Significance
Cancer stem cells (CSCs) frequently acquire the ability to self-renew and persist in their hosts by coopting normal stem cell programs. Blast crisis (BC) chronic myeloid leukemia is a prototypic example, as the acquired activation of β-catenin signaling that enables BC CSC function is also important in normal hematopoietic stem cell maintenance. In identifying eIF4E phosphorylation by the MNK kinases as a necessary step in β-catenin activation in BC CSCs, but not normal hematopoietic stem cells, we define a therapeutic target in BC. Our studies suggest that clinical trials with MNK kinase inhibitors are warranted in BC chronic myeloid leukemia.
Keywords: cancer stem cell, Wnt pathway, xenograft, biomarker
Abstract
Chronic myeloid leukemia responds well to therapy targeting the oncogenic fusion protein BCR-ABL1 in chronic phase, but is resistant to treatment after it progresses to blast crisis (BC). BC is characterized by elevated β-catenin signaling in granulocyte macrophage progenitors (GMPs), which enables this population to function as leukemia stem cells (LSCs) and act as a reservoir for resistance. Because normal hematopoietic stem cells (HSCs) and LSCs depend on β-catenin signaling for self-renewal, strategies to specifically target BC will require identification of drugable factors capable of distinguishing between self-renewal in BC LSCs and normal HSCs. Here, we show that the MAP kinase interacting serine/threonine kinase (MNK)-eukaryotic translation initiation factor 4E (eIF4E) axis is overexpressed in BC GMPs but not normal HSCs, and that MNK kinase-dependent eIF4E phosphorylation at serine 209 activates β-catenin signaling in BC GMPs. Mechanistically, eIF4E overexpression and phosphorylation leads to increased β-catenin protein synthesis, whereas MNK-dependent eIF4E phosphorylation is required for nuclear translocation and activation of β-catenin. Accordingly, we found that a panel of small molecule MNK kinase inhibitors prevented eIF4E phosphorylation, β-catenin activation, and BC LSC function in vitro and in vivo. Our findings identify the MNK–eIF4E axis as a specific and critical regulator of BC self-renewal, and suggest that pharmacologic inhibition of the MNK kinases may be therapeutically useful in BC chronic myeloid leukemia.
Chronic phase chronic myeloid leukemia (CML) is a myeloproliferative disorder that is characterized by the presence of the fusion gene BCR-ABL1 in primitive progenitors (1). BCR-ABL1 encodes a constitutively active tyrosine kinase that is causative for the condition (1), and, accordingly, therapeutic inhibition of BCR-ABL1 kinase elicits excellent responses in chronic phase (2). In contrast, myeloid blast crisis (BC) CML does not respond to BCR-ABL1 tyrosine kinase inhibitors (TKIs), suggesting that additional transforming events contribute to the BC phenotype (3). Several of these factors have recently been identified to be critical to BC pathogenesis, and include the acquisition of a β-catenin–driven self-renewal program in a committed progenitor population known as the granulocyte macrophage progenitor (GMP) (4, 5). BC GMPs are highly enriched for LSCs because they have the capacity to serially transplant immunodeficient mice (6). Importantly, the BC LSC population is also thought to underlie TKI resistance, as well act as a reservoir for the maintenance of the disease in patients (4, 6). That TKIs have minimal clinical activity in BC suggests that the LSC function in GMPs occurs independently of BCR-ABL1, a conclusion that is supported by the inability of BCR-ABL1 per se to confer LSC function on committed progenitors (7).
In the present study, we set out to identify additional factors responsible for conferring stemness to the BC LSC population that might be drugable. We focused in particular on the cell’s translational machinery because our prior work had implicated cap-dependent mRNA translation in TKI resistance in CML (8–10), and because the process of mRNA translation encompasses a series of therapeutic targets that include several protein kinases (11). Specifically, we were interested in determining if there was a direct connection between the overexpression of the mRNA cap-binding protein and translation regulator eIF4E, which has been reported to be overexpressed in myeloid BC cells (12), and BC LSC function.
eIF4E is essential for cap-dependent mRNA translation, which is the means by which the majority of mammalian mRNAs are translated (13). eIF4E recruits the translation initiation machinery to the 5′ cap of mRNAs so that initiation can proceed. This function of eIF4E is rate limiting, and represents a key regulatory node in the control of mRNA translation and protein expression (13, 14). Indeed, overexpression of eIF4E by itself has been shown to contribute directly to cellular transformation (15, 16), and, prognostically, eIF4E overexpression has also been shown to correlate with poorer outcome in a variety of human cancers (17). Mechanistically, the transforming properties of eIF4E have been linked to its ability to promote translation of genes involved in proliferation and survival (18, 19). Recent data have also highlighted the importance of eIF4E phosphorylation at serine 209 (S209) in transformation. These reports included the use of genetic approaches to demonstrate that nonphosphorylatable forms of eIF4E are less efficient in causing in vivo transformation, and also highlighted the therapeutic potential for targeting the MNK1/2 kinases, which phosphorylate eIF4E in vivo, as a way to prevent eIF4E-mediated transformation (15, 20, 21). Exactly how eIF4E phosphorylation contributes to cancer in these models is not entirely clear, although recent work has suggested that eIF4E phosphorylation may be particularly important for the translation of a subset of cancer-promoting mRNAs (15, 22). In the present work, we show that eIF4E is highly phosphorylated in BC GMPs, and that overexpression of eIF4E is sufficient to confer self-renewal function on bone marrow (BM) progenitors in a phosphorylation-dependent manner. Mechanistically, we show that the MNK–eIF4E axis activates Wnt/β-catenin signaling by increasing β-catenin mRNA translation and facilitating its nuclear translocation. Consistent with these findings, we demonstrate that a panel of MNK kinase inhibitors impairs the ability of BC GMPs to function as LSCs, including the capacity to serially transplant immunodeficient mice. The identification of a BC-specific MNK–eIF4E–β-catenin axis may therefore provide a therapeutic window for targeting LSCs without affecting normal HSC function.
Results
eIF4E Overexpression and Phosphorylation Is a Feature of BC GMPs and Confers Stem Cell-Like Properties on Normal BM Progenitors.
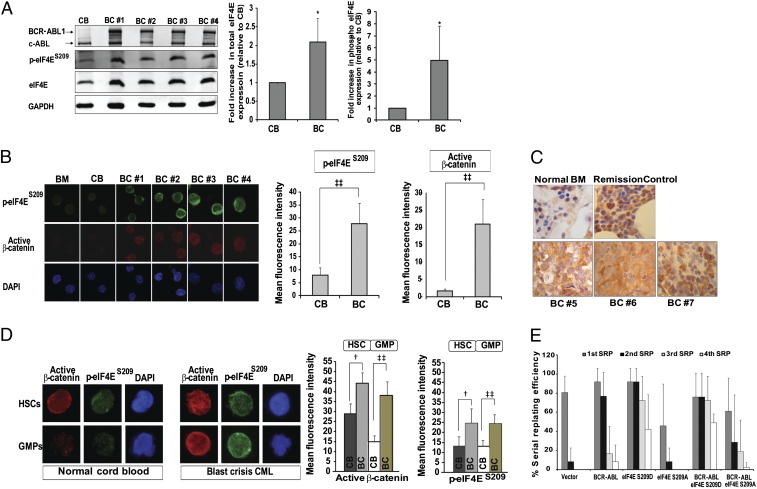
We performed Western blot analysis on cell lysates obtained from primary CD34+ BC cells and found that total and phosphorylated (i.e., S209) eIF4E levels were increased by two and five fold, respectively, compared with normal CD34+ cord blood (CB) controls (Fig. 1A). In addition, by using immunofluorescence, we observed in the same CD34+ BC samples that the increase in eIF4E phosphorylation paralleled nuclear β-catenin levels (Fig. 1B). The presence of phosphorylated eIF4E was also observed in blast cells in situ when BM from individuals with BC was examined (Fig. 1C).
Fig. 1.
eIF4E overexpression and phosphorylation in BC GMPs and eIF4E-mediated induction of serial replating capacity in hematopoietic progenitors. (A) (Left) Representative immunoblot analysis shows increased total and phosphorylated eIF4E levels in BC cells from patients with CML compared with CD34+ normal CB. (Right) Densitometric analysis of Western blots shows mean expression level of total eIF4E and phosphorylated eIF4E in BC (n = 8) relative to CB (n = 4). (B) (Left) Correlation between increased eIF4E phosphorylation and active nuclear β-catenin level in CD34+ BC cells (n = 7) but not normal CB or BM by immunofluorescence staining. (Right) Graphical representation of mean fluorescence intensity of phosphorylated eIF4E and active β-catenin. (C) Immunohistochemical staining demonstrates increased eIF4E phosphorylation in BC BM compared with control BM from a healthy donor (Normal BM) and an individual with acute leukemia in remission (Remission Control). (D) (Left) Confocal fluorescence microscopy demonstrates increased nuclear β-catenin and eIF4E phosphorylation in BC HSCs and GMPs compared with normal HSCs and GMPs from CB controls. (Right) Bar chart of the mean expression levels of active β−catenin and phosphorylated eIF4E in HSCs and GMPs from CB and BC [mean ± SEM; †P ≤ 0.001 and ‡‡P < 0.00001; CB (n = 3), BC (n = 3)]. (E) 5-Fluorouracil–primed BM cells were transduced with the indicated MSCV-based vectors and subjected to four rounds of serial replating in methylcellulose. eIF4ES209D-tranduced, but not BCR-ABL1– or eIF4ES209A-transduced cells, demonstrated replating capacity to the fourth plating.
