Abstract
Clinical and epidemiological synergy exists between the globally important sexually transmitted infections, gonorrhea and HIV. Neisseria gonorrhoeae, which causes gonorrhea, is particularly adept at driving HIV-1 expression, but the molecular determinants of this relationship remain undefined. N. gonorrhoeae liberates a soluble factor that potently induces expression from the HIV-1 LTR in coinfected cluster of differentiation 4-positive (CD4+) T lymphocytes, but this factor is not a previously described innate effector. A genome-wide mutagenesis approach was undertaken to reveal which component(s) of N. gonorrhoeae induce HIV-1 expression in CD4+ T lymphocytes. A mutation in the ADP-heptose biosynthesis gene, hldA, rendered the bacteria unable to induce HIV-1 expression. The hldA mutant has a truncated lipooligosaccharide structure, contains lipid A in its outer membrane, and remains bioactive in a TLR4 reporter-based assay but did not induce HIV-1 expression. Mass spectrometry analysis of extensively fractionated N. gonorrhoeae-derived supernatants revealed that the LTR-inducing fraction contained a compound having a mass consistent with heptose-monophosphate (HMP). Heptose is a carbohydrate common in microbes but is absent from the mammalian glycome. Although ADP-heptose biosynthesis is common among Gram-negative bacteria, and heptose is a core component of most lipopolysaccharides, N. gonorrhoeae is peculiar in that it effectively liberates HMP during growth. This N. gonorrhoeae-derived HMP activates CD4+ T cells to invoke an NF-κB–dependent transcriptional response that drives HIV-1 expression and viral production. Our study thereby shows that heptose is a microbial-specific product that is sensed as an innate immune agonist and unveils the molecular link between N. gonorrhoeae and HIV-1.
Keywords: microbial-associated molecular pattern, pathogen-associated molecular pattern, coinfection, sexually transmitted disease, inflammation
Globally, the majority of HIV-1 transmission occurs during sex. Despite the scourge of this virus, the efficiency of transmission is poor, with infectivity rates as low as one or two cases per 1,000 coital acts (1, 2). A myriad of viral, host, and environmental factors influence the heterogeneity of HIV-1 transmission, infection, and disease progression. A key factor is the existence of coinfecting bacterial, viral, and parasitic microbes that alter the immune status of the host and affect HIV-1 pathogenesis (2, 3). Accompanying copathogens can suppress (4, 5) or enhance (3, 6, 7) transmission and the clinical progression of HIV-1–related disease. Understanding the mechanism of copathogen-induced HIV-1 virulence is crucial because of its impact on the global spread of HIV-1.
Clinical and epidemiological studies revealed a positive correlation between coinfection with sexually transmitted infections (STIs) and increased genital tract HIV-1 shedding and/or susceptibility to HIV-1 (2, 3, 8, 9). Neisseria gonorrhoeae (Ng), the etiological agent of gonorrhea, is one of the most common bacterial STIs in people living with HIV-1 (9, 10). Women with laboratory-diagnosed Ng infections are at a significantly higher risk of HIV-1 acquisition, even when the data have been controlled for demographic and behavioral factors, clinical symptoms, and other STIs (9). Symptomatic Ng infection is associated with increased detection of viral-derived nucleic acids from genital secretions of men and women (11–13), and this effect was reversed upon successful Ng treatment. Concurrent Ng infection is associated with an increase in HIV-1 viremia (14, 15), decreases in HIV-1 target lymphocyte [cluster of differentiation 4-positive (CD4+) T-cell] counts (14), and a decrease in effector [cluster of differentiation 8-positive (CD8+) T-cell] lymphocyte responses (16). Because of the impact of Ng on HIV-1 shedding, coinfection is associated with a two- to fivefold increase in male-to-female transmission rates (3).
These clinical findings drove investigations aimed at understanding the synergistic relationship between Ng and HIV-1 at a molecular level. The earliest report demonstrated that Ng promotes HIV-1 transcription in a CD4+ T-cell line-based model of HIV-1 expression and that Ng culture supernatants were sufficient for induction (17). Subsequently, Ng was shown to enhance HIV-1 replication in an in vitro female genital microenvironment (18).
Invading microbes are first recognized by host innate immune receptors. The best-characterized class of these receptors is the family of Toll-like receptors (TLRs) that, upon recognition of conserved microbial-associated molecular patterns (MAMPs), trigger a cascade of signaling events that modulate both the adaptive and innate immune responses (19). TLR activation modulates HIV-1 infection and/or transmission, and, depending on the specific TLR agonist and the target cell, TLR activation can either promote or inhibit HIV-1 expression in vitro (17, 18, 20–26).
CD4+ T cells are the key HIV-1 target cell and prime latent viral reservoirs (27). Of the TLR ligands, the FimH component of type I pili (TLR4 agonist) and flagellin (TLR5 agonist) directly elicit HIV-1 LTR expression in CD4+ T cells (18). There is ligand specificity in TLR-driven HIV-1 induction, because another TLR4 agonist, LPS, does not induce HIV-1 expression in the same cell line (17, 18). TLR2 agonists including dipalmitoyl-S-glyceryl-L-Cys-Ser-(Lys)4 (Pam2CSK4) and peptidoglycan promote the replication of HIV-1 from resting CD4+ T cells (21), and flagellin has been shown to reactivate latent HIV-1 in CD4+ T cells and to induce viral gene expression in quiescent central memory CD4+ T cells (25).
With regards to Ng-specific ligands, Pam(3)C-Lip, a synthetic Ng-like lipopeptide, enhances HIV-1 infection of resting CD4+ T cells via TLR2 (21). However, Ng still drives HIV-1 LTR expression in T-cell lines that do not express TLR2 (18, 28), indicating that other Ng factors are key to the induction of HIV-1 expression in CD4+ T cells. This study was undertaken to identify the Ng-derived factor that drives HIV-1 LTR expression in coinfected CD4+ T cells.
Results
Neisseria spp. Potently Induce HIV-1 LTR Expression in a TLR5-Independent Manner.
