Abstract
Rapid eye movement (REM) sleep constitutes a distinct “third state” of consciousness, during which levels of brain activity are commensurate with wakefulness, but conscious awareness is radically transformed. To characterize the temporal and spatial features of this paradoxical state, we examined functional interactions between brain regions using fMRI resting-state connectivity methods. Supporting the view that the functional integrity of the default mode network (DMN) reflects “level of consciousness,” we observed functional uncoupling of the DMN during deep sleep and recoupling during REM sleep (similar to wakefulness). However, unlike either deep sleep or wakefulness, REM was characterized by a more widespread, temporally dynamic interaction between two major brain systems: unimodal sensorimotor areas and the higher-order association cortices (including the DMN), which normally regulate their activity. During REM, these two systems become anticorrelated and fluctuate rhythmically, in reciprocally alternating multisecond epochs with a frequency ranging from 0.1 to 0.01 Hz. This unique spatiotemporal pattern suggests a model for REM sleep that may be consistent with its role in dream formation and memory consolidation.
Keywords: EEG, fMRI, thalamus, slow-wave sleep, brain dynamics
Despite over a century of scientific inquiry, the nature of consciousness—the way in which brain activity gives rise to conscious experience—remains a mystery. As currently conceived, consciousness has two essential facets: the level (reflecting arousal or vigilance) and content (reflecting awareness of and intentional interaction with elements of the environment or internal milieu) (1). In healthy subjects, both facets vary systematically across the sleep/wake cycle (2), with daily maxima and minima occurring during wakefulness (WAKE) and nonrapid eye movement (non-REM) sleep, respectively. During REM sleep, however, there is a paradoxical uncoupling of activity and awareness as the latter is typically conceived. The brain is in an activated state, but remains functionally isolated, operating with reflective awareness despite the relative absence of exteroceptive input (2, 3). Accordingly, the contents of consciousness during REM sleep reflect internally generated sensory (particularly visual) activity, producing a hallucinosis that is manifest as dreaming (2). Although dreaming is the behavioral hallmark of this sleep stage, the functional significance of dreams or of any other aspect of REM sleep remains unknown [although evidence is accumulating that REM may somehow facilitate the acquisition of new sensorimotor or cognitive skills, such as procedural learning (4, 5)].
Over the past two decades, functional imaging techniques have been used in an attempt to pinpoint the neural correlates of consciousness. These studies have revealed that activity of, and connectivity within, a set of higher-order heteromodal cortical areas, centered in the posterior cingulate (PCC)/retrosplenial cortex, the medial prefrontal cortex (MPFC), and the inferior parietal lobules (IPL)—the so-called default mode network (DMN)—may play a significant role (6). In the model proposed by Vogt and Laureys (1), the PCC acts as a lynchpin, mediating interactions between arousal systems in the brainstem and thalamus, and regions distributed throughout the cortex. A role for the DMN has been most dramatically demonstrated in clinical conditions characterized by impaired consciousness in which its integrity is interrupted. For example, activity or connectivity of the DMN is severely attenuated in coma and vegetative state, but emerges to a degree in patients who are minimally conscious (7, 8) (SI Text, Note 1). However, it should be noted that certain neurological disorders in which the DMN connectivity is altered may not be associated with impairment of consciousness (e.g., ref. 9).
DMN integrity also plays a role in sleep. Regions that make up the DMN remain coupled at sleep onset and throughout light sleep (10, 11) (although this has not been universally observed; see, for example, ref. 12). However, during slow-wave sleep (SWS, the deepest stage of sleep) DMN connectivity is significantly attenuated, characterized by functional uncoupling of its anterior and posterior nodes (particularly the MPFC and PCC) (12, 13), a pattern that is remarkably similar to that seen in minimally conscious patients. On the basis of these findings, it has been argued that integrity of the DMN and its connections may reflect, and perhaps even dictate, the level of consciousness.
The role of the DMN in REM sleep, however, remains unclear (SI Text, Note 2). Given that REM sleep constitutes a unique “third state” of consciousness (4), it is reasonable to hypothesize that REM is characterized by a correspondingly unique pattern of DMN connectivity.
Results and Discussion
DMN: Reestablishment of Functional Connectivity During REM Sleep.
To address this question, resting-state connectivity methods were applied to blood-oxygen level-dependent (BOLD) functional MRI (fMRI) data to characterize functional connectivity within the DMN—and between the DMN and the rest of the brain—during polysomnographically determined wakefulness, non-REM (predominantly slow wave) sleep, and REM sleep in human subjects. Imaging was carried out following an extended period of sleep deprivation (Methods) to facilitate sleep initiation and maintenance in the scanner environment. Although it is well-established that sleep deprivation changes subsequent “sleep architecture” (e.g., reducing sleep onset latency and promoting SWS), there is no evidence that it in any way changes the physiological features of the subsequent sleep, which should not differ fundamentally from that which follows a more typical diurnal period of continuous wakefulness.
