Abstract
Background
A multicenter NCI-sponsored phase II study was conducted to analyze the safety and efficacy of the combination of ixabepilone with trastuzumab in patients with metastatic HER2-positive breast cancer.
Patients and methods
Two cohorts were enrolled: cohort 1 had received no prior chemotherapy or trastuzumab for metastatic disease and cohort 2 had received 1–2 prior trastuzumab-containing regimens for metastatic disease. Patients in both cohorts received ixabepilone 40 mg/m2 as a 3-h infusion and trastuzumab on day 1 of a 21-day cycle. Tumor biomarkers that may predict response to trastuzumab were explored.
Results
Thirty-nine women entered the study with 15 patients in cohort 1 and 24 patients in cohort 2. Across both cohorts, the overall RR was 44%, with a clinical benefit rate (CR + PR + SD for at least 24 weeks) of 56%. Treatment-related toxic effects included neuropathy (grade ≥2, 56%), leukopenia (grade ≥2, 26%), myalgias (grade ≥2, 21%), neutropenia (grade ≥2, 23%), and anemia (grade ≥2, 18%).
Conclusions
This represents the first study of the combination of ixabepilone with trastuzumab for the treatment of metastatic HER2-positive breast cancer. These results suggest that the combination has encouraging activity as first and subsequent line therapy for metastatic breast cancer.
Keywords: breast, cancer, HER2, ixabepilone, trastuzumab
introduction
Trastuzumab, a humanized monoclonal antibody directed against the extracellular domain of HER2, is a critical component of treatment of HER2-positive breast cancer. Its use in combination with chemotherapy, particularly with taxanes, is well established and results in an improvement in progression-free survival (PFS) and overall survival [1, 2]. However, many patients have tumors that fail to respond to these agents, and nearly all patients with metastatic breast cancer will eventually progress [3]. Novel therapies for metastatic breast cancer are therefore needed.
The epothilones are a new class of antineoplastic agents that act by disrupting microtubule function [4, 5]. These agents bind to β-tubulin subunits and inhibit microtubule depolymerization, leading to mitotic arrest and apoptosis [6, 7]. Ixabepilone (BMS-247550, aza-epothilone B), a semi-synthetic analog of epothilone B, has demonstrated efficacy as monotherapy or in combination with capecitabine in anthracycline- and taxane-pretreated metastatic breast cancers and has recently been approved for use in refractory breast cancer [8].
Several clinical trials have explored the efficacy of ixabepilone. Ixabepilone as a single agent was investigated in a phase II study in 126 patients with metastatic breast cancer resistant to an anthracycline, taxane, and capecitabine [9]. Patients received ixabepilone 40 mg/m2 every 3 weeks and the objective response rate (ORR) was 11.5%, with an additional 50% of patients with stable disease. In a phase III study, 1221 women with anthracycline-pretreated or resistant and taxane-resistant locally advanced or metastatic breast cancer were randomized to ixabepilone (40 mg/m2 every 3 weeks) in combination with capecitabine (2000 mg/m2 daily for 2 weeks, followed by 1 week off) or to capecitabine (2500 mg/m2 given in the same schedule) alone [10]. In this study, ixabepilone–capecitabine was found to prolong PFS compared with capecitabine alone (5.8 versus 4.2 months, P = 0.003). These studies led to the FDA approval of ixabepilone, in combination with capecitabine, for the treatment of patients with metastatic or locally advanced breast cancer after failure of an anthracycline and a taxane and as monotherapy for the treatment of patients with metastatic or locally advanced breast cancer after failure of an anthracycline, taxane, and capecitabine.
Because trastuzumab has demonstrated synergistic activity in combination with several microtubule-stabilizing agents, we designed this trial to explore the safety and efficacy of ixabepilone in combination with trastuzumab. A preliminary analysis to identify tumor biomarkers that may predict sensitivity to trastuzumab was also performed.
patients and methods
patients
Patients ≥18 years of age with measurable metastatic HER2-positive breast cancer were eligible. HER2 positivity was defined as 3+ positive for HER2 overexpression by immunohistochemistry (IHC) or amplified by fluorescence in situ hybridization (FISH) (FISH/CEP17 ≥2.0) by local review. Two cohorts of patients were eligible. Patients in cohort 1 could not have received prior chemotherapy or prior trastuzumab therapy for metastatic breast cancer, but may have received prior chemotherapy and/or trastuzumab therapy in the adjuvant setting, provided that trastuzumab therapy ended at least 12 months before study participation and chemotherapy ended 6 months before study participation. Patients in cohort 2 may have received up to two prior chemotherapy regimens for metastatic breast cancer. Patients in cohort 2 must have received one prior trastuzumab-containing regimen either in the metastatic setting or in the adjuvant setting. Patients with a history of brain metastases were eligible, provided that they had completed the treatment of their brain metastases at least 1 week before enrollment ECOG performance status of ≤2 and life expectancy of ≥6 months were required.
