Abstract
Background
Based upon preclinical evidence for improved antitumor activity in combination, this phase I study investigated the maximum-tolerated dose (MTD), safety, activity, pharmacokinetics (PK), and biomarkers of the mammalian target of rapamycin inhibitor, temsirolimus, combined with sorafenib in hepatocellular carcinoma (HCC).
Patients and methods
Patients with incurable HCC and Child Pugh score ≤B7 were treated with sorafenib plus temsirolimus by 3 + 3 design. The dose-limiting toxicity (DLT) interval was 28 days. The response was assessed every two cycles. PK of temsirolimus was measured in a cohort at MTD.
Results
Twenty-five patients were enrolled. The MTD was temsirolimus 10 mg weekly plus sorafenib 200 mg twice daily. Among 18 patients at MTD, DLT included grade 3 hand–foot skin reaction (HFSR) and grade 3 thrombocytopenia. Grade 3 or 4 related adverse events at MTD included hypophosphatemia (33%), infection (22%), thrombocytopenia (17%), HFSR (11%), and fatigue (11%). With sorafenib, temsirolimus clearance was more rapid (P < 0.05). Two patients (8%) had a confirmed partial response (PR); 15 (60%) had stable disease (SD). Alpha-fetoprotein (AFP) declined ≥50% in 60% assessable patients.
Conclusion
The MTD of sorafenib plus temsirolimus in HCC was lower than in other tumor types. HCC-specific phase I studies are necessary. The observed efficacy warrants further study.
Keywords: hepatocellular carcinoma, mTOR, pharmacokinetics, sorafenib, temsirolimus
introduction
Despite improvements in overall survival with the multikinase inhibitor, sorafenib, outcomes remain grim in advanced hepatocellular carcinoma (HCC) [1]. Multiple randomized, phase III trials of other targeted therapies in HCC have not demonstrated benefit, underscoring the challenges of hepatic dysfunction, chemoresistance, and poorly understood therapeutic targets in this prevalent disease [2–4].
Activation of the mammalian target of rapamycin (mTOR) kinase leads to malignant transformation in HCC cell lines [5–7]. Approximately 50% of human HCC demonstrate aberrant mTOR pathway activation [6]. Inhibition of mTOR in preclinical models of HCC reduces proliferation, impairs angiogenesis, delays metastasis, and improves survival [6, 8–10]. Small phase I and II studies of the allosteric mTOR inhibitors, everolimus and sirolimus, suggest modest single-agent activity in advanced HCC [11, 12]. A meta-analysis of 2950 patients showed significantly reduced HCC recurrence (odds ratio 0.42, 95% confidence interval 0.21–0.83) post liver transplantation with sirolimus-based compared with non-sirolimus-based immunosuppression [13].
Addition of an mTOR inhibitor to sorafenib augments antitumor effects in HCC preclinical studies in vitro and in vivo [14–21], though there are limited clinical data on the combination in HCC [22, 23]. In a phase I study of the combination of everolimus and standard dose sorafenib (400 mg twice daily) in advanced HCC, the maximum-tolerated dose (MTD) of everolimus was 2.5 mg daily (25% of standard dose) [22]. PK studies in other tumor types have not identified an interaction between sorafenib and sirolimus or its analogs [22, 24, 25]. An organ dysfunction PK study of the allosteric mTOR inhibitor, temsirolimus, showed no significant difference in temsirolimus exposure as a single agent in patients with mild-to-moderate liver dysfunction by National Cancer Institute (NCI) Organ Dysfunction Working Group (ODWG) criteria; with severe impairment, however, exposure was significantly increased [26].
We developed a phase I trial to determine the safety and MTD of the combination of temsirolimus and sorafenib as first-line therapy in patients with incurable HCC and normal-to-mild hepatic dysfunction. PK of temsirolimus and its major metabolite, sirolimus, were carried out in an expanded cohort at the MTD to evaluate for interaction with sorafenib. Candidate biomarkers were explored.
patients and methods
All patients provided written informed consent. The trial was approved by the two participating centers’ Institutional Review Boards and the NCCN Temsirolimus Scientific Review Committee. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
patient selection
Patients were recruited and treated at the UCSF Helen Diller Family Comprehensive Cancer Center and the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. The principal inclusion criteria were: radiographic [27] or histologic diagnosis of American Joint Committee on Cancer (AJCC) stage II, III, or IV HCC not eligible for curative therapies; no prior systemic therapy; Child Pugh A or B with ≤7 points; Eastern Cooperative Oncology Group (ECOG) performance status ≤2; measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; ≥6 weeks since prior liver-directed therapy or resection; co-treatment with antiviral therapy if active HBV; and adequate organ function (absolute neutrophil count ≥1500/mcl, platelet count ≥75 000/mcl, total bilirubin ≤1.5 times upper limits of normal (ULN), AST/ALT less than five times ULN, INR ≤1.5 times ULN, albumin ≥2.8 g/dl). The exclusion criteria included mixed histology, transplantation, HIV, and uncontrolled blood pressure, diabetes, or hyperlipidemia.
study design and treatment
Standard 3 + 3 dose escalation was employed. An expansion cohort of nine patients was enrolled for PK and exploratory biomarker testing once the MTD was identified. The primary end points were determination of MTD and recommended phase II dose (RP2D). The secondary end points included safety, PK, response rate, and progression-free survival (PFS).
