Abstract
Characterization of the mature protein complement in cells is crucial for a better understanding of cellular processes on a systems-wide scale. Toward this end, we used single-dimension ultra–high-pressure liquid chromatography mass spectrometry to investigate the comprehensive “intact” proteome of the Gram-negative bacterial pathogen Salmonella Typhimurium. Top-down proteomics analysis revealed 563 unique proteins including 1,665 proteoforms generated by posttranslational modifications (PTMs), representing the largest microbial top-down dataset reported to date. We confirmed many previously recognized aspects of Salmonella biology and bacterial PTMs, and our analysis also revealed several additional biological insights. Of particular interest was differential utilization of the protein S-thiolation forms S-glutathionylation and S-cysteinylation in response to infection-like conditions versus basal conditions. This finding of a S-glutathionylation-to-S-cysteinylation switch in a condition-specific manner was corroborated by bottom-up proteomics data and further by changes in corresponding biosynthetic pathways under infection-like conditions and during actual infection of host cells. This differential utilization highlights underlying metabolic mechanisms that modulate changes in cellular signaling, and represents a report of S-cysteinylation in Gram-negative bacteria. Additionally, the functional relevance of these PTMs was supported by protein structure and gene deletion analyses. The demonstrated utility of our simple proteome-wide intact protein level measurement strategy for gaining biological insight should promote broader adoption and applications of top-down proteomics approaches.
Liquid chromatography-coupled mass spectrometry (LC-MS)-based top-down proteomics is an emerging approach in which whole/intact proteins are separated and fragmented directly in the mass spectrometer to achieve both protein identification and characterization. This is in contrast to the well-established bottom-up LC-MS approaches in which proteins are digested with a protease into smaller peptides and analyzed by tandem mass spectrometry (i.e., MS/MS) to infer protein identity from peptide level information. This approach is presently unsurpassed in its ability to identify large numbers of proteins, but suffers from the loss of information required to distinguish proteoforms (1) that constitute the functionally accurate proteome that is essential for understanding a biological system.
Most top-down measurements have been limited to single proteins or simple protein mixtures due to technical limitations. However, recent advances in instrumentation, separations, and software are now beginning to allow initial applications on much deeper scales. For example, Bunger et al. (2) reported the identification of 322 different proteoforms representing 174 proteins via top-down proteomic analysis of Escherichia coli and another study reported identification of 201 different proteoforms representing 154 Salmonella Typhimurium proteins by top-down analysis (3). Top-down proteomics application to bacteria has been infrequent, with a few exceptions such as noted above. Studies with eukaryotic cells have been more prominent and have demonstrated identification and characterization of up to 1,000 unique proteins via top-down means (4–7). For example, Kellie et al. (4) identified and characterized 530 proteins including 1,103 proteoforms in Saccharomyces cerevisiae. In a seminal contribution, Tran et al. (7) recently reported the identification of 1,043 proteins from HeLa S3 cells that are dispersed into more than 3,000 proteoforms. More recently Ahlf et al. (8) identified 690 unique proteins and over 2,000 proteoforms from H1299 human carcinoma cells. However, the requirement of customized three- and four-dimensional separation systems in the above studies and large amount of starting material present a potential limitation to the widespread application of high-throughput top-down proteomics.
In the present study, we have used a single-dimension ultra–high-pressure liquid chromatography (UPLC) system coupled with a Velos-Orbitrap mass spectrometer to profile the intact proteome of the Gram-negative bacterial pathogen Salmonella Typhimurium (STM). The single-dimension top-down proteomics platform analysis identified 563 proteins including 1,665 proteoforms generated by posttranslational modification (PTMs). Our analysis revealed several intact protein-centric biological insights, while confirming several previously recognized aspects of Salmonella biology. Of particular interest, we report the differential utilization of protein S-thiolation forms in Salmonella in response to growth under infection-like conditions. Protein S-thiolation is a PTM in which free thiol groups on proteins form mixed disulfides with low–molecular-mass thiols such as glutathione (S-glutathionylation) and cysteine (S-cysteinylation). Protein S-thiolation occurs as a cellular response to oxidative stress, preventing the irreversible oxidation of cysteine residues, as well as under basal (physiological) conditions, in which it can affect protein function, making this PTM crucial for protection and regulation of thiol-containing proteins. Although S-glutathionylation is relatively well studied (see reviews in refs. 9 and 10), less well studied is S-cysteinylation. Literature contains only two reports of bacterial S-cysteinylation, both in Gram-positive bacteria (11, 12), and two serial reports on cysteinylation of protein kinase C in mammalian cells (13, 14). Our results report S-cysteinylation in Gram-negative bacteria and report differential utilization of protein S-thiolation forms in a pathogen in response to growth under infection-like conditions. Under infection-like conditions, Salmonella preferentially used S-cysteinylation as a mechanism for thiol protection and/or environmental sensing, whereas under basal conditions the pathogen preferentially used S-glutathiolyation. These observations were corroborated by complementary bottom-up proteomics data that quantified glutathionylated and cysteinylated peptides. Furthermore, the bottom-up proteomics data in combination with prior transcriptomics data revealed strong induction of cysteine biosynthesis genes under in vitro infection-like conditions, as well as during actual infection of host cells, whereas expression of key glutathione biosynthesis enzymes glutamate-cysteine ligase (GshA) and glutathione synthetase (GshB) were either unchanged or decreased under the same conditions, providing a possible mechanism to explain differential utilization of S-thiolation forms in Salmonella.
