Abstract
Background
Epidemiological studies evaluating the association between cruciferous vegetables (CVs) intake and female lung cancer risk have produced inconsistent results.
Patients and methods
This study followed 74 914 Chinese women aged 40–70 years who participated in the Shanghai Women's Health Study. CV intake was assessed through a validated food-frequency questionnaire (FFQ) at baseline and reassessed during follow-up. Hazard ratios (HRs) and 95% confidence interval (CIs) were estimated by using Cox proportional hazards models. Furthermore, we carried out a meta-analysis of all observational studies until December 2011.
Results
After excluding the first 2 years of follow-up, 417 women developed lung cancer over a mean of 11.1 years of follow-up. An inverse association of borderline statistical significance was observed between CV consumption and female lung cancer risk, with HR for the highest compared with the lowest quartiles of 0.73 (95% CI 0.54–1.00, P trend = 0.1607). The association was strengthened in analyses restricting to never smokers, with the corresponding HR of 0.59 (95% CI 0.40–0.87, P trend = 0.0510). The finding of an inverse association between CV intake and lung cancer risk in women was supported by our meta-analysis of 10 included studies.
Conclusions
Our study suggests that CV consumption may reduce the risk of lung cancer in women, particularly among never smokers.
Keywords: cruciferous vegetable, lung cancer, meta-analysis, prospective study, women
introduction
Lung cancer is the most common cancer worldwide with ∼1.6 million newly diagnosed cases and an estimated 1.4 million deaths in 2008 [1]. Tobacco is clearly the dominant risk factor for lung cancer and contributed to 80% of the worldwide lung cancer burden in males and at least 50% in females [1]. In China, some epidemiological studies demonstrated that a high proportion of lung cancer in females may reflect indoor air pollution from unventilated coal-fueled stoves and cooking fumes [2, 3]. Apart from these known risk factors, it is important to identify other factors associated with risk and prevention, such as certain groups of fruits and vegetables, which may also be used for primary prevention of lung cancer [4].
Cruciferous vegetables (CVs), named for their four equal-sized petals in the shape of a ‘crucifer’ cross, including cabbage, broccoli, cauliflower and other members of the family, contain a variety of anticancer constituents such as glucosinolates, the precursors of isothiocyanates (ITCs) as well as indole-3-carbinol (I3C), both of which may contribute to a reduced risk of lung cancer. Evidence from animal studies has suggested that ITCs hinder lung carcinogenesis mainly through inhibition of tobacco smoke procarcinogens, such as polycyclic aromatic hydrocarbons, by phase I enzymes (e.g. cytochrome P450s) and enhancement of detoxification by phase II enzymes (e.g. glutathione S-transferases, GST) [5, 6]. However, a previous meta-analysis failed to carry out the stratified analysis by gender and did not find any significant association between CV consumption and the risk of cases who never smoked [7].
To further clarify whether CV consumption influences female lung cancer risk, we investigated this association in the Shanghai Women's Health Study (SWHS), which is the first and the largest prospective cohort study in Asia to focus on this topic to date. Moreover, we updated the meta-analysis of Lam et al. [7] by adding the data of females from four observational studies (two prospective and two case–control studies) published after publication in 2007 and the results from this current study.
methods
study population, assessment of dietary and follow-up
The Shanghai Women's Health Study (SWHS) is a population-based, prospective cohort study in urban Shanghai, China. All participants provided a written informed consent. The SWHS was approved by the relevant Institutional Review Boards for human research at all participating institutes. Details of the study design, methods, assessment of dietary, follow-up of cohort, adjustment of confounding factors, and etc. are provided in the supplementary Materials, available at Annals of Oncology online.
statistical analysis
All statistical analyses were carried out using SAS software, version 9.2 (SAS Institute, Inc, Cary, NC). All P values were calculated using two-sided tests and were considered statistically significant if the P value was <0.05.
supplementary material
Methods of cohort study, meta-analysis and any associated references are provided under supplementary Methods, available at Annals of Oncology online.
results
the shanghai women's health study
Overall, 417 incident cases of lung cancer occurred over an average follow-up of 11.1 years of observation of the SWHS after excluding the first 2 years of follow-up. Table 1 shows the characteristics of the study population by the quartiles of CV consumption reported in the FFQs. Females with a higher CV consumption tended to have a higher body mass index, have higher total energy consumption, be more physically active and have greater per capita family income. Women with greater CV consumption were also less likely to smoke.
