Abstract
Reactive iron and organic carbon are intimately associated in soils and sediments. However, to date, the organic compounds involved are uncharacterized on the molecular level. At redox interfaces in peatlands, where the biogeochemical cycles of iron and dissolved organic matter (DOM) are coupled, this issue can readily be studied. We found that precipitation of iron hydroxides at the oxic surface layer of two rewetted fens removed a large fraction of DOM via coagulation. On aeration of anoxic fen pore waters, >90% of dissolved iron and 27 ± 7% (mean ± SD) of dissolved organic carbon were rapidly (within 24 h) removed. Using ultra-high-resolution MS, we show that vascular plant-derived aromatic and pyrogenic compounds were preferentially retained, whereas the majority of carboxyl-rich aliphatic acids remained in solution. We propose that redox interfaces, which are ubiquitous in marine and terrestrial settings, are selective yet intermediate barriers that limit the flux of land-derived DOM to oceanic waters.
Keywords: carbon cycle, wetland restoration, carbon sequestration, phenolics
Peatlands store about 30% of the global soil carbon pool (1). Therefore, the export of organic carbon from peatlands plays an important role in the global carbon cycle. Originally, peatlands covered about 495,000 km2 of Europe, or about 5% of the total land area. Today, ∼60% of these peatlands are drained [in some countries, more than 90% (e.g., Great Britain, France, The Netherlands, Germany)], mostly for agricultural use (2). The recent practice of fen rewetting to restore the natural function of the peatlands as a carbon sink, however, has turned many drained mires from oxic into anoxic systems, thereby releasing large amounts of dissolved organic carbon (DOC), phosphorus, and iron (3). Rewetting and water table rise may increase concentrations of nutrients (ammonia and phosphate), as well as DOC, to values of one to three orders of magnitude higher compared with undisturbed mires and may remain at elevated levels for years (3). The intense mobilization of dissolved organic matter (DOM) and phosphate is a result of the reductive dissolution of ferric oxides (3, 4). As a consequence, adjacent surface waters can be subject to increased phosphate and DOC loadings.
The reprecipitation of the mobilized iron at the redox interface leads to an enrichment of reactive iron phases at the surface of the fens (5) and potentially acts as a barrier for DOC and phosphate through coprecipitation or adsorption. Because DOM has a large affinity for freshly precipitating Fe(III) (6) but not for Fe(II) (7), coagulation of Fe with DOM is observed (8). Such iron/organic carbon associations have recently been suggested to promote the long-term stabilization of sedimentary organic matter (9). Although the nature of Fe(III) precipitates has been extensively studied, not much is known about the quality of DOM cycling at such redox interfaces. Because freshly precipitating Fe(hydr)oxides preferentially remove phenolic and condensed aromatic organic matter (10, 11), we propose here that redox interfaces may be important barriers for terrestrially derived DOM.
To test our hypothesis, we studied the interactions of Fe and DOM at the redox interface of two rewetted fens in northeastern Germany, where an intense cycling between Fe and DOM has been observed (12). We retrieved samples of surface and pore waters for analysis of total dissolved iron, organic carbon, and the molecular composition of DOM using ultra-high-resolution MS. We further conducted experiments to test the coagulation potential of Fe and DOM in anoxic fen pore waters on oxygenation. A detailed description of the two study sites, sampling procedures, and analytical methods applied is given in Materials and Methods.
