from previously reported MD simulations (24). Taking advantage of the small number of related compounds that we were interested in, we deliberately used a small rmsd cutoff of 1.3 Å to generate a large number of clusters and used all of the cluster centroids to include infrequently visited K-Ras conformers. Blind docking against these structures was carried out using AutoDock 4.2 (47) and a search area that encompasses the entire Ras structure plus a buffer space of 10 Å in each direction. GTP was retained to exclude nonspecific hits at the catalytic site (see SI Materials and Methods for details and controls).
Binding-Site Identification and Selection of Ligand Poses.
To account for the joint probability that K-Ras samples a given conformation and AutoDock consistently places the ligand into a consensus site, binding sites were identified based on the size of both the ligand and receptor clusters. Therefore, ligand–receptor pairs were ranked by the product of the fraction of Ras conformers within a cluster and the fraction of ligand poses within a ligand cluster (Table 2). Visual inspection of the high-ranked complexes and histograms of contact frequencies was then used to identify the most commonly targeted pockets (SI Materials and Methods).
Molecular Dynamics Simulations.
After docking and site identification, we tested whether binding at the predicted sites is viable by performing MD on selected protein–ligand complexes. The simulation details are similar to those described previously (24) and in SI Materials and Methods. By assigning different initial velocities, we generated two sets of five separate trajectories with SRJ23 bound to K-Ras at either of two preferred sites (p1 and p2).
In Vitro Cell-Based Assays.
Based on initial work that formed the basis of this study (25, 48), AGP, SRJ09, and SRJ23 were prepared as reported earlier (48) (see Tables S1 and S2 for proof of purity). Antibodies against H-Ras (F235; sc-29) and N-Ras (F155; sc-31) were obtained from Santa Cruz Biotechnology. Monoclonal K-Ras antibody (R3400) was obtained from Sigma-Aldrich. Rabbit phospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204) antibody (9101), mouse anti–phospho-EGF receptor (Y1068) antibody (2236), and rabbit total EGF receptor antibody (2232) were purchased from Cell Signaling Technology. Monoclonal anti-Ras antibody (610001) was obtained from BD Transduction Laboratories. For cell culture, baby hamster kidney (BHK) cells were maintained in Dulbecco’s modified Eagle medium (Gibco) supplemented with 10% (vol/vol) donor calf serum and 2 mM l-glutamine. Ras-GTP levels were measured in a GST–Ras-binding domain pull-down assay as described previously (49). Samples were analyzed by quantitative Western immunoblotting using pan-Ras or Ras isoform-specific antibodies.
The potential of AGP, SRJ09, and SRJ23 to induce growth inhibition against PC-3 (prostate), HCT-116 (colon), and MDA-231 (breast) cancer cells was assessed according to Jada et al. (25). Briefly, exponentially growing cells were seeded in flat-bottom 96-well plates at a density of 2,000 cells in 0.18 mL per well and incubated overnight for cell attachment. The cells were then treated with compounds at a final concentration range of 0.1–100 µM (n = 4). The control wells were introduced with 0.1% of DMSO equivalent to the highest amount of DMSO used as a vehicle in the compound-treated wells. After 96 h of incubation in a CO2 incubator, the cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The 50% growth-inhibition values were obtained from dose–response growth-inhibitory curves.
Supplementary Material
Acknowledgments
We thank Dr. Rommie Amaro for kick-starting the collaboration between the J.S. and A.A.G. laboratories. Wong Charng Choon and Wong Hui Chyn (Pharmacotherapeutics Unit, Department of Medicine, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia) are gratefully acknowledged for performing the in vitro anticancer test. H.J.H. is supported by a fellowship from Keck Gulf Coast Consortia Training in Pharmacological Sciences (National Institute of General Medical Sciences Grant T32GM089657). This work is supported in part by grants from National Institutes of Health General Medical Sciences (R01GM10078 to A.A.G.), Cancer Prevention and Research Institute of Texas (RP100483 to J.F.H.), and Ministry of Higher Education, Malaysia (Research University Grant Scheme Grant 04-02-12-2017RU to J.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300016110/-/DCSupplemental.