Next, we sorted populations highly enriched for HSCs [Lineage negative (Lin−) CD34+CD38−] and GMPs (Lin−CD34+CD38+IL3Rα+CD45RA+) from CB and BC samples (4, 23). Here, we found that HSCs and GMPs from patients with BC had elevated levels of active, nuclear β-catenin, and that this was associated with increased eIF4E phosphorylation (Fig. 1D). In contrast, only CB HSCs, and not CB GMPs, expressed nuclear β-catenin, a result that confirms earlier work (4). We also noticed that CB-derived HSCs expressed low levels of phosphorylated eIF4E despite the presence of nuclear β-catenin (Fig. 1D), indicating a lack of association between β-catenin activity and eIF4E phosphorylation in normal HSCs.
Normal murine BM progenitors are unable to serially replate in methylcellulose, but are able to do so when transduced with leukemia oncogenes that confer self-renewal function (4, 6, 7). Accordingly, we used this assay to determine if eIF4E overexpression is able to confer self-renewal properties on normal progenitors. We retrovirally transduced BM cells with murine stem cell virus (MSCV)-based vectors expressing a phosphomimetic form of eIF4E (15), eIF4ES209D, or a nonphosphorylatable form, eIF4ES209A, with or without BCR-ABL1, and plated these cells in methylcellulose. Vector-only controls were unable to serially replate, and, in agreement with an earlier report, we found that BCR-ABL1 by itself was unable to sustain replating (Fig. 1E) (7). In contrast, we observed that eIF4ES209D, but not eIF4ES209A, enabled colonies to be serially replated for as many as four replatings, in the presence or absence of BCR-ABL1 (Fig. 1E). These results demonstrate that eIF4E confers serial replating properties on murine hematopoietic progenitors in a phosphorylation-dependent manner, and suggested that the high levels of phosphorylated eIF4E we observed in BC GMPs might also contribute to LSC function.
eIF4E Overexpression and Phosphorylation Coordinately Regulate β-Catenin Signaling.
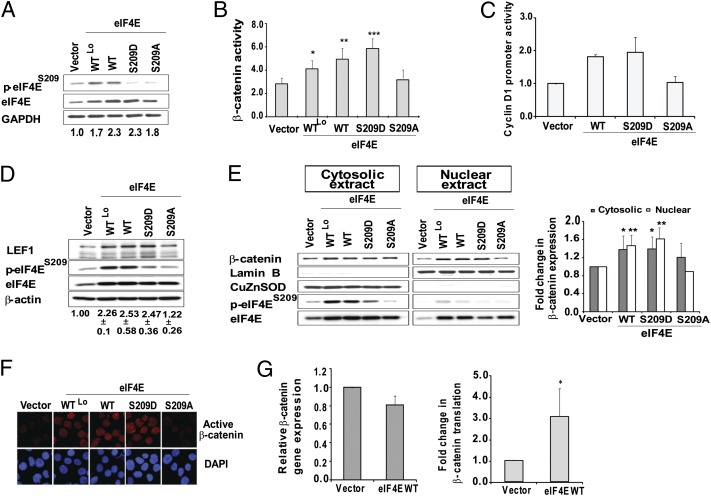
The Wnt/β-catenin self-renewal pathway is activated in BC GMPs, and plays an important role in BC LSC function because specific Wnt inhibitors abrogate LSC function (4). Based on an earlier report that Wnt/β-catenin signaling can be regulated by the cap-dependent translation of β-catenin itself (24), we investigated if activated eIF4E might contribute to BC LSC function via up-regulating the translation of components of the Wnt/β-catenin pathway. To explore this possibility, we used retroviral transduction to generate CML cells overexpressing WT eIF4E at two different levels (K562-eIF4EWTLo and K562-eIF4EWT), the phosphomimetic form of eIF4E (K562-eIF4ES209D) (15), and the nonphosphorylatable form (K562-eIF4ES209A), and achieved a range of total eIF4E levels in transduced cells that reflected the range of increased eIF4E levels in primary BC cells (Fig. 2A). By using a β-catenin reporter assay, we found that overexpression of WT eIF4E activated β-catenin in a dose-dependent manner, and furthermore that this was dependent on phosphorylation at S209 (Fig. 2B). By using two further readouts of β-catenin transcriptional activity, we confirmed that the increase in β-catenin activity resulted in increased Wnt target gene promoter activity (cyclin D1; Fig. 2C) and expression of a Wnt target gene, lymphoid enhancer-binding factor 1 (LEF1) (Fig. 2D) (25). Similar findings were observed in another BC CML cell line (Fig. S1).
Fig. 2.
eIF4E overexpression and phosphorylation is sufficient to activate β-catenin. (A) K562 cells were retrovirally transduced with MSCV-based vectors expressing vector only, eIF4E WT (WTLo and WT, corresponding to subpopulations with increasing eIF4E levels), eIF4E S209D, or eIF4E S209A. Immunoblots for eIF4E levels are shown. The antibody to phosphorylated eIF4E does not detect S209D or S209A. Numbers indicate densitometry readings for eIF4E, averaged from six experiments. (B) SuperTOP/FOPflash reporter assays were performed on K562 cell lines, and demonstrate increased β-catenin activity in an eIF4E-phosphorylation–dependent manner. Results indicate mean ± SEM from four independent experiments (*P < 0.01, **P ≤ 0.002, and ***P ≤ 0.001). (C) The cyclin D1 promoter is activated in an eIF4E-phosphorylation–dependent manner. K562 cells (5 × 105 cells per milliliter) were conucleofected with a β-gal reporter as well as a luciferase reporter for the cyclin D1 promoter; cells were harvested and assayed for reporter activities 24 h posttransfection (mean ± SEM obtained from three independent experiments). (D) Western blot analysis of LEF1 in K562-eIF4EWT cells and phosphomutants. Densitometry readings are for LEF1 levels, averaged from four independent experiments, normalized to β-actin. (E) (Left) Western blots of nuclear/cytoplasmic extracts of K562-vector, -eIF4EWT, and phosphomutants show increased cytoplasmic β-catenin in all eIF4E-overexpressing cells, but increased nuclear β-catenin only in eIF4EWT- and eIF4ES209D-containing cells. Antibodies to Lamin B and CuZnSOD were used to assess nuclear/cytosolic protein separation, respectively. (Right) Densitometric readings for nuclear/cytoplasmic distribution of β-catenin (average of five experiments). Nuclear/cytoplasmic distribution of β-catenin was expressed as fold change relative to vector control (mean ± SEM; *P ≤ 0.005 and **P ≤ 0.002). (F) Immunofluorescent staining demonstrates increased active nuclear β-catenin in K562-eIF4E WTLo, -eIF4E WT, and -eIF4ES209D cells, but not -eIF4ES209A cells. (G) RT-PCR shows no difference in β-catenin transcription (Left), and increased β-catenin protein synthesis in K562-eIF4EWT vs. control cells (Right). Data are mean of four experiments (*P ≤ 0.05).
Because most of the β-catenin in a cell is membrane bound or cytoplasmic, but only nuclear β-catenin is able to participate in transcriptional activation, we used Western blot and immunofluorescence to determine the nuclear/cytoplasmic distribution of β-catenin in control, K562-eIF4EWTLo, -eIF4EWT, -eIF4ES209D, and -eIF4ES209A cells. By using Western blot, we found that cytoplasmic β-catenin was increased in K562-overexpressing cells compared with control cells, but, in contrast, only cells in which eIF4E was overexpressed as well as phosphorylated was nuclear β-catenin increased (Fig. 2E). Immunofluorescence with the use of a different β-catenin antibody that recognizes active β-catenin yielded similar results, with increased nuclear β-catenin in K562-eIF4EWT and -eIF4ES209D cells but not K562-eIF4ES209A cells (Fig. 2F) (26). We also determined that the increase in total cell β-catenin occurred at the level of protein synthesis, as β-catenin transcription was unaffected but translation was increased threefold in eIF4E-overexpressing cells compared with controls (Fig. 2G). Interestingly, MNK1/2 inhibition caused a greater relative decrease in β-catenin protein synthesis compared with global protein synthesis, indicating a specific role for MNK1/2 in promoting β-catenin translation (Fig. S2). Taken together, these observations demonstrate that, although eIF4E overexpression and phosphorylation promote β-catenin translation, a second step that is critically dependent on eIF4E-phosphorylation is required for β-catenin nuclear localization and activation of signaling.