Results and Discussion
At both study sites (“Zarnekow” site and “Wendewiesen” site), we found dissolved iron concentrations in the anoxic pore waters to be one to three orders of magnitude higher than in the oxic surface waters (Table 1). We observed 79–85% and 58–60% lower DOC concentrations in surface waters at both sites, respectively, compared with the pore waters, which is consistent with previous observations for other rewetted fens (12). For a detailed molecular characterization of DOM, we used electrospray ionization (ESI) Fourier transform (FT) ion cyclotron resonance (ICR) MS, which resolves the molecular composition of DOM at the highest possible level to date. The largest fraction of DOM in the investigated fens is composed of organic acids and neutrals (12) making ESI-FT-ICR-MS run in negative ionization mode an ideal tool with which to study DOM molecular composition. Through this method, we obtained >4,000 molecular formulas of individual compounds per sample. Roughly half of all molecular formulas (45–51%) found at Wendewiesen were organic acids containing only carbon, hydrogen, and oxygen atoms (CHO). About one-third contained one or two nitrogen atoms (CHON1 and CHON2, respectively), and 16–19% contained one sulfur atom (CHOS1) (Table 2). To our knowledge, this is the largest contribution of nitrogen- and sulfur-containing compounds in an environmental DOM sample analyzed by FT-ICR-MS reported to date. The abundance of phosphorus-containing formulas was very low (∼1% and less per sample). Hydrogen-to-carbon (H/C) ratios and oxygen-to-carbon (O/C) ratios of all formulas found varied between 0.3 and 2.7 and between 0.01 and 1, respectively. The relative abundances of H/C and O/C ratios sorted by CHO, CHON1, CHON2, and CHOS1 compounds are shown in Figs. S1 and S2. A complete molecular characterization of the DOM is summarized in Table 2.
Table 1.
Dissolved oxygen, Fe, and DOC in surface and pore waters of the investigated fens
| Zarnekow |
Wendewiesen |
|||||
| Parameter | Surface water | Pore water, 5–10 cm | Pore water, 55–60 cm | Surface water | Pore water, 5–10 cm | Pore water, 55–60 cm |
| O2, μM | 215.6 | n.d. | n.d. | 121.9 | n.d. | n.d. |
| Fe, μM | 0.7 | 195.4 | 830.5 | 21.4 | 257.5 | 224.1 |
| DOC, mM | 1.3 | 5.9 | 8.6 | 2.4 | 6.0 | 5.8 |
| DOC/Fe | 1,750 | 30 | 10 | 113 | 23 | 26 |
n.d., not detectable.
Table 2.
Summary of molecular composition of surface and pore water samples from Wendewiesen and the oxygenation experiment from Zarnekow
| Parameter | Surface | Pore water, 5–10 cm | Pore water, 55–60 cm | Pore water, acidified | Pore water, Fe-precipitated |
| O/C ratio | 0.43 | 0.43 | 0.44 | 0.42 | 0.41 |
| H/C ratio | 1.12 | 1.08 | 1.05 | 1.13 | 1.17 |
| N/C ratio | 0.10 | 0.09 | 0.08 | 0.09 | 0.09 |
| S/C ratio | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| P/C ratio | 0.05 | 0.04 | 0.05 | 0.04 | 0.04 |
| CHO, % | 45 | 45 | 51 | 48 | 46 |
| CHON, % | 33 | 35 | 31 | 34 | 32 |
| CHON1, % | 18 | 19 | 20 | 20 | 20 |
| CHON2, % | 10 | 11 | 9 | 10 | 9 |
| CHOS1, % | 17 | 14 | 15 | 13 | 15 |
| CHONS, % | 2 | 3 | 1 | 2 | 3 |
| CHOP, % | 1 | <1 | 1 | 1 | 1 |
| Other, % | 2 | 4 | 2 | 3 | 4 |
| Aromatic, % | 27.9 | 31.9 | 34.8 | 25.7 | 20.0 |
| Condensed aromatic, % | 8.6 | 9.7 | 10.6 | 6.3 | 3.6 |
Elemental ratios are number-averaged for all formulas found per sample. The percentages of aromatic and condensed aromatic compounds per sample were identified using the modified AI (13). CHON, all compounds containing at least one nitrogen (including CHON1–2S1 and CHON1–2P1); CHONS, compounds containing carbon, hydrogen, oxygen, nitrogen, and sulfur; CHOP, compounds containing carbon, hydrogen, oxygen, and phosphorus; N/C ratio, nitrogen-to-carbon ratio; P/C ratio, phosphorus-to-carbon ratio; S/C ratio, sulfur-to-carbon ratio.
Although the O/C ratios of all CHO compounds were similar in surface and pore water, the relative frequency of compounds with ratios below 1–1.5 was lower in the oxic surface water compared with the pore waters (Figs. S1, S2, and S3 A–D ). Similar trends were observed for CHON1 and CHON2 compounds [i.e., there were fewer dissolved organic nitrogen compounds with H/C ratios <1 in the surface water compared with the pore waters]. Dissolved organic sulfur showed no clear trend.