Key exclusion criteria included leptomeningeal carcinomatosis, prior epothilone therapy, motor or sensory neuropathy ≥grade 2 based on National Cancer Institute Common Terminology Criteria for Adverse Events version 3 (CTCAE), uncontrolled intercurrent illness, liver dysfunction [alanine transaminase >5× upper limit of normal (ULN) or total bilirubin >1.5× ULN), or cardiac dysfunction [left ventricular ejection fraction (LVEF) <50%]. The protocol was approved by the institutional review boards of participating institutions, and all patients provided written informed consent.
HERmark® and p95 assays
The HERmark Breast Cancer Assay (Monogram Biosciences, South San Francisco, CA) is an application of the VeraTag® technology platform designed specifically for breast cancer and currently includes two quantitative measurements: total HER2 expression (H2T) and HER2 homodimers (H2D). VeraTag is a proximity-based method designed to accurately and reproducibly quantify protein expression and protein–protein complexes, including cell-surface dimers in formalin-fixed, paraffin-embedded tissue samples. The detailed method of the VeraTag platform technology was published previously [11]. The technical performance of the HERmark Breast Cancer Assay has been validated according to the requirements specified by the Clinical Laboratory Improvement Amendments (CLIA) and was carried out in a laboratory accredited by the College of American Pathologists (CAP) at Monogram Biosciences. Quantitative measurements of p95 (truncated HER2 receptor) protein expression, also assessed using the VeraTag platform and a proprietary p95-specific antibody, were correlated with outcomes for those patients whose tumors expressed HER2 as determined by HERmark above a prespecified cutoff [12].
study design
This was a nonrandomized multicenter (Memorial Sloan Kettering Cancer Center, Dana-Farber/Partners Cancer Center) phase II study that recruited two cohorts of patients with metastatic breast cancer. The trial was designed to evaluate the activity of ixabepilone–trastuzumab in each of the cohorts. The primary objective was to evaluate the ORR, defined as complete response (CR) and partial response (PR) by Response Evaluation Criteria in Solid Tumors (RECIST). Secondary objectives were to assess the clinical benefit rate (CBR), defined as CR + PR + SD > 24 weeks, time to progression (TTP), time to treatment failure (TTF), safety, and toxicity and to analyze various tissue biomarkers and to correlate them with response to treatment.
treatment
Patients in each cohort received the same treatment regimen of ixabepilone with trastuzumab. Patients received ixabepilone 40 mg/m2 as a 3-h continuous infusion on day 1 of a 21-day cycle plus trastuzumab every 21 days. For the initial treatment of trastuzumab, patients received 8 mg/kg IV and 6 mg/kg for all subsequent trastuzumab treatments. Treatment was continued until disease progression or unacceptable toxicity.
Doses were reduced or discontinued based on tolerability. Events necessitating ixabepilone dose reduction (from 40 to 32 to 25 mg/m2) included grade 4 neutropenia lasting more than 7 days, febrile neutropenia, grade 4 thrombocytopenia or grade ≥3 thrombocytopenia with significant bleeding requiring transfusion, grade 3 diarrhea, and grade 2 neuropathy (motor or sensory) lasting ≥7 days or grade 3 neuropathy lasting ≥7 days. In the event that a patient's ixabepilone was held, the patient received trastuzumab therapy at a dose of 2 mg/kg each week that ixabepilone was held. There were no dose or schedule adjustments for trastuzumab based on specific trastuzumab-related toxicity criteria, except cardiac toxicity. Patients underwent cardiac evaluation with an echocardiogram or multi gated acquisition scan at baseline, before study entry, and once again after six cycles (18 weeks) of treatment.