The cycle length was 28 days. Temsirolimus was administered by intravenous (IV) infusion over 30–60 min weekly with diphenhydramine premedication. The starting dose of temsirolimus was 15 mg i.v. weekly (Table 2), one dose level below the standard single agent dose of 25 mg i.v. weekly. Sorafenib was initiated on cycle 1, day 1. The starting dose of sorafenib was 200 mg orally twice daily, also one dose level below standard dose, due to the established high incidence of dose reduction required as a single agent in HCC patients [1, 2]. In the expansion cohort, temsirolimus was administered over a fixed duration of 60 min during cycle 1, with sorafenib initiation day 8.
Table 2.
Patient distribution by dose level and DLTs
| Dose level | Sorafenib dose | Temsirolimus dose | Number enrolled | Number evaluable for DLT | DLT (n) | DLT events |
|---|---|---|---|---|---|---|
| 3 | 400 mg PO BID | 25 mg IV weekly | 0 | 0 | 0 | |
| 2 | 400 mg PO BID | 15 mg IV weekly | 0 | 0 | 0 | |
| 1 (starting dose) | 200 mg PO BID | 15 mg IV weekly | 7 | 5 | 1a | Grade 3 platelets requiring dose reduction |
| −1 (MTD) | 200 mg PO BID | 10 mg IV weekly | 9 | 6 | 1 | Grade 3 HFSR |
| Expansion cohort at −1 (MTD) | 200 mg PO BID | 10 mg IV weekly | 9 | 8 | 1 | Grade 3 platelets plus grade 2 ANC requiring dose reduction |
| −2 | 200 mg PO QD | 10 mg IV weekly | 0 | 0 | 0 |
aDe-escalated to dose level −1 due to cumulative lack of tolerability without meeting the DLT criteria.
safety assessments
Dose-limiting toxicities (DLTs) and adverse events were graded by NCI Common Terminology Criteria for Adverse Events, version 3.0. Patients were continuously monitored for toxicity. Clinical evaluation occurred weekly during cycle 1 and monthly thereafter. Laboratory monitoring was carried out weekly in all cycles. Fasting glucose, cholesterol panel, and hepatitis viral DNA or RNA quantitation (if serology positive) were monitored each cycle. DLT were defined as treatment-related grade 4 hematologic toxicity (except grade 4 leukopenia <7 days), grade 3 thrombocytopenia ≥7 days, or grade ≥3 clinically-relevant, non-hematologic toxicity despite optimal supportive care. Exceptions were grade 3 hypertension if medically optimized within 14 days, hypophosphatemia, or fatigue. Toxicity from chronic liver disease required increase ≥2 grades from baseline to be considered a DLT. DLT included any treatment-related toxicity requiring dose reduction or delay >8 days within cycle 1.
PK analyses
In the expansion cohort at the MTD, PK was carried out using a validated liquid chromatography-mass spectrometry (LC-MS)/MS method for temsirolimus and sirolimus [28]. Whole blood samples were obtained pre-sorafenib in cycle 1 at 0 (pre-dose), 1, 3, 5, 24, 48, and 168 (pre-dose) hours from the first temsirolimus infusion. Sorafenib was started on day 8, with expected steady-state by day 15 [29]. Repeat PK samples were obtained starting cycle 1, day 15, at the same time points. PK for sorafenib was not carried out due to high inter-patient variability and absence of association with toxicity or efficacy [30–33]. PK analyses were carried out by noncompartmental pharmacokinetic (PK) methods using Phoenix WinNonlin®, Version 6.2.1 (Pharsight Corporation, Mountain View, CA).
efficacy assessments
Baseline radiographic tumor assessment by computed tomography or magnetic resonance imaging imaging of chest, abdomen (triphasic recommended), and pelvis was carried out within 28 days of enrollment. Restaging assessments were carried out every 8 weeks (±7 days) using RECIST 1.1 by an independent radiologist [34]. PFS was calculated in months from the first dose of protocol therapy to date of removal from study for clinical or radiographic progression or death. PFS of patients removed for reasons other than progression or death was censored at the date last known to be progression-free.
exploratory biomarker assessments
AFP, AFP-L3, and DCP
Serum AFP was measured at baseline and on day 1 each cycle. In the expansion cohort, AFP-L3 and DCP (LabCorp, Inc.) were measured at baseline and after one and two cycles. AFP levels ≥20 ng/ml, DCP levels ≥7.5 ng/ml, and AFP-L3% of ≥10% were considered elevated [35, 36].
Circulating tumor cells (CTC)
A nonenrichment, high-definition (HD)-CTC assay was carried out in the expansion cohort at baseline and after one and two cycles. The HD-CTC assay was carried out in the Kuhn Laboratory at The Scripps Research Institute using published methods [37].
statistical methods
Frequency and proportions were used for safety, response, and AE. Mean and standard deviation with t-tests were used for PK. The final cohort of 18 patients treated at MTD provided 78% likelihood of observing (at least once) toxicity with a true occurrence rate of at least 15%. Kaplan–Meier methods were used to summarize PFS with 95% confidence interval.
results
patient characteristics
Twenty-five patients were enrolled and received at least one dose of both study drugs between December 2009 and April 2012 (Table 1).
Table 1.