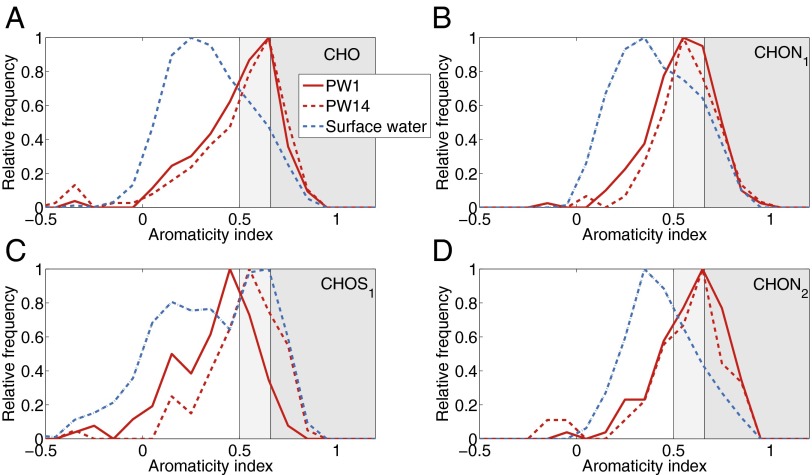
The observed differences in H/C ratios indicated that aromaticity played an important role in the cycling of DOM at the redox interface. Using an aromaticity index (AI) (13), we identified about 28% of all formulas (including nitrogen- and sulfur-containing formulas) in the oxic surface water and up to 35% in the pore waters as aromatic (i.e., phenolic compounds indicative of vascular plant remains). Nine percent to 11% were identified as condensed aromatic compounds that are derived from combustion processes. The relative frequency of aromatic CHO, CHON1, and CHON2 formulas was higher in the anoxic pore waters compared with the oxic surface water (Fig. 1), which is indicative of selective removal of these compounds at the redox interface. For sulfurized molecules, the trend was less clear.
Fig. 1.
Differences in the relative abundance of compounds of different aromaticity at Wendewiesen. All compounds detected in the pore waters at 5–10 cm (PW1) and 55–60 cm (PW14), respectively, that were not detected in the surface water are shown in red. The complete surface water sample is shown in blue for comparison. Aromaticities are indicated as follows: nonaromatic compounds (AI < 0.5, white), aromatic compounds (0.5 < AI < 0.66, light gray), and condensed aromatic compounds (AI > 0.66, dark gray shading). We show normalized abundances to facilitate comparison of the samples. (A) Compounds containing carbon, hydrogen, and oxygen. (B and D) Compounds containing one or two additional nitrogen atoms, respectively. (C) Compounds containing one additional sulfur atom.
A decrease in the abundance of aromatic compounds in the surface water may occur through coagulation with oxidizing Fe (10, 11) diffusing from anoxic pore waters (12), microbial degradation, or photooxidation (14–16). Each of these processes should be identifiable in our data because all leave a distinct molecular fingerprint on the composition of DOM. Photodegradation results in increasing relative abundances of more aliphatic compounds and a shift toward higher O/C ratios because aromatic compounds are transformed into more saturated and oxygenated compounds on irradiation (15, 16). Although we found that aromatic compounds were removed, O/C ratios did not show any significant differences among the samples, except for sulfurized compounds. This suggests that no oxygenation of DOM has occurred (Fig. S2). Because of the molecular evidence, and given that the turbidity of the colored fen waters reduces the penetration of sunlight, we ruled out photodegradation as an important removal pathway above the redox interface.
Microbial degradation may be another possibility. However, microbes tend to degrade more saturated, as well as nitrogen-containing, compounds (17, 18). Because we did not find clear evidence for preferential removal of nitrogen-containing compounds (Table 2), and given that aromatic moieties are generally less susceptible to microbial attack, we may rule this process out as well.
