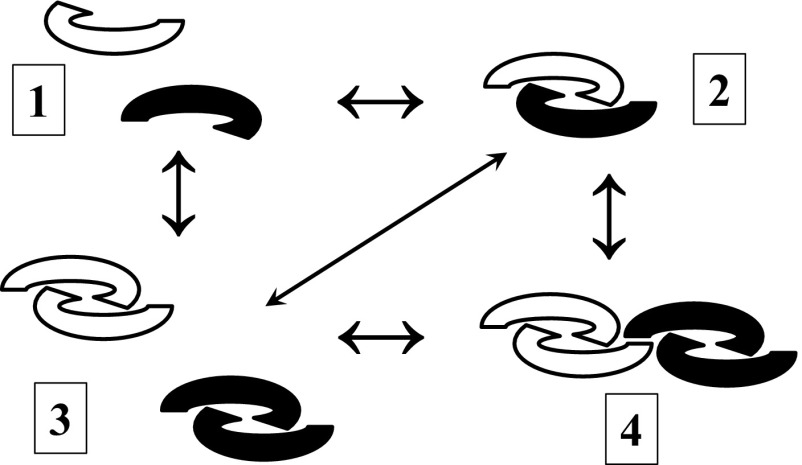
Although the title of the paper by Peng et al. (1) gave the impression that the authors were working with a purified growth differentiation factor 9:bone morphogenetic protein 15 (GDF9:BMP15) heterodimer preparation, at best, what was being tested was a mixture of BMP15 homodimers (75%) and GDF9:BMP15 heterodimers (25%), a point made by the authors early on in the results section. However, this important point was not reiterated, and the hG9:B15 preparation was misleadingly referred to as a GDF9/BMP15 heterodimer. In this context, there is a lack of data on characterization of the hG9:B15 preparation; e.g., no silver-stained SDS/PAGE gels are presented to indicate purity, and no chemical crosslinking data are presented in support of heterodimer formation. Given that GDF9 and BMP15 dimers interact noncovalently, they may be capable of forming monomers; hence, an equilibrium may exist between monomers, heterodimers, and homodimers (Fig. 1). The authors should not have assumed that heterodimerization of GDF9 and BMP15 would take place simply because they were coexpressed in the same cells, and copurification of GDF9 along with BMP15 via affinity chromatography was not sufficient evidence to establish heterodimer formation. For example, a dimer of homodimers (as shown in Fig.1, part 4) would similarly copurify and may well be biologically extremely potent.
Fig. 1.
Potential structural forms of BMP15 (solid) and GDF9 (clear) mature regions: monomers (1), heterodimers (2), homodimers (3), and a dimer of homodimers (4). These different forms may well be in a dynamic equilibrium, given that BMP15 and GDF9 mature regions do not form covalent dimers. A further extension of 4 would be higher-order noncovalent multimers or aggregates. All of these forms would result in the same banding pattern in silver-stained SDS/PAGE gels or Western blots.
Peng et al. (1) referred to previous studies on the cooperative effects of GDF9 and BMP15 and made the assumption that such studies monitor the actions of two homodimers. This is not a valid assumption, for the reasons pictured in Fig. 1. Furthermore, in four previous studies (ref. 2 and references therein), the coaddition of GDF9 and BMP15 resulted in a synergistic response, in contrast to the results of Peng et al., a crucial difference being that the previous studies did not use GDF9 or BMP15 with modified mature regions.
A secondary theme of the study by Peng et al. (1) was species differences in the bioactivity of GDF9 and BMP15. In particular, the following question was raised: “why do mice GDF9 homodimers have significant activity compared with human homodimers?” Some suggestions were presented to help explain these species differences, but the presence of an Arg residue in mouse GDF9 at position 391 (as opposed to a Gly residue for human GDF9) is likely to be the crucial factor when explaining these bioactivity differences (3).
The cell surface receptors used by GDF9 and BMP15 were the central issue of the model presented by Peng et al. (figure 7 in ref. 1). Although the authors referred in the text to the type 1 receptor of GDF9 as activin receptor-like kinase (ALK) 4/5/7, the figure depicted that this receptor was ALK4; however, no experimental evidence was presented to support this. Additionally, published results show that overexpression of ALK4 in human granulosa cells does not affect the GDF9 response, whereas ALK5 overexpression results in a very significant increase in the GDF9 response (4). We do find the suggestion of Peng et al. for the involvement of a BMPR2:ALK4/5/7:ALK6 complex as the signaling mediator of the putative GDF9:BMP15 heterodimer to be very reasonable. In fact, this suggestion has been made previously (5).
Acknowledgments
This work was supported by National Health and Medical Research Council of Australia (NHMRC) Development Grant 1017484 (to D.G.M.), NHMRC Project Grants 1024358 (to C.A.H.) and 1009144 (to P.G.S.), NHMRC Program Grant 494802 (to P.G.S.), Cook Medical project funding (to R.B.G.), the Royal Society of New Zealand (K.P.M.), and the New Zealand Foundation for Research Science and Technology (K.P.M.). C.A.H. is supported by NHMRC Career Development Fellowship 1013533, and R.B.G. is supported by NHMRC Senior Research Fellowship 1023210.
Footnotes
Conflict of interest statement: R.B.G. is a consultant to Cook Medical. The University of Adelaide holds a patent on applications of GDF9 and BMP15 (R.B.G. is an inventor).
References
- 1.Peng J, et al. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci USA. 2013;110(8):E776–E785. doi: 10.1073/pnas.1218020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mottershead DG, Ritter LJ, Gilchrist RB. Signalling pathways mediating specific synergistic interactions between GDF9 and BMP15. Mol Hum Reprod. 2012;18(3):121–128. doi: 10.1093/molehr/gar056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson CM, et al. Activation of latent human GDF9 by a single residue change (Gly 391 Arg) in the mature domain. Endocrinology. 2012;153(3):1301–1310. doi: 10.1210/en.2011-1632. [DOI] [PubMed] [Google Scholar]
- 4.Kaivo-Oja N, et al. Adenoviral gene transfer allows Smad-responsive gene promoter analyses and delineation of type I receptor usage of transforming growth factor-β family ligands in cultured human granulosa luteal cells. J Clin Endocrinol Metab. 2005;90(1):271–278. doi: 10.1210/jc.2004-1288. [DOI] [PubMed] [Google Scholar]
- 5.McNatty KP, et al. The oocyte and its role in regulating ovulation rate: a new paradigm in reproductive biology. Reproduction. 2004;128(4):379–386. doi: 10.1530/rep.1.00280. [DOI] [PubMed] [Google Scholar]