Patient characteristics (n = 25)
| Characteristic | Number or median (range) | % |
|---|---|---|
| Gender | ||
| Male | 19 | 76 |
| Female | 6 | 24 |
| Age (years) | ||
| Median (range) | 60 (45–77) | |
| Baseline body weight (kg) | ||
| Median (range) | 73.6 (54.0–122.5) | |
| Race/ethnicity | ||
| African-American | 2 | 8 |
| Asian | 6 | 24 |
| Caucasian | 16 | 64 |
| Non-Hispanic/Latino | 13 | 52 |
| Hispanic/Latino | 3 | 12 |
| Native American | 1 | 4 |
| ECOG | ||
| 0 | 14 | 56 |
| 1 | 11 | 44 |
| Cause of liver disease | ||
| HCV | 8 | 32 |
| HCV plus cleared HBV | 6 | 24 |
| HBV | 4 | 16 |
| Alcoholic liver disease | 2 | 8 |
| Unknown/idiopathic | 5 | 20 |
| Child pugh class | ||
| A | 24 | 96 |
| B | 1 | 4 |
| Baseline Albumin (g/dL) | ||
| Median (range) | 3.4 (2.6–4.5) | |
| NCI-ODWG category | ||
| Normal | 8 | 32 |
| Mild | 17 | 68 |
| Moderate/severe | 0 | 0 |
| Histologically-confirmed HCC diagnosis | 21 | 84 |
| BCLC Stage | ||
| B (intermediate) | 4 | 16 |
| C (advanced) | 21 | 84 |
| Macroscopic vascular invasion present | 10 | 40 |
| Extrahepatic spread present | 19 | 76 |
| Baseline AFP elevated ≥ 20 ng/mL | 17 | 68 |
| Median AFP (ng/mL) (range) | 77.5 (2.6–89.035) | |
| Previous therapya | ||
| Surgical resection | 8 | 32 |
| Bland embolization | 2 | 8 |
| Chemoembolization (TACE) | 8 | 32 |
| Radioembolization | 6 | 24 |
| Ablation (radiofrequency or microwave) | 2 | 8 |
| None | 6 | 24 |
| Treatment site | ||
| University of California, San Francisco | 17 | 68 |
| Northwestern University | 8 | 32 |
a5 patients had ≥1 prior therapeutic modality.
dose-finding, DLT, and maximum-tolerated dose
Patients were enrolled starting at dose level 1 (Table 2). At dose level 1, two patients were not assessable due to unrelated AE (supplementary Table S3, available at Annals of Oncology online). Due to cumulative and heterogeneous post-DLT toxicity requiring dose reductions after cycle 1 in all assessable patients (supplementary Table S3, available at Annals of Oncology online), dose level 1 was considered non-tolerable, and the study was de-escalated. At dose level 1, 3 were not assessable for toxicity due to unrelated complications (supplementary Table S3, available at Annals of Oncology online). Among six assessable patients, 1 DLT of grade 3 hand–foot skin reaction (HFSR) occurred. Dose level −1 was defined as MTD and RP2D.
Nine additional patients were enrolled to an expansion cohort at dose level −1 (Table 2). One patient was not assessable for toxicity or efficacy (supplementary Table S3, available at Annals of Oncology online). Among eight assessable patients, one experienced grade 3 thrombocytopenia plus grade 2 neutropenia requiring temsirolimus dose reduction within DLT window.
safety and tolerability
Treatment-related AEs are listed in Table 3. Among the 18 patients treated at MTD, grade 3 and 4 events possibly treatment-related and occurring in ≥10% were: hypophosphatemia in 6 (33%), infection with normal or grade 1/2 neutrophils in 4 (22%), abnormal liver function tests in 4 (22%), thrombocytopenia in 3 (17%), HFSR in 2 (11%), and fatigue in 2 (11%). Hypophosphatemia was asymptomatic and responded to phosphorus supplementation. Liver function test abnormalities were asymptomatic. There were no bleeding or transfusion events. Both cases of grade 3 HFSR resolved after dose reduction in sorafenib. There was one event (4%) of grade 1 asymptomatic radiographic pneumonitis attributed to temsirolimus which resolved after drug discontinuation for progressive disease. All four events of grade 3 or 4 infection resulted in serious AE (SAE) and consisted of (1 each): gram-negative urosepsis, cellulitis arising from HFSR, dental abscess from pre-existing caries, and community-acquired pneumonia. All were reversible. There were no events of hepatic decompensation from reactivation or exacerbation of viral hepatitis. Another possibly related SAE of grade 4 tumor rupture (with normal platelet count) manifesting with pain and hypotension occurred at dose level −1 during cycle 6 in the setting of tumor progression and resulted in death within 30 days. Overall, 12 patients (48%) experienced SAE, of which 5 (20%) were at least possibly treatment-related.
Table 3.