assessments
evaluation of tumor response and toxicity assessment
Baseline tumor assessments were carried out within 2 weeks of the start of treatment by computed tomography or magnetic resonance imaging scans of the chest, abdomen, and pelvis. Patients were assessed for tumor response every 9 weeks for the first 27 weeks and then every 12 weeks thereafter. Radiologic assessments were evaluated by independent radiology review (IRR) using RECIST. The selection of target lesions by IRR and tumor assessments was done independently of investigator evaluations. The ORR was defined as the proportion of CR + PR among patients who started therapy, and the CBR was defined as the proportion of CR + PR + SD ≥ 24 weeks. TTP was measured from the time of study entry to time of tumor progression (progressive disease), and TTF was measured from time of study entry to time of patient withdrawal from the study for either disease progression or removal for pre-determined toxicity criteria, whichever occurred first. Adverse events were evaluated at all visits. Toxic effects were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
statistical methods
There were separate statistical designs for each cohort using a one-stage design. The protocol called for enrolling a total of 60 patients (30 patients per cohort). For cohort 1, the regimen was considered worthy of further study if there were at least 13 confirmed responses out of 30 patients. The probability of declaring the regimen worthy of further study was 0.08 if the regimen had a response rate of 30% and was 0.91 if the regimen had a true response rate of 54%. For cohort 2, the regimen was considered worthy of further study if there were at least 6 confirmed responses out of 30 patients. The probability of declaring the regimen worthy of further study was 0.07 if the regimen had a true response rate of 10% and 0.91 if the regimen had a true response rate of 29%. The response rate was defined as the percentage of patients achieving a confirmed CR or PR. Times were censored at date of last tumor assessment. Cohorts were evaluated separately. 95% CIs for the response rate were calculated using the exact methods. TTF and TTP were evaluated using the Kaplan–Meier methods.
results
patient population
Thirty-nine patients were recruited from February 2004 to May 2008 from Dana-Farber/Partners Cancer Center and Memorial Sloan Kettering Cancer Center; 15 patients were in cohort 1 and 24 patients were in cohort 2. The study was closed early due to slow patient accrual. The two cohorts were similar in terms of baseline demographics, including stage at diagnosis, number of metastatic sites, and prior adjuvant chemotherapy (Table 1). Local HER2 FISH tests were available for 13 patients (33%); the remaining patients were enrolled on the basis of IHC results. Patients were treated for a median of 7 cycles (range, 1–29 cycles). Patients in cohort 1 received the treatment of a median of 8 cycles (range, 1–29 cycles) and patients in cohort 2 received the treatment of a median of 6 cycles (range, 1–23 cycles). Toxicity was the most common reason for treatment discontinuation in cohort 1 (n = 8; 53%), whereas disease progression was the predominant reason for treatment discontinuation in cohort 2 (n = 12, 50%).
Table 1.
Patient characteristics by cohort
| Characteristic | Cohort 1 (n = 15) | Cohort 2 (n = 24) |
|---|---|---|
| Age (years) | ||
| Median | 48 | 51 |
| Range | 32–67 | 29–74 |
| ECOG PS [n (%)] | ||
| 0 | 11 (73) | 20 (83) |
| 1 | 4 (27) | 4 (17) |
| Stage at diagnosis [n (%)] | ||
| I | 3 (20) | 6 (25) |
| II | 7 (47) | 9 (38) |
| III | 2 (13) | 3 (12) |
| IV | 3 (20) | 6 (25) |
| Number of metastatic sites [n (%)] | ||
| ≥3 | 6 (40) | 12 (50) |
| 2 | 5 (33) | 8 (33) |
| 1 | 4 (27) | 4 (17) |
| ER/PR status [n (%)] | ||
| ER+ or PR+ | 5 (33) | 12 (50) |
| Prior adjuvant chemotherapy | 9 (60) | 17 (71) |
| Taxane-based | 4 (27) | 7 (29) |
| Prior hormonal therapy | 5 (33) | 9 (38) |
| Prior number of chemotherapy regimens metastatic breast cancer [n (%)] | ||
| 0 | 15 (100) | 0 (0) |
| 1 | 0 (0) | 9 (38) |
| 2 | 0 (0) | 15 (63) |
PS, performance status; ER, estrogen receptor; PR, progesterone receptor.