The third possibility is removal of DOM by Fe(III)-induced coagulation (10, 11). At DOC/Fe molar ratios of >10, aromatic DOM is preferentially removed from solutions (10, 11). At lower DOC/Fe ratios, more oxygenated compounds start to disappear (10) as Fe(hydr)oxides form inner-sphere complexes with carboxyl groups, resulting in relatively stable Fe-DOM flocs (19). With pore water DOC/Fe ratios ranging between 10 and 30 (Table 1) and almost unaffected O/C ratios (Table 2 and Fig. S2), our data are therefore consistent with iron-induced removal of aromatic DOM at the redox interface.
This process is relatively rapid. Aeration of anoxic pore water samples from rewetted fens leads to precipitation of Fe(III) and removal of DOM within a few minutes (12), which is much quicker than microbial mineralization of DOM. To estimate the coagulation potential of dissolved Fe and DOM in the pore waters, we repeated the oxygenation experiments of Zak et al. (12) with pore water samples taken at both study sites during several field trips (additional information is provided in Materials and Methods). Initial molar ratios of DOC to dissolved Fe ranged between 9 and 33 (Table S1). After 24 h of aeration 92–99% (mean ± SD: 97 ± 2%, n = 41) of the dissolved Fe and 10–44% (mean ± SD: 27 ± 7%) of the DOC were removed (Table S1), illustrating the efficiency of this retention process.
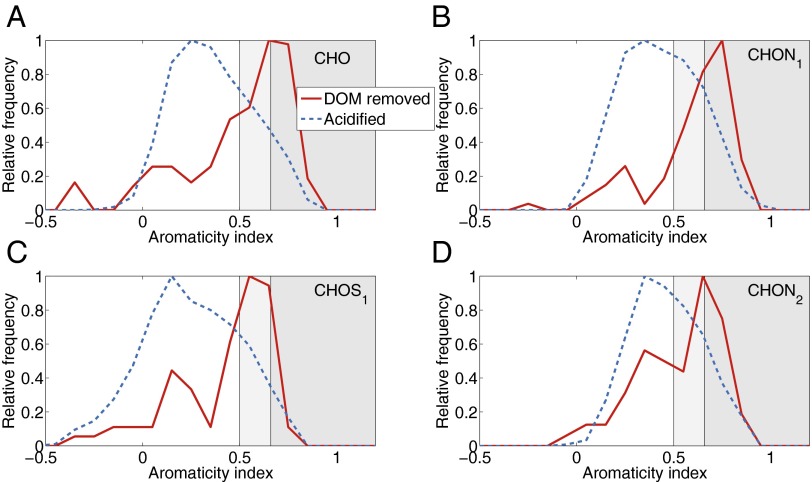
We further analyzed the effect of oxygenation on the molecular composition of pore water DOM. One aliquot of a pore water sample from Zarnekow was acidified to pH 2 with hydrochloric acid to prevent oxidation of Fe(II) and subsequent precipitation as Fe(III). A second aliquot was aerated and allowed Fe(III)-DOM to coagulate. After Fe had precipitated, the supernatant was sampled, acidified, and further processed. The artificially induced oxidation resulted in differences in molecular composition that were comparable to those observed between surface and pore waters. Aromatic moieties were preferentially removed on aeration (Fig. 2, Fig. S3, and Table 2). These findings are consistent with what would be expected based on previous laboratory studies (10, 11).
Fig. 2.
Results of the oxygenation experiment at Zarnekow. Compounds removed after coagulation with Fe following aeration are shown in red. The directly acidified pore water sample is shown in blue for comparison. Shaded areas are as in Fig. 1. (A–D) Compound classes sorted as in Fig. 1.
