Abstract
In the era of effective antiretroviral therapy (ART), epidemiologic studies have found that HIV-infected persons have a higher prevalence and incidence of chronic obstructive pulmonary disease than HIV-uninfected persons. Recently, pulmonary function studies in HIV-infected persons have shown a high prevalence of airway obstruction, bronchodilator reversibility, and impaired diffusing capacity. In comparison to HIV-uninfected persons and those with well-controlled HIV disease, HIV-infected persons with poor viral control or lower CD4 cell count have more airflow obstruction, a greater decline in lung function, and possibly more severe diffusing impairment. This chapter will review the evidence linking HIV infection to obstructive lung disease and discuss management issues related to the treatment of obstructive lung disease in HIV-infected patients.
Keywords: Chronic obstructive pulmonary disease, emphysema, asthma, HIV, AIDS, smoking-related lung disease
Introduction
Obstructive lung disease includes the most common lung conditions in the general population, asthma and chronic obstructive lung disease (COPD). While described as two separate disorders, there is much overlap in their pathology, physiology, and clinical manifestations.1 Both conditions are becoming more common in the developing worlds: asthma likely from multiple factors2, 3, and COPD from smoking.4 Obstructive lung disease is increasingly recognized as a common comorbidity in HIV-infected individuals. Beyond the typical risk factors, HIV infection may itself be an independent risk factor for obstructive lung disease.
This chapter will review the evidence for HIV infection as a risk factor for obstructive lung diseases, namely COPD and asthma. Additionally, studies identifying potential mechanisms leading to airways disease – airflow obstruction and airway hyperreactivity – will be discussed. Finally, we will consider the treatment of HIV-related obstructive lung disease.
Increased chronic obstructive lung disease/emphysema
The Global Initiative on Obstructive Lung Disease (GOLD) group defines COPD as “persistent airflow limitation that is usually progressive”… and ”associated with an enhanced chronic inflammatory response in the airways and the lung.”5 COPD results from emphysema, small airways inflammation, bronchoconstriction, excess mucus in the airways or a combination of these factors. Fixed airflow obstruction is required to diagnose COPD, and is defined by GOLD criteria as a ratio of the forced expiratory volume in one second (FEV1) over the forced vital capacity (FVC) of less than 70%. COPD can also be diagnosed based on a ratio of the FEV1/FVC that is below the lower limit of normal (less than the 5th percentile). Impaired diffusing capacity of the lung (DLco) can be a manifestation of significant emphysema.6, 7
Cigarette smoking is the major risk factor for development of COPD. However, as not all smokers develop COPD, other factors appear to be involved8, including genetic factors such as alpha-one antitrypsin deficiency, and factors such as race and gender. Occupational and environmental factors can also play a significant role.9–12 HIV-infected individuals may represent another population with an increased susceptibility to COPD.
Prior to effective ART, abnormalities of pulmonary function associated with HIV were first noted by the Pulmonary Complications of HIV Infection Study (PCHIS). In longitudinal pulmonary function evaluation of 1,300 participants, HIV-infected persons had more dyspnea compared to HIV-uninfected persons, and injection drug users (IDU) reported more cough and sputum production.13 Lower diffusing capacity for carbon monoxide (DLco) was more common in those with respiratory symptoms and in smokers and IDU, and HIV-infected persons had a lower DLco percent predicted. These findings need to be interpreted with caution since the prevalence of African-Americans was different in the groups, and the DLco prediction equations did not account for race. Also, DLco tended to be lower in those persons with CD4+ cell counts below 200 cells/μl.
Early in the HIV epidemic, it was thought DLco impairment was primarily due to HIV-related inflammation or infection, and DLco was worse with more severe HIV disease.14–17 However, data from Diaz and colleagues demonstrated that emphysema was an important determinant of a decreased DLCO. HIV-infected smokers without a history of pulmonary infections had emphysema by either PFT or CT scans18, and emphysema also occurred in HIV-infected persons who were non-smokers.19 Twenty-three percent of HIV-infected smokers without a history of pulmonary infections had emphysema by either PFT or CT scan compared to only 2% of HIV-uninfected controls matched for age and smoking, and 37% of HIV-infected persons with a greater than 12 pack-year smoking history had emphysema compared to none of the HIV-uninfected controls. Diaz reported a series of 4 HIV-infected non-smokers who had air-trapping, decreased DLco, and emphysema on CT scan.19 These observations suggest that prior to ART, HIV was a risk factor for COPD or interacted with other risk factor(s) in the development of COPD, particularly of the emphysema subtype.19
Since the advent of effective combination ART, several studies have sought to determine the prevalence and risk factors for COPD, and for abnormal pulmonary function associated with HIV infection. Two studies from the Veteran’s Aging Cohort Study (VACS) suggest that HIV infection is an independent risk factor for COPD.20, 21 The first assessed 1014 HIV-infected and 713 HIV-uninfected men enrolled at 5 VACS Sites for diagnoses of COPD determined by ICD-9 diagnostic codes and patient self-report on questionnaire. Unadjusted prevalence of COPD in HIV-infected vs. HIV-uninfected by ICD-9 codes was 10% vs. 9% (p = 0.4) and by participant self-report was 15% vs. 12% (p = 0.04). After adjusting for differences in age, smoking, race/ethnicity and other potential confounders such as injection drug use and alcohol abuse, HIV-infected subjects were approximately 50% to 60% more likely to have COPD than HIV-uninfected subjects by either ICD-9 codes or patient self-report. The second VACS study tracked the incidence of lung disease by ICD-9 diagnosis in 33,420 HIV-infected veterans and 66,840 HIV-uninfected veterans matched by age, sex, race and ethnicity, and site.21 The incidence of new COPD diagnosis was 20.3 per 1000 person-years, and incident COPD was 8% greater in HIV-infected persons 50 years old and 17% greater in HIV-infected persons < 50 years old compared to HIV-uninfected controlling for age, race and ethnicity, sex, alcohol disorders, drug abuse, and hepatitis C infection. In a subgroup with data to control for smoking history, HIV-infected participants had even greater incidence rate compared to HIV-uninfected: 11% greater in persons 50 years old and 25% greater in persons < 50 years old. The difference in rates between HIV-infected and uninfected persons was greater at younger ages suggesting a potential earlier onset of lung disease in HIV-infected persons; competing risk for mortality could also account for a lower relative difference in older HIV-infected compared to uninfected individuals.
