Abstract
With the global proliferation of toxic harmful algal bloom species, there is a need to identify the environmental and biological factors that regulate toxin production. One such species, Karenia brevis, forms nearly annual blooms that threaten coastal regions throughout the Gulf of Mexico. This dinoflagellate produces brevetoxins, which are potent neurotoxins that cause neurotoxic shellfish poisoning and respiratory illness in humans, as well as massive fish kills. A recent publication reported that a rapid decrease in salinity increased cellular toxin quotas in K. brevis and hypothesized that brevetoxins serve a role in osmoregulation. This finding implied that salinity shifts could significantly alter the toxic effects of blooms. We repeated the original experiments separately in three different laboratories and found no evidence for increased brevetoxin production in response to low-salinity stress in any of the eight K. brevis strains we tested, including three used in the original study. Thus, we find no support for an osmoregulatory function of brevetoxins. The original publication also stated that there was no known cellular function for brevetoxins. However, there is increasing evidence that brevetoxins promote survival of the dinoflagellates by deterring grazing by zooplankton. Whether they have other as-yet-unidentified cellular functions is currently unknown.
Keywords: red tides, toxic blooms, HABs
Harmful algal blooms threaten human health and damage coastal ecosystems worldwide. In the Gulf of Mexico, the athecate dinoflagellate Karenia brevis causes nearly annual toxic blooms. The physiology, ecology, and adverse effects of K. brevis have been well studied since the species was described in the 1940s (1). Notably, K. brevis produces brevetoxins, a family of potent polyether neurotoxins that bind to voltage-activated sodium channels in cell membranes, preventing normal nerve and muscle activity (2, 3). A nontoxic polyether that inhibits brevetoxin function, brevenal, is also produced in small amounts by this species (4). In humans, brevetoxins cause neurotoxic shellfish poisoning, and brevetoxin-contaminated aerosols cause respiratory irritation and illness (2, 5). Blooms also cause massive fish kills (6), as well as bird and marine mammal mortalities (7, 8). In 2005, a bloom of K. brevis lasted for more than a year and caused extensive mortalities at all trophic levels in lagoons, coastal ecosystems, and offshore reefs on the west Florida shelf (8, 9).
Because of the extensive damage caused by K. brevis, there has been considerable interest in understanding the function and regulation of brevetoxins. Competition experiments reveal that K. brevis produces allelopathic compounds, which inhibit the growth of competing phytoplankton and thereby help enable this slow-growing species to dominate; however, brevetoxins themselves do not appear to be responsible for this inhibition (10, 11). Recent studies support the hypothesis that brevetoxins serve as grazing deterrents, which promote population survival by decreasing grazing mortality rates (12–14), consistent with a classic defensive role for this neurotoxin. Furthermore, very recently published experiments show that limitation of growth rate by nitrogen and phosphorus increases cellular brevetoxin concentrations by two- to threefold, consistent with the behavior of other grazing defense toxins in terrestrial plants and phytoplankton (15–18). However, before these recent findings, brevetoxins were thought to be constitutively expressed, and the cellular function of these and other algal toxins was debated (19–21). Errera and Campbell (22) recently reported that a rapid decrease in salinity from 35 to 27, which is within the optimal growth range for K. brevis, caused up to a 16-fold increase in cellular brevetoxins in three K. brevis strains (Wilson, TXB4, and SP3). Subsequently, these same authors said their original data were wrong because of a calculation error and that the observed brevetoxin increase resulting from the low-salinity shock was actually 10-fold lower, or 20–53% (23). On the basis of these results, these authors argued that osmoregulation may be the primary cellular function of brevetoxins and that salinity could be a major factor regulating brevetoxins in coastal waters. In previous, longer-term culture experiments from this same research group with the same strains, the authors also reported significantly higher toxin levels per cell for strain TBX4 grown at lower (27) than at higher (35) salinity; however, they reported the same toxin content for the Wilson strain at the two salinities and a sevenfold lower brevetoxin content per cell in strain SP3 grown at the lower salinity (24). This latter result is the opposite of the pattern reported by these authors for this strain in their subsequent short-term salinity stress experiments (22, 23).
In this article, we challenge the conclusions of Errera and Campbell (22, 23). We present research from independent studies conducted at three separate laboratories that show no effect of abrupt decreases in salinity (or longer-term exposure) on cellular brevetoxin content in eight different strains of K. brevis. These findings fail to support the hypothesis that osmotic stress triggers brevetoxin production or that brevetoxins have an osmoregulatory function.
Results
Independent sets of experiments were conducted in three separate laboratories to quantify changes in cellular toxin quotas and volume per cell in eight different K. brevis strains after an abrupt change in salinity from 35 or 36 to 27. These salinity changes were equivalent to those used in the experiments of Errera and Campbell (22), and three of the strains tested here (Wilson, TXB4, and SP3) also were used in those experiments.