References
- 1.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: A changing paradigm. Nat Rev Cancer. 2009;9(3):153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 2.Scheffzek K, et al. The Ras-RasGAP complex: Structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277(5324):333–338. doi: 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- 3.McCormick F, Wittinghofer A. Interactions between Ras proteins and their effectors. Curr Opin Biotechnol. 1996;7(4):449–456. doi: 10.1016/s0958-1669(96)80123-6. [DOI] [PubMed] [Google Scholar]
- 4.Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72(10):2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W, Fang G, Rudolph J. Ras inhibition via direct Ras binding—Is there a path forward? Bioorg Med Chem Lett. 2012;22(18):5766–5776. doi: 10.1016/j.bmcl.2012.07.082. [DOI] [PubMed] [Google Scholar]
- 6.DeGraw AJ, Keiser MJ, Ochocki JD, Shoichet BK, Distefano MD. Prediction and evaluation of protein farnesyltransferase inhibition by commercial drugs. J Med Chem. 2010;53(6):2464–2471. doi: 10.1021/jm901613f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niessner H, et al. The farnesyl transferase inhibitor lonafarnib inhibits mTOR signaling and enforces sorafenib-induced apoptosis in melanoma cells. J Invest Dermatol. 2011;131(2):468–479. doi: 10.1038/jid.2010.297. [DOI] [PubMed] [Google Scholar]
- 8.Whyte DB, et al. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem. 1997;272(22):14459–14464. doi: 10.1074/jbc.272.22.14459. [DOI] [PubMed] [Google Scholar]
- 9.Rowell CA, Kowalczyk JJ, Lewis MD, Garcia AM. Direct demonstration of geranylgeranylation and farnesylation of Ki-Ras in vivo. J Biol Chem. 1997;272(22):14093–14097. doi: 10.1074/jbc.272.22.14093. [DOI] [PubMed] [Google Scholar]
- 10.Blum R, Cox AD, Kloog Y. Inhibitors of chronically active Ras: Potential for treatment of human malignancies. Recent Patents Anticancer Drug Discov. 2008;3(1):31–47. doi: 10.2174/157489208783478702. [DOI] [PubMed] [Google Scholar]
- 11.van der Hoeven D, et al. Fendiline inhibits K-Ras plasma membrane localization and blocks K-Ras signal transmission. Mol Cell Biol. 2013;33(2):237–251. doi: 10.1128/MCB.00884-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho KJ, et al. Staurosporines disrupt phosphatidylserine trafficking and mislocalize Ras proteins. J Biol Chem. 2012;287(52):43573–43584. doi: 10.1074/jbc.M112.424457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant BJ, et al. Novel allosteric sites on Ras for lead generation. PLoS One. 2011;6(10):e25711. doi: 10.1371/journal.pone.0025711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buhrman G, et al. Analysis of binding site hot spots on the surface of Ras GTPase. J Mol Biol. 2011;413(4):773–789. doi: 10.1016/j.jmb.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurer T, et al. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc Natl Acad Sci USA. 2012;109(14):5299–5304. doi: 10.1073/pnas.1116510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Q, et al. Discovery of small molecules that bind to K-Ras and inhibit Sos-mediated activation. Angew Chem Int Ed Engl. 2012;51(25):6140–6143. doi: 10.1002/anie.201201358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spoerner M, Wittinghofer A, Kalbitzer HR. Perturbation of the conformational equilibria in Ras by selective mutations as studied by 31P NMR spectroscopy. FEBS Lett. 2004;578(3):305–310. doi: 10.1016/j.febslet.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Araki M, et al. Solution structure of the state 1 conformer of GTP-bound H-Ras protein and distinct dynamic properties between the state 1 and state 2 conformers. J Biol Chem. 2011;286(45):39644–39653. doi: 10.1074/jbc.M111.227074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muraoka S, et al. Crystal structures of the state 1 conformations of the GTP-bound H-Ras protein and its oncogenic G12V and Q61L mutants. FEBS Lett. 2012;586(12):1715–1718. doi: 10.1016/j.febslet.2012.04.058. [DOI] [PubMed] [Google Scholar]