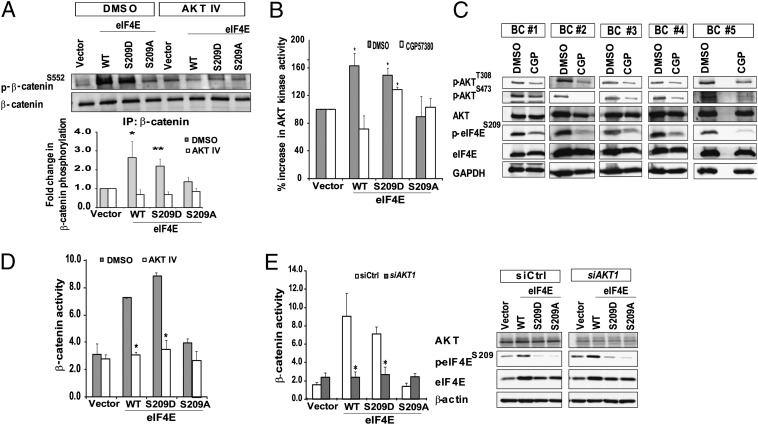
To better understand the role of eIF4E phosphorylation in β-catenin activation, we took note of two earlier findings: first, that eIF4E overexpression can lead to AKT activation (27); and second, that AKT-mediated phosphorylation of β-catenin at serine 552 (S552) is a necessary step in β-catenin nuclear translocation and activation (28). These results suggested a model in which eIF4E first activates AKT, which in turn leads to the phosphorylation and activation of β-catenin. We therefore assessed the phosphorylation status of β-catenin at S552, and, at the same time, determined if phosphorylation at this residue was dependent on AKT activity in vector control, K562-eIF4EWT, -eIF4ES209D, and -eIF4ES209A cells. By using immunoprecipitation (IP)/Western analysis, we found that eIF4E phosphorylation was associated with an increase in β-catenin phosphorylation at S552, and that phosphorylation at this site could be prevented by the AKT inhibitor AKT IV (Fig. 3A). Next, we used an in vitro kinase assay to directly measure AKT activity in the eIF4E-overexpressing K562 cell lines, and determined its dependence on eIF4E phosphorylation. First, we found that K562 cells overexpressing eIF4EWT and eIF4ES209D, but not eIF4ES209A, had increased AKT activity (Fig. 3B). Second, we determined that the increased AKT activity could be abrogated by inhibiting eIF4E phosphorylation with a small-molecule MNK1/2 kinase inhibitor, CGP57380 (Fig. S3) (29), in eIF4EWT but not eIF4ES209D cells (Fig. 3B). Importantly, we found that AKT signaling in primary CD34+ BC cells was also dependent on MNK activity, as CGP57380 treatment decreased AKT activation in these cells (Fig. 3C). Finally, to confirm that eIF4E was indeed activating β-catenin via AKT, we inhibited AKT pharmacologically and genetically, and found that AKT inhibition prevented the ability of eIF4E phosphorylation to activate β-catenin signaling in K562 cells, including those with the S209D phosphomimetic mutant (Fig. 3 D and E). Together, these data are consistent with a model in which eIF4E phosphorylation leads to AKT activation, which in turn promotes β-catenin signaling. Overall, our results show that activation of the MNK–eIF4E axis coordinately activates β-catenin signaling by promoting β-catenin protein synthesis, as well as facilitating its nuclear translocation in an eIF4E phosphorylation- and AKT-dependent manner.
Fig. 3.
eIF4E phosphorylation mediates phosphorylation of β-catenin at serine 552 in an AKT-dependent manner. (A) IP/Western analysis demonstrates an AKT-dependent increase in β-catenin S552 phosphorylation in K562-eIF4EWT and -eIF4ES209D but not -eIF4ES209A cells. Cells were treated with or without an AKT inhibitor (AKT IV, 3.0 μM), harvested, and lysed for IP/Western analysis. IP for total β-catenin was performed, and lysates were probed with antibody recognizing β-catenin phosphorylated at S552. (Lower) Densitometric readings for fold change in S552 β-catenin relative to vector control (averaged from four independent experiments; mean ± SEM; *P < 0.05 and **P < 0.01). (B) Increased AKT activity in K562 cells overexpressing eIF4E is dependent on eIF4E phosphorylation at S209. K562 cells were treated with DMSO or 10 μM CGP57380 for 24 h before harvesting and measurement of AKT kinase activity. Results are displayed as percent change in AKT kinase activity from three independent experiments (*P ≤ 0.05; mean ± SEM). (C) CGP57380 inhibits AKT activation in primary BC cells. CD34+ cells from five different patients with BC were treated with 10 μM of CGP57380 for 24 h before harvesting for Western analysis. (D) Increased eIF4E-phosphorylation–dependent β-catenin activity requires AKT. β-Catenin reporter assays were performed on vector only, K562-eIF4EWT, and phosphomutants treated with DMSO or 3.0 μM AKT IV for 24 h. Results indicate mean ± SEM obtained from three independent experiments (*P < 0.05). (E) Increased eIF4E-phosphorylation–dependent β-catenin activity requires AKT. β-Catenin reporter assays were performed on K562 cell lines 36 h after transfection of control siRNA (siCtrl) or siRNA targeting AKT1 (siAKT1). Results indicate mean ± SEM obtained from three independent experiments (*P < 0.005).
Targeting of MNK Activity Inhibits β-Catenin Signaling in BC.
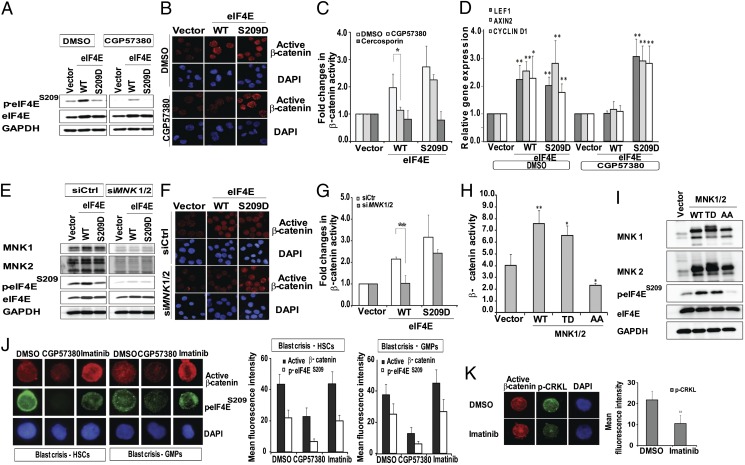
Because the MNK1/2 serine/threonine kinases are required to phosphorylate eIF4E in vivo (30), we asked if the contribution of the MNK kinases to eIF4E phosphorylation was essential for β-catenin activity in BC CML cells. We found that MNK inhibition with CGP57380 decreased eIF4E phosphorylation and prevented the eIF4E mediated increase in nuclear β-catenin in K562-eIF4EWT but not K562-eIF4ES209D cells (Fig. 4 A and B). In reporter assays, CGP57380 abolished β-catenin activity in K562-eIF4EWT but not K562-eIF4ES209D cells, whereas cercosporin (a positive control for Wnt inhibition that acts directly to disrupt TCF/β-catenin complexes) (31) eliminated activity in both cell lines (Fig. 4C). CGP57380 treatment also reduced transcript levels of several Wnt target genes (LEF1, AXIN2, and CYCLIND1) in K562-eIF4EWT but not K562-eIF4ES209D (Fig. 4D). Next, by using siRNA, we found that combined MNK1/2 knockdown prevented β-catenin activation in K562-eIF4EWT cells, but not K562-eIF4ES209D cells (Fig. 4 E– G, and Fig. S4). Parallel to this, we also found that overexpression of WT or constitutively active MNK1/2 (MNK1 T344D; MNK2 T332D) increased eIF4E phosphorylation as well as β-catenin activity in parental K562 cells, whereas overexpression of dominant-negative MNK1/2 (MNK1 T209A, T214A; MNK2 T197A, T202A) (15, 29) caused a decrease in phosphorylation of endogenous eIF4E and β-catenin activity (Fig. 4 H and I).
Fig. 4.