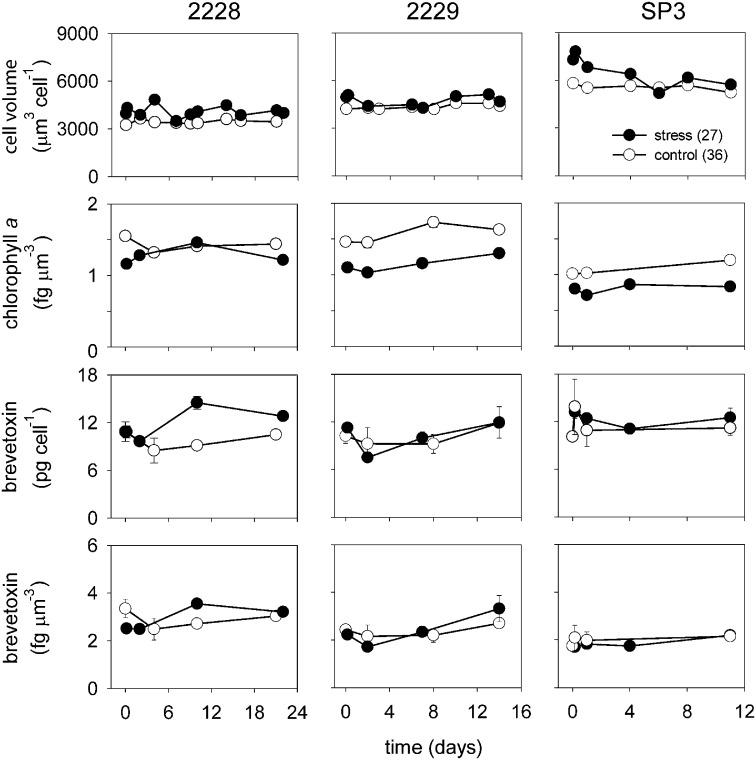
Two sets of experiments were conducted by laboratory A. In the first set, K. brevis strains CCMP 2228, CCMP 2229, and SP3 were grown semicontinuously such that they maintained nutrient-sufficient maximum growth rates throughout the experiment (Fig. S1). Strains CCMP 2228 and 2229 had growth rates of 0.55–0.58 d−1, whereas strain SP3 had rates of 0.32 d−1 and 0.33 d−1 at salinities of 36 and 27, respectively. There was no apparent salinity effect on brevetoxin content per cell in any of the three strains (Fig. 1). Mean brevetoxin per cell values in the control and low-salinity-stressed cultures were 10.6 ± 1.4 and 11.8 ± 2.0 pg per cell, respectively, for strain CCMP 2228; 10.2 ± 1.7 and 10.1 ± 1.2 pg per cell, respectively, for strain CCMP 2229; and 11.1 ± 1.1 and 10.7 ± 1.2 pg per cell, respectively, for strain SP3. Likewise, there was no apparent difference in cellular brevetoxins normalized to cell volume between control and low-salinity treatments for any of the three strains (Fig. 1).
Fig. 1.
Mean cell volume, chlorophyll a normalized to cell volume, and brevetoxins per cell and per unit of cell volume vs. time for strains CCMP 2228, CCMP 2229, and SP3 for controls and low-salinity-stressed cultures (data from experiment 1, laboratory A). Error bars show SDs for triplicate analyses in single cultures. If no error is shown, its dimension was less than the size of the data point.
Mean biovolume per cell increased 18–26% within 3 min of the salinity decreasing from 36 to 27 (Fig. 1). The increases tended to persist in strains CCMP 2228 and 2229 but declined with time in strain SP3. Levels of chlorophyll a per unit of biovolume were higher in the controls than in the low-salinity treatment cultures of strains CCMP 2229 and SP3; at least some of this effect was caused by the lower volume per cell (Fig. 1).
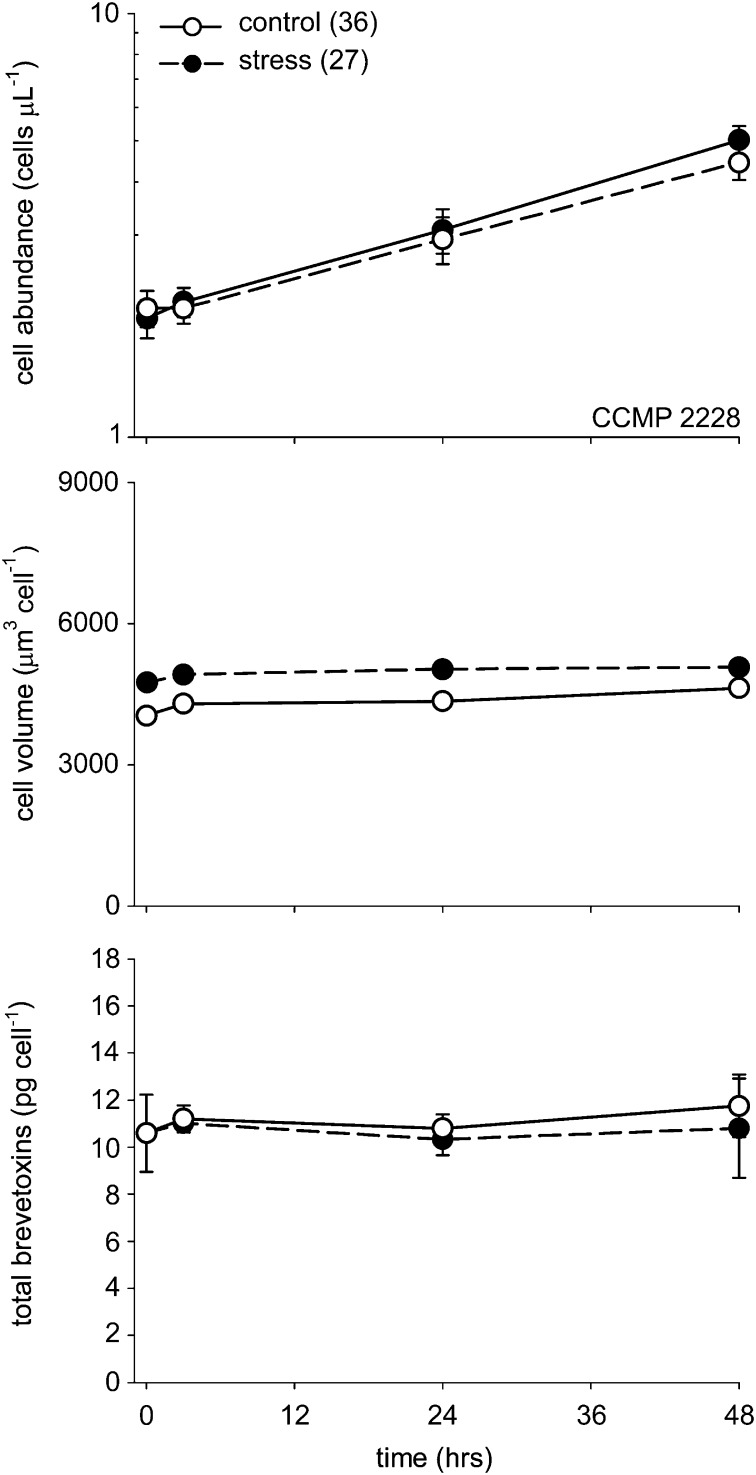
This set of experiments used single control and treatment cultures, which did not allow for valid statistical analysis of the data for each strain. However, in a second experiment conducted with strain CCMP 2228 during a 48-h period, quadruplicate control and low-salinity treatment cultures were used, which allowed for statistical analysis of the results (Fig. 2). A decrease in salinity from 36 to 27 significantly increased the mean cell volume [F(1,31) = 478; P < 0.001, one-way repeated measures ANOVA], with an average increase of 17.4% within the first 3 min and a 9.7% increase after 48 h. As before, the salinity decrease had no effect on toxin content per cell [F(1,31) = 2.36; P = 0.153, one-way repeated measures ANOVA] (Fig. 2). The average specific growth rate was 0.42 ± 0.02 (± SD) d−1 in the control cultures and 0.48 ± 0.01 d−1 in the low-salinity treatments, which is lower than the values in the initial longer-term experiment (0.55 and 0.58 d−1; Fig. S1) but similar to the lower values (0.44 and 0.47 d−1) measured previously at a salinity of 36 (and the same temperature as used here) in nutrient-replete cultures of this K. brevis strain (17, 18).