clinical activity
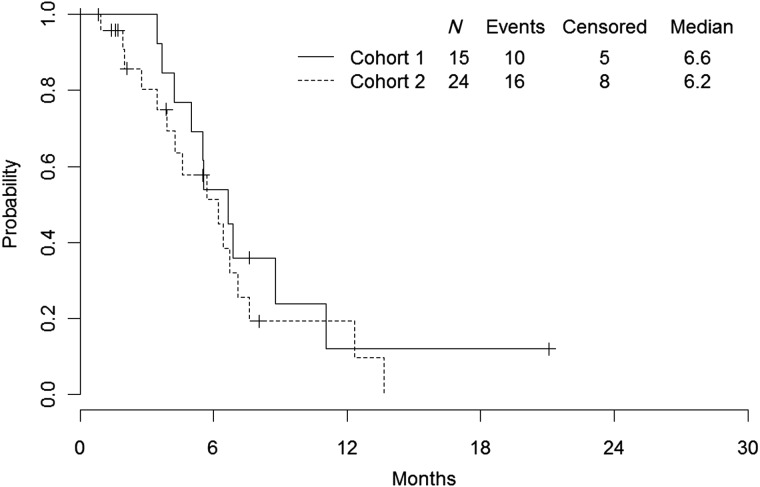
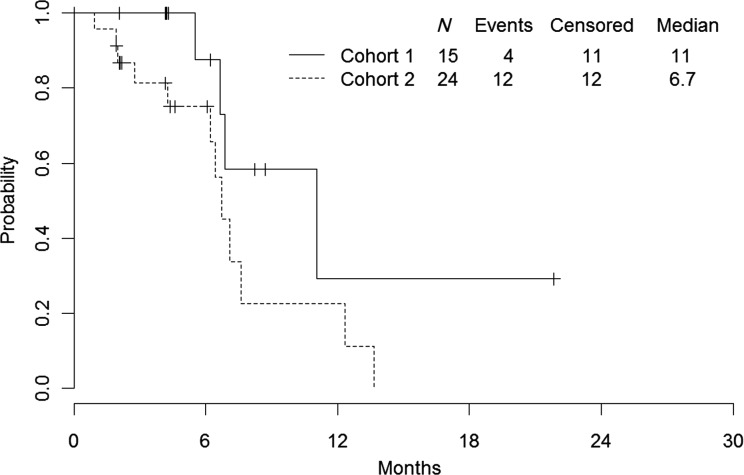
The overall response rate (CR or PR) was 44% in the intent-to-treat population, and the response rate was numerically higher in cohort 1 (73%, 95% CI: 45%–92%) compared with cohort 2 (25%, 95% CI: 10%–47%; Table 2). Approximately half of all patients derived clinical benefit (56% with CR or PR or SD ≥24 weeks), with a CBR of 80% in cohort 1 and 42% in cohort 2. The median TTF was 6.6 months (95% CI: 4.2–11.0) for cohort 1 and 6.2 months (95% CI: 3.4–7.1) for cohort 2 (Figure 1). The median TTP for cohort 1 was 11.0 months. There were insufficient data to determine the CI in cohort 1. The median TTP for cohort 2 was 6.7 months (95% CI: 4.3–12.3; Figure 2).
Table 2.
Patient response rate
| Patient response | Cohort 1 (n = 15) |
Cohort 2 (n = 24) |
All Patients (n = 39) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Best response | ||||||
| CR | 0 | 0 | 0 | 0 | 0 | 0 |
| PR (confirmed) | 11 | 73 | 6 | 25 | 17 | 44 |
| PR (unconfirmed) | 1 | 7 | 2 | 8 | 3 | 8 |
| SD | 1 | 7 | 12 | 50 | 13 | 33 |
| PD | 0 | 0 | 3 | 13 | 3 | 8 |
| Unknown | 2 | 13 | 1 | 4 | 3 | 8 |
| Response rate, % (95% CI) | 73 (45–92) | 25 (10–47) | 44 | |||
| CBR [% (95% CI)] | 80 (52–96) | 42 (22–63) | 56 | |||
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease. Response rate: CR or confirmed PR. CBR: CR or PR (confirmed or unconfirmed) or SD ≥ 24 weeks.
Figure 1.
Time to treatment failure.
Figure 2.
Time to progression.
safety and tolerability
In this study, the most common adverse event of any grade was sensory neuropathy (82% of patients experienced grade ≥1 neuropathy). Neuropathy was mainly grade 1 or 2, with no grade 4 events recorded (Table 3). Other significant grade 2 or higher toxic effects included fatigue (38%), leukopenia (26%), joint pain (26%), neutropenia (21%), and myalgias (21%; Table 3). There were no reported events of the cardiac toxicity of any grade.
Table 3.