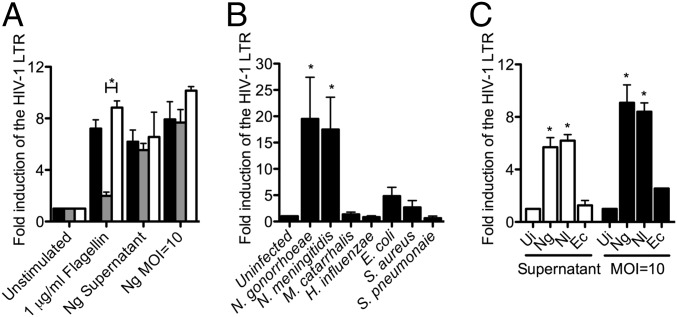
Because a wide variety of bacteria-derived components have the potential to elicit an innate response in mammalian cells, we first tested a spectrum of prototypical MAMPs for their ability to induce the HIV-1 LTR in the Jurkat 1G5 reporter cell line. Of these, only the TLR5 agonist flagellin induced significant expression in our hands (Fig. S1). Although potent TLR5-mediated effects on HIV-1 expression in this cell line have been described (18), Ng does not express flagellin. We tested whether Ng induces HIV-1 expression via a novel TLR5 agonist using a TLR5 neutralization assay. To this end, the CD4+ T cells were incubated with a specific TLR5-blocking antibody before infection, but this incubation did not affect the HIV-1 LTR expression induced by Ng culture supernatants or by live bacteria (Fig. 1A). Combined, these results indicate that Ng induces HIV-1 expression in CD4+ T cells by a mechanism other than a well-described innate immune agonist/receptor interaction.
Fig. 1.
Neisseria spp. potently induce HIV-1 LTR expression in CD4+ T cells. HIV-1 LTR expression in Jurkat 1G5 CD4+ T cells was quantified by luciferase assay. Data represent the fold induction of expression over uninfected cells. (A) TLR5 neutralization. Jurkat 1G5 cells were preincubated with no antibody (black bars), with anti-TLR5 (gray bars), or with isotype control (white bars). Data shown are the average of triplicate samples ± SD, representing three experiments. *P < 0.05; ANOVA, Bonferroni. (B) The potent HIV-1 LTR induction effect is unique to infection with Neisseria spp. MOI = 10. Data shown are the mean of three independent experiments ± SEM. *P < 0.05; ANOVA, Dunnett. (C) The HIV-1 LTR induction effect is a common among Neisseria spp. Jurkat 1G5 cells were either grown in 75% (vol/vol) culture supernatants (white bars) or infected with an MOI = 10 (black bars). Data shown are the mean of three independent experiments ± SEM. *P < 0.05; ANOVA, Bonferroni. Ec, E. coli; Nl, N. lactamica; Ui, uninfected.
To investigate whether Ng induction of HIV-1 LTR expression is caused by a general immune response to live bacterial infection or instead is an Ng phenotype, a panel of other nonflagellated bacteria was evaluated for HIV-inducing activity. In addition to Ng, the closely related Gram-negative species Neisseria meningitidis (Nm), Moraxella catarrhalis, and Haemophilus influenzae, the Gram-positive pathogens Staphylococcus aureus and Streptococcus pneumoniae, and a laboratory-adapted strain of Escherichia coli were tested. Details of bacterial strains used in this study are outlined in Table S1. Strikingly, the Neisseria spp. were the only bacteria that induced significant expression of the HIV-1 LTR, with Ng inducing expression on average 19.5-fold and Nm inducing expression on average 17.5-fold greater than in untreated cells (Fig. 1B). Staphylococcus aureus and Escherichia coli induced expression that was increased on average by 2.7- and 4.8-fold, respectively, but these effects were not found to be significant. Because M. catarrhalis is the bacterium most closely related to Neisseria spp., we tested M. catarrhalis strains further and confirmed the specificity of HIV-1 induction by Neisseria spp. (Fig. S2).
To determine if HIV-1 induction is a virulence trait of the pathogenic Neisseria, the effect of an upper respiratory commensal on expression was tested. Neisseria lactamica, a frequent commensal of infants, potently induced HIV-1 expression (Fig. 1C), indicating that the stimulatory component is a common neisserial trait. Also, culture supernatants from both pathogenic and commensal Neisseria were sufficient to induce expression, but culture supernatant from E. coli was not (Fig. 1C), indicating that the active bacterial component is liberated into the medium during neisserial growth and that the mechanism of HIV-1 induction is common among the Neisseria spp.
Recovery of Neisseria spp. Mutants Defective in HIV-1 LTR Induction.
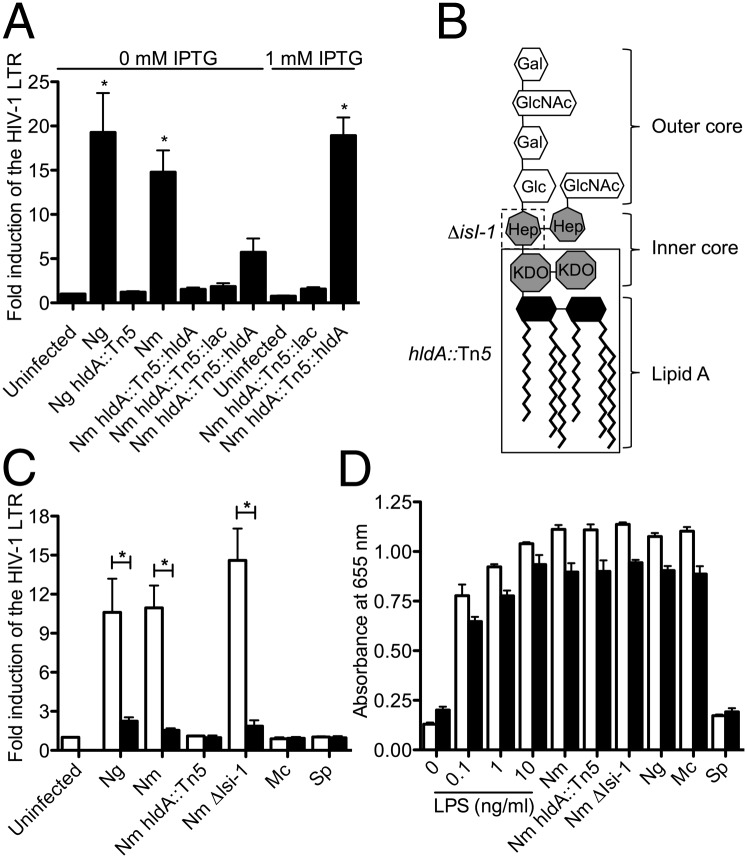
In an attempt to determine the component(s) from Neisseria spp. that trigger HIV-1 LTR expression, we undertook a genome-wide transposon mutagenesis-based approach to generate a mutant library that could be screened for relative HIV-inducing capacity. Nm is less fastidious and more amenable to such a high-throughput approach than Ng and induces similar levels of HIV-1 LTR expression (Fig. 1C). Therefore, a random Tn5-based transposon mutant library was constructed in Nm, and the first 1,800 mutants from this library were individually screened for their capacity to induce HIV-1 LTR using a high-throughput Jurkat 1G5 LTR-luciferase reporter-based assay. One non–HIV-1 LTR–inducing mutant was obtained, and sequence analysis indicated that the transposon had incorporated into a genetic region that aligns with ORF NMB0825 of the published Nm MC58 genome sequence (29). This Nm Tn5 mutant does not induce HIV-1 LTR expression (Fig. 2A), and the lack of induction is not the result of a growth deficit because the mutant does not exhibit any difference in growth compared with the parent strain (Fig. S3). To confirm that NMB0825 was indeed involved in the induction of the HIV-1 LTR, the mutation was complemented with a functional copy of the ORF. A single copy of NMB0825 was reintroduced in trans within the mutant genome under lac promoter control using the pGCC4 vector system (30). In the absence of isopropyl β-d-1-thiogalactopyranoside (IPTG), the complemented strain induced HIV-1 LTR expression at levels an average of 5.7-fold above background (Fig. 2A). However, when IPTG was present to induce expression of NMB0825, the complemented strain induced HIV-1 LTR expression at parental levels (Fig. 2A).