Fig. 2.
Time course of changes in cell abundance (cells per μL), mean volume per cell, and cellular brevetoxins (pg per cell) in control and low-salinity shock treatments of strain CCMP 2228 (data from experiment 2, laboratory A). Error bars show SDs for measurements in four replicate cultures. Error bars for mean volume per cell cannot be seen, as they are smaller than the dimensions of the data points.
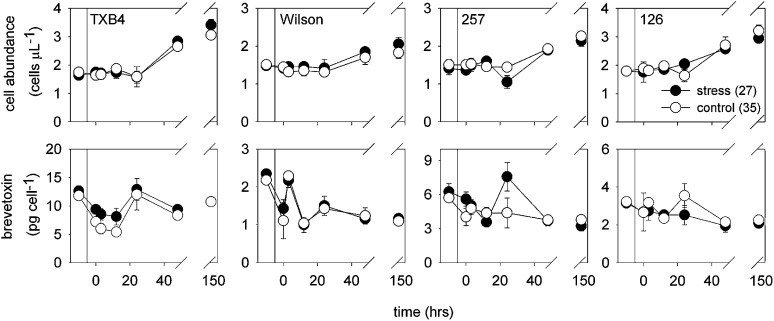
In experiments conducted in laboratory B, K. brevis strains TXB4, Wilson, 257, and 126 were grown in batch cultures. Treatments were diluted more than twofold with low-salinity seawater to lower the salinity from 35 to 27. Control cultures were diluted by an equivalent amount of water with a salinity of 35. There was no effect of salinity on culture growth (Fig. 3). The cultures were treated in late log or early stationary phase, and thus had lower growth rates than in the previous experiments (0.06 d−1 for the Wilson strain and 0.11 d−1 for the other three strains), likely because of nutrient or CO2 limitation of growth rate.
Fig. 3.
Cell concentrations and brevetoxins per cell for strains TX B4, Wilson (low-toxin cell line), 257, and 126 before and at various times after culture dilution and the resulting initiation of osmotic stress (data from laboratory B). Vertical lines indicate the point of culture dilution. Cell concentrations before culture dilution have been adjusted downward by the dilution factor to allow ready comparison with postdilution values. Data represent means of single analyses from four replicate treatment and control flasks. Error bars give associated SDs. If no error is shown, its dimension was less than the size of the data point.
Low-salinity stress did not increase brevetoxin per cell in any of the four strains (Fig. 3; Table S1). The only statistically significant difference in brevetoxins per cell during the experiments was between control and low-salinity stress cultures of strain 126, in which the controls exhibited significantly higher values. However, this increase was driven by a single sampling point. Other transient differences were also noted; for example, an increase in brevetoxin per cell was seen in strain TXB4 at 24 h. There were also differences in cell abundance between control and stressed cultures of the Wilson and 257 strains, but these were small overall (<10%; Fig. 3).