Patients with treatment-related, grade 2 or higher, adverse events that occurred in ≥10% of patients
| Adverse event | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Total |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Percent | n | Percent | n | Percent | n | Percent | n | Percent | |
| Fatigue | 17 | 44 | 13 | 33 | 2 | 5 | 0 | 0 | 32 | 82 |
| Neuropathy, sensorya | 10 | 26 | 15 | 38 | 7 | 18 | 0 | 0 | 32 | 82 |
| Anemia | 22 | 56 | 7 | 18 | 0 | 0 | 0 | 0 | 29 | 74 |
| Leukopenia | 16 | 41 | 6 | 15 | 3 | 8 | 1 | 3 | 26 | 67 |
| Alopecia | 10 | 26 | 14 | 36 | 0 | 0 | 0 | 0 | 24 | 62 |
| Hyperglycemia | 22 | 56 | 1 | 3 | 0 | 0 | 0 | 0 | 23 | 59 |
| Nausea | 16 | 41 | 4 | 10 | 1 | 3 | 0 | 0 | 21 | 54 |
| Myalgias | 12 | 31 | 7 | 18 | 1 | 3 | 0 | 0 | 20 | 51 |
| AST | 13 | 33 | 2 | 5 | 2 | 5 | 0 | 0 | 17 | 44 |
| Diarrhea | 15 | 38 | 2 | 5 | 0 | 0 | 0 | 0 | 17 | 44 |
| Anorexia | 10 | 26 | 6 | 15 | 0 | 0 | 0 | 0 | 16 | 41 |
| Arthralgia | 6 | 15 | 9 | 23 | 1 | 3 | 0 | 0 | 16 | 41 |
| Neutropenia | 6 | 15 | 2 | 5 | 6 | 15 | 1 | 3 | 15 | 38 |
| Alkaline phosphatase | 8 | 21 | 2 | 5 | 3 | 8 | 0 | 0 | 13 | 33 |
| Extremity Pain | 9 | 23 | 4 | 10 | 0 | 0 | 0 | 0 | 13 | 33 |
| ALT | 9 | 23 | 3 | 8 | 0 | 0 | 0 | 0 | 12 | 31 |
| Back pain | 7 | 18 | 2 | 5 | 0 | 0 | 1 | 3 | 10 | 26 |
| Constipation | 9 | 23 | 1 | 3 | 0 | 0 | 0 | 0 | 10 | 26 |
| Hyponatremia | 8 | 21 | 0 | 0 | 2 | 5 | 0 | 0 | 10 | 26 |
| Fever without Neutropenia | 9 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 23 |
| Headache | 7 | 18 | 1 | 3 | 1 | 3 | 1 | 3 | 10 | 26 |
| Stomatitis | 7 | 18 | 1 | 3 | 1 | 3 | 0 | 0 | 9 | 23 |
| Rash | 5 | 13 | 3 | 8 | 1 | 3 | 0 | 0 | 9 | 23 |
| Vomiting | 5 | 13 | 4 | 10 | 0 | 0 | 0 | 0 | 9 | 23 |
| Cough | 6 | 15 | 2 | 5 | 0 | 0 | 0 | 0 | 8 | 21 |
| Dyspnea | 4 | 10 | 3 | 8 | 0 | 0 | 1 | 3 | 8 | 21 |
| Lymphopenia | 8 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 21 |
| Thrombocytopenia | 8 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 21 |
| Allergic reaction | 4 | 10 | 3 | 8 | 0 | 0 | 0 | 0 | 7 | 18 |
| Hematologic-other | 5 | 13 | 0 | 0 | 0 | 0 | 1 | 3 | 6 | 15 |
| Insomnia | 5 | 13 | 1 | 3 | 0 | 0 | 0 | 0 | 6 | 15 |
| Abdominal pain | 3 | 8 | 2 | 5 | 2 | 5 | 0 | 0 | 7 | 18 |
| Bone pain | 3 | 8 | 1 | 3 | 1 | 3 | 0 | 0 | 5 | 13 |
| Dizziness | 4 | 10 | 1 | 3 | 0 | 0 | 0 | 0 | 5 | 13 |
| Hypoalbuminemia | 5 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 13 |
| Hypoalbuminemia | 5 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 13 |
| Hypocalcemia | 4 | 10 | 1 | 3 | 0 | 0 | 0 | 0 | 5 | 13 |
| Nail changes | 2 | 5 | 3 | 8 | 0 | 0 | 0 | 0 | 5 | 13 |
| Dyspepsia | 3 | 8 | 1 | 3 | 0 | 0 | 0 | 0 | 4 | 10 |
| Limb edema | 4 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 10 |
| Influenza-like symptoms | 2 | 5 | 2 | 5 | 0 | 0 | 0 | 0 | 4 | 10 |
| Hot flashes | 3 | 8 | 1 | 3 | 0 | 0 | 0 | 0 | 4 | 10 |
| Hypernatremia | 4 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 10 |
| Hypokalemia | 3 | 8 | 0 | 0 | 1 | 3 | 0 | 0 | 4 | 10 |
| Rigors/chills | 4 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 10 |
| Weight loss | 3 | 8 | 1 | 3 | 0 | 0 | 0 | 0 | 4 | 10 |
aSensory neuropathy was assessed and graded according to symptoms as reported by the patient; neurosensory studies were not carried out.