- 20.Spoerner M, Herrmann C, Vetter IR, Kalbitzer HR, Wittinghofer A. Dynamic properties of the Ras switch I region and its importance for binding to effectors. Proc Natl Acad Sci USA. 2001;98(9):4944–4949. doi: 10.1073/pnas.081441398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shima F, et al. Structural basis for conformational dynamics of GTP-bound Ras protein. J Biol Chem. 2010;285(29):22696–22705. doi: 10.1074/jbc.M110.125161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao J, et al. Two conformational states of Ras GTPase exhibit differential GTP-binding kinetics. Biochem Biophys Res Commun. 2008;369(2):327–332. doi: 10.1016/j.bbrc.2008.01.169. [DOI] [PubMed] [Google Scholar]
- 23.Rosnizeck IC, et al. Metal-bis(2-picolyl)amine complexes as state 1(T) inhibitors of activated Ras protein. Angew Chem Int Ed Engl. 2012;51(42):10647–10651. doi: 10.1002/anie.201204148. [DOI] [PubMed] [Google Scholar]
- 24.Prakash P, Sayyed-Ahmad A, Gorfe AA. The role of conserved waters in conformational transitions of Q61H K-ras. PLoS Comput Biol. 2012;8(2):e1002394. doi: 10.1371/journal.pcbi.1002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jada SR, et al. Benzylidene derivatives of andrographolide inhibit growth of breast and colon cancer cells in vitro by inducing G(1) arrest and apoptosis. Br J Pharmacol. 2008;155(5):641–654. doi: 10.1038/bjp.2008.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behrens M, et al. The human bitter taste receptor hTAS2R50 is activated by the two natural bitter terpenoids andrographolide and amarogentin. J Agric Food Chem. 2009;57(21):9860–9866. doi: 10.1021/jf9014334. [DOI] [PubMed] [Google Scholar]
- 27.Hung SK, et al. Andrographolide sensitizes Ras-transformed cells to radiation in vitro and in vivo. Int J Radiat Oncol Biol Phys. 2010;77(4):1232–1239. doi: 10.1016/j.ijrobp.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Carretta MD, et al. Andrographolide reduces IL-2 production in T-cells by interfering with NFAT and MAPK activation. Eur J Pharmacol. 2009;602(2-3):413–421. doi: 10.1016/j.ejphar.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Lim JCW, et al. Andrographolide and its analogues: Versatile bioactive molecules for combating inflammation and cancer. Clin Exp Pharmacol Physiol. 2012;39(3):300–310. doi: 10.1111/j.1440-1681.2011.05633.x. [DOI] [PubMed] [Google Scholar]
- 30.Levita J, Nawawi A, Mutalib A, Ibrahim S. Andrographolide: A review of its anti-inflammatory activity via inhibition of NF-kappaB activation from computational chemistry aspects. Int J Pharmacol. 2010;6(5):569–576. [Google Scholar]
- 31.He X, et al. Four new andrographolide metabolites in rats. Tetrahedron. 2003;59(34):6603–6607. [Google Scholar]