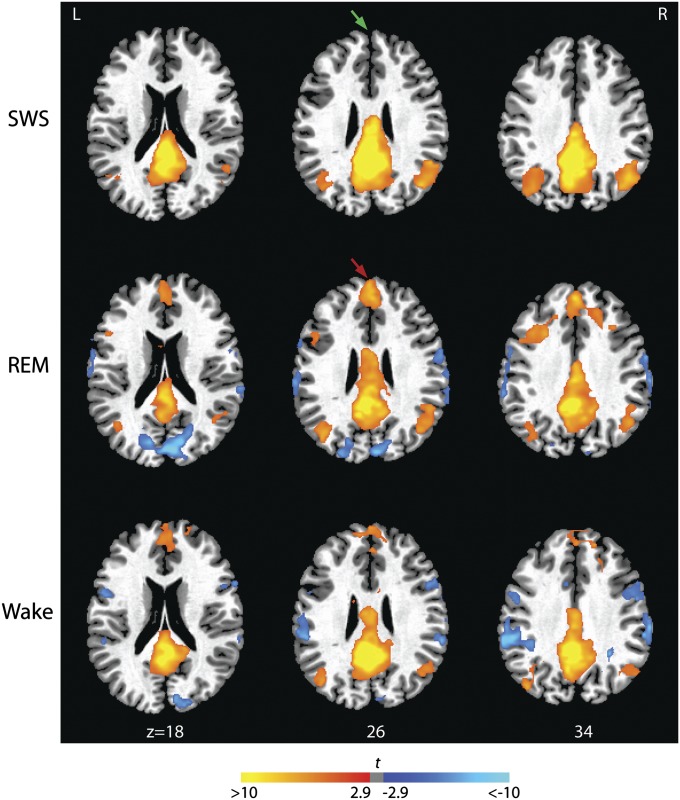
We predicted first that, relative to SWS, REM sleep would be characterized by at least partial reconnection of the anterior and posterior nodes of the DMN, reflecting the relatively increased level of reflective awareness that characterizes dream mentation during this sleep stage (2). To test these hypotheses, we first examined correlations between time-series extracted from PCC and MPFC seed regions (see Methods for details of seed selection) and the remainder of the brain. We found that, relative to SWS, elements of the DMN were functionally recoupled during REM (Fig. 1 and Table S1; see also Fig. 3A). Activity in the PCC was strongly correlated with that in the MPFC as well as other elements of the network, consistent with our first hypothesis. In fact, positive correlations between the PCC and the dorsal portions of the MPFC, and the dorsal IPLs were significantly stronger during REM than during WAKE (Fig. S1). Recoupling within the DMN was confirmed using MPFC seed regions (Figs. S2 and S3). This phenomenon was observed consistently across all subjects who had REM sleep (Fig. S4). Thus, the emergence of REM sleep involves reestablishment of at least some aspects of the connectivity patterns that characterize wakefulness.
Fig. 1.
Functional connectivity between the PCC seed region and other nodes of the DMN during SWS, REM, and WAKE. Brain regions significantly correlated and anticorrelated with the PCC seed region are indicated in orange and blue, respectively (P < 0.05, corrected). REM sleep is characterized by recoupling of the MPFC (anterior midline in each slice) and PCC (posterior midline). Coupling between these anterior and posterior nodes of the DMN is observed during WAKE but is diminished during SWS. Magnitudes of t statistics from the random effects analyses are indicated in the color bar and rendered on a single subject’s T1 image. Axial slices are displayed at the z axis levels shown (in millimeters).
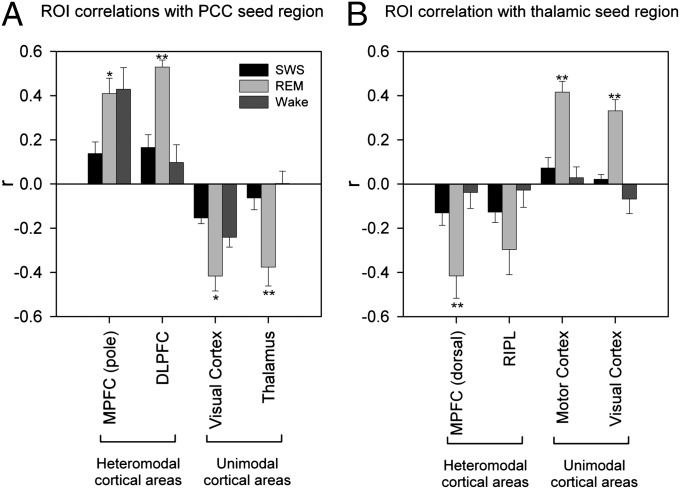
Fig. 3.
Correlations between selected components of DMN, heteromodal and unimodal cortical areas with the PCC (A) and thalamic (B) seed regions. ROI analyses show a dissociated pattern of connectivity between heteromodal and unimodal cortical areas. Activity in the PCC seed region is positive correlated with heteromodal areas and anticorrelated with unimodal areas (A). This pattern is reversed for the thalamic seed region (B). Bars represent mean regional correlation coefficients; SEs are indicated by error bars. Double asterisks indicate significant differences between REM and WAKE and between REM and SWS. Single asterisks indicate significant differences between REM and SWS. RIPL, right IPL.
