Abstract
Background
Whether elevations of urinary biomarkers of tubular injury (urine neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1)) are associated with future risk of kidney disease has not been investigated.
Study Design
1:1 nested case-control study
Setting & Participants
686 participants in the Multi-Ethnic Study of Atherosclerosis (MESA).
Predictor
NGAL and KIM-1 were measured at baseline and expressed as log-transformed continuous variables and categorized into deciles.
Outcomes
Kidney function was estimated by cystatin C using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation. Incident CKD Stage 3 was defined as eGFR <60 ml/min/1.73m2 and a eGFR decline >1 ml/min/1.73m2 per year, and rapid kidney function decline (RKFD) was defined as decline of ≥3 ml/min/1.73m2 per year.
Measurements
Cases were defined as persons with eGFR >60 ml/min/1.73m2 who subsequently developed incident CKD Stage 3 and/or had RKFD by MESA year 5 visit. Controls were matched for age, gender, race, diabetes, and baseline eGFR. We adjusted for age, hypertension and presence of albuminuria (ACR ≥30 mg/g).
Results
Of the 343 cases, 145 had incident CKD Stage 3, 141 had RKFD and 57 had both. Mean eGFR for controls was 81 (±10) ml/min/1.73m2 at baseline and 80 (±10) at follow-up, compared with 82 (±13) and 58 (±10) for cases. Each doubling of KIM-1 (pg/ml) was associated with an OR of 1.15 (95% CI, 1.02-1.29) for incident CKD Stage 3 and/or RKFD. Compared to the lowest 90%, the highest decile of KIM-1 was associated with an OR of 2.02 (95% CI, 1.15-3.56) for the outcome; these associations were independent of albuminuria. NGAL levels (ng/ml) were not associated with incident CKD Stage 3 and/or RKFD (OR, 1.04; 95% CI, 0.99-1.10). Results were similar when KIM-1 and NGAL were standardized for urine creatinine.
Limitations
The case-control design limits ability to account for persons who died or were not available for follow-up.
Conclusions
Urinary KIM-1 is associated with future risk of kidney disease independent of albuminuria. Urinary biomarkers of tubular injury are a promising tool for identifying persons at risk for CKD.
Keywords: KIM-1, NGAL, kidney function decline
Chronic kidney disease (CKD), defined as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2, is highly prevalent(1) and it is associated with increased risk of death, cardiovascular disease and progression to chronic kidney failure.(2) Identification of persons at risk for developing CKD is paramount in designing prevention strategies. Recent work has focused on understanding risk factors for development of CKD.(3) However, clinically available markers detect CKD only when the disease is established, there is extensive kidney damage, and the window for primary prevention has closed. Albuminuria (defined as a urinary albumin-creatinine ratio ≥30 mg/g) has been advocated as an early marker of kidney disease. However, albuminuria primarily reflects glomerular damage,(4) and national data suggest that most of the non-diabetic CKD in the U.S. may be non albuminuric.(1) Among persons with preserved GFR, novel markers of kidney injury could potentially identify persons at risk for development of CKD to allow investigation of targeted prevention strategies.
Urinary biomarkers of kidney injury such as urinary kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) have emerged as predictors of acute kidney injury before reductions in eGFR are detectable.(5-8) High levels of urinary KIM-1 and NGAL are associated with the extent of acute kidney injury, length of hospitalization, outcomes after cardiac surgery, and death.(9-11) Whether or not these urinary markers of acute injury are associated with longer-term kidney function decline in the ambulatory setting is not known. Urinary KIM-1 and NGAL are particularly promising markers to identify persons at risk for CKD because they are expressed by tubular epithelial cells in response to injury and tubulointerstitial damage is a common pathway in the progression of most forms of kidney disease.(12, 13)
We designed this nested case-control study to evaluate the association of urinary KIM-1 and NGAL levels with kidney function decline and incident CKD Stage 3 in the Multi-Ethnic Study of Atherosclerosis (MESA). We hypothesized that higher levels of these biomarkers would predict rapid kidney function decline and the development of CKD.