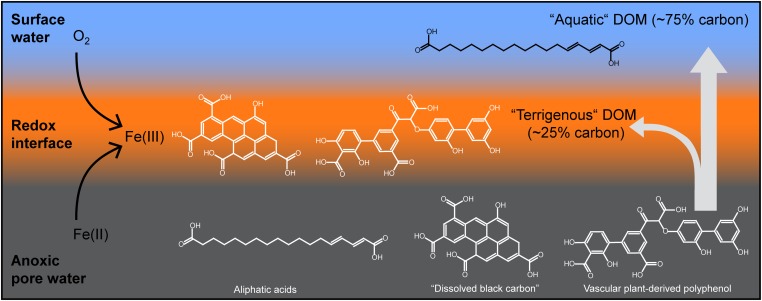
The interactions of Fe with aromatic DOM at the redox interface of the fens described in this study are illustrated in Fig. 3. The reallocation of iron to the oxic surface is well documented in the solid phase because there is threefold more Fe (based on dry mass) in the surface peat layer than in the deeper layers of the fen at Zarnekow (3).
Fig. 3.
Schematic diagram of the iron trap at redox interfaces depicting the export of anoxic peat pore water, oxidation of iron at the oxic surface, and coprecipitation of terrestrial DOM with Fe(III). Examples of molecular structures are shown for representative molecular formulas that were selectively retained at the redox interface or passed into the water column. Although many more possible structural isomers exist, the examples illustrate the selective retention of phenolic acids in conjunction with iron precipitation.
A previous study on pore water DOM of a pristine fen characterized with FT-ICR-MS also reported higher degrees of aromaticity in deeper pore waters (20), indicating that the process we observe may also operate in natural fens.
About 4–11% of all molecular formulas in our study had a condensed aromatic structure resulting from thermal alteration of natural organic matter (21). This compound class is termed pyrogenic or black carbon, and represents a relatively stable pool of organic carbon that accumulates in soils (22) and ocean water (23) because of its apparent recalcitrance. Once considered as being inert, recent research has indicated that partial oxidation turns soil black carbon into dissolved black carbon (DBC) through the addition of oxygen functional groups (24, 25). The presence of soluble nitrogen- and sulfur-containing condensed aromatic compounds has not been reported for peat pore waters before and is highly interesting because it raises questions about the origin and the possibility of dissolved black sulfur and dissolved black nitrogen (DBN). FT-ICR-MS provides only limited structural information, such that we cannot unambiguously identify whether N is ring-bound. However, the similar distribution of the AI for CHO, CHON1, and CHON2 compounds suggests similar processing of these compounds at redox interfaces. The cycling of both DBC and DBN may therefore be coupled to the redox chemistry of Fe. This is an important finding because the sequestration of black carbon with Fe(hydr)oxides has been postulated before (22) but direct field evidence has been lacking to date. The “rusty carbon sink” (9) appears to be a specific sink for aromatic and condensed aromatic compounds.
Because adsorption of aromatic DOM to Fe(hydr)oxides may be reversible under anoxic conditions (4), the iron trap described here may only temporarily retain DBC. For example, variations in depth of the redox interface following water table fluctuations (induced by climatic variations or human activity) (5) may cause either release or reallocation of DBC within the soil profile. Unless DOM forms a “protective layer” that prevents the reductive dissolution of Fe oxides, we propose that redox interfaces should be considered as intermediate pools for DBC.
Although the importance of iron oxides for the cycling of DOM has long been known (e.g., refs. 26, 27), only recently has the role of sedimentary reactive iron phases for organic carbon storage been estimated to be quantitatively important on a global scale (9). Our study now shows that at redox interfaces, Fe selectively traps vascular plant-derived and pyrogenic DOM. Because redox interfaces are ubiquitous in the environment (e.g., in the pore waters of lakes, estuaries, tidal flats, and marine sediments; in the water column of anoxic basins, such as the Black Sea and Chesapeake Bay), the coupled cycling of iron and terrigenous DOM at oxic-anoxic transitions may explain some of the “missing” terrestrially derived DOM in the oceans (28, 29). Given the reactive nature of iron hydroxides, future studies on the age of the organic carbon associated with iron are needed to assess the potential of this sink for long-term storage.
Materials and Methods
Study Sites.
Study sites were Zarnekow (53°52′N and 12°53′E) and Wendewiesen (53°56′N and 12°57′E). Both fens are characterized by groundwater through-flow and strongly decomposed peat in the upper 0.3 m due to the drainage and land use history, followed by up to 10 m of less decomposed peat (30). After rewetting in 2002 and 2004, respectively, the fens were permanently inundated by about 0.1–0.5 m of water. The vegetation of both fens is dominated by dense stands of Typha latifolia, Phragmites australis, and/or tall sedges. The open shallow water areas are mainly vegetated by submerged macrophytes, such as Ceratopyllum demersum.