Inhibition of β-catenin by MNK inhibitors is dependent on eIF4E dephosphorylation. (A) Immunoblot of cell lysates from K562 vector, K562-eIF4EWT, and K562-eIF4ES209D cells treated with DMSO or 10 μM CGP57380 for 24 h. (B) K562 vector, K562-eIF4EWT, and K562-eIF4ES209D cells were treated with the indicated drugs for 24 h, and β-catenin activity was assessed by immunofluorescence. (C) Cells from B were subjected to SuperTOP/FOPflash reporter assays. Bars indicate mean ± SEM from three independent experiments (*P ≤ 0.05). (D) RT-PCR analysis for LEF1, AXIN2, and CYCLIN D1 levels in K562 cells treated with DMSO or 10 μM CGP57380 for 24 h. Bars are mean ± SEM from three independent experiments (*P ≤ 0.02 and **P ≤ 0.005). (E) Immunoblot of cell lysates following siRNA knockdown of MNK1 and MNK2. (F and G) The effects of MNK knockdown on β-catenin activity were assessed as described in B and C. Bars indicate mean ± SEM from three independent experiments (**P ≤ 0.01). (H and I) K562 cells were nucleofected with vector control, WT MNK1/2 (WT), constitutively active MNK1/2 (TD), and dominant-negative MNK1/2 (AA). At 36 h post transfection, cells were harvested for (H) TOPFlash reporter assay and (I) Western analysis. Graph presented is an average of three independent experiments and mean ± SEM (*P ≤ 0.002 and **P ≤ 0.001). (I) Western blot is representative of three independent experiments. (J) BC HSCs and BC GMPs were treated with DMSO, 10 μM CGP57380, or 2 μM IM for 24 h before harvesting for assessment of nuclear β-catenin and eIF4E phosphorylation by fluorescence microscopy. (Right) Graphical illustration of eIF4E phosphorylation and nuclear β-catenin levels following treatment (mean ± SEM obtained from experiments using BC cells from two individuals). (K) Confocal microscopy assessment of p-CrkL in GMPs treated with 2 μM IM for 24 h. (Lower) Bar chart of p-CrkL levels following treatment (mean ± SEM) in BC cells from two individuals (‡‡P ≤ 0.001). (A, B, E, and F) Representative blots/immunofluorescence pictures from four independent experiments.
We then assessed the ability of CGP57380 to inhibit eIF4E phosphorylation and β-catenin signaling in primary BC GMPs. We sorted primary CD34+ BC cells to obtain the HSC and GMP fractions and treated them with CGP57380 or imatinib (IM). We found that CGP57380 effectively inhibited eIF4E phosphorylation as well as β-catenin activation, whereas IM was unable to prevent either despite inhibition of BCR-ABL1 (as readout by CrkL phosphorylation; Fig. 4 J and K). Because BCR-ABL1 may also signal to AKT (32), which we find contributes to β-catenin activation (Fig. 3), we also evaluated the effect of IM and a second-generation TKI dasatinib on AKT phosphorylation and β-catenin activation in primary BC cells. We found that neither inhibitor was able to consistently diminish AKT activation, in agreement with Chu et al. (33), or β-catenin activation (Fig. S5). Together, these findings identify the MNK kinases as a target to extinguish β-catenin signaling in BC CML.
Effect of Panel of MNK Inhibitors on BC LSC Function.
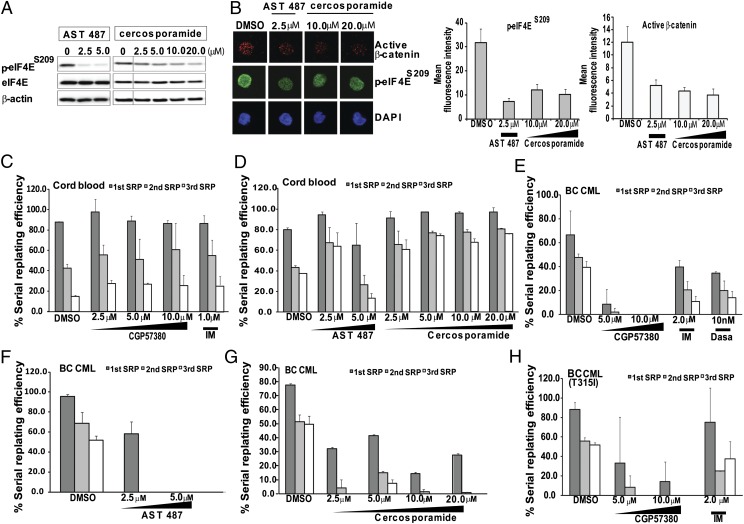
As eIF4E overexpression is able to confer serial-replating function on BM progenitors (Fig. 1E), we went on to perform a series of studies to determine the ability of a panel of recently described and structurally diverse MNK kinase inhibitors [CGP57380, AST 487, and cercosporamide (21, 34)] to extinguish BC LSC function in vitro. First, we confirmed that both AST 487 and cercosporamide inhibited eIF4E phosphorylation as well as β-catenin nuclear localization in CML cell lines and primary BC cells (Fig. 5 A and B). Second, because CGP57380 has been reported to inhibit casein kinase 1 in vitro (35) and casein kinase 1 has been implicated in Wnt/β-catenin activity (36–38), we excluded the possibility that any of the three MNK inhibitors were modulating β-catenin signaling by inhibiting CK1 (Fig. S6 and Table S1). Next, we treated CB and BC CML CD34+ cells with each compound, and used the serial-replating assay as a readout for LSC function. By using normal CB CD34+ cells, we found that control treated cells were capable of serial replating as many as three times (equivalent to >8 wk in vitro), and that the efficiency was reduced with each replating (from ∼85% to <40%; Fig. 5 C and D). We also observed that treatment of CB cells with any of the MNK inhibitors or IM did not significantly alter the efficiency of replating compared with DMSO (except for AST 487 at higher doses; Fig. 5 C and D). For CD34+ BC cells, we found that DMSO-treated cells were able to maintain their replating efficiency to a third replating, and that exposure to CGP57380, AST 487, or cercosporamide potently impaired the ability of BC cells to serially replate (Fig. 5 E–G). In contrast, neither IM nor dasatinib at clinically achievable concentrations were able to reduce the replating efficiency as effectively as CGP57380 (Fig. 5E). We also tested the effect of CGP57380 on BC cells bearing the T315I mutation, which is associated with resistance to all currently Food and Drug Administration-approved TKIs (39). In these samples, we again found that the serial replating capacity of BC cells was significantly diminished by CPG57380 but not by DMSO or IM (Fig. 5H). Altogether, we tested the ability of CGP57380 to impair the serial-replating capacity of BC cells from eight different patients, and, in all cases, CGP57380 significantly reduced serial replating efficiency (Table 1). Together, our findings demonstrate that small--molecule MNK kinase inhibition is able to prevent the serial replating function of BC progenitors.
Fig. 5.
MNK inhibitors impair the serial replating efficiency of BC progenitors. (A) K562 cells were treated with various concentration of AST 487 or cercosporamide for 24 h before harvesting for Western analysis to detect phosphorylated eIF4E. (B) Immunofluorescence analysis of β-catenin and phosphorylated eIF4E levels in BC cells treated with AST 487 or cercosporamide for 24 h. (Right) Quantitative analysis of the mean expression of β-catenin and phosphorylated eIF4E in DMSO- or drug-treated cells. Colony-forming and serial-replating assays were performed on normal CD34+ CB samples and CD34+ BC cells. CD34+ cells from CB (C and D), BC CML with no BCR-ABL1 kinase domain mutation (E–G), and BC CML with the T315I mutation (H) were treated with CGP57380, AST 487, cercosporamide, IM, and/or dasatinib for 48 h. Colonies were enumerated and individually picked for serial replating. Graphs presented are representative of the results obtained from different normal CB or BC samples (CB, n = 5; BC CML, n = 8; Table 1). Bars indicate SD obtained from three replicates.
Table 1.
CGP57380 inhibits the ability of primary BC progenitors to serially replate
| BC CML | DMSO, % | CGP57380 | IM, % | Dasatinib, % |
| CML 1 | 39.5 | ND | 11 | 14.2 |
| CML 2 | 7.1 | ND | 3.9 | — |
| CML 3* | 51.7 | ND | 37.5 | — |
| CML 4 | 10.8 | ND | 7.1 | — |
| CML 5 | 11.8 | ND | 4.3 | — |
| CML 6* | 53.2 | ND | 75 | 45.8 |
| CML7 | 35.2 | ND | 35.6 | 39.9 |
| CML11 | 70.0 | ND | 40.0 | — |
CD34+ BC cells from eight different individuals with BC were treated with DMSO, 10.0 μM CGP57380, 2.0 μM IM, or 150 nM dasatinib for 48 h before harvesting for the CFC assay, and subsequent serial replating assay. Values presented here represent the third serial replating efficiency relative to the first CFC readout. ND, no colonies detected.