Treatment-related toxicity
| Body system general term | Overall (n = 25) | Dose level 1 (n = 7) |
Dose level −1 (n = 18a) |
||
|---|---|---|---|---|---|
| All grades | Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3/4 | |
| Blood/bone marrow | |||||
| Hemolysis | 1 (4%) | 0 | 1 (14%) | 0 | 0 |
| Platelets | 15 (60%) | 2 (29%) | 2 (29%) | 9 (50%) | 3 (17%) |
| Neutrophils/granulocytes | 7 (28%) | 1 (14%) | 0 | 5 (28%) | 1 (6%) |
| Hemoglobin | 7 (28%) | 0 | 0 | 7 (39%) | 0 |
| Cardiac/hypertension | 2 (8%) | 1 (14%) | 0 | 0 | 2 (11%) |
| Constitutional symptoms | |||||
| Fatigue (asthenia, lethargy, malaise) | 16 (64%) | 4 (57%) | 0 | 9 (50%) | 3 (17%) |
| Weight loss/anorexia | 13 (52%) | 5 (71%) | 0 | 9 (50%) | 0 |
| Rigors/chills | 5 ( 20%) | 0 | 0 | 6 (33%) | 0 |
| Dermatology/skin | |||||
| Rash/desquamation/other | 14 (56%) | 6 (86%) | 0 | 8 (44%) | 0 |
| Hand–foot skin reaction | 7 (28%) | 0 | 1 (14%) | 4 (22%) | 2 (11%) |
| Hair loss/alopecia (scalp or body) | 4 (16%) | 1 (14%) | 0 | 3 (17%) | 0 |
| Pruritus/itching/dry skin | 5 (20%) | 0 | 0 | 6 (33%) | 0 |
| Gastrointestinal | |||||
| Diarrhea | 12 (48%) | 5 (71%) | 0 | 5 (28%) | 2 (11%) |
| Mucositis/stomatitis | 7 (28%) | 2 (29%) | 0 | 5 (28%) | 0 |
| Nausea | 7 (28%) | 2 (29%) | 0 | 5 (28%) | 0 |
| Ascites (non-malignant) | 1 (4%) | 0 | 0 | 0 | 1 (6%) |
| Hemorrhage/bleeding | |||||
| Epistaxis | 2 (8%) | 0 | 0 | 2 (11%) | 0 |
| Liver tumor rupture | 1 (4%) | 0 | 0 | 0 | 1 (6%)b |
| Hypersensitivity/infusion reaction | 1 (4%) | 0 | 1 (14%) | 0 | 0 |
| Infection with ≤ Grade 2 neutrophils | 5 (20%) | 0 | 0 | 1 (6%) | 4 (22%)b |
| Lymphatic/edema | 3 (12%) | 0 | 0 | 2 (11%) | 1 (6%) |
| Metabolic/Laboratory | |||||
| Hypophosphatemia | 20 (80%) | 1 (14%) | 5 (71%) | 8 (44%) | 6 (33%) |
| Hypokalemia | 3 (12%) | 1 (14%) | 0 | 0 | 2 (11%) |
| Hypomagnesemia | 4 (16%) | 1 (14%) | 1 (14%) | 2 (11%) | 0 |
| Elevated creatinine | 4 (16%) | 2 (29%) | 0 | 2 (11%) | 0 |
| Hyperglycemia | 3 (12%) | 2 (29%) | 0 | 1 (6%) | 0 |
| Hypertriglyceridemia | 4 (16%) | 3 (43%) | 0 | 1 (6%) | 0 |
| Abnormal liver function tests | 16 (64%) | 2 (29%) | 0 | 10 (56%) | 4 (22%) |
| Neuropathy, sensory | 3 (12%) | 1 (14%) | 0 | 2 (11%) | 0 |
| Pain | 7 (28%) | 0 | 0 | 6 (33%) | 1 (6%) |
aIncludes nine patients in the expansion cohort.
bSerious AEs (SAEs). Only toxic effects attributed as at least possibly treatment-related are included. All grade ≥3 toxic effects are reported. For grade ≤2 toxic effects, only those with ≥10% incidence overall (three or more events) are reported. For overlapping toxic effects (i.e. weight loss and anorexia), only the highest grade and/or most clinically relevant is reported.
Among 18 patients treated at MTD, dose reductions were required in sorafenib in 3 (16.7%), in temsirolimus in 2 (11.1%), and in both drugs in 2 (11.1%). Eleven patients (61.1%) did not require dose reduction. Dose modifications were protocol-defined and according to investigators’ attribution of toxicity.
pharmacokinetics
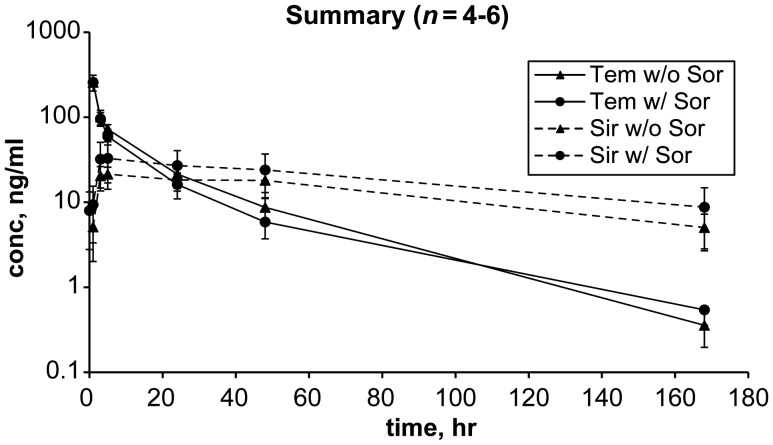
PK profiles for temsirolimus and its metabolite, sirolimus, before and after addition of sorafenib in six patients treated at MTD are shown in Table 4 and Figure 1. One patient was not assessable for PK after cycle 1, day 8 due to removal from study for unrelated complication of gallstone cholecystitis. One patient was not assessable for cycle 1, day 22 time point due to a missing specimen.
Table 4.