Large-Scale Interregional Connectivity During REM Sleep.
The DMN is one of several large-scale functional networks—both heteromodal and unimodal—within the CNS. It is possible that altered relationships between these networks, rather than solely within the elements of the DMN itself, might account for the unique features of this third state. Therefore, we further hypothesized that, beyond restoration of DMN integrity, the most critical differences—those that distinguish REM from both WAKE and SWS—would be manifest not within the DMN, but in its connections with subcortical and cortical regions outside of this network. In this view, the DMN might be seen as one component of a more widespread pattern of interregional connectivity that determines both the level and the contents of consciousness.
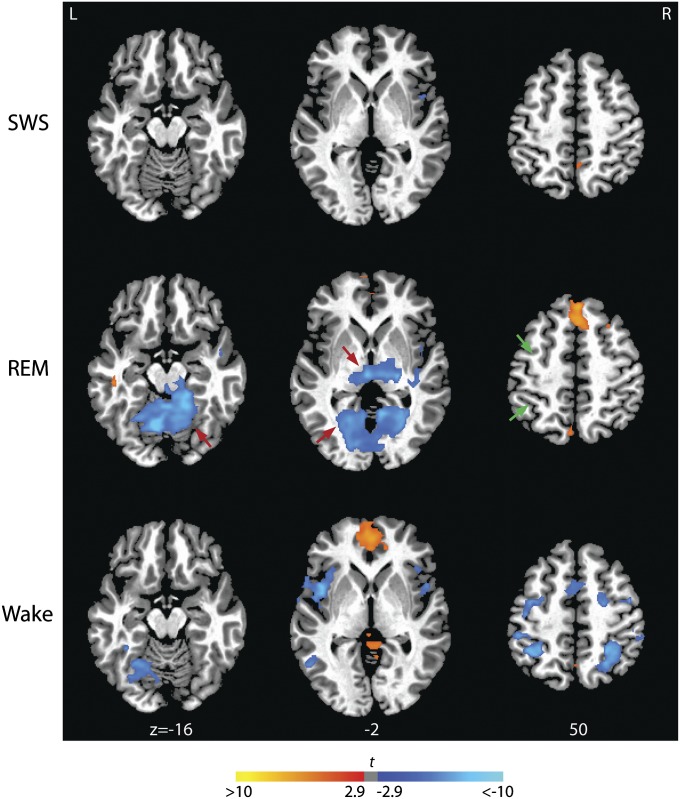
Crucially, however, we found that REM sleep was also characterized by unique patterns of connectivity between elements of the DMN and cortical and subcortical regions outside of this network, and that these patterns involve regions extending throughout the brain. For example, we detected changes in the relationships between the DMN and other so-called resting-state networks, including the dorsal attention network, with which it is typically anticorrelated during resting wakefulness. As expected, we detected negative correlations between the PCC and central elements of the dorsal attention network—including intraparietal sulci and frontal eye fields (Fig. 2 and Table S1)—during wakefulness. In contrast, these negative correlations were absent, or were replaced in some instances by positive correlations (in portions of the frontal eye fields), during REM sleep (Fig. 2 and Table S1). Thus, REM may be differentiated from WAKE by an integration of activity among resting state networks that usually act in opposition (SI Text, Note 3).
Fig. 2.
Functional connectivity between the PCC seed region and brain regions outside the DMN during SWS, REM sleep, and WAKE. Unique to REM sleep, activity in the PCC is significantly anticorrelated with that in thalamus and sensory-motor areas including lingual gyrus and cerebellar vermis (indicated in blue and by red arrows, threshold: P < 0.05, corrected). During REM, anticorrelation between the PCC and both frontal eye field and intraparietal sulcus observed during wakefulness is attenuated (indicated by green arrows). Magnitudes of t statistics from the random effects analyses are indicated in the color bar and rendered on a single subject’s T1 image. Axial slices are displayed at the z axis levels shown (in millimeters).
However, unique patterns of connectivity during REM extended beyond the relationships of these resting-state networks. During REM—and unlike either WAKE or SWS—PCC activity was positively correlated with activity in a wide array of additional regions, including dorsolateral prefrontal and left-lateralized perisylvian (inferior frontal and middle temporal) cortices (Figs. 1, 2, and 3A, and Table S1). The majority of these correlations were significantly stronger during REM than WAKE (Fig. S1 and Table S1).
What do these regions have in common? All of them, like the DMN itself, are heteromodal areas: higher-order association cortices that receive input from multiple brain regions and integrate this information to broadly regulate brain activity. In addition, all are functionally coupled with the DMN during REM sleep.