Table 1.
Baseline characteristics of the study population according to quartile of cruciferous vegetable (CV) consumption, the Shanghai Women's Health Study, 1997–2009
| Characteristic | Quartiles of CV consumption (g/day) |
||||
|---|---|---|---|---|---|
| <58.49 | 58.49–87.27 | 87.27–122.81 | ≥122.81 | P value | |
| No. of participants | 18 271 | 18 269 | 18 271 | 18 270 | |
| Mean age at recruitment (standard deviation, SD), y | 52.3 (9.4) | 51.8 (9.0) | 51.5 (8.8) | 52.4 (9.0) | <0.0001 |
| Mean body mass index (SD), kg/m2 | 23.8 (3.5) | 23.9 (3.4) | 24.0 (3.4) | 24.4 (3.5) | <0.0001 |
| Mean total energy intake (SD), kcal/d | 1519.1 (319.6) | 1605.3 (318.6) | 1674.0 (330.9) | 1776.8 (378.8) | <0.0001 |
| Mean physical activity (SD), MET, h/weeka | 101.4 (44.0) | 105.3 (44.2) | 108.3 (45.0) | 111.4 (46.8) | <0.0001 |
| Education level, n (%) | |||||
| Elementary school or less | 4329 (23.2) | 3685 (20.2) | 3605 (19.7) | 4063 (22.2) | 0.0637 |
| Middle school | 6618 (36.2) | 6859 (37.6) | 7010 (38.4) | 6702 (36.7) | |
| High school | 6581 (36.1) | 6884 (37.6) | 6830 (37.4) | 6624 (36.3) | |
| College or above | 830 (4.5) | 837 (4.6) | 825 (4.5) | 877 (4.8) | |
| Family income, per person per yearb, n (%) | |||||
| Low | 5370 (29.4) | 5000 (27.4) | 4812 (26.3) | 4982 (27.3) | 0.0123 |
| Middle | 6858 (37.6) | 7010 (38.4) | 7242 (39.6) | 7294 (39.9) | |
| High | 6038 (33.0) | 6251 (34.2) | 6215 (34.1) | 5993 (32.7) | |
| Smoking status, n (%) | |||||
| Never smoker | 17 626 (96.5) | 17 804 (97.5) | 17 824 (97.6) | 17 800 (97.4) | <0.0001 |
| Former smoker | 101 (0.5) | 71 (0.4) | 59 (0.3) | 66 (0.4) | |
| Current smoker | 544 (3.0) | 394 (2.1) | 388 (2.1) | 403 (2.2) | |
| Ever drank alcohol, n (%) | 466 (2.6) | 376 (2.1) | 393 (2.2) | 408 (2.2) | 0.0798 |
| Family history of lung cancer, n (%) | 886 (4.9) | 875 (4.8) | 884 (4.8) | 911 (5.0) | 0.5181 |
| Post-menopausal, (%) | 9141 (50.0) | 8724 (47.8) | 8586 (47.0) | 9296 (50.7) | 0.4664 |
aPhysical activity level was measured by metabolic equivalent (MET), h/week/year.
bFamily income level (low income for <5000 yuan/year; medium income for 5000 to <10 000 yuan/year; and high income for ≥10 000 yuan/year).
As shown in Table 2, CV consumption was inversely associated with female lung cancer risk comparing the highest with the lowest quartile of consumption in an age-adjusted model (hazard ratio, HR: 0.74; 95% CI 0.56–0.97, P trend = 0.1036). After adjustment for potential covariates, the inverse association was slightly attenuated and showed borderline significance (HR: 0.73; 95% CI 0.54–1.00, P trend = 0.1607). Additionally, we found a significant protective effect of CV consumption among never smokers (HR: 0.59; 95% CI 0.40–0.87, P trend = 0.0510), which also showed a borderline dose-response trend.
Table 2.