Fig. 2.
HldA is required for the induction of HIV-1 LTR expression. (A) Neisserial hldA mutants do not induce HIV-1 LTR expression. HIV-1 LTR expression in Jurkat 1G5 CD4+ T cells was quantified by luciferase assay. Data represent the fold induction of expression over uninfected cells. Data shown represent the mean of three independent experiments ± SEM. *P < 0.05; ANOVA, Dunnett. Mc, catarrhalis; Sp. S. pneumoniae. (B) Representation of Nm LOS adopted from ref. 53. Truncated mutant chemotypes are indicated by boxes. (C) Neisseria spp. must be viable and have functional ADP-heptose biosynthesis to induce HIV-1 LTR expression. HIV-1 LTR expression in Jurkat 1G5 CD4+ T cells was quantified by luciferase assay. Data represent the fold induction of expression over uninfected cells. White bars represent live bacteria; black bars represent heat-killed bacteria. Data shown are the mean of three independent experiments ± SEM. *P < 0.05; ANOVA, Bonferroni. (D) Detection of bioactive endotoxin. HEK-Blue hTLR4 cells containing a TLR4-induced reporter fusion were stimulated with LPS or bacteria. White bars represent live bacteria at an MOI = 2; black bars represent the heat-killed equivalent. Data shown are mean of triplicate samples ± SD, representing three individual experiments.
The Nm ORF NMB0825 was annotated as encoding for an ADP-heptose synthase that is RfaE- or HldE-like (29). HldE from E. coli is a bifunctional enzyme that functions as a carbohydrate kinase and an adenylyltransferase involved in the biosynthesis of ADP-heptose, a precursor for LPS biosynthesis (31). In Nm, the two catalytic activities of HldE are performed by separate enzymes: HldA as d-beta-d-heptose-7-phosphate kinase and HldC as d-beta-d-heptose-1-phosphate adenosyltransferase (31). The translated NMB0825 sequence from Nm MC58 contains a predicted carbohydrate kinase domain and shares 57% identity (74% similarity) with HldA of Burkholderia cenocepacia J2315 (32), indicating that NMB0825 is an hldA homolog and that the transposon disrupted a key gene in ADP-heptose precursor biosynthesis. To confirm that hldA also is responsible for the HIV-1 induction phenotype of Ng, a directed hldA::Tn5 mutant was constructed in this species. The Ng hldA::Tn5 mutant also did not induce HIV-1 LTR expression (Fig. 2A).
The lipooligosaccharide (LOS) chemotype of an hldA mutant is anticipated to be truncated after the keto-3-deoxy-d-manno-octulosonic acid (KDO) moieties in the inner core (Fig. 2B) (33). The lipid A component of LOS has long been considered as the immunoactive component of the Gram-negative endotoxin (34). However, the hldA mutation is not expected to affect endotoxin expression, and clear evidence for the presence of an intact lipid A moiety was identified in both the wild-type and hldA mutant by mass spectrometry (Fig. S4). To ensure that the lack of HIV-1 induction by the hldA::Tn5 mutants is not caused by a general loss of endotoxic potential, we compared the effect of the mutant and parental strains on a TLR4 reporter cell line. The hldA::Tn5 mutants induced a TLR4-dependent response indistinguishable from that of the parent (Fig. 2D). Therefore, the hldA mutants retain bioactive endotoxin.
To determine if the biosynthesis of ADP-heptose is key to HIV-1 LTR induction by Neisseria spp. or if loss of heptose and the additional carbohydrates in the LOS structure of the hldA mutant resulted in the lack of HIV-1 LTR induction activity, an additional LOS biosynthesis mutant was tested in the Jurkat 1G5 bioassay. The α1,3-heptosyl transferase, Isi-1 (or RfaF) adds the second heptose onto the LOS structure (33); thus Nm Δlsi-1 mutants have functional ADP-heptose biosynthesis and contain one additional heptose beyond the chemotype of the hldA mutants (Fig. 2B). The Nm Δlsi-1 mutant potently induced HIV-1 LTR expression (Fig. 2C), indicating that ADP-heptose biosynthesis or the presence of heptose within the LOS is necessary for HIV-1 induction.
Neisseria spp. Viability Is Essential for HIV-1 LTR Induction.
The composition of the core oligosaccharide in Gram-negative bacteria is highly conserved, and many of these bacteria, including E. coli and H. influenzae, contain heptose (35). However, E. coli and H. influenzae do not induce HIV-1 LTR expression (Fig. 1 B and C). Because the mere presence of heptose within LPS is not sufficient for induction, we hypothesized that Neisseria spp. must either modify LOS to expose heptose at the surface or actively liberate a heptose-containing moiety into the culture medium. Killing the Neisseria spp. by incubation at 65 °C for 1 h before infection eliminated the HIV-inducing activity of the bacteria (Fig. 2C), whereas heat-killing the bacteria did not affect innate endotoxin activity (Fig. 2D). This result is consistent with metabolically active Neisseria spp. liberating a heptose that drives HIV-1 expression in CD4+ T cells.
Ng-Derived Heptose-Monophosphate Induces HIV-1 Expression and Viral Production in CD4+ T Cells.