In stark contrast (Figs. 1, 2, and 3A, and Table S1), during REM the PCC was negatively correlated with unimodal areas of the cortex, including sensory-association regions that process visual (calcarine cortex, cuneus, lingual, and fusiform gyri), somatosensory (postcentral gyri and paracentral lobules), and auditory (superior temporal gyri) stimuli, as well as cortical and subcortical motor areas (precentral gyrus, cerebellum, globus pallidus, and sensorimotor portions of the posterior, granular insula) that play a central role in motor control. PCC activity was also negatively correlated with activity in thalamic nuclei (including medial and lateral pulvinar, ventral, anterior, ventral lateral and ventral posterolateral nuclei, medial and lateral geniculate) that process information from these same sensorimotor areas. Of note, the posterior thalamic cluster in which activity was negatively correlated with the PCC included the nonspecific intralaminar nuclei. Many of these negative correlations were significantly stronger during REM than during either WAKE or SWS (Fig. S1 and Table S1), and observed at the single-subject level (Fig. S4).
Taken together, these findings suggest a broader pattern of interregional connectivity. Although the number of subjects in this study is small and our results need to be interpreted cautiously, it appears that a large-scale functional dissociation of two major brain systems—heteromodal areas on the one hand, unimodal sensory and motor areas on the other—may underlie the unique qualities of consciousness that characterize dream mentation during stage REM sleep.
Thalamic Orchestration of Brain Rhythms During REM Sleep.
What might drive this dissociation? Tract tracing studies indicate that this dissociation is unlikely to be mediated by direct intracortical interactions; the PCC and MPFC, for example, do not project directly to most unimodal areas. A more likely candidate is the pulvinar (Fig. 2 and Table S1), which, as a higher-order thalamic nucleus that plays a critical role in coordinating cortico-cortical communication, has multiple reciprocal connections to both heteromodal and unimodal areas (14), and is on this basis in a key position to orchestrate the dissociated patterns observed during REM (SI Text, Note 4).
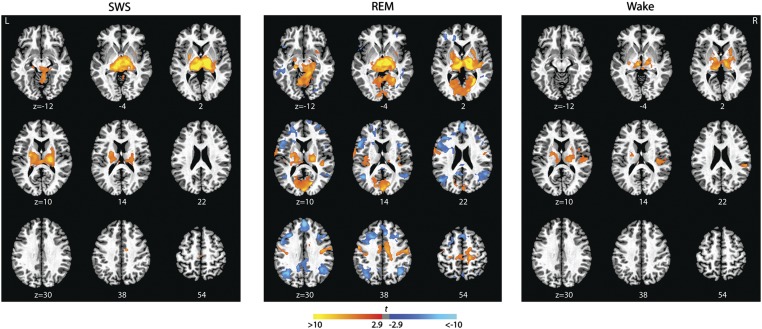
To explore this theory, we used the posterior thalamic cluster itself as a seed (Methods) and characterized the functional connections of this region during each of the three stages of interest. Importantly, the patterns we observed were congruent, but were in this instance even stronger and more widespread (Figs. 3B and 4, and Table S2). (Note that when the thalamus, which is negatively correlated with the PCC, is used as a seed, the sign of the correlation coefficients is reversed compared with Figs. 1 and 2).
Fig. 4.
Functional connectivity of the thalamic seed region during SWS, REM sleep, and WAKE. Brain regions significantly correlated (orange) and anticorrelated (blue) with the thalamic seed during SWS, REM sleep, and WAKE (threshold: P < 0.05, corrected). The thalamic seed region is positively correlated with unimodal areas including the lingual gyrus, vermis, motor areas, auditory areas (indicated in orange), and anticorrelated with elements of the default network (MPFC, PCC/precuneus, IPL) and other heteromodal regions, including inferior frontal gyrus, DLPFC, posterior parietal cortex, and middle temporal gyrus (indicated in blue). Magnitudes of t statistics from the random effects analyses are indicated in the color bar and rendered on a single subject’s T1 image. Axial slices are displayed at the z axis levels shown (in millimeters).
Relatively few significant correlations with the thalamic seed region are evident outside of thalamus during either SWS or WAKE. In contrast, during REM, strong positive correlations are found between activity in the thalamus and regions that mediate early processing of sensory information (principally in occipital and parietal cortices), as well as regions that mediate sensory-guided organization of motor responses (neocortical motor and premotor cortices, posterior insula, cerebellum and striatal regions, including the entire basal ganglia–thalamocortical motor circuit), mesencephalic reticular formation, and hippocampus.
At the same time, thalamic activity is negatively correlated with activity in heteromodal association areas in the frontal, temporal, and parietal cortices, including the DMN, the dorsal attention/executive network, a wide expanse of the dorsolateral prefrontal cortex, and perisylvian language areas, as well as their homologs in the right hemisphere (SI Text, Note 5). Many of these positive and negative correlations were significantly stronger during REM than during either WAKE or SWS (Fig. S5 and Table S1).
These patterns clearly extend beyond the DMN, suggesting that although the DMN may play a role, it operates within a larger context: REM is characterized by a large-scale dissociation—perhaps orchestrated by the posterior thalamus—between activity in sensory and motor systems in the cortex and the subcortical regions to which they project, and activity in higher-order association areas, including the DMN, that ordinarily modulate their activity (SI Text, Note 6).
Temporal Dynamics of the Interactions Between Heteromodal and Unimodal Systems.