Results and Discussion
Top-Down Proteomics Analysis and Coverage.
A high-throughput top-down proteomics pipeline consisting of a single-dimension Waters UPLC system augmented with long (80-cm) nanocapillary LC columns and an extended (250-min) gradient interfaced with a Velos Orbitrap mass spectrometer was used to profile the intact proteome of Salmonella Typhimurium and comprehensively characterize endogenous PTMs and their modulation in response to physiologically relevant conditions. Pathogen growth in Luria–Bertani broth (LB) and in a low-phosphate, low-magnesium, low-pH minimal medium (LPM) represented basal and infection-like conditions, respectively (15–19). In high-resolution (MS and MS/MS), high-throughput mode, the ultrahigh-pressure single-dimension top-down proteomics platform consumed ∼5 µg of intact protein extract sample per analysis. Top-down mass spectra were analyzed using MS-Align+, an algorithm for top-down protein identification that enables searches for unexpected PTMs (20).
We identified 563 unique Salmonella proteins and 1,665 proteoforms at a 5% protein level false-discovery rate (FDR) (4, 7) using the single-dimension top-down platform (Dataset S1). The data represent a broad spectrum of gene products (SI Appendix, Fig. S1) with proteome coverage comparable to the largest top-down eukaryotic datasets reported using three-dimension or four-dimension top-down platforms (SI Appendix, Fig. S2). A number of factors contributed to the improved top-down proteome coverage using our single-dimension top-down platform compared with prior studies. Among these were the use of an UPLC system with long columns (80 cm) and long gradients (250 min) that afforded efficient protein separation, as well as enough time to limit MS/MS undersampling, a well-recognized issue for bottom-up proteomics that can be relaxed using long columns and gradients (21, 22). An additional factor is application of the software tool MS-Align+ for top-down protein identification based on spectral alignment, which has been shown to increase the number of identified spectra compared with other currently available tools (20).
Identification of Distinct Proteoforms.
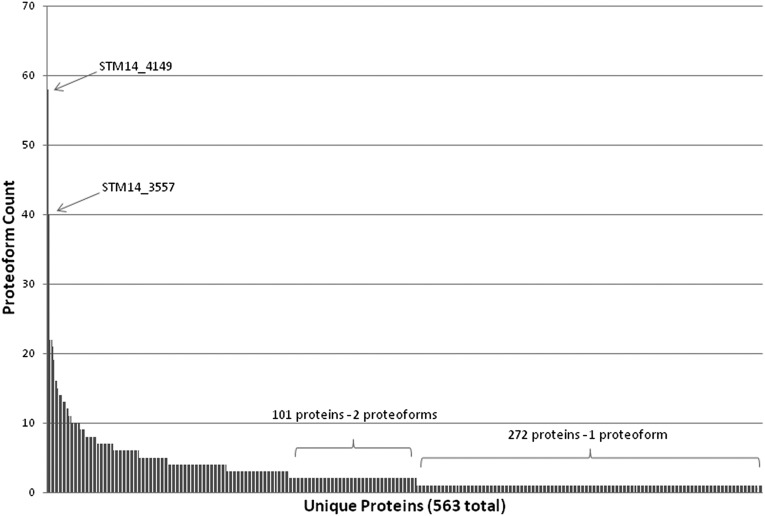
The 563 unique Salmonella proteins identified in this study are dispersed into more than 1,600 proteoforms created by PTMs. Fig. 1 shows a histogram of the number of identified proteoforms per unique protein, with the majority of the 563 unique proteins being represented by either one proteoform (272 unique proteins) or two proteoforms (101 unique proteins). Twenty-six unique proteins display >10 proteoforms each, with a majority of these abundant primary metabolism and translation machinery proteins. Interestingly, prior work by Manes et al. (23) suggested that certain classes of Salmonella proteins, including translation machinery proteins, are preferentially targeted for proteolysis. Proteins in which the start methionine was retained or exhibiting N-terminal methionine cleavage represented ∼30% of the proteins identified (Dataset S1). Proteins with evidence of signal peptide cleavage, using the following conservative criteria—presence of the canonical AXA signal peptide motif (24) and considering only the narrow window of proteins with start residue between amino acids 20 and 30—represented ∼10% of the proteins identified (Dataset S1).
Fig. 1.
Analysis of distinct proteoforms. The counts of total proteoforms per unique protein are shown. A majority of entries have only one form identified.