Sampling Procedure and Oxygenation Experiment.
Anoxic pore water samples were taken using the dialysis sampler technique (31) to analyze different chemical parameters (DOC and Fe). Dialysis samplers are thin Perspex plates (70 × 16 × 2.5 cm) covered by a 0.2-μm polysulfone membrane (HT-Tuffryn 200; Pall Gelman Laboratory) with chambers filled with deionized water. Before insertion, oxygen from the chamber water and the sampler material (Perspex) was displaced by degassing with nitrogen for 24 h. For that purpose, samplers were stored in watertight PVC vessels (diameter of 25 cm and length of 80 cm) filled completely with deionized water. After degassing, vessels were sealed with airtight cups for transportation to the sampling sites. Three dialysis samplers with 14 chambers (total volume of 0.7 L) were always inserted completely into the upper horizon of the peat soil (0–60 cm) in each of the sampling sites. The samplers were used to obtain integrated pore water samples by combining the 14 chambers with a composite sample in a 1-L polyethylene bottle for the measurement of the above-mentioned parameters and to simulate the retention of DOM at the fen surface due to redox change and precipitation of Fe(III) hydroxides (see below). Additionally, subsamples for further analysis (Fe, DOC, and molecular composition of DOM) were obtained from the first and last chambers of the dialysis sampler, representing a soil depth of 5–10 cm and 55–60 cm, respectively. According to the vertical zoning of peat layers, the shallowest chamber was exposed to highly decomposed peat and the deepest chamber was exposed to moderately decomposed peat. The exposure time of the samplers in the water-saturated peat was at least 14 d so that the concentrations of dissolved substances in the pore water could equilibrate with the chamber water (31). After recovering and cleaning the samplers with deionized water, the water in the dialysis chambers was taken rapidly by penetrating the membrane with a 50-mL multipette (Eppendorf). By doing so, oxygen contact with the anoxic pore water samples was negligible at this sampling step. Overall, oxidative changes of the redox-sensitive samples (8) were avoided through fast sampling and immediate chemical fixation of subsamples for Fe and DOM/DOC analysis with 0.5 mL of 14 M HNO3 or 0.5 mL of 1 M HCl, respectively.
The rest of the samples were aerated by occasional shaking to induce oxygen saturation and to simulate the oxidation processes at the anoxic/oxic interface at the peat surface. The time to complete the oxygen-induced precipitation was adjusted according to results from prior investigations (12). Accordingly, a new chemical equilibrium was assumed to become established after approximately 24 h at the latest for samples from fens under investigation.
Analytical Methods.
Determination of total dissolved iron was done using inductively coupled plasma optical emission spectrometry (715-ES; Varian). Fe concentrations of the aerated and nonaerated samples were determined using flame atomic absorption spectrometry (3300; Perkin–Elmer). All DOC concentrations were measured as nonpurgable organic matter via thermocatalytic combustion with a C-Analyzer (TOC 5000; Shimadzu). Precision and accuracy were better than 5% for all the above-mentioned methods.
Details of the ESI-FT-ICR-MS method are given by Riedel et al. (10). Briefly, DOM was purified via solid phase extraction at pH 2 (acidified with ultrapure HCl) using Varian Bond Elute PPL cartridges (100 mg) as the solid phase following the procedure described by Dittmar et al. (32). Samples were ionized via ESI in negative mode. Five hundred broadband scans were accumulated. Peaks were internally calibrated. Differences in samples are based on presence/absence of peaks in the mass spectra. The limit of detection for the applied method was calculated as a signal-to-noise ratio of 9.8 (10). Molecular formulas were assigned using self-written software that basically follows the procedures and suggestions made by Koch et al. (33). Mass spectra were internally calibrated using an in-house mass reference list during each run. Calibrated mass accuracy was better than 300 parts per billion (ppb; the rms error of the mass accuracy per sample is usually below 100 ppb). With the method applied, we routinely obtain number-averaged elemental ratios with an error of less than 1% (expressed as relative SD based on triplicate analysis of samples) in our laboratory. An example of the precision of the method is illustrated in Fig. S4. A modified aromaticity index (AI) to identify aromatic as well as condensed aromatic compounds was calculated, accounting for heteroatoms according to the method of Koch and Dittmar (13). The relative SD of fractions with different aromaticities (AI, %) was <3%.