To ensure selective immune induction, MAMPs typically are invariant bacterial features unique to microorganisms. Heptose is ideally suited for this purpose because it is a carbohydrate that is not produced by mammals but is associated with most bacteria (35). A purification protocol was developed to isolate the active form of heptose from Ng culture supernatants. The presence of heptose in the preparation was preliminarily confirmed by LC-MS. HPLC fractionation of the preparation with a C18-reversed phase column revealed that the HIV-1 LTR-inducing component was not retained on the column, indicating that, like heptose, it is a small and very polar molecule. The active reversed-phase fraction subsequently was submitted to normal-phase HPLC fractionation and MS. The resulting fractions were tested for activity in the 1G5 bioassay, and the active fraction contained a signal at m/z 289.0314 in negative mode, consistent with the theoretical expected value for the deprotonated molecular mass [M-H] of heptose-monophosphate (HMP) (formula: C7H15O10P: 289.0324; mass error: 3.7 ppm) (Fig. S5).
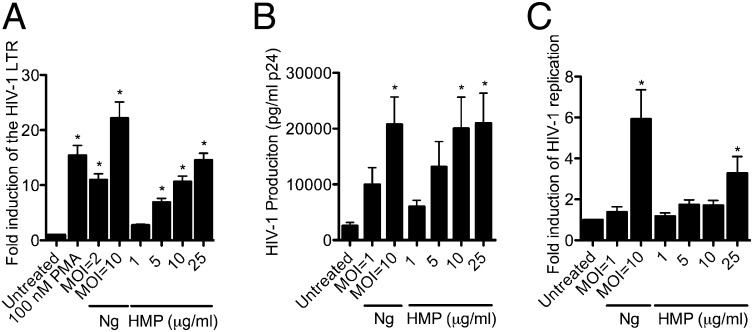
The Ng-derived HMP preparation induced HIV-1 LTR expression in Jurkat 1G5 cells in a dose-dependent manner, with a concentration of 25 μg/mL of preparation inducing expression at a level similar to the positive control (phorbol myristate acetate, PMA) and between that induced by MOI = 2 and MOI = 10 of live Ng (Fig. 3A). Using the HEK-Blue TLR4 reporter cell line, we confirmed that the preparation did not contain detectable levels of bioactive endotoxin (Fig. S6).
Fig. 3.
HMP induces HIV-1 expression and replication. (A) Dose-dependent response of HIV-1 LTR expression to HMP. HIV-1 LTR expression in Jurkat 1G5 cells was quantified by luciferase assay. Data represent the fold induction of expression over untreated cells. Data shown are the mean of three independent experiments ± SEM. *P < 0.05; ANOVA, Dunnett. (B) Effect of HMP on replication of HIV-1 LAV from latently infected CD4+ T cells. HIV-1 LAV replication from ACH-2 cells was measured by ELISA for p24 antigen. Data shown are the mean four independent experiments ± SEM. *P < 0.05; ANOVA, Dunnett. (C) Effect of HMP on replication of HIV-1 NL4-3 from acutely infected CD4+ T cells. HIV-1 NL4-3 replication was measured by ELISA for p24 antigen. Data are represented as fold induction over untreated cells and are the mean of three individual experiments ± SEM. *P < 0.05 comparing MOIs or HMP concentrations versus untreated cells; ANOVA, Dunnett.
To confirm that the effect of HMP on HIV-1 expression observed in the Jurkat 1G5 model translates to the induction of full-length viral replication, we tested the preparation for the induction of HIV-1 virion production. When CD4+ T cells latently infected with replication-competent HIV-1 LAV provirus (36) were treated with increasing concentrations of HMP, a dose-dependent induction of HIV-1 was observed, reflecting an HMP-driven viral reactivation from latency (Fig. 3B). In this assay system, an HMP concentration of 5 μg/mL induced viral production similar to that induced by infection with viable Ng at MOI = 1, whereas 10 and 25 μg/mL of HMP induced levels similar to those induced by Ng at MOI = 10.
To illustrate the effect of HMP on viral production from acutely infected CD4+ T cells, Jurkat cells were infected with the prototypical X4 tropic HIV-1 strain NL4-3 and then either were coinfected with viable Ng or were treated with increasing concentrations of HMP. The HMP preparation significantly induced HIV-1 production from these acutely HIV-1–infected T cells at a concentration of 25 μg/mL (Fig. 3C).
Ng-Derived HMP Induces HIV-1 Expression via Activation of CD4+ T Cells.
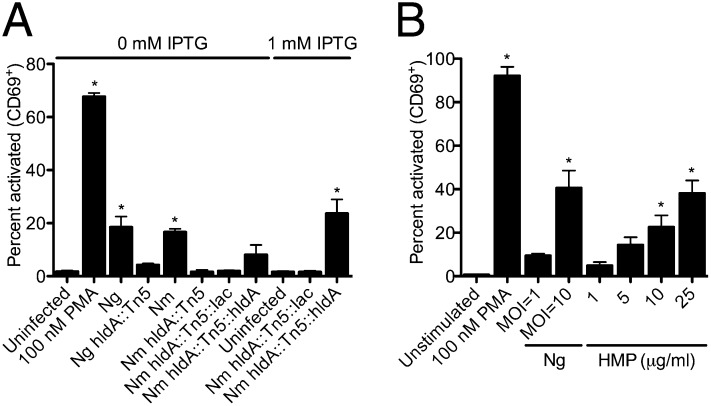
HIV-1 expression depends on T-cell activation (37). Ng previously has been demonstrated to induce expression of the early activation surface marker, cluster of differentiation 69 (CD69), on CD4+ T cells (38). To explore whether the hldA mutants were affected in their ability to activate the T cells, CD69 expression by the infected cells was evaluated by flow cytometry. Although the wild-type strains of Nm and Ng induced CD69 expression, T cells infected with the hldA::Tn5 mutants were indistinguishable from the untreated cells (Fig. 4A). Restoring a functional copy of hldA into the Nm mutant restored the cell activation to parental levels.
Fig. 4.
HMP is required for activation of CD4+ T cells by Neisseria spp. (A) CD69 expression in response to Neisseria spp. hldA mutants and (B) to increasing concentrations of HMP. The proportion of cells expressing CD69 was determined by flow cytometry. Data are the mean of three individual experiments ± SEM. *P < 0.05 versus untreated; ANOVA, Dunnett.
Next, the effect of HMP on T-cell activation was investigated. CD69 expression increased in a dose-dependent manner: 5 μg/mL of HMP activated CD69 expression to an extent corresponding to Ng MOI = 1, and 25 μg/mL of HMP activated CD69 expression to an extent similar to Ng MOI = 10 (Fig. 4B). This response mirrors HIV-1 LTR expression (Fig. 3A) and virion production (Fig. 3 B and C) in response to HMP, verifying a role for the HldA-mediated heptose precursor biosynthesis in the activation of T cells and in the induction of HIV-1 expression and viral replication.