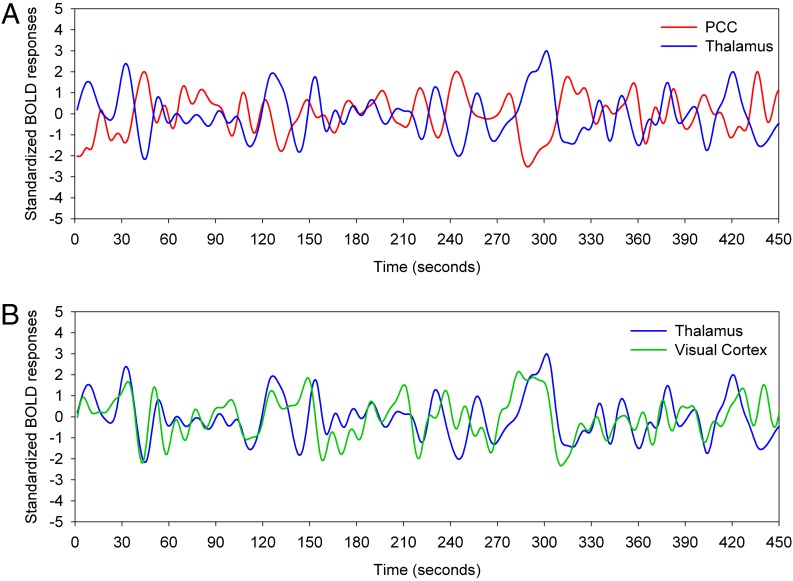
However, there is another dimension to the present findings. Although our results should be interpreted with caution, they nevertheless reveal a pattern that is more complex and potentially more informative. Crucially, there are dynamic temporal fluctuations in the relationships between heteromodal and unimodal systems: The activity in these systems appears to oscillate in time. This pattern is illustrated in Fig. 5 (see also Movie S1): activity in the sensorimotor and heteromodal association cortices alternates systematically in multisecond periods during which sensorimotor areas are active but heteromodal areas are relatively silent, followed by the opposite pattern, and recurring in a cyclic fashion that is repeated throughout, but not beyond the boundaries of, REM sleep (SI Text, Note 7).
Fig. 5.
Standardized BOLD signal time series from a single, continuous REM epoch in a single subject. The thalamus (blue) and PCC (red) are strongly anticorrelated (r = −0.65, P > 0.05) (A) but the thalamus and the visual cortex (green) are strongly correlated (r = 0.45, P > 0.05) (B). Regions correspond to those for which group data are illustrated in Fig. 3 and were selected as outlined in Methods.
Although fluctuations at these frequencies—roughly between 0.1 and 0.01 Hz—are typically described in the BOLD signal at rest (15, 16), they are not BOLD-specific, and have been consistently detected with other dynamic measures of brain activity, including EEG (17) and MEG (18); these occur during sleep (19, 20) and persist into REM (21) (SI Text, Note 8). Interestingly, these fluctuations occur at the same temporal scale as a number of the other phasic phenomena that have been identified during REM (SI Text, Note 9).
It is important to recognize that fluctuations of the BOLD signal at this time scale are present during resting wakefulness and in other sleep stages. What appear to be unique to REM are the strong coherent patterns, including widespread correlations and anticorrelations, that emerge during this sleep stage.
It is possible that this coherent activity is triggered by changes in the functional relationship between CNS neuromodulator systems that is characteristic of (and may initiate) REM sleep (22). The monoamines (norepinephrine and serotonin) and acetylcholine each have independent modulatory effects on naturally occurring slow oscillations within the CNS (23–26). During WAKE and SWS, these systems are all active to some degree, and their independent, uncoordinated effects may be reflected in the broadly asynchronous fluctuations seen during these states. At REM onset however, the monoamingeric nuclei are inhibited, resulting in enhanced, unopposed cholinergic activity that may drive the widespread patterns of coherence we observe.
Converging evidence suggests that in general, slow oscillations may serve a critical carrier function in the CNS, constituting the optimal frequencies at which information can be transmitted over longer distances with minimal decay, and may on this basis play a central role in coordinating activity across multiple corticocortical networks during REM (27) (SI Text, Note 10). Importantly, it has been proposed that, on this basis, large-scale integration of information by slow cortical potentials contributes to conscious awareness (28). If so, the reciprocal, coherent patterns of connectivity observed at these frequencies during REM may reflect or mediate the radical transformation of the contents of consciousness during this sleep stage.
Functional Implications: Dreaming and Memory.
Exactly how then might these physiological fluctuations relate to the cognitive and behavioral features of REM sleep? First, a functional dissociation between sensorimotor areas and the heteromodal systems that monitor and regulate their activity is consistent with many of the features of REM sleep dream mentation: activation of sensory and motor systems in the absence of heteromodal direction or control might explain the fact that dream narratives seem to evolve outside of the dreamer’s sense of agency [i.e., without voluntary planning, abstraction, or coherent time-dependent organization (3, 29)].