Methods
The Multi-Ethnic Study of Atherosclerosis
MESA is a large, NHLBI-sponsored study designed to understand subclinical cardiovascular disease and its progression in a multi-ethnic cohort. Details on recruitment and design have been previously published.(14) Briefly, between 2000 and 2002, MESA recruited 6,814 men and women aged 45 to 84 years old who were free of cardiovascular disease and who self-identified as White, Black, Hispanic or Chinese. Persons were recruited from Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan and the Bronx, New York; and St. Paul, Minnesota. Participants returned for 3 visits, at years 2002-2004 (exam 2), years 2004-2005 (exam 3), and years 2005-2007 (exam 4). Repeat measures of kidney function were done at visits 3 and 4. The institutional review boards at all participating centers approved the study, and all participants gave written informed consent.
Measures of Kidney Function
Kidney function was assessed by creatinine and cystatin C. All assays were performed in frozen serum specimens that were stored at -70°C. Serum creatinine was measured by rate reflectance spectrophotometry using thin-film adaptation of the creatine amidinohydrolase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, NY) at the Collaborative Studies Clinical Laboratory at Fairview-University Medical Center (Minneapolis, MN) and calibrated to Cleveland Clinic. Cystatin C was measured by means of a particle-enhanced immunonephelometricassay (N Latex Cystatin C, Siemens) with a nephelometer (BNII, Siemens) and corrected for assay drift. We used the CKD-EPI equations to estimate GFR from creatinine (eGFRcr) and from cystatin C (eGFRcys=76.7 × [cystatin C]-1.19) for cystatin C.(15)
Selection of Cases and Controls
We excluded persons with eGFRcr<60 ml/min/1.73m2 at the baseline visit, following CKD guideline definitions.(16) Cases were defined as persons with eGFR >60 ml/min/1.73m2 (by both creatinine and cystatin C) at baseline who subsequently developed incident CKD Stage 3 and/or had rapid kidney function decline by the MESA year 5 visit (N=343). Controls were individually matched for age, gender, race, diabetes, and baseline eGFR. Specifically, cases were matched to controls within 10 years of age (45-54, 55-64, 65-74 and 75-84) and within 10 ml/min/1.73m2 of baseline eGFR as 60-69, 70-79, 80-89, 90-99, 100-109, 110-119, and >120 ml/min/1.73m2.
In this study, ncident CKD Stage 3 was defined as both reaching an eGFRcys <60 ml/min/1.73m2 and also having an eGFRcys decline >1 ml/min/1.73m2 per year. We used this definition to reduce misclassification due to small changes around the CKD threshold. Rapid decline was defined as eGFRcys decline of ≥ ml/min/1.73m2 per year. This definition has been associated with increased risk of death and cardiovascular outcomes, independent of baseline eGFR.(17, 18) For both outcomes, we based our definitions based on eGFRcys because we have shown that eGFRcys significantly reduces misclassification of CKD status based on creatinine.(19, 20) Controls were individually matched for age, gender, race, diabetes, and baseline eGFR. Total sample size was 343 cases and 343 controls.
Measurement of Urinary KIM-1 and NGAL
Urinary KIM-1 and NGAL were measured from previously frozen stored urine samples. Urinary soluble KIM-1 and NGAL were measured by a microbead-based assay as previously described.(21) The inter- and intra-assay coefficient of variation for KIM-1 and NGAL was less than 8%. Urine creatinine concentrations were measured by the Jaffé assay using Randox Daytone Analyzer (Randox Laboratories Ltd., UK). The inter- and intra-assay coefficient of variation for creatinine was less than 3%. KIM-1and NGAL concentrations were also standardized for urinary creatinine measured concurrently. All laboratory personnel performing measurements were blinded to case-control status, and all statistical analyses were performed at the MESA coordinating center (by R.K.).