Pulmonary function has recently been measured in several cohorts that include HIV-infected persons (Table 1).22–28 In HIV-infected persons, airflow obstruction is common, possibly more common than would be expected. Spirometry from 234 HIV-infected individuals found 8.6% had an FEV1/FVC below the 5% lower limit of age, race, and gender predicted normal22, and lower FEV1/FVC was associated with older age, smoking, history of bacterial pneumonia, and ART use. In a separate cohort of 167 HIV-infected outpatients, an FEV1/FVC < 0.7 was found in 21.0%, and airflow obstruction was associated with smoking, IDU and ART use.23 Another cohort of 98 individuals had a 16.3% prevalence of obstructive lung disease (FEV1/FVC < 0.70 and an FEV1 < 80% predicted), which was associated with age, smoking, intravenous drug use, and history of Pneumocystis pneumonia.25 Compared to data from the National Health and Nutrition Evaluation Survey (NHANES) III, this HIV cohort appeared to have greater prevalence of obstructive lung disease even with similar prevalence of smoking. In a cohort of intravenous drug abusers, airflow obstruction (FEV1/FVC < 0.70) was present in 16.8% of HIV-infected participants26, and HIV-infected participants with a viral load > 200,000 copies/mL were approximately 3.4 times more likely to have obstructive lung disease than HIV-uninfected participants. HIV-infected participants with controlled viral loads had similar rates of obstruction as HIV-uninfected participants.
Table 1.
Studies of pulmonary function in HIV-infected individuals during the combination ART era
| Author, year | Design and population | Important findings |
|---|---|---|
| George et al., 200922 | Cross-sectional analysis of spirometry data from 234 HIV-infected individuals recruited from an HIV clinic in Los Angeles, CA. |
|
| Gingo et al., 201023 | Cross-sectional analysis of pre-and post-bronchodilator spirometry and DLco data from 167 HIV-infected participants recruited from an HIV clinic at the University of Pittsburgh. |
|
| Cui et al., 201024 | Cross-sectional analysis of spirometry from 119 HIV-infected participants from an HIV-clinic. |
|
| Hirani et al. 201125 | Cross-sectional analysis of spirometry and St. George Respiratory Questionnaire data from 98 consecutive HIV-infected patients seen for routine care at Thomas Jefferson University in Philadelphia, PA. |
|
| Drummond et al., 201226 | Spirometry obtained in 1077 participants from the AIDS Linked to Intravenous Experience study cohort in Baltimore, MD. |
|
| Kristoffersen et al., 201227 | Prospective cohort of 88 HIV-infected participants with repeat spirometry in 63 participants with a mean follow-up of 4.4 years. Denmark. |
|
| Drummond et al., 201328 | Serial spirometry on 1064 AIDS Linked to Intravenous Experience participants |
|
Diffusion impairment (a common manifestation of emphysema) remains a frequent abnormality in the current HIV era. In one recent study, 64% of HIV-infected individuals had a DLco < 80% predicted23, and in another, 43% had a DLco < 1.645 residual standard deviations below predicted values.27 Diffusion impairment, while worse in smokers, is also quite common in never smokers. In these two studies, 48% and 9% of never smokers respectively had impaired diffusing capacity. Reduced diffusing capacity was also associated with use of pneumonia prophylaxis.23 In longitudinal follow-up, DLco declined significantly and to a greater degree than other lung function parameters.27 Additionally, a comparison of lung function between 229 HIV-infected and 213 HIV-uninfected participants from the Multicenter AIDS Cohort and VACS studies found that HIV infection was independently associated with impaired diffusing capacity.29
The decrease in DLco likely has significant clinical relevance. In our experience, lower DLco is a significant predictor of mortality.30 In a cohort of 237 HIV-infected participants followed for 3 years on average, individuals who died had a significantly lower DLco (51.3% predicted versus 66.0% predicted, p = 0.004). The odds of death if the DLco was below 60% predicted were significantly greater independent of age, smoking history, ART use, and CD4 cell count (adjusted odds ratio = 6.31, 95% confidence interval = 1.21–32.9, p = 0.029). These findings suggest that lung diseases in HIV-infected individuals are an important health concern and either directly contribute to mortality or are a marker of an underlying systemic process.