The pattern that follows this activation of heteromodal areas while unimodal activity is attenuated is more difficult to understand in the context of what is experienced by the dreamer. However, it is possible that during these intervals, material generated during periods of sensorimotor activity is tethered together into a coherent dream narrative, an attempt by higher-order cognitive systems to reconstruct or contextualize the dream content, in a process similar to confabulation [and analogous to the mechanism proposed by the earlier activation-synthesis model of dreaming (2)]. On the other hand, intermittent heteromodal activity may have nothing to do with the experiential content of dreams but might instead constitute an unrelated process that is not yet understood. If increased levels of activity reflect increased salience, crucial for the encoding of memory, this might account for the selective intensity of sensorimotor features during dreaming and for the dominance of these features when dreams are, in fact, recalled. At the same time the dreamer should have no memory of how higher-order regions have subsequently processed this information. Furthermore, a significant portion, roughly 50%, of dream mentation will never gain access to waking consciousness.
Because the function of dreams, if any, is unknown, no assumptions can be made about the practical significance of the impact of sensorimotor activation or the heteromodal activity that follows it on dream mentation. However, the fluctuating patterns we report may be related to more tangible cognitive functions that have been proposed for REM. Specifically, the patterns may be consistent with the idea that REM plays a critical role in learning and memory.
It has been proposed, for example, that REM sleep facilitates procedural learning by enabling off-line rehearsal and consolidation of newly acquired sensorimotor or cognitive routines (4, 5) (SI Text, Note 11). Similarly, it has been suggested that REM supports establishment and strengthening of cortical sensorimotor pathways (30)—or that it facilitates rehearsal of genetically encoded sensorimotor programs (31)—that enable the brain to anticipate and effectively respond to sensory or emotional stimuli during subsequent periods of wakefulness.
Any of these functions might conceivably be supported by the patterns we observe. That is, during REM selective activation of sensory (particularly visual) and motor areas (including neocortical motor and premotor cortices, posterior insula, cerebellum, and striatum) might make it possible for rehearsal or “replay” of critical sensorimotor routines to occur in context-free isolation (i.e., in the absence of top down heteromodal control). Following such a period of off-line rehearsal, reactivation of heteromodal areas may enable the incorporation of these responses into the existing behavioral repertoire. Sequential alternation of these substates throughout REM might facilitate systems-level consolidation of sensorimotor performance. Findings from previous imaging studies have supported the notion of sensorimotor “replay” during REM in the context of procedural learning (32, 33).
Another emerging theory of sleep-related memory consolidation (34, 35) suggests that SWS may facilitate the initial encoding of individual episodic memories and incorporation of these into a concrete knowledge base, but REM plays a role in distinguishing general patterns within this newly acquired set of items and extracting abstract sets of schemas or rules from them. This paradigm is not inconsistent with our findings. It is conceivable that concrete knowledge is represented (at least in part) in unimodal sensorimotor areas but the processes of extraction, generalization, and abstraction may be mediated by heteromodal association areas. The reciprocally alternating activity within these systems could reflect an integrative process that underlies this transformation, enabling the REM-dependent generalization and extraction of abstract patterns and synaptic consolidation of these patterns in heteromodal cortices.
Each of these potential mechanisms is clearly speculative. Additional empirical studies will be needed to determine whether the complex temporospatial pattern we described can be modulated in predictable ways, and whether this modulation contributes to successful procedural memory consolidation or derivation of abstract rule sets during REM sleep.
We previously speculated (3, 36) that REM sleep might constitute a state of generalized brain activity with the specific exclusion of regions that play a role in executive control. The findings we report here provide a more complex, multidimensional model of the ways in which brain activity may be reorganized in the absence of such control, and the possible physiological consequences of this reorganization. The brain’s DMN, uncoupled during SWS, is recoupled during REM. However, this appears to be part of a broader process involving brain regions that extend well beyond the DMN, which may reflect the contents of consciousness. Indeed, REM sleep is characterized by a spatially unique and temporally dynamic pattern of interregional connectivity that is as extraordinary as the quality of the dream mentation it underlies.
Methods
Experimental Design.
fMRI and EEG data were acquired simultaneously from 18 subjects (10 female; mean age, 25.3 y, range, 21–31 y, SD, 2.8 y) during sleep. Details of data acquisition and preprocessing steps are provided in SI Methods. Experiments were conducted between 2:00 and 6:00 AM after 44 h of sleep deprivation. The data presented were collected and reported in the previous studies to investigate different aspects of sleep phenomena during SWS (13, 21). Good-quality fMRI and EEG data were obtained from 11 of the 18 subjects studied. Another two datasets were excluded from further analyses because neither SWS nor REM sleep were observed using standard sleep scoring criteria (37). The remaining nine subjects, included in the final analyses, had at least 30 min (600 fMRI volumes) SWS and four subjects had at least 5 min (100 fMRI volumes) REM sleep. The total REM sleep duration for the four subjects included in this study was 32.4 min, corresponding to 648 fMRI volumes; additional measures of their sleep structures are presented in Table S3. Additionally, 16.8-min simultaneous EEG-fMRI data were recorded during resting wakefulness in five of these nine subjects. All subjects had normal neurologic and audiologic evaluations and provided written informed consent in accordance with a protocol approved by the National Institutes of Health and Walter Reed Army Institute of Research Institutional Research Boards. Subjects were compensated for participation according to the protocol.
fMRI Correlation Analysis.