Hazard ratios (HRs) for lung cancer by quartiles of cruciferous vegetables (CVs) in the Shanghai Women's Health Studies, 1997–2009
| Dietary intake (g/day) | All participants |
Never smokers |
||||
|---|---|---|---|---|---|---|
| No. of cases | HR (95% CI)a | HR (95% CI)b | No. of cases | HR (95% CI)a | HR (95% CI)c | |
| CV | ||||||
| <58.59 (58.76) | 123 | 1.00 (ref) | 1.00 (ref) | 106 | 1.00 (ref) | 1.00 (ref) |
| <87.37 (87.54) | 94 | 0.81 (0.62–1.06) | 0.81 (0.62–1.07) | 88 | 0.88 (0.66–1.16) | 0.77 (0.56–1.06) |
| <122.82 (122.92) | 112 | 0.99 (0.77–1.28) | 1.00 (0.76–1.30) | 111 | 1.12 (0.86–1.47) | 0.99 (0.73–1.35) |
| ≥122.82 (122.92) | 88 | 0.74 (0.56–0.97) | 0.73 (0.54–1.00) | 78 | 0.75 (0.56–1.00) | 0.59 (0.40–0.87) |
| P for trend | 0.1036 | 0.1607 | 0.2205 | 0.0510 | ||
| Chinese greensd | ||||||
| <42.81 (42.92) | 113 | 1.00 (ref) | 1.00 (ref) | 99 | 1.00 (ref) | 1.00 (ref) |
| <67.16 (67.24) | 105 | 0.97 (0.74–1.26) | 0.98 (0.75–1.27) | 97 | 1.01 (0.77–1.34) | 0.90 (0.66–1.23) |
| <97.09 (97.13) | 110 | 1.05 (0.80–1.36) | 1.06 (0.81–1.38) | 106 | 1.14 (0.86–1.50) | 0.98 (0.72–1.34) |
| ≥97.09 (97.13) | 89 | 0.79 (0.60–1.04) | 0.80 (0.60–1.08) | 81 | 0.81 (0.61–1.09) | 0.63 (0.44–0.91) |
| P for trend | 0.1653 | 0.2540 | 0.3064 | 0.0376 | ||
| Green cabbaged | ||||||
| <1.13 (1.15) | 127 | 1.00 (ref) | 1.00 (ref) | 112 | 1.00 (ref) | 1.00 (ref) |
| <3.75 (3.78) | 102 | 0.96 (0.74–1.25) | 0.98 (0.75–1.28) | 94 | 1.00 (0.76–1.31) | 0.97 (0.70–1.33) |
| <7.71 (7.75) | 90 | 0.92 (0.70–1.20) | 0.95 (0.72–1.26) | 82 | 0.93 (0.70, 1.24) | 0.97 (0.69–1.34) |
| ≥7.71 (7.75) | 98 | 1.00 (0.77–1.31) | 1.07 (0.81–1.43) | 95 | 1.08 (0.82, 1.43) | 1.21 (0.87–1.70) |
| P for trend | 0.8873 | 0.7348 | 0.7234 | 0.3285 | ||
| Chinese cabbaged | ||||||
| <1.85 (1.86) | 102 | 1.00 (ref) | 1.00 (ref) | 88 | 1.00 (ref) | 1.00 (ref) |
| <4.28 (4.28) | 118 | 1.22 (0.94–1.60) | 1.25 (0.96–1.63) | 112 | 1.34 (1.01–1.77) | 1.40 (1.01–1.93) |
| <8.99 (8.98) | 92 | 0.94 (0.71–1.24) | 0.97 (0.73–1.29) | 84 | 0.99 (0.73–1.33) | 1.06 (0.75–1.50) |
| ≥8.99 (8.98) | 105 | 1.04 (0.79–1.36) | 1.09 (0.82–1.46) | 99 | 1.13 (0.85–1.51) | 1.26 (0.89–1.79) |
| P for trend | 0.7241 | 0.9736 | 0.9108 | 0.5041 | ||
| Cauliflowerd | ||||||
| <0.83 (0.84) | 132 | 1.00 (ref) | 1.00 (ref) | 116 | 1.00 (ref) | 1.00 (ref) |
| <2.44 (2.47) | 109 | 0.97 (0.75–1.25) | 0.98 (0.76–1.27) | 102 | 1.02 (0.78–1.33) | 0.95 (0.70–1.28) |
| <5.15 (5.17) | 94 | 0.90 (0.69–1.17) | 0.92 (0.70–1.21) | 87 | 0.93 (0.70–1.22) | 0.83 (0.61–1.15) |
| ≥5.15 (5.17) | 82 | 0.83 (0.63–1.09) | 0.86 (0.64–1.16) | 78 | 0.88 (0.66–1.17) | 0.74 (0.52–1.06) |
| P for trend | 0.1576 | 0.2925 | 0.3033 | 0.0784 | ||
| White turnip/radishd | ||||||
| <0.39 (0.40) | 119 | 1.00 (ref) | 1.00 (ref) | 107 | 1.00 (ref) | 1.00 (ref) |
| <2.08 (2.10) | 104 | 0.89 (0.68–1.15) | 0.89 (0.68–1.16) | 93 | 0.88 (0.67–1.16) | 0.84 (0.61–1.15) |
| <5.61 (5.64) | 100 | 0.86 (0.66–1.12) | 0.88 (0.67–1.16) | 95 | 0.90 (0.68–1.18) | 0.87 (0.63–1.20) |
| ≥5.61 (5.64) | 94 | 0.78 (0.60–1.03) | 0.80 (0.60–1.07) | 88 | 0.81 (0.61–1.07) | 0.83 (0.59–1.16) |
| P for trend | 0.0802 | 0.1459 | 0.1710 | 0.3298 | ||
aAdjusted for age.