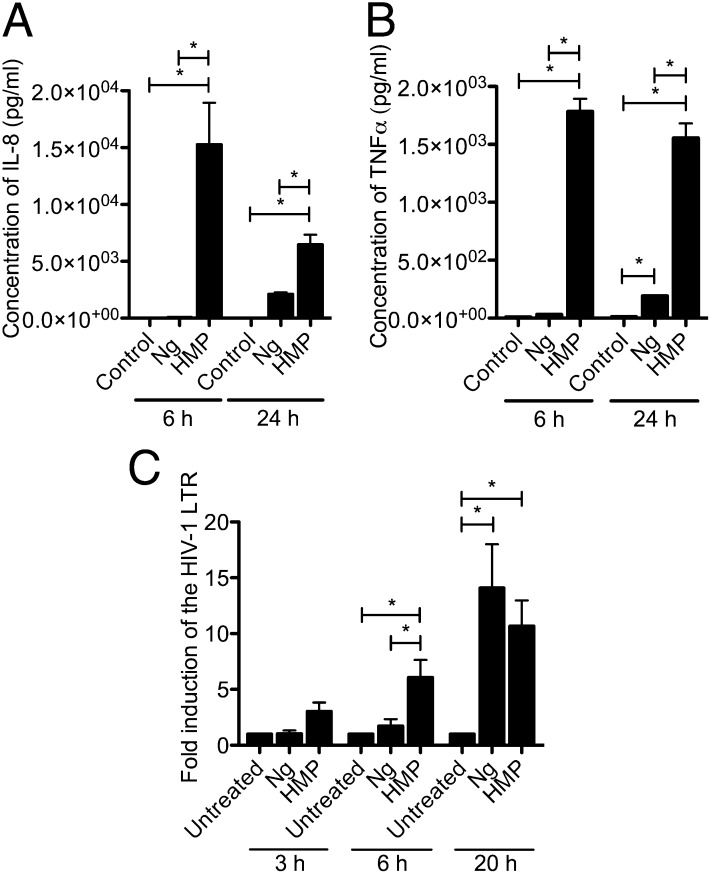
With HMP activating CD4+ T cells, we investigated what other cellular responses it elicits. Using a multiplex ELISA, we observed that HMP induced significant expression of the proinflammatory cytokines IL-8 and TNF-α (Fig. 5 A and B). Induction of IL-8 by HMP is intriguing, because IL-8 drives the massive neutrophil recruitment that manifests as the purulent discharge observed in symptomatic gonorrhea. The induction of IL-8 by HMP was greater at 6 h than at 24 h and even at 24 h was significantly greater than the response triggered by live Ng. This lag in induction by the live bacteria is consistent with a model in which the immunoactive HMP must accumulate in culture during Ng growth to provoke its effect.
Fig. 5.
Ng releases HMP during growth that induces a functional cytokine response from CD4+ T cells. (A) IL-8 and (B) TNF-α response to a MOI = 10 of Ng or 20 μg/mL of HMP. Concentration of cytokines after 6 and 24 h was determined by Multiplex ELISA. Data are the mean of triplicate samples ± SD. *P < 0.05, ANOVA, Bonferroni. (C) Temporal induction of HIV-1 LTR induction by Ng and HMP. Jurkat 1G5 cells were infected with a MOI = 10 of Ng or treated with 20 μg/mL of HMP. HIV-1 LTR expression was quantified by luciferase assay. Data represent the fold induction of expression over untreated cells. Data are the mean of three independent experiments ± SEM. *P < 0.05 ANOVA, Newman–Keuls.
To test this model, we investigated the kinetics of HIV-1 LTR induction by Ng relative to that triggered by the HMP preparation. After 3 h, HMP already induces a notable response from the HIV-1 LTR, and this response becomes significantly greater than that of Ng at 6 h (Fig. 5C). However, by 20 h postinfection, the HIV-1 LTR response to Ng begins to surpass that seen with HMP (Fig. 5C).
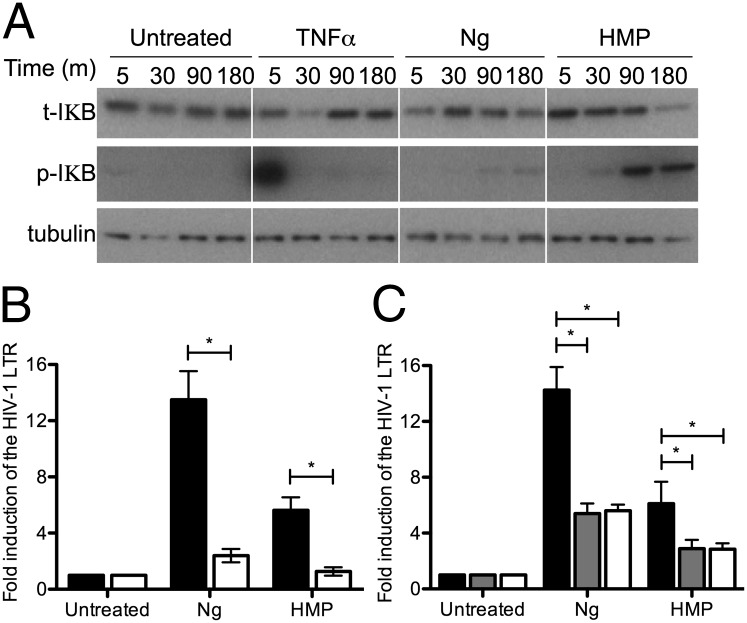
IL-8 and TNF-α expression are controlled by NF-κB (39, 40), and the induction of HIV-1 LTR expression by Ng requires NF-κB activation (17, 18). We sought to confirm activation of the NF-κB pathway biochemically during the HMP response. To this end, the appearance of the phosphorylated inhibitor of NF-κB (IκB) upon exposure to HMP, Ng, or the prototypical agonist TNF-α was examined. Upon phosphorylation, IκB disassociates from the NF-κB and is degraded by the proteosome, allowing free NF-κB to translocate into the nucleus to stimulate transcription. TNF-α is a potent activator of NF-κB and HIV-1 LTR expression (41). After 5 min of treatment with TNF-α, phospho-IκB appeared rapidly, and a subsequent decrease in total IκB was evident after 30 min incubation (Fig. 6A). IκB returned to untreated levels by 90 min. HMP and Ng also trigger phosphorylation of IκB, although the kinetics differed from that observed with TNF-α, with the appearance of phospho-IκB and subsequent IκB degradation occurring at later time points after infection with Ng or exposure to HMP (Fig. 6A). This delay implies that the NF-κB may be activated via a pathway distinct from that induced by TNF-α. Moreover, the earlier and enhanced response to purified HMP relative to viable Ng is consistent with a requirement for the ongoing liberation of HMP by viable Ng to elicit this cellular response. In any case, the sustained presence of phosphorylated IκB in response to HMP has important implications for the kinetics and duration of viral expression.