For each subject, the fMRI volumes corresponding to the epochs of SWS with a minimum duration of 72 s (24 repetition times, TRs) were concatenated. If REM sleep was observed, the corresponding volumes were concatenated to a separate file using the same duration criteria. For each SWS, REM, and WAKE dataset, the first eigenvector of the activity in the left and right PCC, defined anatomically according to a Montreal Neurological Institute (MNI) atlas (38), was extracted and correlated with each voxel in the brain. Correlation coefficients were then converted to a standard normal metric using r-to-z Fisher’s transformation, generating a z-map of each stage. Subjects’ z-maps were entered into a voxel-wise random-effects ANOVA model, implemented in SPM8 with stages as a factor. Student t test was used to determine whether the mean z of each stage was significantly different from zero and to compare whether there were significant differences between stages. The height threshold was set at P < 0.01 and the spatial extent threshold was set at k > 104 voxels, which corresponds to corrected P < 0.05 according to Monte Carlo simulations implemented in AFNI’s 3dClustSim. There is a chance that an effect may be consistent across subjects (compared with the intersubject variability) and therefore captured by a random-effect analysis, but the effect size [i.e., the average correlation coefficient (r) or difference in r between two stages across subjects] may be trivial. To make sure that this was not the case, we masked each t-map generated from a random-effect analysis with the results of the same comparison using a fixed-effect analysis. In the fixed-effect analysis, the average correlation coefficient (r) or difference in r between two stages across subjects were converted to standard z scores (equation 6 in ref. 39). The threshold of the fixed-effects analysis was set at z > 5, corresponded to r > 0.23. In addition to the PCC seed, the correlation patterns associated with the posterior thalamic and the MPFC seeds were investigated using the same analytic method described above. The thalamic seed region was defined based on two criteria. The selected region was within the left and right thalamus according to an MNI atlas (38) and exhibited anticorrelation with the PCC seed region during REM sleep in the group analysis (Fig. 3). The MPFC seed was defined as the area in a 6-mm radius sphere centered at the MNI coordinate (3, 44, 37) that showed peak positive correlation with the PCC seed during REM sleep in the group analysis (Fig. S2).
Region of Interest Correlation Analysis.
To further illustrate the similarities and differences between connectivity of DMN, heteromodal, and unimodal areas during SWS, REM sleep, and WAKE, we defined the coordinates of six regions of interest (ROIs) based on the group results of the PCC and thalamic seeds. These ROIs included the polar (0, 56, 7) and dorsal (0, 29, 49) MPFC, the right IPL (45, −67, 28), the left dorsal lateral PFC (DLPFC) (−51, 5, 28), the visual cortex (0, −88, 10), and the left precentral gyrus (−51, −4, 40). The eigenvector of the voxels within a 6-mm radius sphere centered at each coordinate were extracted and correlated with the PCC and thalamic seeds described above. Correlation coefficients were converted to Fisher’s z values; t tests were used to compare correlations of ROI pairs between stages. To illustrate the temporal interactions between hetermodal and unimodal regions during REM sleep, the standardized BOLD signals of the PCC, thalamic, visual cortex seeds defined above were extracted from a single, continuous REM epoch of one subject and are plotted in Fig. 5. Additionally, a video of the standardized BOLD signals (z) in a 120-s segment of that epoch was generated. The video displaying z values (>1 and <-1 SD) in five axial slices, providing a coarse coverage of the whole brain, is presented in Movie S1. Voxels that were not significantly correlated with the PCC or thalamic seeds during REM sleep in the group analysis have been masked out.
Supplementary Material
Acknowledgments
The authors thank Carmen Brewer, Christopher Zalewski, Teresa Kessinger, and Jim Paterson for key collaboration and support; and Alex Martin and Kelly Barnes for insightful and critical reviews of this manuscript. This work was supported by the Intramural Research Programs of the National Institute on Deafness and Other Communication Disorders, National Institute of Neurological Disorders and Stroke, and the Walter Reed Army Institute of Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217691110/-/DCSupplemental.