bAdjusted for age, body mass index, family income level, education level, total energy intake, physical activity, non-CV intake, smoking status, pack-years of smoking.
cAdjusted for age, body mass index, family income level, education level, total energy intake, physical activity, non-CV intake, husband's smoking status, pack-years of smoking exposed by husband, exposed to passive smoking in the work place, total years exposed to passive smoking at work.
dFurther adjusted for other CVs in the table.
Regarding the individual CVs, consumption of Chinese greens, cauliflower and white turnip/radish were inversely associated with female lung cancer risk (Table 2), but significant results were only observed for Chinese greens among never smokers, which also suggested a dose-response trend (HR: 0.63; 95% CI 0.44–0.91, P trend = 0.0376).
meta-analysis
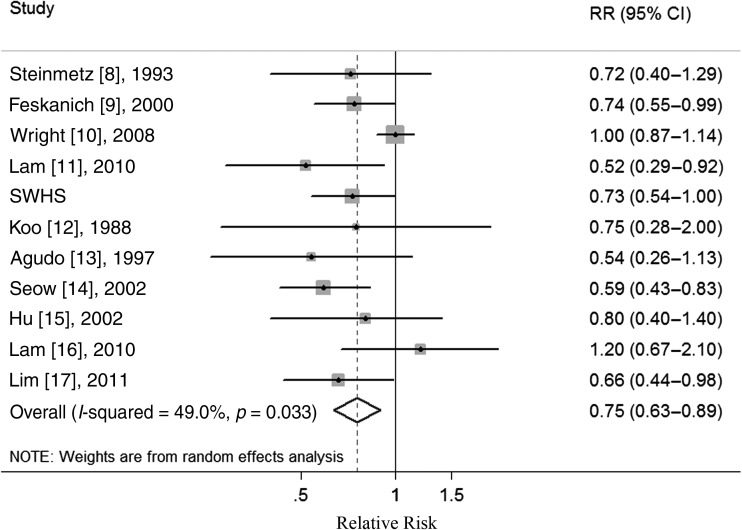
Supplementary Figure S1, available at Annals of Oncology online illustrates the study selection process. An overview of the 10 original publications [8–17] which qualified for our meta-analysis (supplementary Tables S1–S3, available at Annals of Oncology online). In a pooled analysis of these studies, high CV consumption was associated with a significantly reduced risk of female lung cancer (relative risk, RR: 0.75; 95% CI 0.63–0.89) (supplementary Table S4, available at Annals of Oncology online, Figure 1). Moderate heterogeneity was observed (I2 = 49.0%, P = 0.033), but no publication bias was observed either using Begg's test (P = 0.938) or visually inspecting the funnel plot (not shown). In subgroup analyses defined by study design, study quality, geographic location, smoking status and adjustment for confounders, CV consumption was inversely associated with the risk of female lung cancer in all subgroups, with no evidence of significant heterogeneity between subgroups in meta-regression analyses (supplementary Table S4, available at Annals of Oncology online).
Figure 1.
Forest plot (random effects model) of cruciferous vegetables (CVs) consumption and female lung cancer risk in observational studies. Squares indicate study-specific relative risks (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95% CIs; diamond indicates the summary relative risk estimate with its 95% CI. CI: confidence interval; RR: relative risk; SWHS: the Shanghai Women's Health Study.