Mechanistic factors associated with COPD
In addition to smoking, HIV-related factors appear to contribute to COPD/emphysema as the disease appears to be accelerated in HIV-infected smokers and is also seen in non-smokers. Poorly controlled HIV has been associated with worse pulmonary function,21, 26 and greater decline in lung function.28 In the VACS study, lung diseases such as asthma and COPD were less likely in those with lower HIV RNA levels and use of ART at baseline. The AIDS Linked to Intravenous Experience study cohort directly measured pulmonary function and also found that individuals with HIV viral levels above 200,000 copies/ml had worse airway obstruction.26 In a follow-up longitudinal study of this cohort, high viral loads (> 75,000 copies/mL) or low CD4 counts (< 100 cells/uL) were associated with greater decline in FEV1 and FVC over time.28 These studies suggest pathogenesis of COPD in HIV is related to worse HIV control (either a direct viral effect or effects of HIV sequelae such as infections). However, two studies have found that use of ART is also associated with worse lung function.22, 23 Biologic mechanisms to explain this paradox could be related to differences in timing of ART initiation between populations; those in whom ART is initiated at a lower CD4 cell count could experience worsening in lung function on ART, potentially from increases in autoimmunity or renewed immunologic response to low-level lung pathogens.31, 32
Microbial colonization may play a role in the pathogenesis of COPD in HIV. Several studies have implicated colonization with Pneumocystis jirovecii, a common HIV-associated infection, in the pathogenesis of obstructive lung disease in HIV. In a cohort of 42 HIV-infected individuals, those with Pneumocystis jirovecii detectable only by nested PCR of the mitochondrial large subunit rRNA in oral washes or induced sputum had worse airflow and more obstructive lung disease by pulmonary function studies.33 In macaque models of HIV infection, monkeys that become colonized with Pneumocystis (detection of Pneumocystis DNA in lung or airway samples) develop worse airflow obstruction and emphysema.34, 35 A non-immunosuppressed rodent model has also shown development of COPD-like changes in mice exposed to both cigarette smoke and Pneumocystis.36 HIV infection is associated with a greater probability of colonization with Pneumocystis which may explain in part the increased prevalence of obstructive lung disease in HIV-infected persons.37 Several studies have identified the lung microbiome community as unique in HIV-infected persons.38–40 One study found an increase in Trophyrema whipplei in HIV-infected persons, but relationship of T. whipplei to lung function is not known.41
Asthma in HIV infection
Asthma is characterized by airway inflammation and inducible or reversible airway obstruction (airway hyperreactivity). Asthma is associated with morbidity related to episodes of dyspnea and functional impairment and increased mortality in some populations.2, 42 Prior to ART, not all studies showed a significant association, but HIV-infected persons were more likely to have airway hyperreactivity.43, 44 Airway hyperreactivity in the pre-ART era was associated with smoking and atopy. Data are limited in the ART era regarding the association of HIV and asthma. In the VACS cohort, which was predominantly older male smokers, asthma diagnosis was not more common in HIV-infected individuals.21 However, two studies in children have shown that asthma symptoms and inhaler use are more common in HIV-infected children in the ART era and in those children taking ART medications.45–47
Self-reported asthma diagnosis is commonly used in epidemiologic studies. Two recent studies have found 11% and 21% of HIV-infected persons had an asthma diagnosis by history (doctor-diagnosed asthma),24, 48 in contrast to a prevalence of approximately 9% in the general population.49, 50 There was also a 4% and 9% prevalence of bronchodilator reversibility by American Thoracic Society/European Respiratory Society criteria with pulmonary function testing in these studies, respectively, which is potentially higher than expected in the general population.50 In the general population, most adults with asthma are diagnosed as children.51 In contrast, 55% of the HIV-infected individuals reported onset of asthma as an adult, often after diagnosis of HIV.