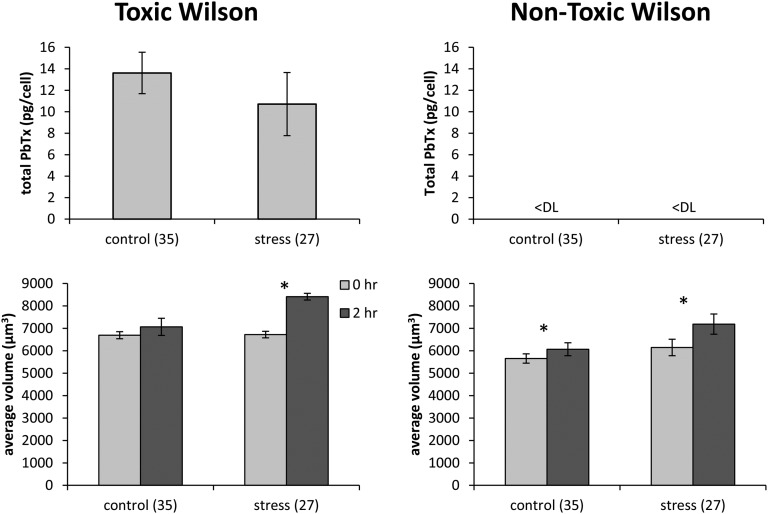
The brevetoxin per cell values for the Wilson strain were 1.0–2.2 pg per cell (Fig. 3), which is an order of magnitude lower than values reported previously in nutrient-sufficient and nitrogen-limited cultures of this strain (17 ± 1 and 22 ± 3 pg per cell, respectively) (17) and values reported for the toxic Wilson strain culture in the experiments of laboratory C (10–14 pg per cell; Fig. 4). However, this strain has been in culture for over 50 y, and various cell lines maintained in different laboratories have been observed to rapidly shift their toxin concentrations up or down and then maintain their new toxin profiles for months or years. The reasons for these abrupt shifts in cellular toxins are unknown and were a main impetus behind the experiments described in laboratory C. The researchers wanted to determine whether a salinity change would induce a nontoxic strain to produce toxin.
Fig. 4.
Brevetoxin per cell and mean volume per cell for toxic and nontoxic cell lines of the Wilson strain before and after the salinity decrease from 35 to 27 (data from laboratory C). Error bars show SDs for five replicate cultures. Asterisks indicate significant differences between treatment pairs (paired t test, P < 0.05). “<DL” indicates measured values below the detection limit.
In the experiments of laboratory C, toxic and nontoxic cell lines of the K. brevis Wilson strain were subjected to an abrupt salinity change from 35 to 27. In the nontoxic Wilson culture, brevetoxin cell quotas remained below the detection limit (0.17 pg per cell) in both the control and stressed cultures 2 h after the salinity shift (Fig. 4). Similarly, osmotic stress did not significantly influence total brevetoxin per cell in the toxic Wilson culture 2 h after the salinity shift (Fig. 4; Table S2).
The toxic Wilson culture exhibited a significant increase in mean volume per cell from 6,722 to 8,410 µm3 after the salinity shift (Fig. 4; Tables S2 and S3). Likewise, there was a significant volume increase from 6,147 to 7,188 µm3 in the nontoxic Wilson strain (Table S3). Significant increases in mean volume per cell (nontoxic isolate only) were also observed in the controls, which experienced turbulence associated with mixing but no osmotic stress (Tables S2 and S3). Cell abundance did not significantly decrease after osmotic stress, indicating that the stress did not kill the cells (Tables S2 and S3).
Discussion
We report the results of experiments at three independent laboratories that investigated the role of osmotic stress on brevetoxin production in eight different K. brevis strains. Despite different experimental designs and culture conditions in the studies at the three laboratories, each set of experiments independently rejected the hypothesis that osmotic stress stimulates brevetoxin production. K. brevis cultures osmotically stressed by a sudden decrease in salinity did not exhibit higher brevetoxin cell quotas than control cultures. These findings were consistent across each of eight K. brevis strains and among strains growing at their nutrient-sufficient maximum rates (0.3–0.6 d−1) and at low, nutrient-limited or CO2-limited growth rates (∼0.1 d−1). Our findings contrast with those of Errera and Campbell (22, 23), who reported that “brevetoxin production increased in response to low salinity stress.” Because of the substantial adverse effects of K. brevis and brevetoxins in the Gulf of Mexico and a desire to understand their regulation, Errera and Campbell’s reported findings have garnered much attention from researchers (e.g., 25–27), as well as media outlets, but we found that their reported findings are not reproducible. Furthermore, their claims that they “close a critical gap in knowledge” are unsubstantiated. In the following discussion, we examine experimental flaws and logical inconsistencies in the previous study that led to inaccurate conclusions.
Errera and Campbell (22, 23) drew their conclusions principally from an experiment in which cultures were osmotically stressed by a rapid salinity decrease from 35 to 27. They reported a 20–53% increase in cellular brevetoxins in the low-salinity-stressed cultures relative to “controls.” However, there was no evidence of true controls in this experiment, such as parallel portions of the cultures that did not receive an osmotic stress. Rather, the authors used previously published data (24) to serve as controls in their 2011 study. This information is found in the figure legends; for example, in the legend to figure 1 of Errera and Campbell (22), they write: “Mean total brevetoxin cell quota for three clones under control [acclimated to salinities of 27 (black) and 35 (white); redrawn from ref. 11] and after hypo-osmotic stress.” The lack of true controls obscured any possible differences resulting from calculation errors or problems in toxin analysis. The inherent value of controls in the design of experiments has been discussed elsewhere at length (e.g., 28–30) and will not be discussed here further.
Errera and Campbell (22) further speculated that brevetoxins may “facilitate osmoregulation through interaction with Na+ channels and allow for ion adjustment.” Although this speculation is interesting, it is not consistent with our current understanding of osmosis or cell biology. Water diffuses from areas of higher water activity (lower concentration of solutes) to areas of lower activity (higher concentration of solutes). For the low-salinity stress experiments, the decrease in salinity results in a higher water activity in the seawater surrounding the cells. As a consequence, water is predicted to move through the cell membrane into the cell, which has a lower intracellular water activity than the surrounding seawater after the salinity decrease. This influx of water causes the cells to swell, as observed in the experiments of laboratory A and laboratory C (where cell size was measured) and those of Errera and Campbell (22). Errera and Campbell hypothesized that brevetoxins facilitate osmoregulation through interactions with Na+ channels, allowing for intracellular ion adjustment. However, there is no known mechanism for this to occur. Brevetoxins bind with the voltage-gated sodium channels of cell membranes and hold these channels in an open configuration (2, 3). This allows sodium ions to flux into the cells, driven by the electrochemical gradient for these ions across the outer cell membrane (31). Unfortunately, such an inward flux of sodium ions would increase the intracellular solute concentration and thereby decrease the water activity, which is the opposite of the needed effect for an effective osmoregulatory response to the lower external seawater salinity.
