Needleless vaccines may immunize patients more efficiently and effectively than injections. But are these new technologies ready for prime time?
At a makeshift clinic in southern Cambodia, with cows lazing on the dirt outside, children take turns sitting in a blue plastic chair, bracing themselves for the sharp pain of a measles vaccine injection. However, time and time again, the children barely flinch, let alone cry. Throughout the morning, more than 200 children are vaccinated. The key to the efficient and tearless vaccination: no needles necessary.
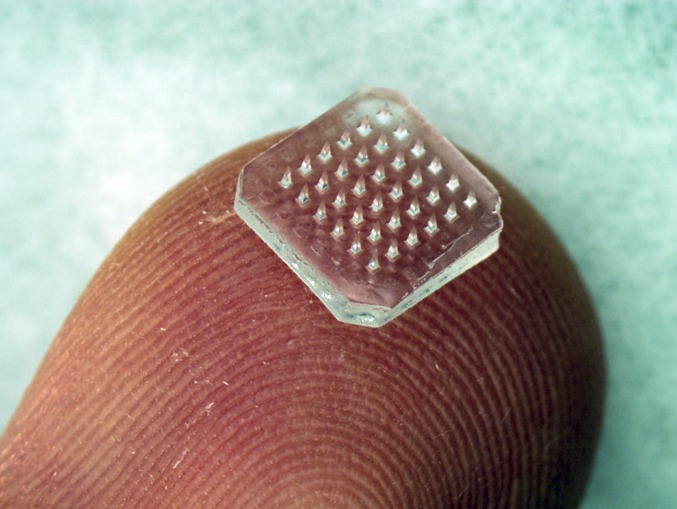
A patch containing 36 dissolving microneedles is shown on a fingertip. The microneedles dissolve within minutes after insertion into skin to release encapsulated drug or vaccine. Each microneedle is 900-μm tall. (Image courtesy of Jeong-Woo Lee, Laboratory for Drug Delivery, Georgia Institute of Technology.)
In this scene captured on video during a Cambodian measles vaccination program, a device called a jet injector shoots a high-speed jet of the drug into the skin. Unlike a typical needle syringe that sticks all of the way into muscle, this device never punctures the skin. The jet injector is just one of a number of technologies being developed to deliver vaccines to patients without using painful muscle-piercing needles: everything from patches with tens of thousands of microscopic needles that painlessly perforate the skin, to ultrasound pulses that temporarily “open” the skin for drug delivery, to special concoctions of drugs engineered to seep into the skin.
A lack of pain isn’t the only advantage of such methods. Vaccines delivered using these technologies may be more effective than intramuscular injections and thus require less of the drug. Moreover, many of these technologies are easier to use in the developing world because they use vaccine formulations that don’t require refrigeration and can be administered with minimal training. The challenge is to make effective vaccines and also drive down the cost of the technologies to deliver them. “At this point, we have a good degree of confidence that we can hit those goals,” says biomedical engineer Mark Kendall of the University of Queensland, in Brisbane, Australia, who has pioneered one of the new delivery approaches.
Look Ma, No Pain
Any vaccine aims to set off a minor immune reaction to essentially teach the body how to fight off a pathogen. Ideally, when inactivated or dead microorganisms, or parts of pathogens, are injected as a vaccine, the immune system not only produces antibodies against that organism but will also “remember” to fight it in the future. If the bacterium or virus is encountered again, the immune system is able to fend it off more quickly and efficiently than before. Historically, vaccines have been given using needles and syringes or oral formulations. In addition, although some oral vaccines have been successful—like those against polio and cholera—many work less effectively because the digestive system breaks down the complex vaccine molecules before they can fully mobilize the immune system. Even injecting vaccines into muscles has started to seem misguided to some researchers.
“If you think about how your body is designed, the immune system is looking for pathogens that enter the body from the outside,” says biomedical engineer Mark Prausnitz of the Georgia Institute of Technology in Atlanta. “It’s not expecting a pathogen to pop into your muscle.”
With this in mind, Prausnitz is among a handful of researchers who are focusing on delivering vaccines to the outermost layers of the skin, which contain a different set of immune cells than muscles, so-called dendritic cells that act as sentinels against intruders from the environment. Prausnitz’s approach is based on something called a microneedle patch, which is far less painful than it sounds. “It’s a technically accurate [name],” says Prausnitz, “but marketing folks don’t like it much since it still has the word ‘needle’.”