Supplementary Material
Acknowledgments
We thank Katrin Klaproth for assistance with the FT-ICR-MS, Petra Schmidt and Ina Ulber for general assistance in the laboratory, and colleagues Antje Lüder and Miriam Schneider (Leibniz-Institut für Gewässerökologie und Binnenfischerei) for fieldwork. Two anonymous reviewers provided comments that greatly improved the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221487110/-/DCSupplemental.
References
- 1.Limpens J, et al. Peatlands and the carbon cycle: From local processes to global implications a synthesis. Biogeosciences. 2008;5:1475–1491. [Google Scholar]
- 2.Joosten H. Mires in Europe: A preliminary status report. International Mire Conservation Group Members Newsletter. 1997;3:10–13. [Google Scholar]
- 3.Zak D, Gelbrecht J. The mobilization of phosphorus, organic carbon and ammonium in the initial stage of fen rewetting. Biogeochemistry. 2007;85:141–151. [Google Scholar]
- 4.Chin Y-P, Traina SJ, Swank CR, Backhus D. Abundance and properties of dissolved organic matter in pore waters of a freshwater wetland. Limnol Oceanogr. 1998;43(6):1287–1296. [Google Scholar]
- 5.Koretsky CM, Haas JR, Ndenga NT, Miller D. Seasonal variations in vertical redox stratification and potential influence on trace metal speciation in minerotrophic peat sediments. Water Air Soil Pollut. 2006;173(1–4):373–403. [Google Scholar]
- 6.Gregory J, Duan J. Hydrolyzing metal salts as coagulants. Pure Appl Chem. 2001;73(12):2017–2026. [Google Scholar]
- 7.Nierop KGJ, Jansen B, Verstraten JM. Dissolved organic matter, aluminium and iron interactions: Precipitation induced by metal/carbon ratio, pH and competition. Sci Total Environ. 2002;300(1-3):201–211. doi: 10.1016/s0048-9697(02)00254-1. [DOI] [PubMed] [Google Scholar]
- 8.Orem WH, Gaudette HE. Organic matter in anoxic marine pore water: Oxidation effects. Org Geochem. 1984;5(4):175–181. [Google Scholar]
- 9.Lalonde K, Mucci A, Ouellet A, Gélinas Y. Preservation of organic matter in sediments promoted by iron. Nature. 2012;483(7388):198–200. doi: 10.1038/nature10855. [DOI] [PubMed] [Google Scholar]
- 10.Riedel T, Biester H, Dittmar T. Molecular fractionation of dissolved organic matter with metal salts. Environ Sci Technol. 2012;46(8):4419–4426. doi: 10.1021/es203901u. [DOI] [PubMed] [Google Scholar]
- 11.Christl I, Kretzschmar R. C-1s NEXAFS spectroscopy reveals chemical fractionation of humic acid by cation-induced coagulation. Environ Sci Technol. 2007;41(6):1915–1920. doi: 10.1021/es062141s. [DOI] [PubMed] [Google Scholar]
- 12.Zak D, Gelbrecht J, Steinberg CEW. Phosphorus retention at the redox interface of peatlands adjacent to surface waters in northeast Germany. Biogeochemistry. 2004;70:357–368. [Google Scholar]
- 13.Koch BP, Dittmar T. From mass to structure: An aromaticity index for high-resolution mass data of natural organic matter. Rapid Commun Mass Spectrom. 2006;20:926–993. [Google Scholar]
- 14.Opsahl S, Benner R. Photochemical reactivity of dissolved lignin in river and ocean waters. Limnol Oceanogr. 1998;43(6):1297–1304. [Google Scholar]
- 15.Gonsior M, et al. Photochemically induced changes in dissolved organic matter identified by ultrahigh resolution fourier transform ion cyclotron resonance mass spectrometry. Environ Sci Technol. 2009;43(3):698–703. doi: 10.1021/es8022804. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt-Koplin P, Hertkorn N, Schulten HR, Kettrup A. Structural changes in a dissolved soil humic acid during photochemical degradation processes under O2 and N2 atmosphere. Environ Sci Technol. 1998;32(17):2531–2541. [Google Scholar]