Covariates
Information on age and self-reported race/ethnicity was obtained using standardized questionnaires. Blood pressure measurements were obtained using the Dinamap® automated blood pressure device (Dinamap Monitor Pro 100®). Three sequential measures were obtained and the average of the second and third measurements was recorded. Hypertension was defined as systolic pressure ≥140 mm Hg, diastolic pressure ≥90 mm Hg, or current use of antihypertensive medication. Diabetes was defined as either a fasting glucose ≥126 mg/dl or use of oral hypoglycemic medication or insulin. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Fasting blood was collected and stored at -70°F until needed for the appropriate assays. High density lipoprotein (HDL) cholesterol was measured using the cholesterol oxidase cholesterol method (Roche Diagnostics). Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation. Urine albumin and creatinine were measured by MESA in a single morning urine sample by nephelometry and the rate Jaffe reaction, respectively, and expressed as albumin-creatinine ratio (ACR) in mg/g. We define albuminuria as ACR ≥30 mg/g.
Analyses
Baseline characteristics were compared for cases and controls using the t-test or Chi-squared test where appropriate. We calculated pair-wise Spearman correlation coefficients among urinary KIM-1, NGAL, and ACR. The distribution of concentrations of urinary KIM-1 and NGAL in the study population was evaluated and discovered to be skewed. Therefore, we used each marker as a log-transformed continuous variable (with and without standardization for creatinine). We also categorized each marker into deciles. We used a decile categorization based on the distributions of the biomarkers and for ease of clinical comparisons with ACR. We examined the distribution of the biomarker levels, and we evaluated differences at the tails of these distributions in order to ensure we would not miss important threshold effects. In addition, since the cutpoint of ACR >30 mg/g corresponded to the highest decile, we explored this cutpoint for the biomarkers. In prior analyses, the association between ACR and incident CKD Stage 3 in MESA was found to be non-linear and primarily observed among participants with ACR>30 mg/g.(22) We investigated the associations of baseline covariates with urinary concentrations of log transformed urinary KIM-1 and NGAL using linear regression, and we compared associations with ACR. Beta coefficients were back transformed to relative differences for ease of interpretation.
Next, we calculated the proportion of cases among persons within each decile of KIM-1 and NGAL respectively. Then, using multivariable conditional logistic regression, we evaluated the associations of KIM-1 and NGAL with the combined outcome of incident CKD Stage 3 and/or rapid decline. We present results for the biomarkers as continuous (per doubling) and for the top decile compared with lower 90th percentile. We adjusted for age, presence of hypertension and presence of albuminuria in staged models. As a sensitivity analysis, we repeated analyses for incident CKD Stage 3 and rapid decline separately.
Results
Participant Characteristics by Case-Control Status
Among 686 participants (343 case-control pairs), mean age was 67±9 years. Participants were well matched for age, gender, race, diabetes, and baseline eGFR. In addition, concentrations of serum lipids, glucose levels, and systolic blood pressure levels were similar for cases and controls. In contrast, there were a higher proportion of persons with albuminuria and hypertension among cases compared with controls (Table 1).
Table 1.
Baseline Characteristics by Case-Control Status
| Controls (n=343) | Cases(n=343) | P | |
|---|---|---|---|
| Age (y)* | 67 ± 9 | 67 ± 9 | 0.8 |
| Female sex* | 176 (51%) | 176 (51%) | 0.9 |
| Race* | 0.9 | ||
| White | 128 (37%) | 128 (37%) | |
| Chinese | 22 (6%) | 22 (6%) | |
| African-American | 103 (30%) | 103 (30%) | |
| Hispanic | 90 (26%) | 90 (26%) | |
| Hypertension | 187 (55%) | 220 (64%) | 0.01 |
| Diabetes* | 63 (18%) | 63 (18%) | 0.9 |
| Systolic Blood Pressure (mmHg) | 133 ± 21 | 134 ± 22 | 0.3 |
| Glucose (mg/dL) | 101 ± 33 | 101 ± 33 | 0.9 |
| Body Mass Index (kg/m2) | 29.1 (5.8) | 29.8 (6.1) | 0.2 |
| LDL Cholesterol (mg/dl) | 115 ± 28 | 116 ± 34 | 0.6 |
| HDL Cholesterol (mg/dl) | 49 ± 13 | 50 ± 14 | 0.5 |
| Lipid-Lowering Medication | 66 (19%) | 62 (18%) | 0.8 |
| ACR ≥30 mg/g | 22 (6%) | 58 (17%) | <0.001 |
| eGFRcys | |||
| Baseline* (ml/min/1.73m2) | 81 ± 10 | 80 ± 10 | 0.7 |
| Follow-Up (ml/min/1.73m2) | 82 ± 13 | 58 ± 10 | <0.001 |
| Annual change** (ml/min/1.73m2/y) | 0.7 ± 3.1 | -6.2 ± 4.0 | <0.001 |
| Urinary creatinine–standardized KIM-1 (pg/mg) | 418 [239-689] | 473 [287-818] | 0.03 |
| Urinary creatinine–standardized NGAL (ng/mg) | 4.33 [1.17-13.00] | 5.69 [1.44-14.79] | 0.2 |
Note: Values for continuous variables given as mean ± SD or median [95th-75th percentile]; values for categorical variables given as number (%).