We found 12 proteins with an N-terminal acetylation modification. In contrast to eukaryotes where the majority of proteins undergo N-terminal acetylation (>80% in humans), N-terminal acetylation is extremely rare in bacteria. Only five E. coli proteins are known to be N-terminally acetylated, including the ribosomal proteins S18, S5, L7, L12, which we identified in our analysis, and elongation factor Tu (25, 26). In addition to the four ribosomal proteins, we also identified eight more bacterial proteins with an N-terminal acetylation modification (Dataset S1) and note that additional N-terminal acetylated bacterial proteins potentially could be identified by using a systematic search. Indeed, Bonnissone et al. (27) analyzing mass spectrometry data from 57 bacterial species suggest N-terminal acetylation is more prevalent than previously appreciated. A total of 20 disulfide-bonded proteins was identified including those previously reported in literature such as glutaredoxin (GrxA) (28) and 50S ribosomal protein L31 (RpmE) (29) (Dataset S1). Additionally, three proteins harboring a methylation modification were identified, 50S ribosomal protein L33 (RpmG), 30S ribosomal protein S11 (RpsK), and the elongation factor Tu protein. In E. coli, methylation at K56 of elongation factor Tu has been suggested as a mechanism for “fine-tuning” of elongation factor–tRNA intermolecular interactions (30). Our data indicate the same modification at K57 of the Salmonella elongation factor protein, the homologous position to K56 of the E. coli protein, suggesting a similar functional role in Salmonella as in E. coli. Other identified chemical modifications included methionine oxidation (14 proteins) and of particular interest protein S-thiolation (25 proteins), which we next discuss in more detail.
Top-Down Proteomics Reveals Differential Utilization of Protein S-Thiolation.
From our top-down measurements across basal (LB) and infection-like (LPM) conditions, we identified 25 proteins dispersed into 34 distinct proteoforms having S-thiolation PTMs. Of these, 16 had the +305-Da glutathionylation PTM, whereas 18 harbored the +119-Da cysteinylation PTM. Interestingly, the glutathionylated proteoforms appeared almost exclusively in the basal LB growth condition, whereas the cysteinylated proteoforms appeared almost exclusively in the infection-like LPM growth condition, which suggests a preferential utilization of glutathionylation in LB and cysteinylation in LPM. In further support of this notion, we identified a subset of proteins, an example of which is shown in Fig. 2, that exhibited both properties of being glutathionylated under basal conditions (LB) or cysteinylated under infection-like conditions (LPM) on the same residues, an intriguing switch in modification forms in response to environmental conditions relevant to Salmonella Typhimurium pathogen biology. Overall, nine unique proteins exhibited this condition-dependent switch in PTM (see Dataset S2 and SI Appendix, Figs. S3–S10, for details on top-down proteomics data).
Fig. 2.
Switch in modification from glutathionylation to cysteinylation in response to environmental conditions. (A) Representative zero charge state spectra from top-down proteomics data showing switch in S-thiolation forms in the hypothetical protein YifE (STM14_4694). Fragmentation maps show specific cysteine residue on which switch occurs. (B) Estimated stoichiometry from intact protein mass spectra summed across corresponding LC peak.
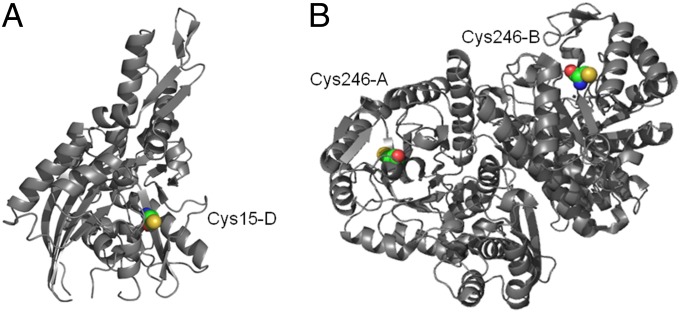
Interestingly, structural analysis of this subset of nine proteins revealed that most of the S-thiolation PTM occurred on buried cysteine residues rather than at readily surface accessible cysteines, suggesting this modification is specific and possibly functionally relevant rather than the result of opportune oxidation of the most accessible thiols in the cell (Fig. 3). Calculation of residue accessibility in eight of the nine proteins (or their close homologs in other organisms) for which structures were available using Naccess software (31) showed six of the eight had relative all-atom accessibilities that were less than 20% of that of the reference peptide Ala-Xxx-Ala, and three of these six had less than 5% relative accessibility (SI Appendix, Fig. S11). Further supporting this notion of functional relevance, structural analysis of enolase whose activity is known to be modified by S-thiolation (32) also shows the S-thiolation PTM occurring on a similarly buried rather than surface-exposed residue (Fig. 3). Notably, in two of the proteins, ribosomal protein L3 and glutaredoxin, the S-thiolated Cys is not conserved in the E. coli homolog, suggesting that in some cases S-thiolation of these proteins could be a specific adaptation in Salmonella.
Fig. 3.