Microneedle patches contain arrays of hundreds or thousands of tiny spikes, each around 750 microns long, about half the thickness of a dime. When a patch of microneedles is pressed onto a person’s skin, the microneedles are long enough to pierce the outermost layer of the skin and deliver the drug, but they don’t dig deep enough to hit blood vessels, or even pain receptors. Different researchers have developed different types of microneedles: some are solid metal or plastic and are coated in drug on the outside; others are hollow and contain a liquid vaccine inside.
The patch that Prausnitz has designed has solid—but dissolvable—microneedles made of a fabricated material containing cellulose and sugar molecules. The microneedles are coated with a dry powder form of a vaccine. In a liquid vaccine, proteins can lose their structure when the temperature fluctuates, requiring refrigeration. Dry powders, however, offer proteins more physical stability and don’t need refrigeration. When the microneedle patch is stuck onto someone’s arm, the needles penetrate the skin and begin dissolving, diffusing the drug into the skin. The team’s initial study of patches for an influenza vaccine has shown that almost 90% of the microneedle material dissolves within five minutes (1). The punctures made by the microneedles are so minuscule they can only be spotted with a microscope, and heal entirely within two hours of removing a patch (2).
At first, the success of microneedles was simply that they got a vaccine into the body. However, when researchers began comparing the effectiveness of microneedle-delivered vaccines with classic, intramuscular injections for the same vaccine, they started to notice other benefits. For example, Prausnitz’s team found that mice that were given flu vaccines using a microneedle patch had better and longer-lasting protection against the influenza virus than if they were vaccinated using intramuscular injections (3).
Although the scientific rationale for this increased efficacy is still being studied, researchers think it’s success might be because of the high numbers of dendritic cells in the skin that are uniquely receptive to vaccines. However, that’s not all. “What we’ve seen so far is that it’s not only the vaccine itself causing a response, but the physical actions of the projections as well,” says Kendall.
Around every microneedle that punctures the skin—and Kendall’s NanoPass patch has 20,000 microneedles per square centimeter—Kendall observed an increase in localized cell death. When cells die and release their contents, the immune system is alerted to the turmoil, causing immune molecules to flood the site. “The biology and engineering really feed off each other,” says Samir Mitagotri of the University of California at Santa Barbara. “Now that people have developed these technologies to deliver drugs, we’re asking questions we didn’t ask before: How does the skin heal? How does the immune system react?”
In a recent PNAS report, researchers from King’s College School of Medicine in London made inroads into understanding this biology when they followed the path of a live adenovirus injected into mice using a microneedle array (4). The virus, they found, moved through the epidermis into the deeper dermis, and interacted with dendritic cells there. The team discovered that a unique subset of dendritic cells, found only in the skin, were required to confer immunity after the vaccine.
Even as these questions continue to be studied, researchers are moving forward on applying microneedle patches to different applications. Prausnitz has tested his patch for not only influenza, but for vaccines against rotavirus (5) and measles (6). Now, he has a grant from the Gates Foundation to apply the technique to a polio vaccine. Kendall, who has launched Vaxxas, a company to market his Nanopatch microneedle patch, is focusing on delivering a human papillomavirus (HPV) vaccine. Many of his initial trials have been conducted in Papua New Guinea, which has one of the highest rates of HPV infections in the world but limited or no access to the current vaccine because of cost. This year, Kendall’s team shipped their patches across the ocean to Papua New Guinea, testing to see how they held up during shipping and at higher temperatures. They also noticed the ease with which people figured out how to use the patches. Kendall is also collaborating with Merck to study the delivery of other vaccines—both existing and new—using microneedle patches.

Microscope image shows dissolving microneedles encapsulating a pink dye used to simulate how a drug or vaccine would be incorporated into the needles. Each microneedle is 650-μm tall. (Image courtesy of Sean Sullivan, Laboratory for Drug Delivery, Georgia Institute of Technology.)
All Sound, No Fury
Other research teams have turned entirely away from puncturing skin, and are instead making gels that naturally seep into the skin. Existing drug patches—like those containing hormones for birth control or nicotine for smoking cessation—rely on the fact that small molecules with certain chemical properties will diffuse through the upper layers of the skin. However, vaccines contain larger, hydrophilic molecules, which don’t move smoothly through the outermost, hydrophobic layer of the skin.
“The patches that are already out there have a narrow range of drug properties. They are all small and hydrophobic and have a very easy time getting into the skin,” Mitagotri says. “So we want to know how we can expand that range of properties,” he adds.