PK of temsirolimus and sirolimus after a 10 mg dose of temsirolimus
| Without sorafenib (n = 6) |
With sorafenib at steady state (n = 4 or 5) |
|||
|---|---|---|---|---|
| TEM | SIR | TEM | SIR | |
| Cmax (µg/l) | 257 ± 54.2 | 22.0 ± 5.32 | 257 ± 29.2 | 33.1 ± 15.8 |
| Tmax (h) | 1 | 17.8 ± 17.8 | 1 | 8 ± 9 |
| T1/2 (h) | 22. ± 4.42 | 73.4 ± 17.5 | 18.3 ± 5.16 | 133 ± 115 |
| AUC0-168 h (h·µg/l) | 2220 ± 415 | 2062 ± 582 | 1664 ± 435 | 2495 ± 1329 |
| AUCinf (h·µg/l) | 2356 ± 435 | 2635 ± 908 | 1761 ± 390a | 3707 ± 2198 |
| AUCsum (h·µg/l) | – | 4991 ± 861 | 5521 ± 2257 | |
| CL (l/h) | 4.37 ± 0.808 | 4.21 ± 1.54 | 5.86 ± 1.01a | 4.93 ± 5.41 |
| Vdss (l) | 86.6 ± 23.0 | 454.3 ± 183 | 61.3 ± 32.5 | 335 ± 192 |
aStatistical t-test, P < 0.05. TEM = temsirolimus; SIR = sirolimus.
Figure 1.
Sorafenib effect on temsirolimus and sirolimus concentration. Temsirolimus AUCinf was lower and clearance more rapid in the presence of sorafenib by a statistical t-test (P < 0.05) though interpretation is limited by small sample size (n = 4–6). The 72 and 96 h time points were optional and not obtained in any patient.
Peak drug concentration (Cmax) for temsirolimus was similar before and after addition of sorafenib. In this small sample, clearance of temsirolimus was more rapid after addition of sorafenib (4.37 l/h versus 5.86 l/h, statistical t-test, P < 0.05), with a nonsignificant decrease in temsirolimus half-life, and the mean area under concentration–time curve (AUCinf) for temsirolimus appeared to be lower (statistical t-test, P < 0.05). There was a nonsignificant trend toward increase in Cmax and AUCinf for sirolimus after addition of sorafenib. There was significant interpatient variability for Cmax and AUCinf for sirolimus across time points (Table 4, Figure 1).
efficacy
Four of 25 (16%) were not assessable for clinical or radiographic response due to removal from study for non-progression-related and non-treatment-related adverse events or withdrawal of consent before post-baseline imaging. Two patients (8%) (both at MTD) experienced a confirmed partial response (PR) , while 15 (60%) had stable disease (SD) as best response, with an overall disease control rate of 68% (Table 5). One additional patient demonstrated PR (unconfirmed) after data cut-off resulting in 3 of 25 (12%) with PR; all had HCV and were treated at dose level −1 (MTD). Formal testing for association between response and demographic characteristics was not possible due to small sample. Overall, tumor regression from baseline was observed in 12 patients (48%) (supplementary Figure S2, available at Annals of Oncology online). PR or SD was observed for ≥6 months on study in five patients at MTD (20% overall). Among the 18 patients treated at dose level −1, the median PFS was 5.65 months (95% confidence interval (CI): 5.38–6.45) with range 1.3–22.8 months. A median of 3.1 cycles were completed in the MTD cohort. Two patients remained on study at the time of data cut-off.
Table 5.
Patient disposition and best response
| Outcome | Number of patients (n = 25) | % |
|---|---|---|
| Best response (RECIST version 1.1) | ||
| Confirmed partial response (PR)a | 2 | 8% |
| Stable disease (SD) | 15 | 60% |
| Progressive disease (PD) | 4 (1 radiographic, 3 clinical) | 16% |
| Not assessableb | 4 | 16% |
| Reason for discontinuationc | ||
| Progressive disease (radiographic or clinical) | 12 | 48% |
| Related toxicity | 3 | 12% |
| Unrelated toxicity | 3 | 12% |
| Withdrawal of consent/noncompliance | 5 | 20% |
aOne additional unconfirmed PR occurred after data cut-off (not included).
bNot assessable because: removed for unrelated toxicity (2), removed for withdrawn consent/inability to comply (2).
cTwo patients remained on study at the time of data cut-off on 20 July 2012.
tumor markers: AFP, AFP-L3, and DCP
At baseline, 17 of 25 (68%) had elevated AFP values ≥20 ng/ml, with median 77.5 (range 2.6–89,035) ng/ml. AFP decline of ≥50% on study was observed in 9 of 15 assessable (60%) (supplementary Figure S3, available at Annals of Oncology online). Baseline levels of AFP (dichotomized and continuous) were not associated with PFS by Pearson's correlation test (P = 0.40). AFP decline ≥50% showed a nonsignificant trend toward longer PFS by a log-rank test (P = 0.15).
Among the nine patients tested in the expansion cohort, six (67%) and seven (78%) had elevated AFP-L3 ≥10% and DCP ≥7.5 ng/ml at baseline. Baseline and changes in levels on therapy were not significantly associated with PFS by Pearson's correlation test, but interpretation is limited by the small sample.
circulating tumor cells
More than one CTCs per millilitre were detected in seven (78%) of nine patients in the expansion cohort (supplementary Figure S4, available at Annals of Oncology online). Due to small sample, no formal testing for association with PFS or response was possible.
discussion
This phase 1 study demonstrates that temsirolimus combined with sorafenib has an acceptable safety profile in patients with incurable HCC and normal or mild hepatic dysfunction, with similar rates of SAE and grade ≥3 AE as reported for single-agent sorafenib [1, 2, 4]. The MTD and RP2D of this combination regimen are temsirolimus 10 mg IV weekly plus sorafenib 200 mg twice daily. The most common treatment-related adverse events were hypophosphatemia, abnormal liver function tests, fatigue, thrombocytopenia, rashes (including HFSR), anorexia/weight loss, and diarrhea, with the majority responsive to supportive care or dose modification. Of note, there were four events (16% overall) of grade 3 or 4 bacterial infection (without ≥grade 2 neutropenia). While bacterial infections are frequent in patients with underlying liver dysfunction independent of therapy [38] and uncommon in other studies of mTOR inhibitors combined with sorafenib [24, 25, 39, 40], an increased risk of bacterial infection from this regimen in HCC patients cannot be excluded.