BC CML samples harboring the T315I mutation.
Effect of in Vivo MNK and Abl Kinase Inhibitors on BC LSC Function.
Our findings suggested that MNK inhibition might effectively control BC CML because it extinguishes BC LSC function in vitro. To test this possibility in an in vivo BC model, we determined the effect of CGP57380 on the ability of BC GMPs to serially transplant immunodeficient nonobese diabetic/SCID IL2Rγ-deficient mice [NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice] (6). In preliminary studies, we found that a brief 48-h in vitro exposure to CGP57380 was able to delay the engraftment of BC LSCs in NSG mice, as well as reduce the leukemia cell burden in engrafted animals (Fig. S7 A and B), while leaving the normal CD34+ cell engraftment untouched (Fig. S7 C and D). These results encouraged us to determine if the self-renewing capacity of BC LSCs could be targeted in vivo by small-molecule MNK inhibitors.
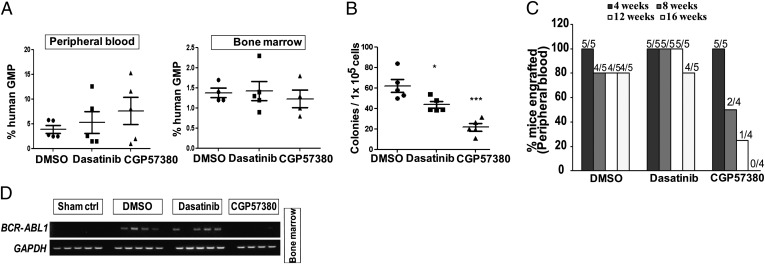
Here, we FACS-sorted GMPs from a BC sample as previously described (6), and injected them intrafemorally into 8- to 12-wk-old female NSG mice (Fig. S8A). At 6 wk posttransplantation, engrafted mice were treated for 3 wk with DMSO, CGP57380, or dasatinib (n = 5 mice per treatment group). At the end of the treatment period, all the mice were killed, and human cells were obtained from hematopoietic tissues by using immunomagnetic beads. We found no difference in the percentage of CD45+ human cells in the peripheral blood or BM of each of the treatment groups (Fig. 6A). However, we observed that dasatinib and CGP57380 had specific activity against committed BC progenitors, as they significantly reduced the number of colony forming units detected in BM (P ≤ 0.05 and P ≤ 0.005, respectively) compared with control, although the effect of CGP57380 was greater (Fig. 6B). Human cells obtained from the primary mice were then transplanted into secondary recipients, and engraftment monitored by flow cytometry over a 16-wk period. By 4 wk, we were able to detect engraftment in all animals in each of the three treatment groups (Fig. 6C). In DMSO- or dasatinib-treated animals, engraftment was maintained at 80% (i.e., four of five animals) throughout the whole experimental time frame of 16 wk, but, in contrast, none of the CGP57380-treated mice were able to maintain long-term engraftment (Fig. 6C). At 16 wk, mice were euthanized, and BM was examined for the presence of BCR-ABL1. BCR-ABL1 transcripts were detectable in each of the animals treated with DMSO or dasatinib (i.e., four of five animals for each treatment group), whereas only a very faint band was detected in one of the four animals in the CGP57380-treated group (Fig. 6D). This experiment was repeated by using CD34+ BC cells from a different individual, and similar result were obtained (Fig. S8 B–J). Taken together, our findings demonstrate that in vivo MNK inhibition can potently extinguish the ability of BC CML cells to serially transplant-immunodeficient mice and function as LSCs.
Fig. 6.
In vivo CGP57380 treatment prevents BC GMPs from serially transplanting immunodeficient mice. BC cells were injected intrafemorally into sublethally irradiated NSG mice and allowed to engraft, after which animals were treated with DMSO or drugs. (A) Dasatinib and CGP57380 do not affect engraftment in primary recipients. The percent engraftment in peripheral blood (Left) and BM (Right) after treatment with DMSO, CGP57380 (40 mg⋅kg−1⋅d−1), or dasatinib (5 mg⋅kg−1⋅d−1) for 3 wk. (B) Committed BC progenitors are decreased by dasatinib and CGP57380. Human CD45+ cells were obtained from the mice at the time of euthanasia, and plated in a colony-forming assay to enumerate committed progenitors. Colonies were counted at 2 wk. (*P ≤ 0.05 and ***P ≤ 0.005). (C) CGP57380 but not dasatinib prevents serial transplantation of BC progenitors. Flow cytometry was performed on peripheral blood to detect human CD45+ cells in secondary transplant recipients at 4-wk intervals over 16 wk. The numbers above each bar describe the number of CD45+ mice/number of transplanted mice. One of the five animals in the CGP57380 treatment group died during a peripheral blood draw at 8 wk. (D) RT-PCR for BCR-ABL1 transcripts in the BM of sham-transplanted mice and mice treated with DMSO, dasatinib, or CGP57380.
Discussion
In the present study, we found that eIF4E overexpression and phosphorylation at S209 is a consistent feature in 15 of 15 BC samples examined (Fig. 1), and that activation of the MNK–eIF4E axis in BC GMPs is critical for this population to serially transplant immunodeficient mice and function as LSCs. Because the LSC function of BC GMPs is thought to underlie their resistance to standard therapy (4, 6), the ability to extinguish LSC function may contribute to improved control of BC in patients (5).
The observations that activation of the MNK–eIF4E axis confers stem cell-like properties to committed progenitors, and that MNK inhibitors have little apparent toxicity to the normal HSC compartment, is also of interest. This is because direct inhibition of the β-catenin signaling pathway may not be possible given that normal HSCs depend on β-catenin to self-renew (40), and suggests that the MNK–eIF4E–β-catenin axis does not regulate critical functions in normal HSCs. This conclusion is consistent with the lack of association between eIF4E phosphorylation and β-catenin activation in normal HSCs compared with BC GMPs, and the finding that mice lacking Mnk1/2 have an apparently normal hematopoietic system (30). Together, these observations suggest that targeting MNK and eIF4E phosphorylation may preferentially impair BC LSC vs. normal stem cell function, whereas direct β-catenin inhibition may not.
Our work also addresses the mechanisms by which overexpression of eIF4E activates β-catenin. In CML cells, we show that eIF4E overexpression increases total cellular β-catenin levels, and that eIF4E phosphorylation is necessary for β-catenin activation and nuclear localization. By using pharmacologic and genetic means, we found that down-regulation of MNK kinase prevented β-catenin activation, and confirmed this was dependent on S209 dephosphorylation. These experiments included the use of three structurally distinct MNK1/2 kinase inhibitors, siRNA-mediated MNK1/2 knockdown, as well as the use of constitutively active and dominant-negative forms of MNK1/2. Together, our combined approaches demonstrate that the MNK kinases can activate β-catenin signaling via eIF4E phosphorylation. These data add to the recent evidence supporting an important role for eIF4E phosphorylation in transformation (15, 20, 21, 41–45). Our studies also add β-catenin to the growing list of eIF4E-regulated cancer-promoting genes (15, 22). Experiments that used polysomal mRNA profiling have suggested that eIF4E phosphorylation, per se, directly affects the translation efficiency of such genes (22), although the precise means by which phosphorylation regulates the translation of specific mRNAs is unclear because eIF4E phosphorylation is dispensable for global mRNA translation (30, 46). That eIF4E phosphorylation is induced in untransformed cells under stress conditions (30, 47, 48) suggests that the physiologic MNK–eIF4E connection has been usurped by cancer cells to compensate for and/or gain a growth advantage while under stress, including oncogene-induced senescence (49). Conversely, our finding that MNK–eIF4E activates self-renewal in BC leads us to speculate if equivalent pathways exist in normal stem cells to preserve self-renewal capacity during physiologic stress.
By linking eIF4E to β-catenin activation, we also uncovered a facet of eIF4E’s transforming properties, namely that of enabling β-catenin–dependent self-renewal. Consistent with these results, we found that overexpression of phosphomimetic forms of eIF4E was sufficient to confer serial-replating function on BM progenitors. Thus, we are able to infer that eIF4E phosphorylation at S209 is likely to represent an important target for therapeutic intervention in BC CML, and that the ability of MNK inhibitors to impair BC LSC function may depend on their effectiveness in decreasing eIF4E phosphorylation. These results also suggest that eIF4E phosphorylation should be considered as a potential biomarker for effective target inhibition in patients with BC treated with MNK kinase inhibitors.