Structures of E. coli homologs of Salmonella proteins found to be S-thiolated. (A) DnaK (1dkg). (B) Enolase dimer (1e9i). Modified Cys residues are shown as spheres and the residue number and chain ID indicated. Relative accessibilities of the modified Cys residues calculated with Naccess are 1.3 and 2.1, respectively.
This study reports differential utilization of protein S-thiolation forms, previously unappreciated most likely due to shortcomings of prior methods used (see ref. 33 for review). Briefly, in a typical approach, protein synthesis in the organism under study is inhibited using cycloheximide unavoidably perturbing cell physiology followed by incorporation of radiolabeled cysteine, which does not allow discrimination between the different protein S-thiolation forms, with S-glutathionylation being assumed, and subsequently the system is stimulated with diamide or hydrogen peroxide to stimulate oxidative stress and thus precluding access to proteins glutathionylated or cysteinylated under basal/physiological conditions.
Bottom-Up Proteomics Data Corroborates Top-Down Proteomics Observations.
To further validate the observed switch in PTM in response to physiologically relevant conditions, we reexamined data from an independent bottom-up proteomics analysis of Salmonella grown under growth conditions identical to the top-down analysis. The bottom-up proteomics analysis was performed as previously described without any reduction and alkylation (15, 34, 35). Tandem mass spectra were searched against a database of the Salmonella Typhimurium proteome allowing for a modification of +305 and +119 on cysteine residues, and the results filtered as described in SI Appendix, SI Methods. Examining the global overlap of unique protein identifications between top-down and bottom-up approaches showed 497 of the 563 proteins identified using top-down measurements (88%) were also identified by bottom-up (SI Appendix, Fig. S12). Of the 66 proteins not identified by bottom-up, 24 proteins (36%) were <150 aa in length, which may result in fewer ideal peptides for bottom-up analysis.
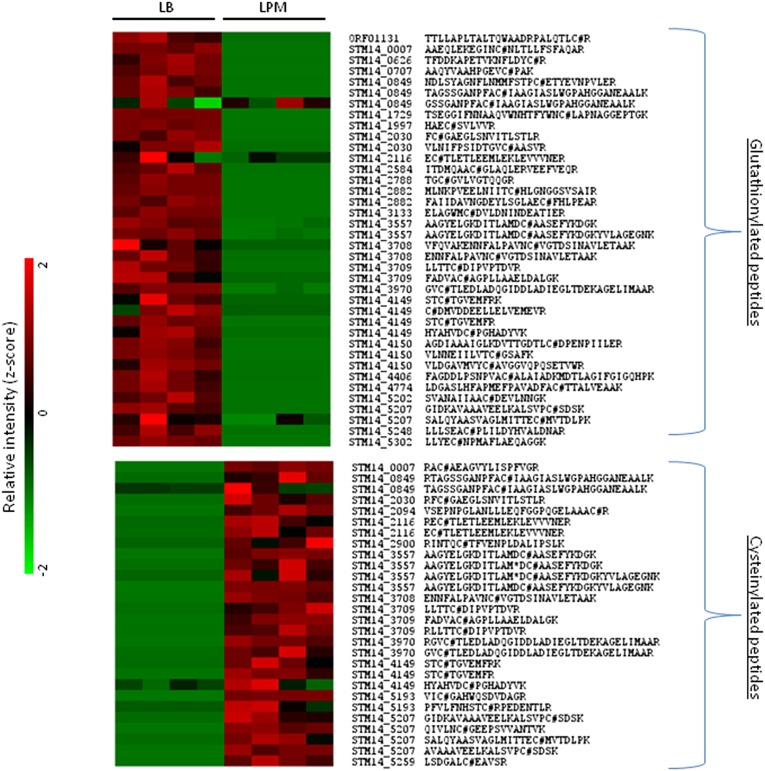
Next, we used a spectral counting approach to quantify the glutathionylated proteins and cysteinylated proteins in the bottom-up dataset, which revealed 84 unique glutathionylated peptides representing 38 proteins and 62 unique cysteinylated peptides representing 26 proteins. In agreement with the top-down data, the glutathionylated proteins appeared almost exclusively in the basal (LB) growth condition, whereas the cysteinylated proteins appeared almost exclusively in the infection-like (LPM) growth condition, i.e., preferential utilization of glutathionylation in LB and cysteinylation in LPM (SI Appendix, Fig. S13). Further analysis of the bottom-up dataset using a peptide intensity-based approach (36) quantified 38 glutathionylated peptides representing 25 proteins and 28 cysteinylated peptides representing 14 proteins (Fig. 4). A comprehensive ANOVA scheme included in the software program DAnTE (37) was used to assess differences in glutationylated or cysteinylated peptide intensity between LB and LPM in a statistically rigorous manner and fold changes calculated as shown in Dataset S3. Consistent with both top-down data and bottom-up spectral count observations, the glutathionylated peptides/proteins appeared almost exclusively and in high abundance in the basal growth condition [the average fold difference between LB and LPM was ∼10-fold (P < 0.05)] and the cysteinylated peptides/proteins appeared almost exclusively and in high abundance in the infection-like growth condition [the average fold difference between LB and LPM was ∼5-fold (P < 0.05)]. Furthermore, a subset of these peptides/proteins exhibited a condition-dependent switch in PTMs as described above (i.e., a glutathionylated protein under basal condition switching PTMs to cysteinylation under infection-like conditions). These included phosphoglycerate kinase (STM14_3709) and elongation factor (STM14_4149), which have previously been reported to be glutathionylated (32) and enolase (STM14_3557) whose activity is known to be modified by S-thiolation (32). To control for the possibility that the abundance increases reported for glutathionylated peptides and cysteinylated peptides above were simply due to just increased protein abundance, we quantified changes in protein abundance using the corresponding non–S-thiolated peptides identified for a representative sampling of the proteins in Fig. 4. As shown in SI Appendix, Fig. S14, the switch from LB to LPM does not result in any significant increase in protein abundance, and in some cases there is a decrease in the overall protein abundance, whereas specific S-cysteinylated peptides showed increases in abundance.