As expected, the Cmax for both temsirolimus and sirolimus after a 10 mg dose of temsirolimus was lower than historical controls of healthy volunteers and cancer patients without hepatic dysfunction treated with a 25 mg dose [26, 41]. Unexpectedly, however, the AUC of both temsirolimus and sirolimus, after a 10 mg dose of temsirolimus before starting sorafenib, was similar to non-HCC subjects treated with a 25 mg dose, and clearance appeared to be slower [26]. This finding suggests that metabolism may be diminished in HCC patients compared with historical controls matched by NCI–ODWG criteria. Differences in plasma protein binding in the setting of hepatic dysfunction could also impact exposure and clearance. In the presence of sorafenib, temsirolimus clearance was more rapid and exposure decreased, and there was a nonsignificant trend toward a mild increase in sirolimus AUC. The mechanism of increased clearance of temsirolimus is unknown; sorafenib has not been shown to induce CYP3A [42]. It is noteworthy that the tolerable doses for the combination of temsirolimus and sorafenib in this study are lower than each single-agent MTD as well as the combination MTD identified in melanoma patients without hepatic dysfunction, which was temsirolimus 25 mg IV weekly plus sorafenib 400 mg in the mornings, 200 mg in the evenings [39].
The PK observations from this study are limited by small sample size and require confirmation in a larger, independent cohort. Nonetheless, they provide a basis for the lower combination MTD identified and suggest that NCI-ODWG criteria, defined by AST and bilirubin levels and applied to all-cause hepatic dysfunction, may not accurately recapitulate the cumulative impact of cirrhosis on hepatic metabolism across drugs and metabolic pathways. This hypothesis supports that disease-specific phase I or Ib studies should be undertaken in HCC, in addition to all-cause organ dysfunction populations, before embarking upon efficacy or combination studies which may be confounded by dose delays, reductions, and toxicity.
A subset of patients in this study demonstrated promising responses, including durable PR in 2 patients treated at MTD and overall tumor regression by RECIST 1.1 in 12 of 25 response-assessable patients (48%). Among 15 assessable subjects with baseline elevations in AFP, a decline of at least 50% was observed in 60%, a signal associated with treatment response and survival [35, 43, 44].
Despite encouraging tumor regression and AFP response rates in this first-line combination study, however, the median PFS was only 5.65 months in PFS-assessable patients treated at the RP2D, similar to outcomes from single-agent sorafenib in the SHARP population though superior to outcomes in an Asia-Pacific study of sorafenib [1, 45]. The high degrees of prognostic and biologic heterogeneity in HCC limit the conclusions which can be drawn from cross-study comparisons. Further confounding interpretation of time-to-event end points in HCC, background liver fibrosis and residua of prior liver-directed therapies can obscure measurement of radiographic response and progression. Novel imaging techniques such as liver tumor volumetry and modified RECIST criteria are promising approaches to improve discernment of therapeutic efficacy, but require validation to determine their surrogacy for overall survival.
Our phase 1 trial is timely in heralding an expanding body of data surrounding the role of mTOR inhibition across stages in HCC [11–13]. The randomized, phase III EVOLVE (NCT01035229) and SILVER (NCT00355862) trials will report on the efficacy of mTOR inhibition in advanced and post-transplant HCC populations, respectively, and novel dual TORC1/2 inhibitors are in development. These emerging data mandate parallel efforts to identify biomarkers of response to mTOR inhibition as well as to sorafenib.
Fundamental questions beyond the scope of this phase 1 trial include whether there is a benefit from the combination of mTOR inhibition with sorafenib over single agent or sequential administration, whether dual TORC1/2 inhibitors will prove more effective than rapamycin analogs, and how to identify which HCC tumors will respond. A phase II trial of temsirolimus plus sorafenib at the RP2D is now underway to characterize the tolerability and efficacy of this regimen in an expanded cohort with correlative biomarker analyses.
funding
This work was supported by the National Comprehensive Cancer Network (NCCN) with general research support provided by Pfizer, Inc. Temsirolimus was provided by Pfizer, Inc. RKK's effort was supported by a Young Investigator Award (YIA) from the American Society of Clinical Oncology (ASCO) in 2011. A support for specimen banking was provided by the Bili Project Foundation. A support for circulating tumor cell testing was provided by National Institutes of Health U54CA143906 to PK. Research funding is reported by: PNM, Bayer/Onyx and Pfizer Oncology; PK, Epic Sciences; ABB and APV, Bayer/Onyx.
disclosure
RKK, HSN, MTV, YH, C-ML, JH, MFM, BMY, JAG, LS-M, WMK, and AHK report no conflicts of interest relevant to the subject matter under consideration in this article. PK is a founder and shareholder and MSL is a shareholder of Epic Sciences which is commercializing the circulating tumor cell technology used in this study. EKB served as a scientific advisor to Pfizer Oncology (uncompensated). ABB served as a scientific advisor to Bayer/Onyx (compensated).