Asthma in HIV infection is common, and there may be distinct phenotypes of asthma associated with HIV. Airway inflammation in HIV could have unique mechanisms related to allergy/atopy, metabolic disease, and chronic inflammation possibly stimulated by underlying infections.50 In the study by Gingo et al, doctor-diagnosed asthma was associated with female sex, being obese, not being on ART, and a history of bacterial or Pneumocystis pneumonia, and there was a strong association between doctor-diagnosed asthma and high sputum eosinophil counts. In addition, approximately 10% of the cohort had high sputum eosinophil counts, suggesting an increase in Th2 type inflammation in the airways of HIV-infected persons. Doctor-diagnosed asthma and bronchodilator reversibility were also associated with cytokines that can be elevated in chronic HIV infection: RANTES and macrophage inflammatory protein-1α and -1β.52
Treatment considerations for obstructive lung disease in HIV
Although there are a few studies of smoking cessation therapy in HIV-infected persons, there are no studies of therapy specific to obstructive lung disease in HIV. In the absence of other data, the general treatment guidelines from various respiratory societies should be followed as with other patient populations. However, several factors are important to keep in mind when approaching HIV-infected patients with obstructive lung disease. The first is the high prevalence of smoking and significant impact smoking has on mortality in the HIV population. Rates of current smoking are nearly 2-fold higher in most HIV-infected compared to HIV-uninfected populations.53 However, health care providers of HIV-infected patients may be less aware of current smoking and less confident in their ability to counsel their patients regarding smoking cessation.54 Current smoking is associated with increased respiratory symptoms, COPD, bacterial pneumonia and decreased quality of life among HIV-infected patients.55 Additionally, the population-attributable risk of death associated with smoking is twice in HIV-infected individuals what it is in HIV-uninfected persons, and HIV-infected smokers lose more life-years to smoking than to HIV infection.56 These findings highlight the need to increase efforts at smoking cessation among patients with HIV. Smoking cessation interventions can be effectively applied in HIV-infected populations, and are reviewed in detail in the paper “Tobacco use and cessation in HIV-infected individuals,” by Browning and colleagues in this issue of Clinics in Chest Medicine.57–60
Respiratory medications commonly recommended for obstructive lung diseases warrant careful consideration in HIV-infected populations. Inhaled corticosteroids (ICS) in particular may pose increased risk of complications in HIV-infected individuals. ICS are associated with oral candidiasis, bacterial pneumonia61, and tuberculosis in the HIV-uninfected population.62 These complications of ICS could be augmented by HIV infection, particularly as bacterial pneumonia continues to be a major comorbidity in HIV, even with ART and relatively controlled CD4 counts.63, 64 Additionally, there are certain ICS (fluticasone in particular) whose metabolism is slowed by the presence of certain antiretroviral medications (ritonavir). This interaction leads to increased levels of systemic steroids and side effects of chronic steroid therapy such as osteoporosis and Cushing’s disease.65–67 Further studies are needed to assess the safety and/or effectiveness of ICS in this population.
Pulmonary rehabilitation programs may be of increased importance in HIV-patients with COPD. Obstructive lung disease (COPD and/or asthma) was independently associated with self-reported increased physical disability among HIV-infected veterans,68 and the combination of HIV infection and COPD had significant impact on physical functioning.69 Pulmonary rehabilitation significantly improves physical functioning in HIV-uninfected patients with COPD.70 HIV may exaggerate the systemic and skeletal manifestations associated with decrements in physical capacity encountered in HIV-uninfected COPD. There are skeletal muscle dysfunction and mitochondrial abnormalities related to HIV infection and its treatment.71 Peak aerobic capacity was 41% decreased in HIV-infected patients,72 and respiratory muscle function was decreased in otherwise healthy HIV-infected patients compared to HIV-uninfected patients.73 In HIV-infected patients, exercise training is safe and has potential benefits,74, 75 but studies to determine the role and optimal type of exercise training in HIV-infected patients, particularly those with COPD, are needed.
Conclusion
HIV-infected individuals appear to have an increased risk for obstructive lung diseases, although whether this represents increased emphysema, chronic bronchitis, asthma, or a combination of these disorders has not been fully evaluated. Although some of the increased obstructive lung disease, particularly COPD, may be related to smoking and drug abuse, the apparent risk for COPD remains elevated in HIV-infected persons even after controlling for these and other potential confounders.18, 20, 21 Recent studies of pulmonary function in HIV-infected persons have elucidated some factors that may be important in the pathogenesis of obstructive lung disease in HIV – poor HIV control contributing to COPD and decline in lung function, and metabolic disease and inflammation associated with asthma and airway hyperreactivity (Figure 1). There may be further mechanisms currently being elucidated such as chronic immune activation and immune senescence leading to early aging in HIV as described in the paper “Future Directions -- Lung Aging, Inflammation, and HIV” by Fitzpatrick and colleague in this issue of Clinics in Chest Medicine.
Figure 1.
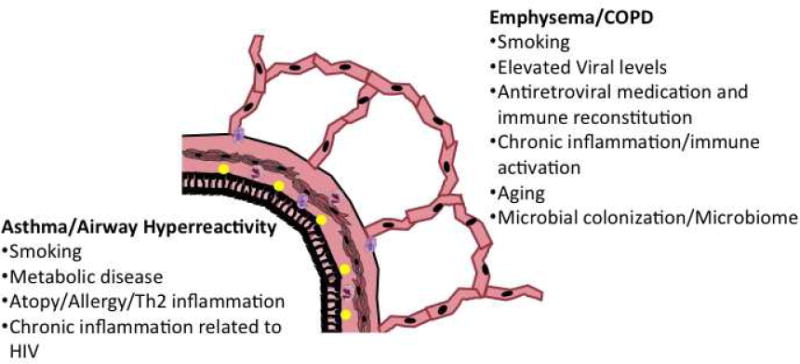
Mechanisms related to obstructive lung disease in HIV-infected persons grouped by association with COPD/emphysema and association with asthma/airway hyperreactivity.