predictive biomarkers
In an effort to identify molecular biomarkers that were predictive of outcome in patients treated with ixabepilone and trastuzumab, we assessed H2D, H2T, and p95 protein levels using sensitive and quantitative proximity-based assays. Archival tumor tissue was available from 20 patients (7 from cohort 1 and 13 from cohort 2) and all 20 samples were evaluated with assays for H2T, H2D, and p95. H2T, H2D, and p95 data were obtained for all seven cases from cohort 1. Only six p95 cases from cohort 1 were used for correlation with outcomes, with one case excluded because of subthreshold total HER2 levels. Useful H2T and H2D data were obtained for all 13 cases from cohort 2. Only 11 cases had sufficient tumor to generate a p95 result; however, only 9 of these were used for correlation with outcomes, with missing 2 cases excluded for low HER2 levels.
Similar to what has been observed in previous studies of patients treated with trastuzumab-based regimens [13, 14], higher levels of H2D were associated with best overall response, although this was of borderline significance (P = 0.061 using the Jonckheere–Terpstra test). Patients whose cancers had higher levels of H2D and H2T had longer TTP [hazard ratio (HR): 0.54 and 0.49, respectively] but this relationship was not statistically significant. The fact that these markers did not definitively separate responding patients from non-responders suggests the presence of other factors that influence sensitivity to ixabepilone and trastuzumab. One candidate is p95, a truncated form of HER2 that has constitutive catalytic activity and lacks the trastuzumab-binding domain [15]. Recent retrospective studies [12, 16] have suggested that elevated p95 levels predict poor outcome in patients with metastatic HER2 + breast cancer treated with trastuzumab containing regimens. In the current study, tumors with higher levels of p95 expression did have a numerically shorter median TTP, but this was not statistically significant (HR = 3.71, P = 0.39). However, we hypothesized that the relationship between p95 may be more apparent when controlling for the positive influence of H2T, as p95 is thought to negate the benefit gained by trastuzumab in HER2-positive breast cancer. Indeed, when using a bivariate continuous Cox analysis to independently assess the influences of p95 and H2T, p95 levels were associated with shorter TTP (HR = 3.6, P = 0.042).
discussion
This phase II study demonstrates the clinical activity of ixabepilone in combination with trastuzumab for metastatic HER2-positive breast cancer. The overall response rate was 44%, with a numerically higher response rate in cohort 1 compared with cohort 2 (73% versus 25%). The higher response rate observed in cohort 1 is likely a reflection of the fact that these patients had not received any prior chemotherapy or trastuzumab for metastatic breast cancer. Of patients in cohort 2, ∼40% had received one prior chemotherapy regimen in the metastatic setting, whereas ∼60% had received two prior regimens. In addition, all of these patients had received prior trastuzumab. Nonetheless, 25% of these pretreated patients experienced a PR to ixabepilone in combination with trastuzumab. Responses occurred rapidly, with most being reported by the first assessment at 9 weeks. Furthermore, the median TTF was 6.6 months for cohort 1 and 6.2 months for cohort 2.
The response rate of ixabepilone in combination with trastuzumab as first-line therapy for metastatic breast cancer (73%) is similar to response rates seen using trastuzumab–taxane combinations as first-line treatment. Studies have reported response rates of 50%–70% for trastuzumab in combination with docetaxel [17–19] and 62%–81% for trastuzumab in combination with paclitaxel [20–22]. Currently, the standard first-line therapy for metastatic HER2-positive breast cancer is a taxane in combination with trastuzumab and pertuzumab. Data suggest an 80.2% response rate for first line docetaxel–trastuzumab–pertuzumab, slightly better than that seen with ixabepilone and trastuzumab in this study [23].
The clinical utility of any new therapy can be improved if there are biomarkers that can prospectively predict which patients are likely to respond and which are not allowing the therapy to be directed specifically to those patients with the greatest chance of deriving benefit. The need for such biomarkers is particularly acute in HER2 + breast cancer, where there are already a number of available targeted therapies and combinations from which to choose, with several additional agents likely to become available in the near future. Unfortunately, it has so far proven difficult to identify such markers. For trastuzumab, one potential marker of sensitivity is p95. Preclinical studies and retrospective clinical studies in patients with metastatic HER2 + disease suggest that those tumors with high levels of p95 are less likely to respond to trastuzumab-based therapy compared with those with minimal or no p95 expression [12, 16, 24]. However, this hypothesis has been called into question by recent data from three preoperative studies using very different IHC-based assays [25–27]. It is not known to what extent cross-reactivity with full-length HER2 in the p95 IHC assays contributed to this result.