Fig. 4.
Quantification of glutathionylated and cysteinylated peptides. Heat map representation of peak intensity-based quantification of glutathionylated (Upper) and cysteinylated (Lower) peptides identified from bottom-up proteomics data under LB and LPM growth conditions.
We note that validation by Western blot was thwarted by the lack of a commercially available cysteinylation antibody. Furthermore, low sensitivity and specificity of the glutathionylation antibody limits the number of glutathionylated proteins detected under basal conditions (up to four total proteins detected), which constrains their utility for unbiased proteomics studies (33, 38–40). Limited success has been achieved only after stimulating glutathionylation with diamide or hydrogen peroxide and not for detecting endogenous/basal protein S-thiolation as monitored in this study (33, 41, 42). In our hands, the commercially available anti-glutathionylation antibody was not successful in detecting endogenous/basal gluthionylation in Salmonella, as has been reported for other systems (see ref. 33 for review).
Proposed Biological Mechanism for the Observed Differential Utilization of S-Thiolation.
Literature reports strong induction of cysteine biosynthesis genes for both Gram-negative and -positive bacteria in response to infection-mimicking conditions, including oxidative stress (43, 44). We hypothesized that, under infectious or infectious-like conditions, Salmonella uses cysteinylation instead of glutathionylation to protect thiol groups and/or sense the environment in the intracellular compartment, possibly because it is energetically more favorable to synthesize the simpler cysteine moiety or perhaps because it provides faster response to changing environmental conditions.
To evaluate a possible global regulatory hypothesis, we examined the expression patterns of cysteine and glutathione biosynthesis genes under LB and LPM growth conditions (data from this study) as well as in vivo during infection of host cells [data from a previously published dataset (45)]. If the hypothesis were true, then we should observe an up-regulation of cysteine biosynthesis genes in the infection-like condition (LPM) relative to the basal condition (LB) and, similarly, an up-regulation during infection of host cells, whereas glutathione biosynthesis genes would remain unchanged or may even decrease. Protein expression of glutathione and cysteine biosynthesis genes under in vitro conditions and previously published transcriptomics data of Salmonella gene expression during host macrophage cell infection (45) shown in SI Appendix, Fig. S15, support this hypothesis. We observed an up-regulation of cysteine biosynthesis genes following infection relative to basal conditions, whereas the glutathione biosynthesis genes showed no significant change in expression in line with our expectation. As a control for a general program of increased biosynthesis required for growth on LPM vs. richer LB media, we also examined the responses for biosynthetic genes for non–S-containing metabolites like nucleotides. Specifically, we examined purine biosynthesis and pyrimidine biosynthesis genes required for nucleotide biosynthesis. As shown in SI Appendix, Fig. S16 (Inset, Right), essentially no response in the 17 nucleotide biosynthetic genes examined was observed for growth on LPM vs. richer LB media. Additionally, we also examined metabolic genes for other S-containing metabolites, specifically methionine and biotin, to further assess specificity of the cellular response. Although methionine and biotin biosynthesis genes appeared to respond similarly to cysteine biosynthesis genes under in vitro conditions (SI Appendix, Fig. S16, Inset, Left), under more physiologically relevant in vivo conditions (i.e., an actual infection), only the cysteine biosynthesis genes showed significant up-regulation (SI Appendix, Fig. S16, main figure) as would be predicted if the up-regulation being observed were a specific cellular response.
Based on these observations, we have developed an initial model of the differential utilization of protein S-thiolation in Salmonella during infection (Fig. 5). Under free living conditions (e.g., in rich media such as LB), there is minimal stress and Salmonella can afford to synthesize and use the large tripeptide glutathione for thiol protection and environmental sensing. However, in the harsher intracellular infectious environment, the pathogen must quickly adapt to the change in environmental conditions and reduced availability of nutrients so it synthesizes and uses cysteine rather than the larger tripeptide glutathione. The intriguing possibility that this PTM provides a switch for global regulation of different activities suited to the different environments is particularly intriguing and will be the subject of further study.
