Autoimmune destruction of self tissue is the consequential result of a convergence of several factors, both genetic and environmental, that effectively dislocates the immune system’s ability to tolerate self-antigens but simultaneously retains its focus on those perceived as foreign. Although many of these autoimmune targeted self-antigens are known, immune system triggers can be more elusive. Nevertheless, once autoimmunity has been initiated, what is clear is the role that immune complexes (IC) play as a significant driving force in maintaining chronic inflammation and subsequently pathology in many of these conditions. ICs are antibody/antigen assemblies, usually IgG antibody subtype, sometimes found systemically but primarily at the site of autoimmune self-antigen recognition. Nandakumar et al. have revealed a unique approach to the disruption of these pathogenic complexes (1).
Nandakumar et al. used the mouse anticollagen type II (anti-CII) mediated-arthritis model to show that deglycosylated IgG, regardless of Fab antigen specificity, reduces inflammation in a dose-dependent manner (1). This finding suggests that deglycosylated IgG has a dominant suppressive effect on inflammation and points to a unique class of therapeutic immunoglobulins for the treatment of autoimmunity.
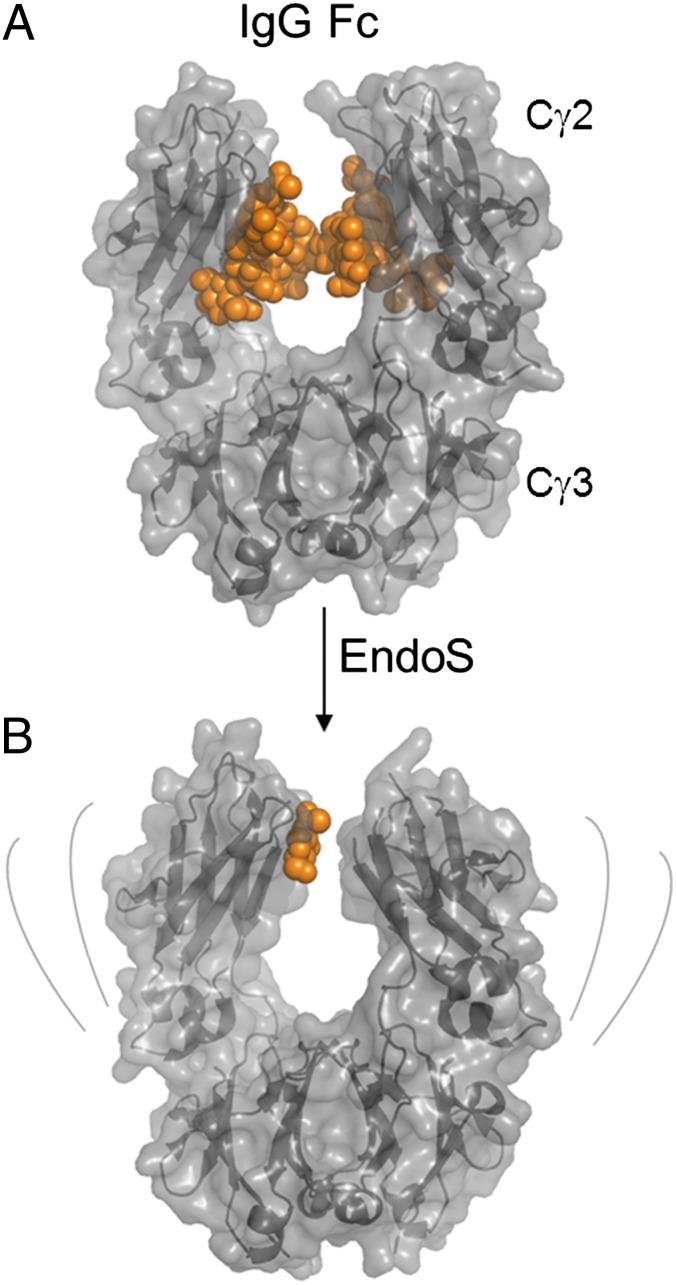
Antibody deglycosylation was achieved by in vitro digestion with EndoS (endo-β-N-acetylglucosaminidase). EndoS is an immune evasion factor from Streptococcus pyogenes, which functions by cleaving the glycans from the fragment crystallizable (Fc) domain of IgG molecules (2, 3). EndoS is highly unusual in its lack of cross-reactivity to other glycoproteins and has a tight specificity for the biantennary glycans found on serum IgG (4). Hydrolysis of the Fc glycans by EndoS significantly reduces the binding to cellular receptors of the immune system to IgG (5). These Fc γ-receptors (FcγR) bind asymmetrically across the lower hinge region and the tips of the Cγ2 domains (6). Crystallographic analysis of the endoglycosidase-treated human IgG Fc shows a closed configuration incompatible with receptor binding (7); solution-phase biophysical analysis shows an increased radius of gyration (8). Taken together these studies demonstrate that the quaternary architecture of the Fc is perturbed upon deglycosylation (Fig. 1).