Errera and Campbell (22) also stated that the function of brevetoxins was unknown. However, there is increasing evidence that brevetoxins promote the survival of K. brevis by deterring grazing by zooplankton (12–14). Studies investigating the functional role of toxins in organisms have a long history, and one that is deep-seated in terrestrial systems, including numerous terrestrial plants (e.g., 16, 32, 33). Because the defensive function of toxic secondary metabolites in organisms is indisputable, it is often accepted that toxin production has largely evolved to defend prey against predators (e.g., 16, 34–36). Within aquatic systems, there is substantial evidence in support of the evolution of chemical defenses (37, 38), and this evidence extends to both macro- and microalgae (13, 39–41). As such, productive lines of inquiry regarding the function of brevetoxins not only should include how production changes with environmental factors but also must consider the role of biological interactions, especially those between phytoplankton and grazing organisms.
Methods
Experiments were conducted independently in each of three laboratories to quantify changes in toxin cell quotas and cell size after a sudden decrease in salinity. Eight K. brevis strains were tested, including three of the four isolates used by Errera and Campbell (22, 23) (Table S4). Because the culture conditions and experimental methods differed in detail among laboratories, the methods are presented separately for each laboratory, designated laboratory A (D.R.H. and W.G.S.), laboratory B (C.B., J.W., L.J.F., and A.A.C.), and laboratory C (J.S.M., Z.W., and F.M.V.D.).
Laboratory A.
Two sets of experiments were conducted by laboratory A to determine the effect of an abrupt salinity shift from 36 to 27. In all of the experiments the cells were first preacclimated for 30–50 generations at their maximum growth rates in 36-salinity medium, using semicontinuous batch culture methods (17). A first set of experiments examined the response of three K. brevis strains (CCMP 2228, CCMP 2229, and SP3), using single control and low-salinity treatment cultures. For each strain, a 1-L culture of exponentially growing cells was split into separate sterilized polycarbonate bottles, which became the control and osmotic stress treatments. The osmotic stress culture was diluted with sterile Milli-Q water (containing the same nutrient enrichment as the 36-salinity medium) to adjust the salinity from 36 to 27. The 36-salinity control either received no further treatment [strain SP3, the one previously used by Errera and Campbell (22, 23)] or was diluted by four- to sixfold with fresh 36-salinity culture medium to maintain exponential growth of the cells. Treatment and control cultures were diluted with fresh media at appropriate times to ensure that the growth of the cells was not limited by nutrients or carbon dioxide (Fig. S1). These cultures were analyzed in triplicate for cell numbers, mean cell volume, brevetoxins, and chlorophyll a during an 11–22-d period (Fig. 1 and Fig. S1).
A second experiment used the same methods and procedures except that it examined a single strain (CCMP 2228) during a shorter period (48 h; Fig. 2) and used quadruplicate control and low-salinity shock treatments. Here a 1-L control culture was subdivided into eight 125-mL cultures; four of these cultures were diluted with nutrient-enriched Milli-Q water to adjust the salinity from 36 to 27, and the other four served as controls and were diluted with an equal volume of 36-salinity medium. Each control and treatment culture was then measured in duplicate for cell numbers and mean volume per cell and was measured singly for brevetoxins after 3 min and 3, 24, and 48 h. Because of the large number of replicate treatments and smaller culture volume, these cultures were not measured for chlorophyll a.
Cells in both experiments were cultured at 23°C on a 14 h:10 h daily light:dark cycle. Light was provided at an intensity of 120 µmol photons m−2 sec−1 from Duro-Test Vita Light fluorescent bulbs. Media consisted of 0.2-µm-filtered Gulf Stream seawater (salinity 36 or 27) amended with 64 µM NaNO3, 4 µM NaH2PO4, vitamins (0.074 nmol/L vitamin B12, 0.4 nmol/L biotin, and 60 nmol/L thiamine), 10 nmol/L Na2SeO3, and an EDTA-trace metal buffer system (42). The 36-salinity medium and nutrient-enriched Milli-Q water used for salinity adjustment were sterilized by microwave treatment (43). Culture pH was monitored throughout the experiments (17) to ensure no carbon dioxide limitation occurred. The pH ranged from 8.1 to 8.3.
Culture salinity in all experiments was measured using a Thermo Scientific Orion conductivity meter (model 135A), equipped with an Orion conductivity cell (model 013010A). Culture samples were measured for cell concentrations, mean volume per cell, and total cell volume per liter of culture with a Beckman Coulter Multisizer 3 electronic particle counter (17). Specific growth rates in the first set of experiments were computed from linear regressions of the natural log of total cell volume (per liter of culture) vs. time after correcting for culture dilution (44). Specific growth rates in the second experiment were determined from similar linear regressions of the natural log of cell concentration vs. time. Chlorophyll a was measured by filtering cells onto 25-mm GF/F filters (Whatman), extracting them with a 90:10 (vol/vol) acetone:water mixture, and measuring the extracted chlorophyll a with a Turner Design 10-AU fluorometer (45).