Given the increasing age of HIV-infected individuals and the high prevalence of smoking, health care providers are likely to encounter a substantial number of HIV-infected patients with obstructive lung diseases. Undiagnosed airway obstruction is associated with impaired health and functional status, thus making identification of COPD and asthma important.76 Studies to determine whether the pharmacologic and non-pharmacologic management strategies for obstructive lung diseases should differ among HIV-infected versus HIV-uninfected patients are needed. A better understanding of treatments to appropriately manage obstructive lung disease in HIV-infected patients is essential to optimize health benefits such as decreased symptoms and exacerbations of disease, and to improve exercise capacity, quality of life and to increase smoking cessation.77, 78
Key Points:
Obstructive lung disease is common among HIV-infected person, and HIV infection appears to be an independent risk factor for diagnosis of chronic obstructive lung disease (COPD).
Early and progressive emphysema and COPD likely contribute to significant morbidity in HIV-infected persons.
In addition to smoking, other likely contributors to the pathogenesis of COPD in HIV infection include microbial colonization, elevated HIV viral levels, and possible immune reconstitution with antiretrovirals.
Asthma is a phenotype of obstructive lung disease that is commonly diagnosed in HIV-infected persons and may have unique mechanisms related to metabolic disease in HIV and inflammation related to chronic HIV infection.
Smoking cessation, monitoring for infectious complications of inhaled corticosteroids, and pulmonary rehabilitation are aspects of treatment for obstructive lung disease that are important considerations in HIV-infected persons.
Acknowledgments
Funding: National Institutes of Health / National Heart, Lung, and Blood Institute K23 HL108697 (MG); R01 HL083461, R01 HL 090339, and HL083461S (AM); R01 HL 090342 (KC)
Footnotes
Contact Information: Matthew R. Gingo, M.D, M.S., University of Pittsburgh School of Medicine, 628 NW MUH, 3459 Fifth Avenue, Pittsburgh, PA 15213, 412-624-3045, Fax: 412-624-7383, gingomr@upmc.edu
Alison Morris, MD, MS, University of Pittsburgh School of Medicine, 628 NW MUH, 3459 Fifth Avenue, Pittsburgh, PA 15213, 412-692-2210, Fax: 412-692-2260, morrisa@upmc.edu
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64(8):728–735. doi: 10.1136/thx.2008.108027. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012;94:1–8. [PubMed] [Google Scholar]
- 3.Kazani S, Israel E. Update in asthma 2011. Am J Respir Crit Care Med. 2012;186(1):35–40. doi: 10.1164/rccm.201204-0634UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease, GOLD executive summary. Am J Respir Crit Care Med. 2012 doi: 10.1164/rccm.201204-0596PP. Aug 9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Haraguchi M, Shimura S, Hida W, et al. Pulmonary function and regional distribution of emphysema as determined by high-resolution computed tomography. Respiration. 1998;65(2):125–129. doi: 10.1159/000029243. [DOI] [PubMed] [Google Scholar]
- 7.Park KJ, Bergin CJ, Clausen JL. Quantitation of emphysema with three-dimensional CT densitometry: comparison with two-dimensional analysis, visual emphysema scores, and pulmonary function test results. Radiology. 1999;211(2):541–547. doi: 10.1148/radiology.211.2.r99ma52541. [DOI] [PubMed] [Google Scholar]
- 8.Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121(5 Suppl):121S–126S. doi: 10.1378/chest.121.5_suppl.121s. [DOI] [PubMed] [Google Scholar]
- 9.Sandford AJ, Silverman EK. Chronic obstructive pulmonary disease. 1: Susceptibility factors for COPD the genotype-environment interaction. Thorax. 2002;57(8):736–741. doi: 10.1136/thorax.57.8.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman KR. Chronic obstructive pulmonary disease: are women more susceptible than men? Clin Chest Med. 2004;25(2):331–341. doi: 10.1016/j.ccm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Silverman EK, Weiss ST, Drazen JM, et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(6):2152–2158. doi: 10.1164/ajrccm.162.6.2003112. [DOI] [PubMed] [Google Scholar]
- 12.Petty TL, Weinmann GG. Building a national strategy for the prevention and management of and research in chronic obstructive pulmonary disease. National Heart, Lung, and Blood Institute Workshop Summary. Bethesda, Maryland, August 29–31, 1995. JAMA. 1997;277(3):246–253. doi: 10.1001/jama.277.3.246. [DOI] [PubMed] [Google Scholar]
- 13.Rosen MJ, Lou Y, Kvale PA, et al. Pulmonary function tests in HIV-infected patients without AIDS. Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med. 1995;152(2):738–745. doi: 10.1164/ajrccm.152.2.7633736. [DOI] [PubMed] [Google Scholar]