In the current study, p95 levels by the VeraTag method were associated with shorter TTP (HR = 3.6, P = 0.042), but this relationship was only clearly observed when controlling for the effect of H2T levels. This analysis also utilized a novel highly sensitive and specific assay for p95 that is distinct from the assays used in the preoperative studies. It is unclear whether differences in the statistical approach, assay technology, and/or patient populations account for the conflicting results observed in the studies to date. Additional studies utilizing a uniform analysis technique are needed.
Although the combination of ixabepilone and trastuzumab demonstrated an acceptable safety profile in this study, sensory neuropathy was a significant problem, with about half of patients experiencing grade 2 or higher toxicity. Although there were no grade 4 neuropathy events, seven patients (18%) had grade 3 neuropathy. This incidence of neuropathy is not dissimilar to that which has been reported in other studies of ixabepilone. The phase II study of ixabepilone monotherapy in patients with advanced breast cancer resistant to an anthracycline, taxane, and capecitabine reported 14% of patients with grade 3 or 4 sensory neuropathy [9], and the phase III study of ixabepilone–capecitabine for metastatic breast cancer that progressed after an anthracycline and a taxane reported a 21% incidence of grade 3 or 4 sensory neuropathy in the combination arm [10].
Other notable toxic effects included leukopenia (21% grade ≥ 2) and neutropenia (21% grade ≥ 2); however, there were only two cases (5%) of febrile neutropenia. There were also a significant number of patients with grade 2 or higher myalgias (21%), which is similar to that reported in the phase II study of ixabepilone monotherapy [9].
It has been demonstrated that cardiotoxicity is associated with trastuzumab therapy [28]. When trastuzumab is administered as monotherapy in the metastatic setting, the incidence of cardiac dysfunction has been calculated to be 7%, reaching 28% when administered in combination with an anthracycline and a cyclophosphamide [29]. In the current study, no symptomatic LVEF reductions were reported.
This is the first study of ixabepilone in combination with trastuzumab and demonstrates that the combination is both safe and effective. Further studies are needed to explore response in taxane-resistant breast cancer and to compare efficacy to taxanes in combination with trastuzumab.
funding
This work was supported by the Cancer Therapy Evaluation Program (CTEP) of the Division of Cancer Treatment and Diagnosis, National Cancer Institute (NCI) (no grant number).
disclosure
The authors have declared no conflicts of interest.
acknowledgements
The authors wish to acknowledge four contributors from Monogram Biosciences: Mojgan Haddad and Agnes Paquet for data management and Jodi Weidler and Yolanda Lie for logistical support.
references
- 1.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 2.Seidman AD, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26:1642–1649. doi: 10.1200/JCO.2007.11.6699. [DOI] [PubMed] [Google Scholar]
- 3.Fumoleau P, Largillier R, Clippe C, et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004;40:536–542. doi: 10.1016/j.ejca.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Cortes J, Baselga J. Targeting the microtubules in breast cancer beyond taxanes: the epothilones. Oncologist. 2007;12:271–280. doi: 10.1634/theoncologist.12-3-271. [DOI] [PubMed] [Google Scholar]
- 5.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 6.Lee FY, Borzilleri R, Fairchild CR, et al. BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res. 2001;7:1429–1437. [PubMed] [Google Scholar]
- 7.Wartmann M, Altmann KH. The biology and medicinal chemistry of epothilones. Curr Med Chem Anticancer Agents. 2002;2:123–148. doi: 10.2174/1568011023354489. [DOI] [PubMed] [Google Scholar]
- 8.Thomas E, Tabernero J, Fornier M, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol. 2007;25:3399–3406. doi: 10.1200/JCO.2006.08.9102. [DOI] [PubMed] [Google Scholar]
- 9.Perez EA, Lerzo G, Pivot X, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007;25:3407–3414. doi: 10.1200/JCO.2006.09.3849. [DOI] [PubMed] [Google Scholar]