Brevetoxins were analyzed as described previously (17). For toxin analysis, cultures were gently mixed and 20-mL aliquots were removed and extracted using liquid/liquid separations with ethyl acetate. Before separations, culture aliquots were combined 1:1 by volume with ethyl acetate and the mixture was sonicated for 3 min to ensure total release of brevetoxins (17). Collected ethyl acetate fractions were desalted with Milli-Q water and concentrated with a rotovap. Concentrated fractions were measured for brevetoxins using an Agilent 1100 liquid chromatograph coupled to a Thermo-Finnigan TSQ Quantum triple quadrupole mass spectrometer with an electrospray ion source interface. The analytical conditions have been previously described in detail (46, 47). An external standard curve of purified brevetoxins 1, 2, and 3 (World Ocean Solutions) was used to quantify amounts of extracted brevetoxins.
Laboratory B.
Batch cultures of K. brevis clones TXB4, 257, 126, and a low-toxin culture line of the Wilson strain were grown at 23°C in 35-salinity GP/2 medium (48). The cultures were grown on a 12 h:12 h light:dark cycle with light provided from cool white fluorescent bulbs at an intensity of ∼215 µmol photons m−2 s−1. For each strain, a batch culture in late exponential phase was subdivided into eight portions. Four of these subcultures were stressed osmotically by adding dilute sterile seawater to lower the salinity from 35 to 27. Dilutions were made such that the initial cell abundance in all cultures was equal. This resulted in a more than twofold dilution of cells in the cultures and likely imposed a nutrient limitation, as the dilution water did not contain added nutrients. An equivalent amount of sterile 35-salinity seawater was added to the remaining four subcultures of each strain, which served as controls. Salinity was measured by a handheld refractometer (Fisher Scientific). Samples for cell counts and brevetoxin measurements were collected from the control and treatment cultures before and at the following times after the salinity decrease: 0.5 h, 3 h, 12 h, 1 d, 3 d, and 6 d.
To determine cell abundance, culture samples were preserved with Lugol’s solution (49) and stored in the dark until analysis. For each sample, at least 400 cells were counted in a Sedgewick-Rafter Chamber using a Zeiss Axiovert 100S inverted microscope. To analyze brevetoxins, duplicate 10-mL samples were filtered onto Whatman GF/F filters and extracted overnight in 2 mL of methanol at −80°C. Samples were then centrifuged (10 min at 3,200 g), and supernatants were stored in glass vials at −80°C until analysis. Brevetoxins were detected using an Acquity ultrahigh performance liquid chromatograph (UPLC) system (Waters) coupled to a Quattro micro API triple quadrupole mass spectrometer (Waters) operated in positive ionization mode. Chromatographic separations were performed on an Acquity UPLC BEH C18 column (100 × 2.1 mm, 1.7 µm), using a mobile phase consisting of water and acetonitrile with 0.1% acetic acid by volume. The elution gradient was 35% (vol/vol) acetonitrile with 0.1% acetic acid for 1.2 min, increasing to 80% at 8.6 min and 95% at 10 min, holding at 95% for 2 min, and returning to 35% at 12.5 min, with a 1.5-min equilibration before the next injection. Brevetoxins were detected via multiple-reaction monitoring, using optimized cone voltages and collisions energies for each of the following transitions: PbTx-1 m/z 867.4 > 849.5; PbTx-2 m/z 895.5 > 877.4; PbTx-3 m/z 897.5 > 725.3; PbTx-7 m/z 869.5 > 779.4; and oxidized-PbTx-2 m/z 911.8 > 875.3. Toxins were quantified using a 6-point calibration of pure brevetoxins (PbTx-1, -2, -3, -7, and oxidized-PbTx-2) purchased from Marbionc. PbTx-1 and PbTx-2 accounted for ∼98% of total brevetoxins. As a consequence, total cellular brevetoxins are expressed as the sum of PbTx-1 and PbTx-2.
Laboratory C.
Toxic and nontoxic cell lines of the Wilson strain of K. brevis were cultured at 25°C on a 16 h:8 h light:dark cycle with ∼190 µmol photons m−2 s−1 illumination from cool white fluorescent lights. Batch cultures were maintained in 250-mL DeLong flasks supplied with autoclaved, 20-µm-filtered, 35-salinity seawater amended with f/2 medium enrichments (50) with the following modifications: equimolar ferric sequestrene (Alpha Products, Danvers, MA) was used in place of EDTA and FeCl3, and 0.01 µM Na2SiO3 was added.
To initiate the experiments, sterile Milli-Q water was added to adjust the culture salinity to 27. An equivalent amount of salinity-35 seawater was added to the control cultures. The amended cultures were then gently rocked to mix. Salinity was measured using a refractometer (Fisher Scientific). For analysis of cell abundance and average volume per cell, 3-mL subsamples were removed from each replicate (n = 5) flask and fixed with glutaraldehyde just before and after the salinity shock, and again 2 h after the shock. The fixed samples were stored at 4°C until analysis. A Beckman Coulter Multisizer 3 Coulter Counter was used for triplicate measurements of cell concentrations and mean volume.
Samples for toxin analyses were collected 2 h after the salinity shift. Fifty-milliliter aliquots of culture were centrifuged at 600 × g for 10 min, and the cell pellet was resuspended in 500 µL methanol and stored at −20°C overnight. Liquid chromatography coupled with mass spectometry was performed on an Agilent 1100 HPLC (Agilent) coupled to an Applied Biosystems/MDS Sciex 4000 Q TRAP hybrid triple quadrupole/linear ion trap mass spectrometer equipped with a Turbo VTM source (Applied Biosystems), using selected ion monitoring or multiple-reaction monitoring in positive ion mode, as previously described (51). The detection limit using selected ion monitoring was ∼1.2 ng/mL for PbTx-1, 0.8 ng/mL for PbTx-2, and 1.2 ng/mL for PbTx-3 with a 10-µL injection. Using the multiple-reaction monitoring method, the detection limit was ∼0.9 ng/mL for PbTx-3 and 2 ng/mL for PbTx-1 and -2, with a 5-µL injection. The mass of all congeners was summed and divided by the measured cell concentration to give picograms brevetoxins per cell.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217716110/-/DCSupplemental.