- 32.Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44(1):235–249. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 33.Brenke R, et al. Fragment-based identification of druggable ‘hot spots’ of proteins using Fourier domain correlation techniques. Bioinformatics. 2009;25(5):621–627. doi: 10.1093/bioinformatics/btp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorfe AA, Grant BJ, McCammon JA. Mapping the nucleotide and isoform-dependent structural and dynamical features of Ras proteins. Structure. 2008;16(6):885–896. doi: 10.1016/j.str.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grant BJ, Gorfe AA, McCammon JA. Ras conformational switching: Simulating nucleotide-dependent conformational transitions with accelerated molecular dynamics. PLoS Comput Biol. 2009;5(3):e1000325. doi: 10.1371/journal.pcbi.1000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukman S, Grant BJ, Gorfe AA, Grant GH, McCammon JA. The distinct conformational dynamics of K-Ras and H-Ras A59G. PLoS Comput Biol. 2010;6(9):e1000922. doi: 10.1371/journal.pcbi.1000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boriack-Sjodin PA, Margarit SM, Bar-Sagi D, Kuriyan J. The structural basis of the activation of Ras by Sos. Nature. 1998;394(6691):337–343. doi: 10.1038/28548. [DOI] [PubMed] [Google Scholar]
- 38.Margarit SM, et al. Structural evidence for feedback activation by Ras⋅GTP of the Ras-specific nucleotide exchange factor SOS. Cell. 2003;112(5):685–695. doi: 10.1016/s0092-8674(03)00149-1. [DOI] [PubMed] [Google Scholar]
- 39.Krengel U, et al. Three-dimensional structures of H-ras p21 mutants: Molecular basis for their inability to function as signal switch molecules. Cell. 1990;62(3):539–548. doi: 10.1016/0092-8674(90)90018-a. [DOI] [PubMed] [Google Scholar]
- 40.Polakis P, McCormick F. Structural requirements for the interaction of p21ras with GAP, exchange factors, and its biological effector target. J Biol Chem. 1993;268(13):9157–9160. [PubMed] [Google Scholar]
- 41.Eccleston JF, Moore KJ, Morgan L, Skinner RH, Lowe PN. Kinetics of interaction between normal and proline 12 Ras and the GTPase-activating proteins, p120-GAP and neurofibromin. The significance of the intrinsic GTPase rate in determining the transforming ability of ras. J Biol Chem. 1993;268(36):27012–27019. [PubMed] [Google Scholar]
- 42.Gideon P, et al. Mutational and kinetic analyses of the GTPase-activating protein (GAP)-p21 interaction: The C-terminal domain of GAP is not sufficient for full activity. Mol Cell Biol. 1992;12(5):2050–2056. doi: 10.1128/mcb.12.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung HH, Benson DR, Cornish VW, Schultz PG. Probing the role of loop 2 in Ras function with unnatural amino acids. Proc Natl Acad Sci USA. 1993;90(21):10145–10149. doi: 10.1073/pnas.90.21.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neal SE, Eccleston JF, Hall A, Webb MR. Kinetic analysis of the hydrolysis of GTP by p21N-ras. The basal GTPase mechanism. J Biol Chem. 1988;263(36):19718–19722. [PubMed] [Google Scholar]
- 45.Lin J-H, Perryman AL, Schames JR, McCammon JA. Computational drug design accommodating receptor flexibility: The relaxed complex scheme. J Am Chem Soc. 2002;124(20):5632–5633. doi: 10.1021/ja0260162. [DOI] [PubMed] [Google Scholar]
- 46.Amaro RE, Baron R, McCammon JA. An improved relaxed complex scheme for receptor flexibility in computer-aided drug design. J Comput Aided Mol Des. 2008;22(9):693–705. doi: 10.1007/s10822-007-9159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris GM, et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19(14):1639–1662. [Google Scholar]
- 48.Jada SR, et al. Semisynthesis and cytotoxic activities of andrographolide analogues. J Enzyme Inhib Med Chem. 2006;21(2):145–155. doi: 10.1080/14756360500499988. [DOI] [PubMed] [Google Scholar]
- 49.Roy S, et al. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat Cell Biol. 1999;1(2):98–105. doi: 10.1038/10067. [DOI] [PubMed] [Google Scholar]
- 50.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc Natl Acad Sci USA. 2001;98(18):10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.