Fig. 1.
Deglycosylation of IgG Fc by EndoS. Crystal structure of human IgG1 Fc containing (A) native complex-type glycans (19) and (B) the residual glycan following hydrolysis of the glycan by an endoglycosidase (6). The protein is depicted in gray cartoon with transparent surface; the carbohydrate moiety is depicted as orange spheres. The gray lines indicate the increased flexibility of the deglycosylated Fc (4).
Administration of EndoS has emerged as a promising route to the deactivation of autoimmune antibodies and the alleviation of autoimmunity (9–13). However, a recent study by Tradtrantip et al. has suggested that the administration of pathogenic antibodies directed to autoimmune epitopes that have undergone prior deglycosylation ex vivo can also inhibit antibody-dependent cellular cytotoxicity (14). Similar observations have been made in a murine model of fetal/neonatal alloimmune thrombocytopenia. Bakchoul et al. demonstrated that administration of maternal alloantibodies that have been deglycosylated ex vivo prevent destruction of fetal platelets by endogenous glycosylated autoantibodies (15). Nandakumar et al. have made a surprising and significant step by establishing that the deglycosylated antibody does not need to be epitope-specific (1).
However, epitope-independent anti-inflammatory activity of deglycosylated IgG, although highly unusual, is upon first inspection somewhat contradictory with the observation of the loss of anti-inflammatory activity of intravenous Ig upon deglycosylation in the mouse serum-transfer arthritis model (16). Intravenous Ig consists of pooled human serum IgG and is used as a broad anti-inflammatory. However, different animal strains and the mechanism used for disease induction will both have an impact on which component of immune dysfunction is being tested. The anti-CII mediated-arthritis model adopted by Nandakumar et al. (1) allows for accurate timing of the administration of deglycosylated antibody at the point where IC formation is known to be occurring. It would be interesting to see if these effects could be reproduced within a CII model where endogenous autoantibodies are produced, and which might more closely resemble the challenges faced in the clinic.
The anti-inflammatory mechanism of deglycosylated antibodies is of considerable interest. Although in the epitope-directed study by Tradtrantip et al. (14) the effect on antibody-dependent cell-mediated cytotoxicity might be ascribed to a competition with deactivated Fc, the epitope-independent effect on IC formation is less clear. Nandakumar et al. (1) cite early observations that elimination of the Fc (i.e., using Fab′2) impedes IC formation and thus establishes a role for Fc–Fc interactions as a contributing factor to complex formation and immune precipitation (17, 18). Nandakumar et al. look to Fc–Fc interactions observed crystallographically to indicate potential interactions occurring within ICs (1). The capacity for self-association of the Fc domain at high concentration is captured by its very name: Fragment crystallizable. Whether any of the lattice interactions observed by X-ray crystallization of the Fc mimic those interactions within the IC is unknown. Similarly, it is unclear how disruption of the quaternary architecture of the Fc domain can impede the association between two glycosylated Fc domains. If the parallels with crystallographic studies hold, then the presence of any derivative of the Fc, which has a different or reduced tendency to form packing interactions, might be expected to have similar effects on IC formation as deglycosylated IgG. For example, amino acid substitutions on the protein surface or even the addition of further N-linked glycosylation sites could offer additional candidates for recombinant anti-inflammatory molecules. Finally, it would be interesting to test whether the disruption to IC formation can be captured simply by the isolated Fc fragment that has been deglycosylated or otherwise modified.
The anti-inflammatory activity of deglycosylated antibodies may also contribute to the amelioration of autoimmunity by the administration of EndoS. Although EndoS is thought to act principally through the disruption of Fc–FcγR interactions (20), the observations by Nandakumar et al. (1) may point to a more pronounced anti-inflammatory effect than solely predicted by disrupted Fc–FcγR interactions. Evidence to support this might be found in an earlier study by Albert et al., which investigated the effect of EndoS on BXSB mice, which spontaneously develop autoantibodies and lupus-like disease, with and without the FcγR γ-chain (BXSB-γ−/−) (9). Although EndoS had no discernible impact on spontaneous autoantibody production, it did have a significant protective effect on autoantibody-mediated mortality beyond that afforded by the FcγR γ-chain.
Further efforts to explore the full potential of deglycosylated antibodies for the treatment of autoimmune disease, together with the molecular dissection of the mechanisms underpinning their efficacy, will no doubt illuminate routes to the production of effective recombinant antibody-based therapies.