- 14.Diaz PT, King MA, Pacht ER, et al. The pathophysiology of pulmonary diffusion impairment in human immunodeficiency virus infection. Am J Respir Crit Care Med. 1999;160(1):272–277. doi: 10.1164/ajrccm.160.1.9812089. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell DM, Fleming J, Pinching AJ, et al. Pulmonary function in human immunodeficiency virus infection. A prospective 18-month study of serial lung function in 474 patients. Am Rev Respir Dis. 1992;146(3):745–751. doi: 10.1164/ajrccm/146.3.745. [DOI] [PubMed] [Google Scholar]
- 16.Nieman RB, Fleming J, Coker RJ, et al. Reduced carbon monoxide transfer factor (TLCO) in human immunodeficiency virus type I (HIV-I) infection as a predictor for faster progression to AIDS. Thorax. 1993;48(5):481–485. doi: 10.1136/thx.48.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw RJ, Roussak C, Forster SM, et al. Lung function abnormalities in patients infected with the human immunodeficiency virus with and without overt pneumonitis. Thorax. 1988;43(6):436–440. doi: 10.1136/thx.43.6.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz PT, King MA, Pacht ER, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med. 2000;132(5):369–372. doi: 10.7326/0003-4819-132-5-200003070-00006. [DOI] [PubMed] [Google Scholar]
- 19.Diaz PT, Clanton TL, Pacht ER. Emphysema-like pulmonary disease associated with human immunodeficiency virus infection. Ann Intern Med. 1992;116(2):124–128. doi: 10.7326/0003-4819-116-2-124. [DOI] [PubMed] [Google Scholar]
- 20.Crothers K, Butt AA, Gibert CL, et al. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130(5):1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 21.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183(3):388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George MP, Kannass M, Huang L, et al. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One. 2009;4(7):e6328. doi: 10.1371/journal.pone.0006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gingo MR, George MP, Kessinger CJ, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182(6):790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui Q, Carruthers S, McIvor A, et al. Effect of smoking on lung function, respiratory symptoms and respiratory diseases amongst HIV-positive subjects: a cross-sectional study. AIDS Res Ther. 2010;7:6. doi: 10.1186/1742-6405-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirani A, Cavallazzi R, Vasu T, et al. Prevalence of obstructive lung disease in HIV population: a cross sectional study. Respir Med. 2011;105(11):1655–1661. doi: 10.1016/j.rmed.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Drummond MB, Kirk GD, Astemborski J, et al. Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax. 2012;67(4):309–314. doi: 10.1136/thoraxjnl-2011-200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristoffersen US, Lebech AM, Mortensen J, et al. Changes in lung function of HIV-infected patients: a 4.5-year follow-up study. Clin Physiol Funct Imaging. 2012;32(4):288–295. doi: 10.1111/j.1475-097X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 28.Drummond MB, Merlo CA, Astemborski J, et al. AIDS. 2013. Jan 5, The effect of HIV infection on longitudinal lung function decline among injection drug users: a prospective cohort. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crothers K, Kleerup EC, Wongtrakool C, et al. CROI. 2012. HIV infection is associated with impaired pulmonary diffusing capacity. Seattle 2012 (Meeting Abstracts) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gingo MR, Morris A. Current HIV/AIDS reports. 2012. Oct 19, Pathogenesis of HIV and the Lung. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crothers K, Huang L. Pulmonary complications of immune reconstitution inflammatory syndromes in HIV-infected patients. Respirology. 2009;14(4):486–494. doi: 10.1111/j.1440-1843.2008.01468.x. [DOI] [PubMed] [Google Scholar]
- 32.Mori S, Levin P. A brief review of potential mechanisms of immune reconstitution inflammatory syndrome in HIV following antiretroviral therapy. Int J STD AIDS. 2009;20(7):447–452. doi: 10.1258/ijsa.2009.008521. [DOI] [PubMed] [Google Scholar]
- 33.Morris A, Alexander T, Radhi S, et al. Airway obstruction is increased in pneumocystis-colonized human immunodeficiency virus-infected outpatients. J Clin Microbiol. 2009;47(11):3773–3776. doi: 10.1128/JCM.01712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kling HM, Shipley TW, Patil SP, et al. Relationship of Pneumocystis jiroveci humoral immunity to prevention of colonization and chronic obstructive pulmonary disease in a primate model of HIV infection. Infect Immun. 2010;78(10):4320–4330. doi: 10.1128/IAI.00507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shipley TW, Kling HM, Morris A, et al. Persistent Pneumocystis colonization leads to the development of chronic obstructive pulmonary disease in a nonhuman primate model of AIDS. J Infect Dis. 2010;202(2):302–312. doi: 10.1086/653485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensen PJ, Preston AM, Ling T, et al. Pneumocystis murina infection and cigarette smoke exposure interact to cause increased organism burden, development of airspace enlargement, and pulmonary inflammation in mice. Infect Immun. 