Mitagotri screened a massive library of short proteins to find ones that are especially adept at moving through the skin, and has homed in on one that he thinks can help vaccines (7). Mitagotri’s dubbed it SPACE, for “skin penetrating and cell entering,” and found that when it’s attached to molecules that wouldn’t normally move through the skin, it helps transport them across. Now, he’s trying to figure out why SPACE slips through the skin so easily; he thinks that it may bind or associate with keratin, a key structural protein in skin. Mitagotri’s also on the lookout for other molecules, or other properties of molecules, that can chaperone drugs across the skin (8).
Meanwhile, at the Massachusetts Institute of Technology in Cambridge, chemical engineer Daniel Blankschtein is trying out yet another technique to get drugs into the skin: rather than make tiny holes in the skin using microneedles, he’s using ultrasound waves to make the skin temporarily more permeable so that vaccines can pass through unaided.
“If you don’t pretreat your skin with ultrasound, you’ll get negligible amounts of the drugs we study through,” says Carl Schoellhammer, a graduate student in Blankschtein’s laboratory. “But a few minutes of ultrasound pretreatment, and the underlying skin can then take up the drug in meaningful amounts,” he says.
Ultrasound causes tiny air bubbles to form in the outermost layer of skin. As the bubbles grow, they become unstable and implode. This causes tiny abrasions, temporarily weakening the skin and making it easier for drugs to slip in.
Blankschtein and Schoellhammer have discovered a way to maximize this permeability. Low-frequency ultrasound tends to make bubbles more unstable, causing them to implode, whereas high-frequency ultrasound leads to more bubbles. By combining the two, the researchers found that they can create both more bubbles and more implosions. Glucose applied to pig skin after the dual treatment was absorbed 10-times better than when they used a single frequency of ultrasound (9).
Now, Blankschtein and Schoellhammer intend to test the system in humans and with vaccines. Like microneedles, an ultrasound could both help deliver the drug and recruit immune molecules to the site of vaccination by abrading the skin. Furthermore, ultrasound could be easily applied to vaccination efforts in the developing world. Minimal training is needed to use handheld ultrasound devices, and vaccines can be stored and transported as gels, which are more stable than liquids in the absence of refrigeration.
Silicon Salvation
Exciting as these ideas are, they need to be commercially viable. Kendall hopes to make the Nanopatch competitive by making his microneedle patch from silicon wafers, which are already mass-produced for other purposes. “We’re using a deep-etching process highly utilized by the semiconductor industry,” says Kendall. He estimates that producing five million such patches a year would bring the cost per application down to less than a dollar.
Jet injectors of the type used in the Cambodia measles vaccination program would also be easy to scale up. Jet injectors were popular for mass vaccinations of smallpox and other pathogens in the mid-20th century, but were designed to be reused in patient after patient, so concerns about cross-contamination led to a decline in their use. However, new jet injectors, such as the PharmaJet Stratis used in Cambodia, have disposable components, alleviating such concerns. Darin Zehrung, the team leader of vaccine delivery technologies at the Seattle, Washington-based nonprofit Program for Appropriate Technology in Health, says that his organization is conducting trials of the PharmaJet injector for vaccination programs in Brazil.
“This is all about saving more lives and increasing access,” says Zehrung.
References
- 1.Sullivan SP, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16(8):915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. J Control Release. 2011;154(2):148–155. doi: 10.1016/j.jconrel.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koutsonanos DG, et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep. 2012;2:357. doi: 10.1038/srep00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachy V, et al. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc Natl Acad Sci USA. 2013;110(8):3041–3046. doi: 10.1073/pnas.1214449110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon S, et al. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edens C, Collins ML, Ayers J, Rota PA, Prausnitz MR. Measles vaccination using a microneedle patch. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu T, Mitragotri S. Delivery of siRNA and other macromolecules into skin and cells using a peptide enhancer. Proc Natl Acad Sci USA. 2011;108(38):15816–15821. doi: 10.1073/pnas.1016152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pino CJ, Gutterman JU, Vonwil D, Mitragotri S, Shastri VP. Glycosylation facilitates transdermal transport of macromolecules. Proc Natl Acad Sci USA. 2012;109(52):21283–21288. doi: 10.1073/pnas.1200942109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoellhammer CM, et al. Rapid skin permeabilization by the simultaneous application of dual-frequency, high-intensity ultrasound. J Control Release. 2012;163(2):154–160. doi: 10.1016/j.jconrel.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