Footnotes
The author declares no conflict of interest.
See companion article on page 10252.
References
- 1.Nandakumar KS, et al. Dominant suppression of inflammation by glycan-hydrolysed IgG. Proc Natl Acad Sci USA. 2013;110:10252–10257. doi: 10.1073/pnas.1301480110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Collin M, Olsén A. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001;20(12):3046–3055. doi: 10.1093/emboj/20.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collin M, Olsén A. Effect of SpeB and EndoS from Streptococcus pyogenes on human immunoglobulins. Infect Immun. 2001;69(11):7187–7189. doi: 10.1128/IAI.69.11.7187-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodfellow JJ, et al. An endoglycosidase with alternative glycan specificity allows broadened glycoprotein remodelling. J Am Chem Soc. 2012;134(19):8030–8033. doi: 10.1021/ja301334b. [DOI] [PubMed] [Google Scholar]
- 5.Allhorn M, Olin AI, Nimmerjahn F, Collin M. Human IgG/FcγR interactions are modulated by streptococcal IgG glycan hydrolysis. PLoS One. 2008;3(1):e1413. doi: 10.1371/journal.pone.0001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406(6793):267–273. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- 7.Baruah K, et al. Selective deactivation of serum IgG: a general strategy for the enhancement of monoclonal antibody receptor interactions. J Mol Biol. 2012;420(1–2):1–7. doi: 10.1016/j.jmb.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrok MJ, Jung ST, Kang TH, Monzingo AF, Georgiou G. Revisiting the role of glycosylation in the structure of human IgG Fc. ACS Chem Biol. 2012;7(9):1596–1602. doi: 10.1021/cb300130k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albert H, Collin M, Dudziak D, Ravetch JV, Nimmerjahn F. In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc Natl Acad Sci USA. 2008;105(39):15005–15009. doi: 10.1073/pnas.0808248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benkhoucha M, et al. IgG glycan hydrolysis by EndoS inhibits experimental autoimmune encephalomyelitis. J Neuroinflammation. 2012;9:209. doi: 10.1186/1742-2094-9-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirose M, et al. Enzymatic autoantibody glycan hydrolysis alleviates autoimmunity against type VII collagen. J Autoimmun. 2012;39(4):304–314. doi: 10.1016/j.jaut.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Lood C, et al. IgG glycan hydrolysis by endoglycosidase S diminishes the proinflammatory properties of immune complexes from patients with systemic lupus erythematosus: A possible new treatment? Arthritis Rheum. 2012;64(8):2698–2706. doi: 10.1002/art.34454. [DOI] [PubMed] [Google Scholar]
- 13.Nandakumar KS, et al. Endoglycosidase treatment abrogates IgG arthritogenicity: Importance of IgG glycosylation in arthritis. Eur J Immunol. 2007;37(10):2973–2982. doi: 10.1002/eji.200737581. [DOI] [PubMed] [Google Scholar]
- 14.Tradtrantip L, Ratelade J, Zhang H, Verkman AS. Enzymatic deglycosylation converts pathogenic neuromyelitis optica anti-aquaporin-4 immunoglobulin G into therapeutic antibody. Annals of Neurology. 2013;73(1):77–85. doi: 10.1002/ana.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakchoul T, et al. Inhibition of HPA-1a alloantibody-mediated platelet destruction by a deglycosylated anti-HPA-1a monoclonal antibody: towards targeted treatment of fetal-alloimmune thrombocytopenia. Blood. 2013 doi: 10.1182/blood-2012-11-468561. 10.1182/blood-2012-11-468561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 17.Møller NP. Fc-mediated immune precipitation. I. A new role of the Fc-portion of IgG. Immunology. 1979;38(3):631–640. [PMC free article] [PubMed] [Google Scholar]
- 18.Easterbrook-Smith SB, Vandenberg RJ, Alden JR. The role of Fc:Fc interactions in insoluble immune complex formation and complement activation. Mol Immunol. 1988;25(12):1331–1337. doi: 10.1016/0161-5890(88)90048-x. [DOI] [PubMed] [Google Scholar]
- 19.Matsumiya S, et al. Structural comparison of fucosylated and nonfucosylated Fc fragments of human immunoglobulin G1. J Mol Biol. 2007;368(3):767–779. doi: 10.1016/j.jmb.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Allhorn M, Collin M. Sugar-free antibodies–The bacterial solution to autoimmunity? Annals of the New York Academy of Sciences. 2009;1173:664–669. doi: 10.1111/j.1749-6632.2009.04739.x. [DOI] [PubMed] [Google Scholar]