2008;76(8):3481–3490. doi: 10.1128/IAI.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris A, George MP, Crothers K, et al. HIV and chronic obstructive pulmonary disease: is it worse and why? Proc Am Thorac Soc. 2011;8(3):320–325. doi: 10.1513/pats.201006-045WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ireland AW, Ghedin E, Pop M, et al. Comparison of the respiratory microbiome in HIV-infected and HIV-uninfected individuals. Am J Respir Crit Care Med. 2012;185:A4045. Meeting Abstracts. [Google Scholar]
- 39.Twigg HL, Nelson D, Dong Q, et al. Analysis of the respiratory microbiome using bronchoalveolar lavage from HIV-infected and uninfected subjects. Am J Respir Crit Care Med. 2010;181:A5629. Meeting Abstracts. [Google Scholar]
- 40.Twigg HL, Nelson D, Dong Q, et al. Analysis of the respiratory microbiome using bronchoalveolar lavage from HIV-infected and uninfected subjects. Am J Respir Crit Care Med. 2011;183:A6257. Meeting Abstracts. [Google Scholar]
- 41.Lozupone C, Cota-Gomez A, Palmer BE, et al. Tropheryma whipplei is common in the lung of HIV-infected subjects and decreases with ART. Am J Respir Crit Care Med. 2013 (in press) [Google Scholar]
- 42.Pac A, Tobiasz-Adamczyk B, Brzyska M, et al. The role of different predictors in 20-year mortality among Krakow older citizens. Arch Gerontol Geriatr. 2012 doi: 10.1016/j.archger.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Poirier CD, Inhaber N, Lalonde RG, et al. Prevalence of bronchial hyperresponsiveness among HIV-infected men. Am J Respir Crit Care Med. 2001;164(4):542–545. doi: 10.1164/ajrccm.164.4.2010019. [DOI] [PubMed] [Google Scholar]
- 44.Wallace JM, Stone GS, Browdy BL, et al. Nonspecific airway hyperresponsiveness in HIV disease. Pulmonary Complications of HIV Infection Study Group. Chest. 1997;111(1):121–127. doi: 10.1378/chest.111.1.121. [DOI] [PubMed] [Google Scholar]
- 45.Foster SB, Paul ME, Kozinetz CA, et al. Prevalence of asthma in children and young adults with HIV infection. J Allergy Clin Immunol. 2007;119(3):750–752. doi: 10.1016/j.jaci.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Foster SB, McIntosh K, Thompson B, et al. Increased incidence of asthma in HIV-infected children treated with highly active antiretroviral therapy in the National Institutes of Health Women and Infants Transmission Study. J Allergy Clin Immunol. 2008;122(1):159–165. doi: 10.1016/j.jaci.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutin F, Butt A, Alame W, et al. Asthma in immune-competent children with human immunodeficiency virus. Ann Allergy Asthma Immunol. 2009;102(5):438. doi: 10.1016/S1081-1206(10)60518-2. [DOI] [PubMed] [Google Scholar]
- 48.Gingo MR, Wenzel SE, Steele C, et al. Asthma diagnosis and airway bronchodilator response in HIV-infected patients. J Allergy Clin Immunol. 2012;129(3):708–714. e708. doi: 10.1016/j.jaci.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bridevaux PO, Probst-Hensch NM, Schindler C, et al. Prevalence of airflow obstruction in smokers and never-smokers in Switzerland. Eur Respir J. 2010;36(6):1259–1269. doi: 10.1183/09031936.00004110. [DOI] [PubMed] [Google Scholar]
- 50.Appleton SL, Adams RJ, Wilson DH, et al. Spirometric criteria for asthma: adding further evidence to the debate. J Allergy Clin Immunol. 2005;116(5):976–982. doi: 10.1016/j.jaci.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 51.Miranda C, Busacker A, Balzar S, et al. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113(1):101–108. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 52.Cocchi F, DeVico AL, Garzino-Demo A, et al. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270(5243):1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 53.Cigarette smoking among adults--United States, 2004. MMWR Morb Mortal Wkly Rep. 2005;54(44):1121–1124. [PubMed] [Google Scholar]
- 54.Crothers K, Goulet JL, Rodriguez-Barradas MC, et al. Decreased awareness of current smoking among health care providers of HIV-positive compared to HIV-negative veterans. J Gen Intern Med. 2007;22(6):749–754. doi: 10.1007/s11606-007-0158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crothers K, Griffith TA, McGinnis KA, et al. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J Gen Intern Med. 2005;20(12):1142–1145. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helleberg M, Afzal S, Kronborg G, et al. Mortality attributable to smoking among HIV-1-Infected Individuals: a nationwide, population-based cohort study. Clin Infect Dis. 2012 Dec 18; doi: 10.1093/cid/cis933. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Cummins D, Trotter G, Moussa M, et al. Smoking cessation for clients who are HIV-positive. Nurs Stand. 2005;20(12):41–47. doi: 10.7748/ns2005.11.20.12.41.c4016. [DOI] [PubMed] [Google Scholar]