References
- 1.Davis CC. Gymnodinium brevis sp. nov., a cause of discolored water and animal mortality in the Gulf of Mexico. Bot Gaz. 1948;109:358–360. [Google Scholar]
- 2.Baden DG, Bourdelais AJ, Jacocks H, Michelliza S, Naar J. Natural and derivative brevetoxins: Historical background, multiplicity, and effects. Environ Health Perspect. 2005;113(5):621–625. doi: 10.1289/ehp.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poli MA, Mende TJ, Baden DG. Brevetoxins, unique activators of voltage-sensitive sodium channels, bind to specific sites in rat brain synaptosomes. Mol Pharmacol. 1986;30(2):129–135. [PubMed] [Google Scholar]
- 4.Bourdelais AJ, Jacocks HM, Wright JLC, Bigwarfe PM, Jr, Baden DG. A new polyether ladder compound produced by the dinoflagellate Karenia brevis. J Nat Prod. 2005;68(1):2–6. doi: 10.1021/np049797o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming LE, Backer LC, Baden DG. Overview of aerosolized Florida red tide toxins: Exposures and effects. Environ Health Perspect. 2005;113(5):618–620. doi: 10.1289/ehp.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landsberg JH. The effects of harmful algal blooms on aquatic organisms. Rev Fish Sci. 2002;10:113–390. [Google Scholar]
- 7.Flewelling LJ, et al. Brevetoxicosis: Red tides and marine mammal mortalities. Nature. 2005;435(7043):755–756. doi: 10.1038/nature435755a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landsberg JH, Flewelling LJ, Naar J. Karenia brevis red tides, brevetoxins in the food web, and impacts on natural resources: Decadal advancements. Harmful Algae. 2009;8:598–607. [Google Scholar]
- 9.Heil CA, Steidinger KA. Monitoring, management and mitigation of Karenia blooms in the eastern Gulf of Mexico. Harmful Algae. 2009;8:611–617. [Google Scholar]
- 10.Kubanek J, Hicks MK, Naar J, Villareal TA. Does the red tide dinoflagellate Karenia brevis use allelopathy to outcompete other phytoplankton? Limnol Oceanog. 2005;50(3):883–895. [Google Scholar]
- 11.Prince EK, Myers TL, Kubanek J. Effects of harmful algal blooms on competitors: allelopathic mechanisms of the red tide dinoflagellate Karenia brevis. Limnol Oceanogr. 2008;53(2):531–541. [Google Scholar]
- 12.Cohen JH, Tester PA, Forward RB., Jr Sublethal effects of the toxic dinoflagellate Karenia brevis on marine copepod behavior. J Plank Res. 2007;29(3):301–315. [Google Scholar]
- 13.Waggett RJ, Hardison DR, Tester PA. Toxicity and nutritional inadequacy of Karenia brevis: Synergistic mechanisms disrupt top-down grazer control. Mar Ecol Prog Ser. 2012;444:15–30. [Google Scholar]
- 14.Hong J, et al. Algal toxins alter copepod feeding behavior. Plos ONE. 2012;7:e36845. doi: 10.1371/journal.pone.0036845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sunda WG, Graneli E, Gobler CJ. Positive feedback and the development and persistence of ecosystem disruptive algal blooms. J Phycol. 2006;42(5):963–974. [Google Scholar]
- 16. Lambers H, Chapin FS, Pons TL (2008) Plant Physiological Ecology (Springer, New York), p 610.
- 17.Hardison RD, Sunda WG, Wayne Litaker R, Shea D, Tester PA. Nitrogen limitation increases brevetoxins in Karenia brevis (Dinophyceae): Implications for bloom toxicity. J Phycol. 2012;48:844–858. doi: 10.1111/j.1529-8817.2012.01186.x. [DOI] [PubMed] [Google Scholar]
- 18.Hardison DR, Sunda WG, Shea D, Litaker RW. Increased toxicity of Karenia brevis during phosphate limited growth: Ecological and evolutionary implications. PLoS ONE. 2013;8(3):e58545. doi: 10.1371/journal.pone.0058545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lekan DK, Tomas CR. The brevetoxin and brevenal composition of three Karenia brevis clones at different salinities and nutrient conditions. Harmful Algae. 2010;9:39–47. [Google Scholar]
- 20.Plumley FG. Marine algal toxins: biochemistry, genetics, and molecular biology. Limnol Oceanogr. 1997;42(5):1252–1264. [Google Scholar]
- 21. Wright JLC (2004) Harmful Algae 2002, eds Steidinger KA, Landsberg JH, Tomas CR, Vargo GA (Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO, St. Petersburg, FL), pp 3–8.