References
- 1.Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: Cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: Neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3(9):679–693. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- 3.Braun AR, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 4.Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning, and dreams: Off-line memory reprocessing. Science. 2001;294(5544):1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- 5.Stickgold R, Walker MP. Sleep and memory: The ongoing debate. Sleep. 2005;28(10):1225–1227. doi: 10.1093/sleep/28.10.1225. [DOI] [PubMed] [Google Scholar]
- 6.Vanhaudenhuyse A, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133(Pt 1):161–171. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noirhomme Q, et al. Brain connectivity in pathological and pharmacological coma. Front Syst Neurosci. 2010;4:160. doi: 10.3389/fnsys.2010.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boly M, et al. Intrinsic brain activity in altered states of consciousness: How conscious is the default mode of brain function? Ann N Y Acad Sci. 2008;1129:119–129. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brier MR, et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer’s disease progression. J Neurosci. 2012;32(26):8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horovitz SG, et al. Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG-fMRI study. Hum Brain Mapp. 2008;29(6):671–682. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson-Prior LJ, et al. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci USA. 2009;106(11):4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sämann PG, et al. Development of the brain’s default mode network from wakefulness to slow wave sleep. Cereb Cortex. 2011;21(9):2082–2093. doi: 10.1093/cercor/bhq295. [DOI] [PubMed] [Google Scholar]
- 13.Horovitz SG, et al. Decoupling of the brain’s default mode network during deep sleep. Proc Natl Acad Sci USA. 2009;106(27):11376–11381. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guillery RW. Anatomical evidence concerning the role of the thalamus in corticocortical communication: A brief review. J Anat. 1995;187(Pt 3):583–592. [PMC free article] [PubMed] [Google Scholar]
- 15.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 16.Fukunaga M, et al. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson Imaging. 2006;24(8):979–992. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 17.He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc Natl Acad Sci USA. 2008;105(41):16039–16044. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Fukunaga M, de Zwart JA, Duyn JH. Large-scale spontaneous fluctuations and correlations in brain electrical activity observed with magnetoencephalography. Neuroimage. 2010;51(1):102–111. doi: 10.1016/j.neuroimage.2010.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achermann P, Borbély AA. Low-frequency (< 1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81(1):213–222. doi: 10.1016/s0306-4522(97)00186-3. [DOI] [PubMed] [Google Scholar]
- 20.Nir Y, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70(1):153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picchioni D, et al. Infraslow EEG oscillations organize large-scale cortical-subcortical interactions during sleep: A combined EEG/fMRI study. Brain Res. 2011;1374:63–72. doi: 10.1016/j.brainres.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pace-Schott EF, Hobson JA. The neurobiology of sleep: Genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3(8):591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- 23.Quattrochi JJ, Mamelak AN, Binder D, Williams J, Hobson JA. Dose-related suppression of REM sleep and PGO waves by the serotonin-1 agonist eltoprazine. Neuropsychopharmacology. 1993;8(1):7–13. doi: 10.1038/npp.1993.2. [DOI] [PubMed] [Google Scholar]
- 24.Werhun K, Lewandowski MH. The effects of muscarinic cholinergic receptor antagonist on slow bursting neuronal activity in the rat intergeniculate leaflet. Folia Biol (Krakow) 2009;57(3-4):187–192. doi: 10.3409/fb57_3-4.187-192. [DOI] [PubMed] [Google Scholar]
- 25.Lörincz ML, Geall F, Bao Y, Crunelli V, Hughes SW. ATP-dependent infra-slow (<0.1 Hz) oscillations in thalamic networks. PLoS ONE. 2009;4(2):e4447. doi: 10.1371/journal.pone.0004447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filippov IV, Williams WC, Krebs AA, Pugachev KS. Dynamics of infraslow potentials in the primary auditory cortex: Component analysis and contribution of specific thalamic-cortical and non-specific brainstem-cortical influences. Brain Res. 2008;1219:66–77. doi: 10.1016/j.brainres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Buzsaki G. Rhythms of the Brain. 1 Ed. New York: Oxford Univ Press; 2006. [Google Scholar]
- 28.He BJ, Raichle ME. The fMRI signal, slow cortical potential and consciousness. Trends Cogn Sci. 2009;13(7):302–309. doi: 10.1016/j.tics.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desseilles M, Dang-Vu TT, Sterpenich V, Schwartz S. Cognitive and emotional processes during dreaming: A neuroimaging view. Conscious Cogn. 2011;20(4):998–1008. doi: 10.1016/j.concog.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Frank MG. Sleep and developmental plasticity not just for kids. Prog Brain Res. 2011;193:221–232. doi: 10.1016/B978-0-444-53839-0.00014-4. [DOI] [PubMed] [Google Scholar]
- 31.Jouvet M. Paradoxical sleep as a programming system. J Sleep Res. 1998;7(Suppl 1):1–5. doi: 10.1046/j.1365-2869.7.s1.1.x. [DOI] [PubMed] [Google Scholar]
- 32.Peigneux P, et al. Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid-eye-movements sleep. Neuroimage. 2003;20(1):125–134. doi: 10.1016/s1053-8119(03)00278-7. [DOI] [PubMed] [Google Scholar]
- 33.Maquet P, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3(8):831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- 34.Walker MP, Stickgold R. Overnight alchemy: Sleep-dependent memory evolution. Nat Rev Neurosci. 2010;11(3):218, author reply 218. doi: 10.1038/nrn2762-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 36.Braun AR, et al. Dissociated pattern of activity in visual cortices and their projections during human rapid eye movement sleep. Science. 1998;279(5347):91–95. doi: 10.1126/science.279.5347.91. [DOI] [PubMed] [Google Scholar]
- 37.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Bethesda, MD: US National Institute of Neurological Diseases and Blindness, Neurological Information Network; 1968. [Google Scholar]
- 38.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 39.Field AP. Meta-analysis of correlation coefficients: A Monte Carlo comparison of fixed- and random-effects methods. Psychol Methods. 2001;6(2):161–180. doi: 10.1037/1082-989x.6.2.161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.