Recent reports have emphasized that β-catenin signaling in CML can be activated by at least three different mechanisms. These include BCR-ABL1–dependent tyrosine phosphorylation of β-catenin itself, missplicing and inactivation of GSK3β, and PP2A inactivation (6, 50–52). The existence of several paths to β-catenin activation begs the question of whether MNK1/2 inhibition alone would be able to antagonize β-catenin given multiple modes of activation. Importantly, our finding that MNK1/2 inhibition prevents the nuclear localization of β-catenin in CML cell lines suggested that MNK1/2 inhibition may be broadly effective, given that this represents a relatively distal step in the β-catenin signaling pathway. With respect to GSK3β inactivation, found in 50% of patients with BC (6), we mimicked this situation by using the GSK3β inhibitor (2'Z,3'E)-6-Bromoindirubin-3'-oxime (BIO), and, as expected, saw a further activation of β-catenin signaling in BC cells overexpressing phosphorylation-competent eIF4E (Fig. S9). By using this system, we show that MNK1/2 inhibition completely abrogated the cooperativity between GSK3β inactivation and eIF4E, and indicated that eIF4E functions downstream of GSK3β in the canonical Wnt/β-catenin pathway. Thus, our results, together with data from others, underscore β-catenin activation as a critical pathogenic feature of BC, as several mechanisms are now documented to activate this pathway. At the same time, our findings highlight the MNK–eIF4E axis as a drugable target to extinguish LSC function. Moreover, because MNK–eIF4E activation influences distal β-catenin signaling, our results suggest that MNK inhibition may effectively curtail β-catenin activation produced by the diverse upstream activators of this pathway
Although β-catenin might not be the exclusive downstream target of MNK–eIF4E, our findings nevertheless demonstrate that MNK–eIF4E confers self-renewal capacity to LSC and that inhibition of this pathway effectively prevents self-renewal of LSCs. In conclusion, we describe a path to β-catenin activation in BC GMPs that proceeds via MNK-dependent eIF4E phosphorylation, and demonstrate that a panel of MNK kinase inhibitors can inhibit β-catenin activity and BC LSC function without affecting normal HSC function. These results suggest that MNK kinase inhibitors may have utility in treating patients with BC CML.
Materials and Methods
CB and Patient Samples.
CB samples were purchased from the Singapore Cord Blood bank. MNCs were obtained by using Ficoll separation, and CD34+ cells were selected by immunomagnetic beads (Miltenyi Biotech). CML samples were obtained from patients seen at the University of California, Irvine; Duke University Medical Center; and the Singapore General Hospital after signed informed consent as approved by the UCI and Duke University Health System Institutional Review Boards and the SingHealth Centralised Institutional Review Board. All animal experiments were approved and performed in accordance with the guidelines required by the SingHealth Institutional Animal Care and Use Committee.
Cell Culture, Generation of Cell Lines, and Chemicals.
K562 and KCL22 cell lines were obtained from American Type Culture Collection and grown in Roswell Park Memorial Institute-1640 media supplemented with 10% (vol/vol) FCS, L-glutamine, and penicillin/streptomycin. Cell lines overexpressing eIF4E and its mutant forms were generated by retroviral transduction by using MSCV-internal ribosome entry site (IRES)-GFP constructs as previously described (53). Viral particles were generated as previously described (53). GFP+ cells were then sorted by FACS into GFP high and low subpopulations, which corresponded to high and low eIF4E overexpression levels. CGP57380, AKT inhibitor IV, and cercosporamide were purchased from CalBiochem, and cercosporin was purchased from Sigma-Aldrich and dissolved in DMSO. IM was obtained from tablets and dissolved as previously described (10). Dasatinib was purchased from LC Laboratories and dissolved in citric acid (pH 2.1) to make up stock solution. Dasatinib was diluted in citric acid (pH 3.1) for gavage.
In Vitro Nonradioactive Protein Translation Assay.
K562 cells (4 × 105/mL) were grown in methionine-free media for 2 h and then labeled with l-azidohomoalanine for 1 h (Click-iT, Life Technologies). At 1 h after labeling, cells were harvested and azide-modified proteins were further labeled with biotin alkyne. Biotin azide-modified proteins were pulled down with high-capacity streptavidin beads (Thermo Fisher Scientific), and Western analysis was performed. Anti–β-catenin antibodies were used to probe for the presence of newly synthesized β-catenin protein.
Western Blotting.
Exponentially growing cells were plated at 2 × 105 cells per milliliter, and whole cells were extracted by using 1× RIPA lysis buffer (50 mM Tris⋅HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 1 mM Na3VO4, 1 mM NaF, and 1× protease inhibitor). Nuclear and cytoplasmic extracts were obtained by using the NE-PER kit (Thermo Fisher Scientific), and then processed for Western blot analysis by using antibodies recognizing eIF4E, phospho-eIF4E, ABL, phospho-ABL, LEF1, GAPDH (Cell Signaling Technology), anti–β-catenin (BD Bioscience), CuZnSOD, and Lamin B. All antibodies were from Santa Cruz Biotechnology unless otherwise stated.
In Vitro AKT Kinase Assay.
Kinase assays were performed as described in the manufacturer’s protocol (Cell Signaling Technology). In brief, K562 cells were treated with DMSO or 10.0 μM CGP57380 for 24 h before harvesting. The cells were lysed and immunoprecipitated with immobilized phospho-AKT (ser473) overnight. The immunoprecipitates were extensively washed with cell lysis buffer and kinase buffer and were subjected to the kinase assay. Kinase assays were performed by incubating the immune complexes in kinase reaction buffer containing 1 μg GSK3 fusion protein and 200 μM ATP for 30 min at 30 °C. The reaction was terminated with 20 μL of 3× SDS sample buffer, and proteins were electrophoresed on a 12% SDS/PAGE gel. Phosphorylation of GSK3α/β was measured by Western blot analysis by using phosphor- GSK3α/β (ser21/9) antibody (Cell Signaling Technology).
IP.
For phospho–β-catenin experiments, cells were harvested and lysed in 0.5 mL of lysis buffer [50 mM Tris-HCl (pH 7.5), 1% Triton X-100, 150 mM NaCl, 0.5 mM EDTA, 0.1 mM PMSF, 100 mM Na3VO4, 1 mM NaF, and 1× protease inhibitor]. Protein concentrations of cell lysate were normalized by using Quick Start Bradford protein assay kit (Bio-Rad) before incubation with anti–β-catenin (Cell Signaling Technology) overnight at 4 °C. Cell extracts were incubated with 20 μL of protein A/G agarose beads for 3 h at 4 °C. Immunoprecipitates were collected by centrifugation and washed three times with lysis buffer. Immunoprecipitates were resuspended in SDS loading buffer and processed for Western blot analysis by using antibodies recognizing β-catenin (BD Biosciences).
siRNA-Mediated Gene Knockdown.
Specific knockdown of MNK1, MNK2, or AKT1 was accomplished by transfecting cells with siRNAs against MNK1, MNK2, AKT1, or a control siRNA (Ambion) via nucleofection. Cells were harvested for Western blot, immunofluorescence, or reporter assays 36 h after transfection. siRNA oligonucleotide sequences used were as follows: siAKT1 sense, 5′-GAA CAA UCC GAU UCA CGU Att-3′; siAKT1 antisense, 5′-UAG GUG AAU CGG AUU GUU Ctg-3′; siMNK1 (no. 1) sense, 5′-GGA GUA GGG UGU UUC GAG Att-3′; siMNK1(no. 1) antisense, 5′-UCU CGA AAC ACC CUA CUC Cga-3′; siMNK2 (no. 1) sense, 5′-GCC UUG GAC UUU CUG CAU Att-3′; and siMNK2 (no. 1) antisense, 5′-UAU GCA GAA AGU CCA AGG Cgc-3′.
Immunohistochemistry.
Tissue sections 4 mm thick were cut from paraffin blocks, mounted on positively charged slides, and allowed to dry at room temperature. Tissue sections were deparaffinized and rehydrated by incubating twice in xylene and then in decreasing concentrations of alcohol. Heat-induced antigen retrieval was applied by boiling in 0.01 M Tris-EDTA, pH 9, in a microwave oven (500 W) for 12 min. The slides were then cooled to room temperature and washed in PBS solution. Following antigen retrieval, endogenous peroxidase was blocked by using 3% H2O2 . Primary antibody (1:400) against phospho-eIF4E (EP2151Y; Abcam) was added and incubated overnight at room temperature. Antibody binding was detected by the two-stage peroxidase-based EnVision (Dako) method, and sections were counterstained with hematoxylin before mounting.
RNA Extraction, Nested RT-PCR, and Real-Time PCR.