In a sensitivity analysis, we sequentially removed one study at a time and re-analyzed the data to determine whether any one study was influencing the results. The 11 study-specific RRs ranged from a low value of 0.70 (95% CI 0.61–0.80) after omitting the study by Wright et al. [10] to a high value of 0.79 (95% CI 0.67–0.93) after omitting the study by Seow et al. [14], but all showed a significant inverse association. Additionally, we removed two studies [12, 14] in which RRs and 95% CIs were not reported but calculated from raw data and the results (RR: 0.78; 95% CI 0.66–0.93) were similar.
discussion
In this study of Chinese women in Shanghai, we observed a borderline statistically significant inverse association between CV consumption and the incidence of lung cancer. When our current findings were included in an updated meta-analysis, the inverse association was again observed and statistically significant. In both of our cohort study and the updated meta-analysis, the association between CV consumption and female lung cancer was stronger among never smokers. In our cohort study, the consumption of Chinese greens also showed a significant dose-response trend among never smokers.
When component CVs were considered individually, the consumption of Chinese greens had a significant inverse association with the risk of female lung cancer in never smokers after controlling the potential confounders. However, it is unclear why we found no clear indication for a reduced risk of female lung cancer in subjects with a high consumption of green cabbage and Chinese cabbage. This may be explained in part by the fact that different CVs have different precursors of glucosinolates, which were the major anticarcinogenic properties of CVs [18]. On the other hand, certain cooking methods, including boiling, steaming and microwaving at high power (850 W–900 W), which inactivate myrosinase, catalyze glucosinolates hydrolysis and decrease the bioactivity of anticancer constituents of CV [19, 20], may also play a role in these associations. However, only one study [21] has separated CVs into raw or cooked or by cooking method. Furthermore, there is increasing evidence that genetic variants may influence the effects of CV consumption on cancer risk. London et al. [22] first reported the interaction between ITCs derived from CV consumption and variants of glutathione-S-transferases among lung cancer cases in Shanghai. Since then several epidemiological studies have supported these gene–diet interactions in lung cancer [23–25]. Future studies should focus on the individual CVs, cooking method and susceptibility genes which may play an important role in the metabolism of CVs.
The significant inverse effect of CV consumption in our meta-analysis, which was not observed in the previous meta-analysis by Lam et al. [7], may be attributed to the additional published research in the past 4 years. Studies by Wright et al. [10], Lam et al. [11], Lim et al. [17] and Lam et al. [16], including a total of 2948 female lung cancer cases and 195 396 non-cases, accounted for over 71% of the participants in the 10 published studies. These additional studies provided a sufficient sample size to detect the putative association between CV consumption and lung cancer risk among females. Moreover, the results of our meta-analysis remained significant after carrying out various sensitivity analyses. The new analyses conducted here within the SWHS also supported the hypothesis that consumption of CV is inversely associated with female lung cancer risk, especially in never smokers which showed a borderline dose-response trend. Therefore, our results support this inverse association between CV consumption and the risk of female lung cancer.
The inverse association between CV consumption and risk of female lung cancer is biologically plausible. CVs are unique in that they are a good source of glucosinolates, which can be hydrolyzed by the plant enzyme myrosinase into biologically active compounds, including ITC and I3C. Compared with the chemopreventive characteristics of I3C and other phytochemicals and nutrients in CVs, most published research has attributed the multifaceted anticancer properties to ITCs such as the induction of carcinogen-detoxification phase II enzymes, arrest of cell cycle progression and induction of apoptosis [18, 26] Additionally, modulation of metabolism of smoking-related carcinogens by ITCs has been documented in both in vivo and in vitro studies [6, 27], as well as in humans [28]. ITCs have also been shown to inhibit lung tumorigenesis induced by tobacco-specific carcinogens in animal models [29, 30]. Several studies have also demonstrated that I3C inhibits the transcription of estrogen-responsive genes stimulated by 17β-estradiol [31, 32], which might play a role in the prevention of female lung cancer.