- 10.Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–5217. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, Huang W, Tan Y, et al. A novel proximity assay for the detection of proteins and protein complexes: quantitation of HER1 and HER2 total protein expression and homodimerization in formalin-fixed, paraffin-embedded cell lines and breast cancer tissue. Diagn Mol Pathol. 2009;18:11–21. doi: 10.1097/PDM.0b013e31818cbdb2. [DOI] [PubMed] [Google Scholar]
- 12.Sperinde J, Jin X, Banerjee J, et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin Cancer Res. 2010;16:4226–4235. doi: 10.1158/1078-0432.CCR-10-0410. [DOI] [PubMed] [Google Scholar]
- 13.Lipton A, Kostler WJ, Leitzel K, et al. Quantitative HER2 protein levels predict outcome in fluorescence in situ hybridization-positive patients with metastatic breast cancer treated with trastuzumab. Cancer. 2010;116:5168–5178. doi: 10.1002/cncr.25430. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh R, Narasanna A, Wang SE, et al. Trastuzumab has preferential activity against breast cancers driven by HER2 homodimers. Cancer Res. 2011;71:1871–1882. doi: 10.1158/0008-5472.CAN-10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen K, Angelini PD, Laos S, et al. A naturally occurring HER2 carboxy-terminal fragment promotes mammary tumor growth and metastasis. Mol Cell Biol. 2009;29:3319–3331. doi: 10.1128/MCB.01803-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scaltriti M, Rojo F, Ocana A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 17.Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 18.Montemurro F, Choa G, Faggiuolo R, et al. Safety and activity of docetaxel and trastuzumab in HER2 overexpressing metastatic breast cancer: a pilot phase II study. Am J Clin Oncol. 2003;26:95–97. doi: 10.1097/01.COC.0000017087.19671.05. [DOI] [PubMed] [Google Scholar]
- 19.Tedesco KL, Thor AD, Johnson DH, et al. Docetaxel combined with trastuzumab is an active regimen in HER-2 3+ overexpressing and fluorescent in situ hybridization-positive metastatic breast cancer: a multi-institutional phase II trial. J Clin Oncol. 2004;22:1071–1077. doi: 10.1200/JCO.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 20.Fountzilas G, Tsavdaridis D, Kalogera-Fountzila A, et al. Weekly paclitaxel as first-line chemotherapy and trastuzumab in patients with advanced breast cancer. A Hellenic Cooperative Oncology Group phase II study. Ann Oncol. 2001;12:1545–1551. doi: 10.1023/a:1013184301155. [DOI] [PubMed] [Google Scholar]
- 21.Seidman AD, Fornier MN, Esteva FJ, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol. 2001;19:2587–2595. doi: 10.1200/JCO.2001.19.10.2587. [DOI] [PubMed] [Google Scholar]
- 22.Bullock K, Blackwell K. Clinical efficacy of taxane-trastuzumab combination regimens for HER-2-positive metastatic breast cancer. Oncologist. 2008;13:515–525. doi: 10.1634/theoncologist.2007-0204. [DOI] [PubMed] [Google Scholar]
- 23.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villasboas JC, Hurley J, Weidler JM, et al. Correlation of quantitative p95HER2 and HER2 protein expression with pathologic complete response (pCR) in HER2-positive breast cancer patients treated with neoadjuvant trastuzumab-containing therapy. Journal of Clinical Oncology. 2012;30 (Supp): abstract 608. [Google Scholar]
- 25.Loibl S, Bruey J, Von Minckwitz G, et al. Validation of p95 as a predictive marker for trastuzumab-based therapy in primary HER2-positive breast cancer: a translational investigation from the neoadjuvant GeparQuattro study. J Clin Oncol. 2011;29:abstract 530. [Google Scholar]
- 26.Gianni L, Pienkowski T, Im YH, et al. Neoadjuvant pertuzumab (P) and trastuzumab (H): Antitumor and safety analysis of a randomized phase II study ‘Neosphere. In 32nd Annual San Antonio Breast Cancer Symposium; 9–13 December 2009; San Antonio, TX. Abstract S5-1. [Google Scholar]
- 27.Guaneri V, Bpttomo A, Generali DG. Final results of a phase II randomized trial of neoadjuvant anthracycline-taxane chemotherapy plus lapatinib, trastuzumab, or both in HER2-positive breast cancer (CHER-LOB trial) J Clin Oncol. 2011;29:abstract 507. [Google Scholar]
- 28.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 29.Suter TM, Cook-Bruns N, Barton C. Cardiotoxicity associated with trastuzumab (Herceptin) therapy in the treatment of metastatic breast cancer. Breast. 2004;13:173–183. doi: 10.1016/j.breast.2003.09.002. [DOI] [PubMed] [Google Scholar]