Fig. 6.
Activation of HIV-1 expression by HMP requires NF-κB. (A) Phosphorylation and degradation of cytoplasmic IκB in response to HMP. A3.01 cells were treated with 10 ng/mL TNF-α or 20 μg/mL HMP or were infected with an MOI = 10 of Ng. Whole-cell lysates were immunoblotted with antibodies directed against IκBα (t-IκB) and its phosphorylated form (p-IκB); α/β tubulin was used as a loading control. (B) Effect of the proteosome inhibitor, MG-132 on HIV-1 LTR induction by Ng and HMP. Jurkat 1G5 cells were preincubated with 0 nM (black bars) or 250 nM (white bars) MG-132 before infection with MOI = 10 of Ng or 25 μg/mL of HMP, and HIV-1 LTR expression was quantified by luciferase assay. Data represent the fold induction of expression over untreated cells. Data are the mean of five independent experiments ± SEM. *P < 0.05; ANOVA, Bonferroni. (C) Effect of RNAi of NFKB1 and RelA on HIV-1 induction by Ng and HMP. Jurkat 1G5 cells were transfected with scramble shRNA (black bars) as a control or with shRNAs targeting NFKB1 (gray bars) or RelA (white bars). Cells were infected with a MOI = 10 of Ng or were treated with 20 μg/mL of HMP and HIV-1 LTR expression was quantified by luciferase assay. Data the mean of three independent experiments ± SEM. *P < 0.05; ANOVA, Bonferroni.
To investigate further the potential involvement of the NF-κB pathway in the HIV response to HMP, Jurkat 1G5 cells were treated with the proteosome inhibitor MG-132, which prevents active NF-κB from being translocated to the nucleus (42). MG-132 treatment significantly abrogated the induction of HIV-1 LTR expression by both Ng and HMP (Fig. 6B).
To establish firmly the requirement for NF-κB signaling in HIV-1 LTR induction by HMP, RNAi of the two most common inducible NF-κB subunits, protein 50 (p50) and protein 65 (p65) [encoded by NFKB1 and reticuloendotheliosis viral oncogene homolog (RelA), respectively (43)], was performed in the Jurkat 1G5 cells. Heterodimers of these subunits form a functional NF-κB transcription factor. After transduction with targeting shRNAs, NFKB1 expression was reduced by an average of 78%, and RelA expression was reduced by an average of 70% (Fig. S7). Induction of the HIV-1 LTR by Ng and HMP was decreased significantly when either NFKB1 or RelA expression was knocked down (Fig. 6C), confirming that the NF-κB initiates transcription from the HIV-1 promoter in response to HMP.
Discussion
Despite an increasing appreciation of the diversity and biological significance of the bacterial glycome, investigations into the role of these glycans during host–pathogen interactions are still in their infancy (44). The data herein show that Neisseria-derived HMP activates CD4+ T cells, triggering a proinflammatory cytokine response and HIV-1 expression in an NF-κB–dependent manner, providing a direct molecular link between the well-established effect of gonorrhea on HIV-1 transmission in humans (2, 3, 8, 9). Aside from their implications in HIV-1, these results show that bacterial-derived heptose elicits an innate response in mammalian cells. This result is intuitively satisfying, because heptose is not part of the mammalian glycome (35) and thus would provide an obvious signal of microbial infection.
Given that bacteria coexpress numerous MAMPs, the specific contribution of neisserial HMP to HIV-1 expression is most striking when one considers viral expression in response to the hldA mutants. Loss of HldA function eliminates HIV-1 LTR induction by the bacteria. The mere presence of heptose in the outer membrane is not sufficient for HIV-1 LTR induction by bacteria, because both H. influenzae and E. coli express heptose in their LPS core (35) but do not elicit an HIV-1 response. It also is important that the surface exposure of heptose on Neisseria spp. is not sufficient to elicit a host cellular response, because heat-killed Ng have no HIV-inducing effect regardless of whether they express heptose in their LOS. Instead, it is the liberation of HMP that determines the HIV-1 response to Ng, because live Ng must be incubated in coculture with CD4+ T cells for longer than 6 h to allow enough HMP to accumulate in the culture medium to elicit an observable HIV-1 LTR response. The liberation of immunoactive HMP by Neisseria spp. is a unique characteristic among the array of bacteria tested. Indeed, although viable E. coli induced a detectable HIV-1 LTR response, supernatants prepared from E. coli had no such effect.
Why Ng liberates immunoactive HMP remains puzzling. This activity is not a virulence mechanism, because both pathogenic and commensal Neisseria spp. potently induce the HIV-1 LTR. It is notable in this regard that Ng actively releases outer membrane blebs that modulate the host immune system (45) and other MAMPs, including immunoactive peptidoglycan (46, 47). Although neither of these factors appears to contribute to the effect of Ng on HIV-1, it seems likely that they work in concert to make the mucosal tissues receptive to infection by and/or the persistence of the bacteria. Supporting this model, released peptidoglycan fragments are selectively toxic to mucosal ciliary cells so as to stop mucus flow and provide the bacteria access to the subepithelial space (48). Future efforts must consider the cumulative effect of these factors on the mucosal epithelia and their respective roles during Ng infection.
Until now the marked effect of Ng on HIV-1 transmission has seemed strange, because gonorrhea is not an ulcerative disease. The primary site of infection for both Ng and HIV is the human female endocervical mucosa and male urethra. The local accumulation of Ng-derived HMP would explain Ng’s boosting effect on viral shedding and, thereby, on HIV-1 transmission, because the direct effect of HMP on NF-κB–mediated transcription would drive HIV-1 expression and viral production at coinfected sites. However, it also is important to consider that the increased NF-κB–mediated expression of IL-8 and TNF-α also would lead to a local accumulation of these HIV-1–activating cytokines (49, 50), an indirect effect of HMP that could further augment HIV-1 expression in the reproductive tract.
Because Ng enters the subepithelial space, HMP also may have a systemic effect. This effect would be akin to the translocation of microbial products from the gut of HIV patients as the result of HIV-induced enteropathy (51). The systemic immunomodulatory effects of these microbial components contribute to chronic immune activation and disease progression in people living with HIV (51). In this context, it also is enticing to speculate that this persistent innate activation could provide the impetus to initiate the uncontrolled inflammation that is the hallmark of gonorrhea, suggesting that Ng has a curious lifestyle finely balanced between asymptomatic colonization and inflammatory disease.