This study has some important strengths. To our knowledge, ours is the first study using a large, prospective cohort study in Asia to consider the association between CV consumption and the risk of female lung cancer. Since this study was based on a prospective design, our findings are unlikely to be explained by recall bias and selection bias. We also carried out sensitivity analyses in our cohort and meta-analysis, and the findings were generally robust. Moreover, our cohort is the only prospective study considering the chronic disease or other cancers studied among the follow-up surveys which may change the dietary habits of the population of SWHS. Since tobacco exposure is such a strong risk factor for lung cancer, we additionally characterized environmental tobacco smoke exposure over the lifetime in the analysis of never smokers of the SWHS. By utilizing information on the passive smoking status at the work place, husband's smoking status and pack-years of smoking, we were better able to potentially adjust for this confounding factor. Furthermore, our cohort included a substantial number of females who were never smokers, accounting for over 97.8% of all participants, which provided sufficient power to detect an association without possible residual confounding by cigarette smoking, as it is well known that cigarette smoking is highly associated with dietary patterns, including fruit and vegetable intakes [33, 34].
Our study also has several limitations. First, our study did not include many current or former smokers. Although the number of people who use tobacco and the number of cigarettes consumed per person have increased substantially in China, the prevalence of smoking among females is not as high as in developed countries [35]. So although Lam et al. [11] and Wright et al. [10] provided evidence for a protective role of CV consumption among current and former smokers, we were unable to test this association. A second limitation was that our study lacked statistical power to stratify by histological subtypes of lung cancer. However, only a case–control study from Japan [36] suggested a significant inverse association between cabbage consumption and small-cell histological type. A third limitation is that both the SWHS and the meta-analysis relied on FFQ data for dietary intake, which may be subject to measurement errors which may attenuate estimates for the dietary-disease associations. Finally, due to different methods and categorizations used to report CV consumption within the studies included in the meta-analysis, we were unable to carry out a dose-response analysis between CV consumption and female lung cancer risk in our meta-analysis.
In summary, in this large, prospective cohort study and updated meta-analysis, we found convincing evidence for an inverse association between CV consumption and the risk of female lung cancer with stronger associations among never smokers.
funding
This work was supported by the funds of State Key Project Specialized for Infectious Diseases of China (No. 2008ZX10002-015 and 2012ZX10002008-002) and grants from the United States National Institutes of Health (R37 CA070867, R01 CA82729 and R01 HL095931). EV was also supported by the Fogarty International Clinical Research Scholars and Fellows Support Center at the Vanderbilt Institute for Global Health, funded by the Fogarty International Center, NIH, through an R24 Training Grant (Grant number: R24TW007988-5), and the Cancer Prevention and Control Training Program at the University of Alabama at Birmingham funded through the National Institutes of Health (5R25 CA047888).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
Q-JW, Y-BX designed research; Q-JW, LX and Y-BX conducted research; Q-JW, LX analyzed data; Q-JW wrote the first draft; all authors read, reviewed and approved the final manuscript. Y-BX had primary responsibility for the final content. We would like to thank the participants and the staffs from the Shanghai Women's Health Studies for their contribution to this research.
references
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. doi:10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Lam WK, White NW, Chan-Yeung MM. Lung cancer epidemiology and risk factors in Asia and Africa. Int J Tuberc Lung Dis. 2004;8:1045–1057. [PubMed] [Google Scholar]