Total RNA was used for nested RT-PCR and quantitative RT-PCR for the analysis of mRNA expression. β-Actin and GAPDH levels were used for normalization. For nested RT-PCR for BCR-ABL1, first-step PCR primers used were as follows: NB1+, 5′-GAG CGT GCA GAG TGG AGG GAG AAC A-3′; Abl3−, 5′-GGT ACC AGG AGT GTT TCT CCA GAC TG-3′; and for the second-step PCR, B2A, 5′-TTC AGA AGC TTC TCC CTG ACA T-3′; and CA3−, 5′-TGT TGA CTG GCG TGA TGT AGT TGC TTG G-3′. The quantitative RT-PCR primer sequences for WNT-regulated genes were as follows: LEF1 forward, 5′-GAC GAG ATG ATC CCC TTC AA-3′; LEF1 reverse, 5′-AGG GCT CCT GAG AGG TTT GT-3′; AXIN2 forward, 5′-CTC CCC ACC TTG AAT GAA GA-3′; AXIN2 reverse, 5′-TGG CTG GTG CAA AGA CAT AG-3′; CYCLIN D1 forward, 5′-TGT CCT ACT ACC GCC TCA CA-3′; CYCLIN D1 reverse, 5′-CAG GGC TTC GAT CTG CTC-3′; β-CATENIN forward, 5′-TCT GAT AAA GGC TAC TGT TGG ATT GA-3′; and β-CATENIN reverse, 5′-TCA CGC AAA GGT GCA TGA TT-3′.
Flow Cytometric Analysis and FACS.
HSC cells and GMPs were obtained as previously described (23). Briefly, samples were lineage-depleted by immunomagnetic beads by using a mixture of biotin-conjugated antibodies (CD2, CD3, CD4, CD7, CD8, CD10, CD11b, CD14, CD19, CD20, CD56, and glycophorin A (GPA); BD Biosciences). The Lin− population was then collected and stained with antibodies to CD34, CD38, CD45RA, and IL3Rα before sterile FACS (FACSAriaII; BD Biosciences). HSC (CD34+CD38−CD45RA−IL3Rα−) and GMP (CD34+CD38+CD45RA+IL3Rα+) populations, were then prepared for immunofluorescence analysis or recovered overnight in media before treatment with vehicle or drugs for 48 h.
Reporter Assays.
Transfections were carried out in K562 and KCL22 cells by using nucleofection (Lonza). The 16× SuperTopFlash reporter was used to assess the effects of eIF4E on β-catenin transcriptional activity (54). K562 and KCL22 cells were nucleofected with 5 μg of SuperTopFlash or FOPFlash and 5 μg β-gal. At 24 h after transfection, cells were harvested by using the Luciferase Reporter Assay System (Promega) or the β-gal Enzyme Assay System (Promega). β-Catenin activity was calculated by the SuperTOP/FOPflash ratio after normalization to β-gal.
Immunofluorescence Analysis.
Cells (1 × 105) were cytospun onto glass slides, fixed with 4% (wt/vol) paraformaldehyde, and stained with mouse monoclonal antibodies against activated β-catenin (clone 8E7; Millipore) or rabbit monoclonal antibodies against phospho-eIF4E S209 (EP2151Y; Abcam). Slides were then stained with PE-conjugated anti-mouse or FITC-conjugated anti-rabbit antibodies. Images were obtained with the use of a fluorescence microscope (IX71S1F3; Olympus) at 40× magnification or by confocal microscopy (LSM710; Carl Zeiss), and fluorescence intensity was quantified with ImageJ software.
Serial Replating Assay.
CD34-enriched CB and BC cells were thawed and allowed to recover overnight in serum-free StemPro media (Invitrogen), supplemented with human growth factors (33) and 1× nutrient supplement (Invitrogen) (9). Cells were then subjected to drug treatment for 48 h, harvested, washed, and seeded in methylcellulose (H4434; Stemcell Technologies). Colonies were enumerated after 2 wk, individually picked, and replated in fresh methylcellulose in a 96-well format, and counted at 2 wk. Three rounds of serial replating (representing >8 wk in culture) were performed.
Serial Transplantation Model of Human BC and in Vivo Treatment.
CD34+ cells (5 × 105) or GMPs (1 × 105) were resuspended in 25 μL 1% FBS/PBS solution and injected into the right femur of 8- to 10-wk-old sublethally irradiated (200 cGy) female mice (n = 5 mice per group). Mice injected with 1% FBS/PBS solution served as a sham control for each experiment. Beginning at 4 wk posttransplantation, mice were monitored for engraftment of human cells by flow cytometry. At 6 wk after transplantation, engrafted mice were treated with vehicle alone, dasatinib (5 mg⋅kg−1⋅d−1) by gavage, or CGP57380 (40 mg⋅kg−1⋅d−1) intraperitoneally for 3 wk (n = 5 mice per group). At the end of treatment, mice were euthanized, and CD45+ cells were isolated from BM and spleen by using anti-human CD45-specific immunomagnetic microbeads. An aliquot of 1 × 105 human CD45+ cells was seeded into methylcellulose (H4434; Stemcell Technologies) for the colony forming cell (CFC) assay, and colonies were enumerated after 2 wk. All of the remaining human cells from each primary transplant recipient were then transplanted by intrafemoral injection into secondary recipients, and human engraftment was monitored at 2-wk intervals beginning at 4 wk. At the end of 16 wk, all mice were euthanized. Engraftment in BM and blood was assessed by flow cytometry, and BCR-ABL1 transcripts were detected by RT-PCR.
Retroviral Transduction of Murine BM Cells in Vitro.
NSG mice were treated with 5-fluorouracil (150 mg/kg) intraperitoneally for 3 d before euthanasia. BM cells were flushed and washed and red cells were lysed with red blood cell lysis buffer. BM cells were incubated overnight in Iscove's Modified Dulbecco's Medium (IMDM) supplemented with 20% (vol/vol) FBS, 100 ng/mL mIL-11, mSCF, and 10 ng/mL mIL-3 and mIL-6, and transduced the next day. BM cells were transduced with MSCV-based BCR-ABL1, eIF4E S209D, eIF4ES209A, or vector control. For transduction, cells were plated in IMDM [supplemented with 20% (vol/vol) FBS, 100 ng/mL murine IL-11 and SCF, and 10 ng/ml of murine IL-3 and IL-6] in 12-well plates coated with RetroNectin (Takara). Retroviral supernatant was added along with 4 μg/mL Polybrene, and cells were incubated overnight at 37 °C in 5% CO2 incubator. Cells were subjected to three rounds of transduction on three consecutive days before harvesting for colony forming and serial replating assay.
Statistical Analysis.
After a Student t test was performed, values were considered statistically significant at P values <0.05.
Supplementary Material
Acknowledgments
The authors acknowledge the many helpful comments and discussions with our colleagues, in particular Drs. Jit Kong Cheong, Alexandra Pietersen, Shirish Shenolikar, and David Virshup; and the gifts of eIF4E and MNK constructs from Dr. Scott Lowe (Memorial Sloan-Kettering Cancer Center, New York) and BCR-ABL1 construct from Dr. Owen Witte (University of California, Los Angeles). This work was supported by the Duke–National University of Singapore Signature Research Program funded by the Agency for Science, Technology, and Research (Singapore); the Ministry of Health (Singapore); and National Research Foundation Singapore Clinician Scientist Award (CSA) National Medical Research Council (NMRC)/CSA/005/2008 administered by the Singapore Ministry of Health’s NMRC.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1301838110/-/DCSupplemental.