Fig. 5.
Working model of differential thiol utilization in Salmonella during infection. C, cysteine; CSSC, cysteine disulfide (cystine); GSH, glutathione; GSSG, glutathione disulfide.
Although the essentiality of cysteine biosynthesis genes prevents an assessment of the effect of knockout mutants on Salmonella physiology (46), a Salmonella Typhimurium mutant strain with a deletion of the important glutathione biosynthesis gene gshA was defective in glutathione synthesis, exhibited sensitization to oxidative stress in vitro, as might be expected, as well as a reduction in virulence in mice (47).
Summary
The present analysis using a single-dimension UPLC-MS platform represents proteome coverage comparable to the largest eukaryotic top-down datasets obtained using three or four-dimensional LC-MS platforms. Our global analysis of intact proteins across Salmonella basal and infection-like conditions confirmed prior observations with respect to Salmonella biology and provided additional insights, of particular note the differential utilization of protein S-thiolation forms. The differential utilization of protein S-thiolation forms in response to growth under infection-like conditions corroborated by changes in corresponding biosynthetic pathways reveals a unique protein S-thiolation switch in the bacterial pathogen in response to infection-like conditions and underscores metabolic mechanisms that modulate changes in cellular signaling.
Methods
Sample Preparation.
Salmonella Typhimurium 14028 was grown in LB medium to log phase or LPM medium for 20 h as previously described (48, 49). Intact protein lysate was prepared by resuspending cell pellets in 100 mM NH4HCO3, pH 8.4, buffer with 1 mM PMSF protease inhibitor and lysed by using 0.1 mM zirconia/silica beads in a 2.0-mL Cryovial with vigorous vortexing for a total of 3 min with cooling steps. Details are described in SI Appendix, SI Methods.
LC-MS/MS Analysis.
Samples were analyzed using a LTQ Orbitrap Velos MS (Thermo Scientific) coupled to a Waters UPLC platform. Details of the separation and mass spectrometer parameters are described in SI Appendix, SI Methods.
Data Analysis.
Top-down mass spectra were analyzed using MS-Align+ (20) to obtain protein identifications. We restricted our analysis to deconvoluted spectra that had a precursor mass ≥2,500 Da and the fragment mass tolerance was set at 15 ppm. FDR was estimated as previously described (4, 7). Details are described in SI Appendix, SI Methods.
Supplementary Material
Acknowledgments
We gratefully acknowledge the contribution of Nikola Tolić, Tujin Shi, and Matthew Monroe for assistance in preparing this manuscript. Research described was partly supported by the National Institute of General Medicine, National Institutes of Health (NIH) through Grant GM094623 and National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH)/Department of Health and Human Services, through Interagency Agreement Y1-AI-8401. Project website with data and protocols: www.sysbep.org. Proteomics capabilities were developed under support from the Department of Energy (DOE) Office of Biological and Environmental Research (BER), NIH Grant 5P41RR018522-10, and National Institute of General Medical Sciences Grant 8 P41 GM103493-10. Work was performed in the W. R. Wiley Environmental Molecular Sciences Laboratory (EMSL), a DOE-BER national scientific user facility located at Pacific Northwest National Laboratory (PNNL), and partly supported by funds from EMSL intramural research projects and EMSL capability development projects. PNNL is a multiprogram national laboratory operated by Battelle Memorial Institute for the DOE under Contract DE-AC05-76RLO 1830.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.Y. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221210110/-/DCSupplemental.