- 3.Thun MJ, Hannan LM, Adams-Campbell LL, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5:e185. doi: 10.1371/journal.pmed.0050185. doi:10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vainio H, Weiderpass E. Fruit and vegetables in cancer prevention. Nutr Cancer. 2006;54:111–142. doi: 10.1207/s15327914nc5401_13. doi:10.1207/s15327914nc5401_13. [DOI] [PubMed] [Google Scholar]
- 5.Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3:233–255. doi: 10.2174/1389200023337496. doi:10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- 6.Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32:395–411. doi: 10.1081/dmr-100102342. doi:10.1081/DMR-100102342. [DOI] [PubMed] [Google Scholar]
- 7.Lam TK, Gallicchio L, Lindsley K, et al. Cruciferous vegetable consumption and lung cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev. 2009;18:184–195. doi: 10.1158/1055-9965.EPI-08-0710. doi:10.1158/1055-9965.EPI-08-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinmetz KA, Potter JD, Folsom AR. Vegetables, fruit, and lung cancer in the Iowa Women's Health Study. Cancer Res. 1993;53:536–543. [PubMed] [Google Scholar]
- 9.Feskanich D, Ziegler RG, Michaud DS, et al. Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J Natl Cancer Inst. 2000;92:1812–1823. doi: 10.1093/jnci/92.22.1812. doi:10.1093/jnci/92.22.1812. [DOI] [PubMed] [Google Scholar]
- 10.Wright ME, Park Y, Subar AF, et al. Intakes of fruit, vegetables, and specific botanical groups in relation to lung cancer risk in the NIH-AARP Diet and Health Study. Am J Epidemiol. 2008;168:1024–1034. doi: 10.1093/aje/kwn212. doi:10.1093/aje/kwn212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam TK, Ruczinski I, Helzlsouer KJ, et al. Cruciferous vegetable intake and lung cancer risk: a nested case-control study matched on cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2010;19:2534–2540. doi: 10.1158/1055-9965.EPI-10-0475. doi:10.1158/1055-9965.EPI-10-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koo LC. Dietary habits and lung cancer risk among Chinese females in Hong Kong who never smoked. Nutr Cancer. 1988;11:155–172. doi: 10.1080/01635588809513983. doi:10.1080/01635588809513983. [DOI] [PubMed] [Google Scholar]
- 13.Agudo A, Esteve MG, Pallares C, et al. Vegetable and fruit intake and the risk of lung cancer in women in Barcelona, Spain. Eur J Cancer. 1997;33:1256–1261. doi: 10.1016/s0959-8049(97)00050-6. doi:10.1016/S0959-8049(97)00050-6. [DOI] [PubMed] [Google Scholar]
- 14.Seow A, Poh WT, Teh M, et al. Diet, reproductive factors and lung cancer risk among Chinese women in Singapore: evidence for a protective effect of soy in nonsmokers. Int J Cancer. 2002;97:365–371. doi: 10.1002/ijc.1615. doi:10.1002/ijc.1615. [DOI] [PubMed] [Google Scholar]
- 15.Hu J, Mao Y, Dryer D, et al. Risk factors for lung cancer among Canadian women who have never smoked. Cancer Detect Prev. 2002;26:129–138. doi: 10.1016/s0361-090x(02)00038-7. doi:10.1016/S0361-090X(02)00038-7. [DOI] [PubMed] [Google Scholar]
- 16.Lam TK, Rotunno M, Lubin JH, et al. Dietary quercetin, quercetin-gene interaction, metabolic gene expression in lung tissue and lung cancer risk. Carcinogenesis. 2010;31:634–642. doi: 10.1093/carcin/bgp334. doi:10.1093/carcin/bgp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim WY, Chuah KL, Eng P, et al. Meat consumption and risk of lung cancer among never-smoking women. Nutr Cancer. 2011;63:850–859. doi: 10.1080/01635581.2011.589961. doi:10.1080/01635581.2011.589961. [DOI] [PubMed] [Google Scholar]
- 18.Higdon JV, Delage B, Williams DE, et al. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. doi:10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verkerk R, van der Gaag MS, Dekker M, et al. Effects of processing conditions on glucosinolates in cruciferous vegetables. Cancer Lett. 1997;114:193–194. doi: 10.1016/s0304-3835(97)04661-2. doi:10.1016/S0304-3835(97)04661-2. [DOI] [PubMed] [Google Scholar]
- 20.McNaughton SA, Marks GC. Development of a food composition database for the estimation of dietary intakes of glucosinolates, the biologically active constituents of cruciferous vegetables. Br J Nutr. 2003;90:687–697. doi: 10.1079/bjn2003917. doi:10.1079/BJN2003917. [DOI] [PubMed] [Google Scholar]