Supplementary Material
acknowledgements
We would like to express our deep appreciation to the patients who participated in this clinical trial, along with their families, for their donation of time and self to medical oncology research. We also extend our gratitude to The Bili Project Foundation for supporting the blood and tumor specimen banking in this study for future biomarker research, and to the phase I staff and social workers at the University of California, San Francisco Helen Diller Family Comprehensive Cancer Center and Robert H. Lurie Comprehensive Cancer Center of Northwestern University, for their great contributions of effort and expertise to this study and its patients.
references
- 1.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. doi:10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 2.Cheng A, Kang Y, Lin D, Park J, et al. Phase III trial of sunitinib (Su) versus sorafenib (So) in advanced hepatocellular carcinoma (HCC). In American Society of Clinical Oncology Annual Meeting. Chicago, IL. J Clin Oncol. 2011 29(15-suppl): 4000. [Google Scholar]
- 3.Llovet JM, Decaen T, Raoul J-L, Boucher E, et al. Brivanib versus placebo in patients with advanced hepatocellular carcinoma (HCC) who failed or were intolerant to sorafenib: results from the phase 3 BRISK-PS study. International Liver Congress, European Association for the Study of the Liver; Barcelona, Spain. 2012. [Google Scholar]
- 4.Zhu RO, Evans J, Ross P, et al. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with hepatocellular carcinoma (HCC). 37th ESMO Congress, European Society of Medical Oncology; Vienna, Austria. 2012. [DOI] [PubMed] [Google Scholar]
- 5.Menon S, Yecies JL, Zhang HH, et al. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci Signal. 2012;5:ra24. doi: 10.1126/scisignal.2002739. doi:10.1126/scisignal.2002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villanueva A, Chiang DY, Newell P, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972–1983, 1983 e1971–1911. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou L, Huang Y, Li J, Wang Z. The mTOR pathway is associated with the poor prognosis of human hepatocellular carcinoma. Med Oncol. 2010;27:255–261. doi: 10.1007/s12032-009-9201-4. doi:10.1007/s12032-009-9201-4. [DOI] [PubMed] [Google Scholar]
- 8.Huynh H, Chow KP, Soo KC, et al. RAD001 (everolimus) inhibits tumor growth in xenograft models of human hepatocellular carcinoma. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semela D, Piguet AC, Kolev M, et al. Vascular remodeling and antitumoral effects of mTOR inhibition in a rat model of hepatocellular carcinoma. J Hepatol. 2007;46:840–848. doi: 10.1016/j.jhep.2006.11.021. doi:10.1016/j.jhep.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Zhou J, Fan J, et al. Sirolimus inhibits the growth and metastatic progression of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2008 doi: 10.1007/s00432-008-0506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decaens T, Luciani A, Itti E, et al. Phase II study of sirolimus in treatment-naive patients with advanced hepatocellular carcinoma. Dig Liver Dis. 2012;44:610–616. doi: 10.1016/j.dld.2012.02.005. doi:10.1016/j.dld.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Zhu AX, Abrams TA, Miksad R, et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011;117:5094–5102. doi: 10.1002/cncr.26165. doi:10.1002/cncr.26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang W, Wang D, Ling X, et al. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012;18:62–69. doi: 10.1002/lt.22441. doi:10.1002/lt.22441. [DOI] [PubMed] [Google Scholar]
- 14.Huynh H, Ngo VC, Koong HN, et al. Sorafenib and rapamycin induce growth suppression in mouse models of hepatocellular carcinoma. J Cell Mol Med. 2009;13:2673–2683. doi: 10.1111/j.1582-4934.2009.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jasinghe VJ, Xie Z, Zhou J, et al. ABT-869, a multi-targeted tyrosine kinase inhibitor, in combination with rapamycin is effective for subcutaneous hepatocellular carcinoma xenograft. J Hepatol. 2008;49:985–997. doi: 10.1016/j.jhep.2008.08.010. doi:10.1016/j.jhep.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Zhou J, Fan J, et al. Effect of rapamycin alone and in combination with sorafenib in an orthotopic model of human hepatocellular carcinoma. Clin Cancer Res. 2008;14:5124–5130. doi: 10.1158/1078-0432.CCR-07-4774. doi:10.1158/1078-0432.CCR-07-4774. [DOI] [PubMed] [Google Scholar]
- 17.Piguet AC, Saar B, Hlushchuk R, et al. Everolimus augments the effects of sorafenib in a syngeneic orthotopic model of hepatocellular carcinoma. Mol Cancer Ther. 2011;10:1007–1017. doi: 10.1158/1535-7163.MCT-10-0666. doi:10.1158/1535-7163.MCT-10-0666. [DOI] [PubMed] [Google Scholar]
- 18.Li QL, Gu FM, Wang Z, et al. Activation of PI3K/AKT and MAPK pathway through a PDGFRbeta-dependent feedback loop is involved in rapamycin resistance in hepatocellular carcinoma. PLoS One. 2012;7:e33379. doi: 10.1371/journal.pone.0033379. doi:10.1371/journal.pone.0033379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newell P, Toffanin S, Villanueva A, et al. Ras pathway activation in hepatocellular carcinoma and anti-tumoral effect of combined sorafenib and rapamycin in vivo. J Hepatol. 2009;51:725–733. doi: 10.1016/j.jhep.2009.03.028. doi:10.1016/j.jhep.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gedaly R, Angulo P, Chen C, et al. The role of PI3K/mTOR inhibition in combination with sorafenib in hepatocellular carcinoma treatment. Anticancer Res. 2012;32:2531–2536. [PubMed] [Google Scholar]