- 58.Vidrine DJ, Arduino RC, Lazev AB, et al. A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. AIDS. 2006;20(2):253–260. doi: 10.1097/01.aids.0000198094.23691.58. [DOI] [PubMed] [Google Scholar]
- 59.Wewers ME, Neidig JL, Kihm KE. The feasibility of a nurse-managed, peer-led tobacco cessation intervention among HIV-positive smokers. J Assoc Nurses AIDS Care. 2000;11(6):37–44. doi: 10.1016/S1055-3290(06)60353-1. [DOI] [PubMed] [Google Scholar]
- 60.Ferketich AK, Diaz P, Browning KK, et al. Safety of varenicline among smokers enrolled in the Lung HIV Study. Nicotine Tob Res. 2013;15(1):247–254. doi: 10.1093/ntr/nts121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 62.Brassard P, Suissa S, Kezouh A, et al. Inhaled corticosteroids and risk of tuberculosis in patients with respiratory diseases. Am J Respir Crit Care Med. 2011 Mar 1;183(5):675–8. doi: 10.1164/rccm.201007-1099OC. [DOI] [PubMed] [Google Scholar]
- 63.Hirschtick RE, Glassroth J, Jordan MC, et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. N Engl J Med. 1995;333(13):845–851. doi: 10.1056/NEJM199509283331305. [DOI] [PubMed] [Google Scholar]
- 64.Segal LN, Methe BA, Nolan A, et al. HIV-1 and bacterial pneumonia in the era of antiretroviral therapy. Proc Am Thorac Soc. 2011;8(3):282–287. doi: 10.1513/pats.201006-044WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foisy MM, Yakiwchuk EM, Chiu I, et al. Adrenal suppression and Cushing’s syndrome secondary to an interaction between ritonavir and fluticasone: a review of the literature. HIV Med. 2008;9(6):389–396. doi: 10.1111/j.1468-1293.2008.00579.x. [DOI] [PubMed] [Google Scholar]
- 66.Kaviani N, Bukberg P, Manessis A, et al. Iatrogenic osteoporosis, bilateral hip osteonecrosis, and secondary adrenal suppression in an HIV-infected patient receiving inhaled corticosteroids and ritonavir-boosted HAART. Endocr Pract. 2010:1–16. doi: 10.4158/EP09288.CR. [DOI] [PubMed] [Google Scholar]
- 67.Kedem E, Shahar E, Hassoun G, et al. Iatrogenic cushing’s syndrome due to coadministration of ritonavir and inhaled budesonide in an asthmatic human immunodeficiency virus infected patient. J Asthma. 2010 Sep;47(7):830–1. doi: 10.3109/02770903.2010.485666. [DOI] [PubMed] [Google Scholar]
- 68.Oursler KK, Goulet JL, Leaf DA, et al. Association of comorbidity with physical disability in older HIV-infected adults. AIDS Patient Care STDS. 2006;20(11):782–791. doi: 10.1089/apc.2006.20.782. [DOI] [PubMed] [Google Scholar]
- 69.Oursler KK, Goulet JL, Crystal S, et al. Association of age and comorbidity with physical function in HIV-infected and uninfected patients: results from the Veterans Aging Cohort Study. AIDS Patient Care STDS. 2011;25(1):13–20. doi: 10.1089/apc.2010.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173(12):1390–1413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 71.Authier FJ, Chariot P, Gherardi RK. Skeletal muscle involvement in human immunodeficiency virus (HIV)-infected patients in the era of highly active antiretroviral therapy (HAART) Muscle Nerve. 2005;32(3):247–260. doi: 10.1002/mus.20338. [DOI] [PubMed] [Google Scholar]
- 72.Oursler KK, Sorkin JD, Smith BA, et al. Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS Res Hum Retroviruses. 2006;22(11):1113–1121. doi: 10.1089/aid.2006.22.1113. [DOI] [PubMed] [Google Scholar]
- 73.Schulz L, Nagaraja HN, Rague N, et al. Respiratory muscle dysfunction associated with human immunodeficiency virus infection. Am J Respir Crit Care Med. 1997;155(3):1080–1084. doi: 10.1164/ajrccm.155.3.9116990. [DOI] [PubMed] [Google Scholar]
- 74.Nixon S, O’Brien K, Glazier RH, et al. Aerobic exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev. 2010 Aug 4;(8) doi: 10.1002/14651858.CD001796.pub3. CD 001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Brien K, Nixon S, Glazier RH, et al. Progressive resistive exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev. 2004 Oct 18;(4) doi: 10.1002/14651858.CD004248.pub2. CD 004248. [DOI] [PubMed] [Google Scholar]
- 76.Coultas DB, Mapel D, Gagnon R, et al. The health impact of undiagnosed airflow obstruction in a national sample of United States adults. Am J Respir Crit Care Med. 2001;164(3):372–377. doi: 10.1164/ajrccm.164.3.2004029. [DOI] [PubMed] [Google Scholar]
- 77.Sin DD, McAlister FA, Man SF, et al. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA. 2003;290(17):2301–2312. doi: 10.1001/jama.290.17.2301. [DOI] [PubMed] [Google Scholar]
- 78.Tomas LH, Varkey B. Improving health-related quality of life in chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2004;10(2):120–127. doi: 10.1097/00063198-200403000-00006. [DOI] [PubMed] [Google Scholar]