References
- 1.Quintás-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood. 2009;113(8):1619–1630. doi: 10.1182/blood-2008-03-144790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochhaus A, et al. IRIS Investigators Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23(6):1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 3.Radich JP. The Biology of CML blast crisis. Hematology (Am Soc Hematol Educ Program) 2007:384–391. doi: 10.1182/asheducation-2007.1.384. [DOI] [PubMed] [Google Scholar]
- 4.Jamieson CH, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351(7):657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 5.Perrotti D, Jamieson C, Goldman J, Skorski T. Chronic myeloid leukemia: Mechanisms of blastic transformation. J Clin Invest. 2010;120(7):2254–2264. doi: 10.1172/JCI41246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abrahamsson AE, et al. Glycogen synthase kinase 3beta missplicing contributes to leukemia stem cell generation. Proc Natl Acad Sci USA. 2009;106(10):3925–3929. doi: 10.1073/pnas.0900189106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huntly BJ, et al. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell. 2004;6(6):587–596. doi: 10.1016/j.ccr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M, et al. Inhibition of polysome assembly enhances imatinib activity against chronic myelogenous leukemia and overcomes imatinib resistance. Mol Cell Biol. 2008;28(20):6496–6509. doi: 10.1128/MCB.00477-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prabhu S, et al. A novel mechanism for Bcr-Abl action: Bcr-Abl-mediated induction of the eIF4F translation initiation complex and mRNA translation. Oncogene. 2007;26(8):1188–1200. doi: 10.1038/sj.onc.1209901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ly C, Arechiga AF, Melo JV, Walsh CM, Ong ST. Bcr-Abl kinase modulates the translation regulators ribosomal protein S6 and 4E-BP1 in chronic myelogenous leukemia cells via the mammalian target of rapamycin. Cancer Res. 2003;63(18):5716–5722. [PubMed] [Google Scholar]
- 11.Hagner PR, Schneider A, Gartenhaus RB. Targeting the translational machinery as a novel treatment strategy for hematologic malignancies. Blood. 2010;115(11):2127–2135. doi: 10.1182/blood-2009-09-220020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topisirovic I, et al. Aberrant eukaryotic translation initiation factor 4E-dependent mRNA transport impedes hematopoietic differentiation and contributes to leukemogenesis. Mol Cell Biol. 2003;23(24):8992–9002. doi: 10.1128/MCB.23.24.8992-9002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: Effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 14.Sonenberg N, Dever TE. Eukaryotic translation initiation factors and regulators. Curr Opin Struct Biol. 2003;13(1):56–63. doi: 10.1016/s0959-440x(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 15.Wendel HG, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21(24):3232–3237. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruggero D, et al. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004;10(5):484–486. doi: 10.1038/nm1042. [DOI] [PubMed] [Google Scholar]
- 17.Mamane Y, et al. eIF4E—from translation to transformation. Oncogene. 2004;23(18):3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- 18.Mamane Y, et al. Epigenetic activation of a subset of mRNAs by eIF4E explains its effects on cell proliferation. PLoS ONE. 2007;2(2):e242. doi: 10.1371/journal.pone.0000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsson O, et al. Eukaryotic translation initiation factor 4E induced progression of primary human mammary epithelial cells along the cancer pathway is associated with targeted translational deregulation of oncogenic drivers and inhibitors. Cancer Res. 2007;67(14):6814–6824. doi: 10.1158/0008-5472.CAN-07-0752. [DOI] [PubMed] [Google Scholar]
- 20.Ueda T, et al. Combined deficiency for MAP kinase-interacting kinase 1 and 2 (Mnk1 and Mnk2) delays tumor development. Proc Natl Acad Sci USA. 2010;107(32):13984–13990. doi: 10.1073/pnas.1008136107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konicek BW, et al. Therapeutic inhibition of MAP kinase interacting kinase blocks eukaryotic initiation factor 4E phosphorylation and suppresses outgrowth of experimental lung metastases. Cancer Res. 2011;71(5):1849–1857. doi: 10.1158/0008-5472.CAN-10-3298. [DOI] [PubMed] [Google Scholar]
- 22.Furic L, et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci USA. 2010;107(32):14134–14139. doi: 10.1073/pnas.1005320107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manz MG, Miyamoto T, Akashi K, Weissman IL. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci USA. 2002;99(18):11872–11877. doi: 10.1073/pnas.172384399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karni R, Gus Y, Dor Y, Meyuhas O, Levitzki A. Active Src elevates the expression of beta-catenin by enhancement of cap-dependent translation. Mol Cell Biol. 2005;25(12):5031–5039. doi: 10.1128/MCB.25.12.5031-5039.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hovanes K, et al. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28(1):53–57. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- 26.van Noort M, Weerkamp F, Clevers HC, Staal FJ. Wnt signaling and phosphorylation status of beta-catenin: Importance of the correct antibody tools. Blood. 2007;110(7):2778–2779. doi: 10.1182/blood-2007-05-092445. [DOI] [PubMed] [Google Scholar]
- 27.Culjkovic B, et al. The eIF4E RNA regulon promotes the Akt signaling pathway. J Cell Biol. 2008;181(1):51–63. doi: 10.1083/jcb.200707018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang D, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282(15):11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knauf U, Tschopp C, Gram H. Negative regulation of protein translation by mitogen-activated protein kinase-interacting kinases 1 and 2. Mol Cell Biol. 2001;21(16):5500–5511. doi: 10.1128/MCB.21.16.5500-5511.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol. 2004;24(15):6539–6549. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lepourcelet M, et al. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5(1):91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 32.Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96(10):3343–3356. [PubMed] [Google Scholar]
- 33.Chu S, Holtz M, Gupta M, Bhatia R. BCR/ABL kinase inhibition by imatinib mesylate enhances MAP kinase activity in chronic myelogenous leukemia CD34+ cells. Blood. 2004;103(8):3167–3174. doi: 10.1182/blood-2003-04-1271. [DOI] [PubMed] [Google Scholar]
- 34.Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26(1):127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 35.Bain J, et al. The selectivity of protein kinase inhibitors: A further update. Biochem J. 2007;408(3):297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakanaka C. Phosphorylation and regulation of beta-catenin by casein kinase I epsilon. J Biochem. 2002;132(5):697–703. doi: 10.1093/oxfordjournals.jbchem.a003276. [DOI] [PubMed] [Google Scholar]
- 37.Tsai IC, et al. Disease-associated casein kinase I delta mutation may promote adenomatous polyps formation via a Wnt/beta-catenin independent mechanism. Int J Cancer. 2007;120(5):1005–1012. doi: 10.1002/ijc.22368. [DOI] [PubMed] [Google Scholar]
- 38.Greer YE, Rubin JS. Casein kinase 1 delta functions at the centrosome to mediate Wnt-3a-dependent neurite outgrowth. J Cell Biol. 2011;192(6):993–1004. doi: 10.1083/jcb.201011111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Hare T, Deininger MW, Eide CA, Clackson T, Druker BJ. Targeting the BCR-ABL signaling pathway in therapy-resistant Philadelphia chromosome-positive leukemia. Clin Cancer Res. 2011;17(2):212–221. doi: 10.1158/1078-0432.CCR-09-3314. [DOI] [PubMed] [Google Scholar]
- 40.Reya T, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 41.Wheater MJ, Johnson PW, Blaydes JP. The role of MNK proteins and eIF4E phosphorylation in breast cancer cell proliferation and survival. Cancer Biol Ther. 2010;10(7):728–735. doi: 10.4161/cbt.10.7.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korneeva NL, et al. Mnk mediates integrin α6β4-dependent eIF4E phosphorylation and translation of VEGF mRNA. Mol Cancer Res. 2010;8(12):1571–1578. doi: 10.1158/1541-7786.MCR-10-0091. [DOI] [PubMed] [Google Scholar]
- 43.Hay N. Mnk earmarks eIF4E for cancer therapy. Proc Natl Acad Sci USA. 2010;107(32):13975–13976. doi: 10.1073/pnas.1008908107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva RL, Wendel HG. MNK, EIF4E and targeting translation for therapy. Cell Cycle. 2008;7(5):553–555. doi: 10.4161/cc.7.5.5486. [DOI] [PubMed] [Google Scholar]
- 45.Bianchini A, et al. Phosphorylation of eIF4E by MNKs supports protein synthesis, cell cycle progression and proliferation in prostate cancer cells. Carcinogenesis. 2008;29(12):2279–2288. doi: 10.1093/carcin/bgn221. [DOI] [PubMed] [Google Scholar]
- 46.Scheper GC, Proud CG. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur J Biochem. 2002;269(22):5350–5359. doi: 10.1046/j.1432-1033.2002.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, et al. The phosphorylation of eukaryotic initiation factor eIF4E in response to phorbol esters, cell stresses, and cytokines is mediated by distinct MAP kinase pathways. J Biol Chem. 1998;273(16):9373–9377. doi: 10.1074/jbc.273.16.9373. [DOI] [PubMed] [Google Scholar]
- 48.Morley SJ, Naegele S. Phosphorylation of eukaryotic initiation factor (eIF) 4E is not required for de novo protein synthesis following recovery from hypertonic stress in human kidney cells. J Biol Chem. 2002;277(36):32855–32859. doi: 10.1074/jbc.C200376200. [DOI] [PubMed] [Google Scholar]
- 49.Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27(20):2801–2809. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- 50.Coluccia AM, et al. Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 2007;26(5):1456–1466. doi: 10.1038/sj.emboj.7601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neviani P, et al. Activation of PP2A by FTY720 inhibits survival and self-renewal of the Ph(+) chronic myelogenous leukemia (CML) CD34+/CD38– stem cell through the simultaneous suppression of BCR/ABL and BCR/ABL– independent signals. Blood. 2009;114(22):Abstract 2168. [Google Scholar]
- 52.Neviani P, et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell. 2005;8(5):355–368. doi: 10.1016/j.ccr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 53.Kharas MG, et al. Phosphoinositide 3-kinase signaling is essential for ABL oncogene-mediated transformation of B-lineage cells. Blood. 2004;103(11):4268–4275. doi: 10.1182/blood-2003-07-2193. [DOI] [PubMed] [Google Scholar]
- 54.DasGupta R, Kaykas A, Moon RT, Perrimon N. Functional genomic analysis of the Wnt-wingless signaling pathway. Science. 2005;308(5723):826–833. doi: 10.1126/science.1109374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.