- 22.Errera RM, Campbell L. Osmotic stress triggers toxin production by the dinoflagellate Karenia brevis. Proc Natl Acad Sci USA. 2011;108(26):10597–10601. doi: 10.1073/pnas.1104247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Errera RM, Campbell L. Correction for Errera and Campbell, Osmotic stress triggers toxin production by the dinoflagellate Karenia brevis. Proc Natl Acad Sci USA. 2012;109:17723–17724. doi: 10.1073/pnas.1104247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Errera RM, et al. Variation in brevetoxin and brevenal content among clonal cultures of Karenia brevis may influence bloom toxicity. Toxicon. 2010;55(2-3):195–203. doi: 10.1016/j.toxicon.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Brand LE, Campbell L, Bresnan E. Karenia: The biology and ecology of a toxic genus. Harmful Algae. 2012;14:156–178. doi: 10.1016/j.hal.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burkholder JM, Marshall HG. Toxigenic Pfiesteria species—Updates on biology, ecology, toxins, and impacts. Harmful Algae. 2012;14:196–230. [Google Scholar]
- 27.Turner JT, et al. Biogeographic effects of the Gulf of Mexico red tide dinoflagellate Karenia brevis on Mediterranean copepods. Harmful Algae. 2012;16:63–73. [Google Scholar]
- 28.Quinn GP, Keough MJ. Experimental Design and Data Analysis for Biologists. Cambridge, UK: Cambridge Univ Press; 2002. p. 537. [Google Scholar]
- 29.Valiela I. Doing Science: Design, Analysis, and Communication of Scientific Research. New York: Oxford Univ Press; 2001. [Google Scholar]
- 30.Ford ED. Scientific Method for Ecological Research. Cambridge, UK: Cambridge Univ Press; 2000. [Google Scholar]
- 31.da Silva JJR, Williams RJP. The Biological Chemistry of the Elements. Oxford: Clarendon Press; 1991. [Google Scholar]
- 32.Ehrlich PR, Raven PH. Butterflies and plants: A study in coevolution. Evolution. 1964;18:586–608. [Google Scholar]
- 33.Brodie EDIII, Brodie EDJ. Tetrodotoxin resistance in garter snakes: An evolutionary response of predators to dangerous prey. Evolution. 1990;44:651–659. doi: 10.1111/j.1558-5646.1990.tb05945.x. [DOI] [PubMed] [Google Scholar]
- 34.Geffeney S, Brodie ED, Jr, Ruben PC, Brodie ED., 3rd Mechanisms of adaptation in a predator-prey arms race: TTX-resistant sodium channels. Science. 2002;297(5585):1336–1339. doi: 10.1126/science.1074310. [DOI] [PubMed] [Google Scholar]
- 35.Agrawal AA. Natural selection on common milkweed (Asclepias syriaca) by a community of specialized insect herbivores. Evol Ecol Res. 2005;7:651–667. [Google Scholar]
- 36.Hayes RA, Crossland MR, Hagman M, Capon RJ, Shine R. Ontogenetic variation in the chemical defenses of cane toads (Bufo marinus): Toxin profiles and effects on predators. J Chem Ecol. 2009;35(4):391–399. doi: 10.1007/s10886-009-9608-6. [DOI] [PubMed] [Google Scholar]
- 37.Duffy JE, Hay ME. Herbivore resistance to seaweed chemical defense - the roles of mobility and predation risk. Ecology. 1994;75:1304–1319. [Google Scholar]
- 38.Lindquist N. Chemical defense of early life stages of benthic marine invertebrates. J Chem Ecol. 2002;28(10):1987–2000. doi: 10.1023/a:1020745810968. [DOI] [PubMed] [Google Scholar]
- 39.Turner JT, Tester PA. Toxic marine phytoplankton, zooplankton grazers, and pelagic food webs. Limnol Oceanogr. 1997;42:1203–1214. [Google Scholar]
- 40.Teegarden GJ, Campbell RG, Durbin EG. Zooplankton feeding behavior and particle selection in natural plankton assemblages containing toxic Alexandrium spp. Mar Ecol Prog Ser. 2001;218:213–226. [Google Scholar]
- 41.Ianora A, et al. New trends in marine chemical ecology. Est Coasts. 2006;29(4):531–551. [Google Scholar]
- 42.Sunda WG, Price N, Morel F. In: Algal Culturing Techniques. Andersen RA, editor. London: Elsevier; 2005. pp. 35–63. [Google Scholar]
- 43.Keller MD, Bellows WK, Guillard RRL. Microwave treatment for sterilization of phytoplankton culture media. J Exp Mar Biol Ecol. 1988;117:279–283. [Google Scholar]
- 44.Sunda WG, Hardison R. Ammonium uptake and growth limitation in marine phytoplankton. Limnol Oceanogr. 2007;52:2496–2506. [Google Scholar]
- 45.Welschmeyer NA. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol Oceanogr. 1994;39:1985–1992. [Google Scholar]
- 46.Mendoza WG, Mead RN, Brand LE, Shea D. Determination of brevetoxin in recent marine sediments. Chemosphere. 2008;73(8):1373–1377. doi: 10.1016/j.chemosphere.2008.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng YS, et al. Characterization of marine aerosol for assessment of human exposure to brevetoxins. Environ Health Perspect. 2005;113(5):638–643. doi: 10.1289/ehp.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blackburn SI, Bolch CJS, Haskard KA, Hallegraeff GM. Reproductive compatibility among four global populations of the toxic dinoflagellate Gymnodinium catenatum (Dinophyceae) Phycologia. 2001;40:78–87. [Google Scholar]
- 49.Throndsen J. In: Phytoplankton Manual. Sournia A, editor. Paris: United Nations Educational, Scientific and Cultural Organization; 1978. [Google Scholar]
- 50.Guillard RRL. In: Handbook of Phycological Methods - Culture Methods, and Growth Measurements. Stein JR, editor. Cambridge, UK: Cambridge Univ Press; 1973. pp. 69–85. [Google Scholar]
- 51.Monroe EA, Johnson JG, Wang Z, Pierce RK, Van Dolah FM. Characterization and expression of nuclear-encoded polyketide synthases in the brevetoxin-producing dinoflagellate Karenia brevis. J Phycol. 2010;46:541–552. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.