Materials and Methods
A complete description of the materials and methods used in this study is provided in SI Materials and Methods.
Jurkat 1G5 Bioassay for HIV-1 LTR Expression.
Jurkat 1G5 cells contain a stably integrated HIV-1 LTR-luciferase reporter (52). At 18 h after treatment/infection, the cells were lysed, and promoter activity was determined using a luciferase assay kit (Promega). Counts per second of luminescence were recorded in a Wallac Victor 2 (Perkin-Elmer) or a Tecan Infinite M200 (Tecan) luminometer.
Supplementary Material
Acknowledgments
We thank D. P. Speert of the University of British Columbia for providing laboratory space and reagents; N. Leung for excellent technical assistance; R. Whittal at the University of Alberta for LC-MS analysis; A. Cochrane and R. Kaul for helpful discussions during the preparation of this manuscript, and Jianjun Li and Jacek Stupak at the National Research Council for MALDI-MS analysis. R.J.M. received fellowship funding from the Ontario HIV Treatment Network (OHTN) and the Canadian Institutes of Health Research (CIHR). W.N.D.-B. received scholarship funding from CIHR. J.L.H. received a studentship from the University of British Columbia Faculty of Medicine. B.O.K. received an Establishment Award from the Cystic Fibrosis Research Institute. This work was supported by operating funds from CIHR Grants MOP-15499 and HET-85518 and OHTN Grant ROGB-208 (to S.D.G.-O.) and by a trainee award from the British Columbia Proteomics Network (to R.J.M. and B.O.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.A.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303738110/-/DCSupplemental.
References
- 1.Hughes JP, et al. Partners in Prevention HSV/HIV Transmission Study Team Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis. 2012;205(3):358–365. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2(1):33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 3.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watt G, Kantipong P, Jongsakul K. Decrease in human immunodeficiency virus type 1 load during acute dengue fever. Clin Infect Dis. 2003;36(8):1067–1069. doi: 10.1086/374600. [DOI] [PubMed] [Google Scholar]
- 5.Moss WJ, et al. Suppression of human immunodeficiency virus replication during acute measles. J Infect Dis. 2002;185(8):1035–1042. doi: 10.1086/340027. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MS. HIV and sexually transmitted diseases: Lethal synergy. Top HIV Med. 2004;12(4):104–107. [PubMed] [Google Scholar]
- 7.Firoz Mian M, Ashkar AA. Induction of innate immune responses in the female genital tract: Friend or foe of HIV-1 infection? Am J Reprod Immunol. 2011;65(3):344–351. doi: 10.1111/j.1600-0897.2010.00945.x. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MS. Sexually transmitted diseases enhance HIV transmission: No longer a hypothesis. Lancet. 1998;351(Suppl 3):5–7. doi: 10.1016/s0140-6736(98)90002-2. [DOI] [PubMed] [Google Scholar]
- 9.Mlisana K, et al. Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J Infect Dis. 2012;206(1):6–14. doi: 10.1093/infdis/jis298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page KR, Moore RD, Wilgus B, Gindi R, Erbelding EJ. Neisseria gonorrhoeae and Chlamydia trachomatis among human immunodeficiency virus-infected women. Sex Transm Dis. 2008;35(10):859–861. doi: 10.1097/OLQ.0b013e31817bbcb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen MS, et al. AIDSCAP Malawi Research Group Reduction of concentration of HIV-1 in semen after treatment of urethritis: Implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349(9069):1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 12.Ghys PD, et al. The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Côte d’Ivoire. AIDS. 1997;11(12):F85–F93. doi: 10.1097/00002030-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Moss GB, et al. Human immunodeficiency virus DNA in urethral secretions in men: Association with gonococcal urethritis and CD4 cell depletion. J Infect Dis. 1995;172(6):1469–1474. doi: 10.1093/infdis/172.6.1469. [DOI] [PubMed] [Google Scholar]
- 14.Anzala AO, et al. Acute sexually transmitted infections increase human immunodeficiency virus type 1 plasma viremia, increase plasma type 2 cytokines, and decrease CD4 cell counts. J Infect Dis. 2000;182(2):459–466. doi: 10.1086/315733. [DOI] [PubMed] [Google Scholar]
- 15.Nkengasong JN, et al. Human immunodeficiency virus Type 1 (HIV-1) plasma virus load and markers of immune activation among HIV-infected female sex workers with sexually transmitted diseases in Abidjan, Côte d’Ivoire. J Infect Dis. 2001;183(9):1405–1408. doi: 10.1086/319855. [DOI] [PubMed] [Google Scholar]
- 16.Kaul R, et al. Gonococcal cervicitis is associated with reduced systemic CD8+ T cell responses in human immunodeficiency virus type 1-infected and exposed, uninfected sex workers. J Infect Dis. 2002;185(10):1525–1529. doi: 10.1086/340214. [DOI] [PubMed] [Google Scholar]
- 17.Chen A, Boulton IC, Pongoski J, Cochrane A, Gray-Owen SD. Induction of HIV-1 long terminal repeat-mediated transcription by Neisseria gonorrhoeae. AIDS. 2003;17(4):625–628. doi: 10.1097/00002030-200303070-00019. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira VH, et al. Endometrial epithelial cell responses to coinfecting viral and bacterial pathogens in the genital tract can activate the HIV-1 LTR in an NFkappaB-and AP-1-dependent manner. J Infect Dis. 2011;204(2):299–308. doi: 10.1093/infdis/jir260. [DOI] [PubMed] [Google Scholar]
- 19.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Brichacek B, et al. Contrasting roles for TLR ligands in HIV-1 pathogenesis. PLoS ONE. 2010;5(9) doi: 10.1371/journal.pone.0012831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding J, et al. Neisseria gonorrhoeae enhances HIV-1 infection of primary resting CD4+ T cells through TLR2 activation. J Immunol. 2010;184(6):2814–2824. doi: 10.4049/jimmunol.0902125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobson-Belaire WN, et al. Neisseria gonorrhoeae effectively blocks HIV-1 replication by eliciting a potent TLR9-dependent interferon-α response from plasmacytoid dendritic cells. Cell Microbiol. 2010;12(12):1703–1717. doi: 10.1111/j.1462-5822.2010.01502.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, et al. Gonococcal lipooligosaccharide suppresses HIV infection in human primary macrophages through induction of innate immunity. J Infect Dis. 2006;194(6):751–759. doi: 10.1086/506360. [DOI] [PubMed] [Google Scholar]