References
- 1.Smith LM, Kelleher NL. Consortium for Top Down Proteomics Proteoform: A single term describing protein complexity. Nat Methods. 2013;10(3):186–187. doi: 10.1038/nmeth.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunger MK, Cargile BJ, Ngunjiri A, Bundy JL, Stephenson JL., Jr Automated proteomics of E. coli via top-down electron-transfer dissociation mass spectrometry. Anal Chem. 2008;80(5):1459–1467. doi: 10.1021/ac7018409. [DOI] [PubMed] [Google Scholar]
- 3.Tsai YS, et al. Precursor ion independent algorithm for top-down shotgun proteomics. J Am Soc Mass Spectrom. 2009;20(11):2154–2166. doi: 10.1016/j.jasms.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Kellie JF, et al. Robust analysis of the yeast proteome under 50 kDa by molecular-mass-based fractionation and top-down mass spectrometry. Anal Chem. 2012;84(1):209–215. doi: 10.1021/ac202384v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JE, et al. A robust two-dimensional separation for top-down tandem mass spectrometry of the low-mass proteome. J Am Soc Mass Spectrom. 2009;20(12):2183–2191. doi: 10.1016/j.jasms.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth MJ, Parks BA, Ferguson JT, Boyne MT, 2nd, Kelleher NL. “Proteotyping”: Population proteomics of human leukocytes using top down mass spectrometry. Anal Chem. 2008;80(8):2857–2866. doi: 10.1021/ac800141g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran JC, et al. Mapping intact protein isoforms in discovery mode using top-down proteomics. Nature. 2011;480(7376):254–258. doi: 10.1038/nature10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahlf DR, et al. Evaluation of the compact high-field orbitrap for top-down proteomics of human cells. J Proteome Res. 2012;11(8):4308–4314. doi: 10.1021/pr3004216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalle-Donne I, Rossi R, Colombo G, Giustarini D, Milzani A. Protein S-glutathionylation: A regulatory device from bacteria to humans. Trends Biochem Sci. 2009;34(2):85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43(6):883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Hochgräfe F, et al. S-cysteinylation is a general mechanism for thiol protection of Bacillus subtilis proteins after oxidative stress. J Biol Chem. 2007;282(36):25981–25985. doi: 10.1074/jbc.C700105200. [DOI] [PubMed] [Google Scholar]
- 12.Pöther DC, et al. Diamide triggers mainly S thiolations in the cytoplasmic proteomes of Bacillus subtilis and Staphylococcus aureus. J Bacteriol. 2009;191(24):7520–7530. doi: 10.1128/JB.00937-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu F, Ward NE, O’Brian CA. Potent inactivation of representative members of each PKC isozyme subfamily and PKD via S-thiolation by the tumor-promotion/progression antagonist glutathione but not by its precursor cysteine. Carcinogenesis. 2001;22(8):1221–1229. doi: 10.1093/carcin/22.8.1221. [DOI] [PubMed] [Google Scholar]
- 14.Chu F, Ward NE, O’Brian CA. PKC isozyme S-cysteinylation by cystine stimulates the pro-apoptotic isozyme PKC delta and inactivates the oncogenic isozyme PKC epsilon. Carcinogenesis. 2003;24(2):317–325. doi: 10.1093/carcin/24.2.317. [DOI] [PubMed] [Google Scholar]
- 15.Ansong C, et al. Global systems-level analysis of Hfq and SmpB deletion mutants in Salmonella: Implications for virulence and global protein translation. PLoS One. 2009;4(3):e4809. doi: 10.1371/journal.pone.0004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cirillo DM, Valdivia RH, Monack DM, Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol. 1998;30(1):175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- 17.Coombes BK, Brown NF, Valdez Y, Brumell JH, Finlay BB. Expression and secretion of Salmonella pathogenicity island-2 virulence genes in response to acidification exhibit differential requirements of a functional type III secretion apparatus and SsaL. J Biol Chem. 2004;279(48):49804–49815. doi: 10.1074/jbc.M404299200. [DOI] [PubMed] [Google Scholar]
- 18.Deiwick J, Nikolaus T, Erdogan S, Hensel M. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol Microbiol. 1999;31(6):1759–1773. doi: 10.1046/j.1365-2958.1999.01312.x. [DOI] [PubMed] [Google Scholar]
- 19.Yoon H, McDermott JE, Porwollik S, McClelland M, Heffron F. Coordinated regulation of virulence during systemic infection of Salmonella enterica serovar Typhimurium. PLoS Pathog. 2009;5(2):e1000306. doi: 10.1371/journal.ppat.1000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, et al. 2012. Protein identification using top-down. Mol Cell Proteomics 11(6):M111.008524.
- 21.Nagaraj N, et al. 2012. System-wide perturbation analysis with nearly complete coverage of the yeast proteome by single-shot ultra HPLC runs on a bench top Orbitrap. Mol Cell Proteomics 11(3):M111.013722.
- 22.Thakur SS, et al. 2011. Deep and highly sensitive proteome coverage by LC-MS/MS without prefractionation. Mol Cell Proteomics 10(8):M110.003699.