- 17.Kim S, Kaplan LA, Hatcher PG. Biodegradable dissolved organic matter in a temperate and a tropical stream determined from ultra-high resolution mass spectrometry. Limnol Oceanogr. 2006;51(2):1054–1063. [Google Scholar]
- 18.Meng F, Huang G, Li Z, Li S. Microbial transformation of structural and functional makeup of human-impacted riverine dissolved organic matter. Ind Eng Chem Res. 2012;51(17):6212–6218. [Google Scholar]
- 19.Henneberry YK, Kraus TEC, Nico PS, Horwarth WR. Structural stability of coprecipitated natural organic matter and ferric iron under reducing conditions. Org Geochem. 2012;48:81–89. [Google Scholar]
- 20.D’Andrilli J, Chanton JP, Glaser PH, Cooper WT. Characterization of dissolved organic matter in northern peatland soil porewaters by ultra high resolution mass spectrometry. Org Geochem. 2010;41:791–799. [Google Scholar]
- 21.Knicker H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry. 2007;85:91–118. [Google Scholar]
- 22.Czimczik CI, Masiello CA. Controls on black carbon storage in soils. Global Biogeochem Cycles. 2007;21(3):GB3005. [Google Scholar]
- 23.Dittmar T, Paeng J. A heat-induced molecular signature in marine dissolved organic matter. Nat Geosci. 2009;2:175–179. [Google Scholar]
- 24.Haumaier L, Zech W. Black carbon—Possible source of highly aromatic components of soil humic acids. Org Geochem. 1995;23:191–196. [Google Scholar]
- 25.Cheng C-H, Lehmann J, Engelhard MH. Natural oxidation of black carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochim Cosmochim Acta. 2008;72(6):1598–1610. [Google Scholar]
- 26.Tipping E. The adsorption of aquatic humic substances by iron oxides. Geochim Cosmochim Acta. 1980;45(2):191–199. [Google Scholar]
- 27.McKnight D, et al. Sorption of dissolved organic carbon by hydrous aluminum and iron oxides occurring at the confluence of Deer Creek with the Snake River, Summit County, Colorado. Environ Sci Technol. 1992;26(7):1388–1396. [Google Scholar]
- 28.Hedges JI, Keil RG, Benner R. What happens to terrestrial organic matter in the ocean? Org Geochem. 1997;27:195–212. [Google Scholar]
- 29.Bianchi TS. The role of terrestrially derived organic carbon in the coastal ocean: A changing paradigm and the priming effect. Proc Natl Acad Sci USA. 2011;108(49):19473–19481. doi: 10.1073/pnas.1017982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zak D, Wagner C, Payer B, Augustin J, Gelbrecht J. Phosphorus mobilization in rewetted fens: The effect of altered peat properties and implications for their restoration. Ecol Appl. 2010;20(5):1336–1349. doi: 10.1890/08-2053.1. [DOI] [PubMed] [Google Scholar]
- 31.Steinmann P, Shotyk W. Sampling anoxic porewaters in peatlands using ‘peeper’ for in situ-filtration. Fresenius J Anal Chem. 1996;354:709–713. doi: 10.1007/s0021663540709. [DOI] [PubMed] [Google Scholar]
- 32.Dittmar T, Koch BP, Hertkorn N, Kattner G. A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater. Limnol Oceanogr Methods. 2008;6:230–235. [Google Scholar]
- 33.Koch BP, Dittmar T, Witt M, Kattner G. Fundamentals of molecular formula assignment to ultrahigh resolution mass data of natural organic matter. Anal Chem. 2007;79(4):1758–1763. doi: 10.1021/ac061949s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.