- 24.Thibault S, Fromentin R, Tardif MR, Tremblay MJ. TLR2 and TLR4 triggering exerts contrasting effects with regard to HIV-1 infection of human dendritic cells and subsequent virus transfer to CD4+ T cells. Retrovirology. 2009;6:42. doi: 10.1186/1742-4690-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thibault S, Imbeault M, Tardif MR, Tremblay MJ. TLR5 stimulation is sufficient to trigger reactivation of latent HIV-1 provirus in T lymphoid cells and activate virus gene expression in central memory CD4+ T cells. Virology. 2009;389(1-2):20–25. doi: 10.1016/j.virol.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, et al. Neisseria gonorrhoeae enhances infection of dendritic cells by HIV type 1. J Immunol. 2005;174(12):7995–8002. doi: 10.4049/jimmunol.174.12.7995. [DOI] [PubMed] [Google Scholar]
- 27.Stebbing J, Gazzard B, Douek DC. Where does HIV live? N Engl J Med. 2004;350(18):1872–1880. doi: 10.1056/NEJMra032395. [DOI] [PubMed] [Google Scholar]
- 28.Flo TH, et al. Differential expression of Toll-like receptor 2 in human cells. J Leukoc Biol. 2001;69(3):474–481. [PubMed] [Google Scholar]
- 29.Tettelin H, et al. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287(5459):1809–1815. doi: 10.1126/science.287.5459.1809. [DOI] [PubMed] [Google Scholar]
- 30. Dillard JP (2005) Genetic manipulation of Neisseria gonorrhoeae. Current Protocols in Microbiology, (John Wiley & Sons, Inc., New York), pp 4A.2.1–4A.2.19. [DOI] [PubMed]
- 31.Desroy N, et al. Towards Gram-negative antivirulence drugs: New inhibitors of HldE kinase. Bioorg Med Chem. 2009;17(3):1276–1289. doi: 10.1016/j.bmc.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Winsor GL, et al. The Burkholderia Genome Database: Facilitating flexible queries and comparative analyses. Bioinformatics. 2008;24(23):2803–2804. doi: 10.1093/bioinformatics/btn524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahler CM, Stephens DS. Genetic basis for biosynthesis, structure, and function of meningococcal lipooligosaccharide (endotoxin) Crit Rev Microbiol. 1998;24(4):281–334. doi: 10.1080/10408419891294216. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Quinn PJ. Lipopolysaccharide: Biosynthetic pathway and structure modification. Prog Lipid Res. 2010;49(2):97–107. doi: 10.1016/j.plipres.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Herget S, et al. Statistical analysis of the Bacterial Carbohydrate Structure Data Base (BCSDB): Characteristics and diversity of bacterial carbohydrates in comparison with mammalian glycans. BMC Struct Biol. 2008;8:35. doi: 10.1186/1472-6807-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clouse KA, et al. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989;142(2):431–438. [PubMed] [Google Scholar]
- 37.Lassen K, Han Y, Zhou Y, Siliciano J, Siliciano RF. The multifactorial nature of HIV-1 latency. Trends Mol Med. 2004;10(11):525–531. doi: 10.1016/j.molmed.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat Immunol. 2002;3(3):229–236. doi: 10.1038/ni769. [DOI] [PubMed] [Google Scholar]
- 39.Kunsch C, Rosen CA. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993;13(10):6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: Involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990;10(4):1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poli G, et al. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci USA. 1990;87(2):782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsubuki S, Saito Y, Tomioka M, Ito H, Kawashima S. Differential inhibition of calpain and proteasome activities by peptidyl aldehydes of di-leucine and tri-leucine. J Biochem. 1996;119(3):572–576. doi: 10.1093/oxfordjournals.jbchem.a021280. [DOI] [PubMed] [Google Scholar]
- 43.Caamaño J, Hunter CA. NF-kappaB family of transcription factors: Central regulators of innate and adaptive immune functions. Clin Microbiol Rev. 2002;15(3):414–429. doi: 10.1128/CMR.15.3.414-429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reid CW, Fulton KM, Twine SM. Never take candy from a stranger: The role of the bacterial glycome in host-pathogen interactions. Future Microbiol. 2010;5(2):267–288. doi: 10.2217/fmb.09.103. [DOI] [PubMed] [Google Scholar]
- 45.Lee HS, et al. Neisserial outer membrane vesicles bind the coinhibitory receptor carcinoembryonic antigen-related cellular adhesion molecule 1 and suppress CD4+ T lymphocyte function. Infect Immun. 2007;75(9):4449–4455. doi: 10.1128/IAI.00222-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cloud-Hansen KA, Hackett KT, Garcia DL, Dillard JP. Neisseria gonorrhoeae uses two lytic transglycosylases to produce cytotoxic peptidoglycan monomers. J Bacteriol. 2008;190(17):5989–5994. doi: 10.1128/JB.00506-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinha RK, Rosenthal RS. Release of soluble peptidoglycan from growing conococci: Demonstration of anhydro-muramyl-containing fragments. Infect Immun. 1980;29(3):914–925. doi: 10.1128/iai.29.3.914-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melly MA, McGee ZA, Rosenthal RS. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J Infect Dis. 1984;149(3):378–386. doi: 10.1093/infdis/149.3.378. [DOI] [PubMed] [Google Scholar]
- 49.Lane BR, et al. Interleukin-8 stimulates human immunodeficiency virus type 1 replication and is a potential new target for antiretroviral therapy. J Virol. 2001;75(17):8195–8202. doi: 10.1128/JVI.75.17.8195-8202.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okamoto T, et al. Augmentation of human immunodeficiency virus type 1 gene expression by tumor necrosis factor alpha. AIDS Res Hum Retroviruses. 1989;5(2):131–138. doi: 10.1089/aid.1989.5.131. [DOI] [PubMed] [Google Scholar]
- 51.Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26(1):2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aguilar-Cordova E, Chinen J, Donehower L, Lewis DE, Belmont JW. A sensitive reporter cell line for HIV-1 tat activity, HIV-1 inhibitors, and T cell activation effects. AIDS Res Hum Retroviruses. 1994;10(3):295–301. doi: 10.1089/aid.1994.10.295. [DOI] [PubMed] [Google Scholar]
- 53.Gorter AD, et al. Involvement of lipooligosaccharides of Haemophilus influenzae and Neisseria meningitidis in defensin-enhanced bacterial adherence to epithelial cells. Microb Pathog. 2003;34(3):121–130. doi: 10.1016/s0882-4010(02)00193-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.