- 23.Manes NP, et al. Targeted protein degradation by Salmonella under phagosome-mimicking culture conditions investigated using comparative peptidomics. Mol Cell Proteomics. 2007;6(4):717–727. doi: 10.1074/mcp.M600282-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(10):785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 25.Jones JD, O’Connor CD. Protein acetylation in prokaryotes. Proteomics. 2011;11(15):3012–3022. doi: 10.1002/pmic.201000812. [DOI] [PubMed] [Google Scholar]
- 26.Polevoda B, Sherman F. N-terminal acetyltransferases and sequence requirements for N-terminal acetylation of eukaryotic proteins. J Mol Biol. 2003;325(4):595–622. doi: 10.1016/s0022-2836(02)01269-x. [DOI] [PubMed] [Google Scholar]
- 27.Bonissone S, Gupta N, Romine M, Bradshaw RA, Pevzner PA. N-terminal protein processing: A comparative proteogenomic analysis. Mol Cell Proteomics. 2013;12(1):14–28. doi: 10.1074/mcp.M112.019075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foloppe N, Nilsson L. The glutaredoxin -C-P-Y-C- motif: Influence of peripheral residues. Structure. 2004;12(2):289–300. doi: 10.1016/j.str.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Eistetter AJ, Butler PD, Traut RR, Fanning TG. Characterization of Escherichia coli 50S ribosomal protein L31. FEMS Microbiol Lett. 1999;180(2):345–349. doi: 10.1111/j.1574-6968.1999.tb08816.x. [DOI] [PubMed] [Google Scholar]
- 30.L’Italien JJ, Laursen RA. Location of the site of methylation in elongation factor Tu. FEBS Lett. 1979;107(2):359–362. doi: 10.1016/0014-5793(79)80407-x. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard SJ, Thornton JM. NACCESS 2.1.1 Computer Program. London: Department of Biochemistry and Molecular Biology, University College London; 1996. [Google Scholar]
- 32.Fratelli M, et al. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc Natl Acad Sci USA. 2002;99(6):3505–3510. doi: 10.1073/pnas.052592699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao XH, Bedhomme M, Veyel D, Zaffagnini M, Lemaire SD. Methods for analysis of protein glutathionylation and their application to photosynthetic organisms. Mol Plant. 2009;2(2):218–235. doi: 10.1093/mp/ssn072. [DOI] [PubMed] [Google Scholar]
- 34.Adkins JN, et al. Analysis of the Salmonella typhimurium proteome through environmental response toward infectious conditions. Mol Cell Proteomics. 2006;5(8):1450–1461. doi: 10.1074/mcp.M600139-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Yoon H, et al. Systems analysis of multiple regulator perturbations allows discovery of virulence factors in Salmonella. BMC Syst Biol. 2011;5:100. doi: 10.1186/1752-0509-5-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmer JS, Monroe ME, Qian WJ, Smith RD. Advances in proteomics data analysis and display using an accurate mass and time tag approach. Mass Spectrom Rev. 2006;25(3):450–482. doi: 10.1002/mas.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polpitiya AD, et al. DAnTE: A statistical tool for quantitative analysis of -omics data. Bioinformatics. 2008;24(13):1556–1558. doi: 10.1093/bioinformatics/btn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craghill J, Cronshaw AD, Harding JJ. The identification of a reaction site of glutathione mixed-disulphide formation on gammaS-crystallin in human lens. Biochem J. 2004;379(Pt 3):595–600. doi: 10.1042/BJ20031367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newman SF, et al. An increase in S-glutathionylated proteins in the Alzheimer’s disease inferior parietal lobule, a proteomics approach. J Neurosci Res. 2007;85(7):1506–1514. doi: 10.1002/jnr.21275. [DOI] [PubMed] [Google Scholar]
- 40.West MB, Hill BG, Xuan YT, Bhatnagar A. Protein glutathiolation by nitric oxide: An intracellular mechanism regulating redox protein modification. FASEB J. 2006;20(10):1715–1717. doi: 10.1096/fj.06-5843fje. [DOI] [PubMed] [Google Scholar]
- 41.Findlay VJ, et al. A novel role for human sulfiredoxin in the reversal of glutathionylation. Cancer Res. 2006;66(13):6800–6806. doi: 10.1158/0008-5472.CAN-06-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Townsend DM, et al. A glutathione S-transferase pi-activated prodrug causes kinase activation concurrent with S-glutathionylation of proteins. Mol Pharmacol. 2006;69(2):501–508. doi: 10.1124/mol.105.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leichert LI, Scharf C, Hecker M. Global characterization of disulfide stress in Bacillus subtilis. J Bacteriol. 2003;185(6):1967–1975. doi: 10.1128/JB.185.6.1967-1975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang S, et al. Transcriptomic responses of Salmonella enterica serovars Enteritidis and Typhimurium to chlorine-based oxidative stress. Appl Environ Microbiol. 2010;76(15):5013–5024. doi: 10.1128/AEM.00823-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hautefort I, et al. During infection of epithelial cells Salmonella enterica serovar Typhimurium undergoes a time-dependent transcriptional adaptation that results in simultaneous expression of three type 3 secretion systems. Cell Microbiol. 2008;10(4):958–984. doi: 10.1111/j.1462-5822.2007.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thiele I, et al. A community effort towards a knowledge-base and mathematical model of the human pathogen Salmonella Typhimurium LT2. BMC Syst Biol. 2011;5:8. doi: 10.1186/1752-0509-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bjur E, Eriksson-Ygberg S, Aslund F, Rhen M. Thioredoxin 1 promotes intracellular replication and virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2006;74(9):5140–5151. doi: 10.1128/IAI.00449-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown RN, et al. A comprehensive subcellular proteomic survey of Salmonella grown under phagosome-mimicking versus standard laboratory conditions. Int J Proteomics. 2012;2012:123076. doi: 10.1155/2012/123076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niemann GS, et al. Discovery of novel secreted virulence factors from Salmonella enterica serovar Typhimurium by proteomic analysis of culture supernatants. Infect Immun. 2011;79(1):33–43. doi: 10.1128/IAI.00771-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.