- 21.Tang L, Zirpoli GR, Jayaprakash V, et al. Cruciferous vegetable intake is inversely associated with lung cancer risk among smokers: a case–control study. BMC Cancer. 2010;10:162. doi: 10.1186/1471-2407-10-162. doi:10.1186/1471-2407-10-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.London SJ, Yuan JM, Chung FL, et al. Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms, and lung-cancer risk: a prospective study of men in Shanghai, China. Lancet. 2000;356:724–729. doi: 10.1016/S0140-6736(00)02631-3. doi:10.1016/S0140-6736(00)02631-3. [DOI] [PubMed] [Google Scholar]
- 23.Brennan P, Hsu CC, Moullan N, et al. Effect of cruciferous vegetables on lung cancer in patients stratified by genetic status: a mendelian randomisation approach. Lancet. 2005;366:1558–1560. doi: 10.1016/S0140-6736(05)67628-3. doi:10.1016/S0140-6736(05)67628-3. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Spitz MR, Schabath MB, et al. Association between glutathione S-transferase p1 polymorphisms and lung cancer risk in Caucasians: a case-control study. Lung Cancer. 2003;40:25–32. doi: 10.1016/s0169-5002(02)00537-8. doi:10.1016/S0169-5002(02)00537-8. [DOI] [PubMed] [Google Scholar]
- 25.Spitz MR, Duphorne CM, Detry MA, et al. Dietary intake of isothiocyanates: evidence of a joint effect with glutathione S-transferase polymorphisms in lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9:1017–1020. [PubMed] [Google Scholar]
- 26.Ahmad A, Sakr WA, Rahman KM. Anticancer properties of indole compounds: mechanism of apoptosis induction and role in chemotherapy. Curr Drug Targets. 2010;11:652–666. doi: 10.2174/138945010791170923. doi:10.2174/138945010791170923. [DOI] [PubMed] [Google Scholar]
- 27.Hecht SS. Chemoprevention of cancer by isothiocyanates, modifiers of carcinogen metabolism. J Nutr. 1999;129:768S–774S. doi: 10.1093/jn/129.3.768S. [DOI] [PubMed] [Google Scholar]
- 28.Hecht SS, Carmella SG, Murphy SE. Effects of watercress consumption on urinary metabolites of nicotine in smokers. Cancer Epidemiol Biomarkers Prev. 1999;8:907–913. [PubMed] [Google Scholar]
- 29.Morse MA, Eklind KI, Amin SG, et al. Effects of alkyl chain length on the inhibition of NNK-induced lung neoplasia in A/J mice by arylalkyl isothiocyanates. Carcinogenesis. 1989;10:1757–1759. doi: 10.1093/carcin/10.9.1757. doi:10.1093/carcin/10.9.1757. [DOI] [PubMed] [Google Scholar]
- 30.Wattenberg LW. Inhibitory effects of benzyl isothiocyanate administered shortly before diethylnitrosamine or benzo[a]pyrene on pulmonary and forestomach neoplasia in A/J mice. Carcinogenesis. 1987;8:1971–1973. doi: 10.1093/carcin/8.12.1971. doi:10.1093/carcin/8.12.1971. [DOI] [PubMed] [Google Scholar]
- 31.Ashok BT, Chen Y, Liu X, et al. Abrogation of estrogen-mediated cellular and biochemical effects by indole-3-carbinol. Nutr Cancer. 2001;41:180–187. doi: 10.1080/01635581.2001.9680630. [DOI] [PubMed] [Google Scholar]
- 32.Meng Q, Goldberg ID, Rosen EM, et al. Inhibitory effects of indole-3-carbinol on invasion and migration in human breast cancer cells. Breast Cancer Res Treat. 2000;63:147–152. doi: 10.1023/a:1006495824158. doi:10.1023/A:1006495824158. [DOI] [PubMed] [Google Scholar]
- 33.Dallongeville J, Marecaux N, Fruchart JC, et al. Cigarette smoking is associated with unhealthy patterns of nutrient intake: a meta-analysis. J Nutr. 1998;128:1450–1457. doi: 10.1093/jn/128.9.1450. [DOI] [PubMed] [Google Scholar]
- 34.Stram DO, Huberman M, Wu AH. Is residual confounding a reasonable explanation for the apparent protective effects of beta-carotene found in epidemiologic studies of lung cancer in smokers? Am J Epidemiol. 2002;155:622–628. doi: 10.1093/aje/155.7.622. doi:10.1093/aje/155.7.622. [DOI] [PubMed] [Google Scholar]
- 35.Spitz M, Wu X, Wilkinson A, et al. Cancer of the Lung. In: Schottenfeld D, Fraumeni JJ, editors. Cancer epidemiology and prevention. 3rd edition. New York: Oxford University Press; 2006. pp. 638–658. [Google Scholar]
- 36.Gao CM, Tajima K, Kuroishi T, et al. Protective effects of raw vegetables and fruit against lung cancer among smokers and ex-smokers: a case–control study in the Tokai area of Japan. Jpn J Cancer Res. 1993;84:594–600. doi: 10.1111/j.1349-7006.1993.tb02018.x. doi:10.1111/j.1349-7006.1993.tb02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.