- 21.Gedaly R, Angulo P, Hundley J, et al. PKI-587 and sorafenib targeting PI3K/AKT/mTOR and Ras/Raf/MAPK pathways synergistically inhibit HCC cell proliferation. J Surg Res. 2012;176:542–548. doi: 10.1016/j.jss.2011.10.045. doi:10.1016/j.jss.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 22.Finn RS, Poon RP, Yao T, et al. Phase I study of everolimus in combination with sorafenib in patients with advanced hepatocellular carcinoma (HCC). In American Society of Clinical Oncology Annual Meeting. Chicago, IL; J Clin Oncol; 2011. in press. [Google Scholar]
- 23.Gomez-Martin C, Bustamante J, Castroagudin JF, et al. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012;18:45–52. doi: 10.1002/lt.22434. doi:10.1002/lt.22434. [DOI] [PubMed] [Google Scholar]
- 24.Gangadhar TC, Cohen EE, Wu K, et al. Two drug interaction studies of sirolimus in combination with sorafenib or sunitinib in patients with advanced malignancies. Clin Cancer Res. 2011;17:1956–1963. doi: 10.1158/1078-0432.CCR-10-2061. doi:10.1158/1078-0432.CCR-10-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harzstark AL, Small EJ, Weinberg VK, et al. A phase 1 study of everolimus and sorafenib for metastatic clear cell renal cell carcinoma. Cancer. 2011;117:4194–4200. doi: 10.1002/cncr.25931. doi:10.1002/cncr.25931. [DOI] [PubMed] [Google Scholar]
- 26.Sarantopoulos J, Lenz H, LoRusso P, Shibata S, et al. Phase I pharmacokinetic study of temsirolimus (CCI-779) in patients with advanced malignancies and normal and impaired liver function: An NCI Organ Dysfunction Working Group (ODWG) study; 2011. American Society of Clinical Oncology Annual Meeting; 2011. Chicago, IL. J Clin Oncol 29: 2011 (suppl; abstr 3072) [Google Scholar]
- 27.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. doi:10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Louie A, Lee XH, Shi R, Kelley RK, Huang Y. A simple and sensitive LC-MS/MS method for simultaneous determination of temsirolimus and its major metabolite in human whole blood. Chromatographia. 2012;75:1405–1413. [Google Scholar]
- 29.Bayer. Investigator's Brochure BAY 43–9006. Version 12.0 Edition. 2011; 192. [Google Scholar]
- 30.Abou-Alfa GK, Amadori D, Santoro A, et al. Safety and efficacy of sorafenib in patients with hepatocellular carcinoma (HCC) and Child-Pugh A versus B cirrhosis. Gastrointest Cancer Res. 2011;4:40–44. [PMC free article] [PubMed] [Google Scholar]
- 31.Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol. 2009;27:1800–1805. doi: 10.1200/JCO.2008.20.0931. doi:10.1200/JCO.2008.20.0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strumberg D, Clark JW, Awada A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–437. doi: 10.1634/theoncologist.12-4-426. doi:10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 33.Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43–9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–972. doi: 10.1200/JCO.2005.06.124. doi:10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 34.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. doi:10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 35.Personeni N, Bozzarelli S, Pressiani T, et al. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2012;57:101–107. doi: 10.1016/j.jhep.2012.02.016. doi:10.1016/j.jhep.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Sterling RK, Jeffers L, Gordon F, et al. Utility of lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxy prothrombin, alone or in combination, as biomarkers for hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7:104–113. doi: 10.1016/j.cgh.2008.08.041. doi:10.1016/j.cgh.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 37.Cho EH, Wendel M, Luttgen M, et al. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Phys Biol. 2012;9:016001. doi: 10.1088/1478-3975/9/1/016001. doi:10.1088/1478-3975/9/1/016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bunchorntavakul C, Chavalitdhamrong D. Bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. World J Hepatol. 2012;4:158–168. doi: 10.4254/wjh.v4.i5.158. doi:10.4254/wjh.v4.i5.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davies MA, Fox PS, Papadopoulos NE, et al. Phase I study of the combination of sorafenib and temsirolimus in patients with metastatic melanoma. Clin Cancer Res. 2012;18:1120–1128. doi: 10.1158/1078-0432.CCR-11-2436. doi:10.1158/1078-0432.CCR-11-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Margolin KA, Moon J, Flaherty LE, et al. Randomized phase II trial of sorafenib with temsirolimus or tipifarnib in untreated metastatic melanoma (S0438) Clin Cancer Res. 2012;18:1129–1137. doi: 10.1158/1078-0432.CCR-11-2488. doi:10.1158/1078-0432.CCR-11-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torisel Full Prescribing Information. 2012. Administration FaD (ed) [Google Scholar]
- 42.Nexavar Highlights of Prescribing Information. 2011. Bayer/Onyx (ed) [Google Scholar]
- 43.Chan SL, Mo FK, Johnson PJ, et al. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009;27:446–452. doi: 10.1200/JCO.2008.18.8151. doi:10.1200/JCO.2008.18.8151. [DOI] [PubMed] [Google Scholar]
- 44.Kuzuya T, Asahina Y, Tsuchiya K, et al. Early decrease in alpha-fetoprotein, but not des-gamma-carboxy prothrombin, predicts sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncology. 2011;81:251–258. doi: 10.1159/000334454. doi:10.1159/000334454. [DOI] [PubMed] [Google Scholar]
- 45.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. doi:10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.