Indicates variables matched in case-control
over follow-up period
Abbreviations: Albumin-creatinine ratio (ACR); estimated glomerular filtration rate by cystatin C (eGFRcys); NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule 1; HDL, high-density lipoprotein; LDL, low-density lipoprotein
Among the 343 cases, 145 persons had incident CKD Stage 3, 141 had rapid kidney function decline, and 57 had both outcomes. Mean eGFR at baseline was similar for cases and controls by both cystatin C (Table 1) and creatinine (eGFRcr = 75 ± 11 ml/min/1.73m2 for both groups). Persons classified as cases had a 22 ml/min/1.73m2 lower eGFR at end of follow-up and their mean decline was over six-fold compared with controls. (Table 1) Persons identified as cases by incident CKD Stage 3 alone had a baseline eGFRcys of 78±15 ml/min/1.73m2 and eGFRcys of 51±8 ml/min/1.73m2 at year 5, with a mean decline of 7.5±5.2 ml/min/1.73m2 over the follow up period. Cases meeting criteria by rapid decline alone had a mean eGFRcys of 83±4 at baseline and 67±5 ml/min/1.73m2 at follow-up, with a mean decline of 4.4±1.3 ml/min/1.73m2.
Associations of Baseline Characteristics with KIM-1 and NGAL
Urinary KIM-1 and NGAL were only modestly correlated with each other and with ACR; Correlation coefficients were 0.1 (p-value 0.009) for KIM-1 with NGAL; 0.19 (p <0.001) for KIM-1 with ACR and 0.11 (p-value 0.01) for NGAL with ACR. Older age was significantly associated with higher levels of urinary creatinine–standardized KIM-1 and NGAL, but neither hypertension nor diabetes was associated with levels of either marker. Among other covariates, only HDL was significantly associated with NGAL. In contrast, higher BMI, higher glucose levels and the presence of diabetes and hypertension were significantly associated with higher ACR levels (Table 2).
Table 2.
Predictors of Urinary Levels of KIM-1, NGAL, and ACR at Baseline
| Covariate** | KIM-1* | NGAL* | ACR |
|---|---|---|---|
| Age, SD=9 | 16 (9, 22) | 20 (2, 41) | 3 (-6, 13) |
| Body Mass Index, SD=5.9 | 2 (-4, 8) | 5 (-11, 23) | 15 (5, 26) |
| LDL cholesterol, SD=31 | -6 (-12, -1) | -5 (-20, 11) | -4 (-13, 5) |
| HDL cholesterol, SD=14 | 6 (-1, 13) | 35 (14, 58) | -4 (-13, 5) |
| Systolic Blood Pressure, SD=21 | 2 (-4, 8) | 1 (-15, 18) | 30 (19, 42) |
| Glucose, SD=33 | 7 (0, 13) | -18 (-30, -4) | 40 (28, 52) |
| Diabetes | 16 (-1, 35) | -21 (-49, 18) | 173 (116, 239) |
| Hypertension | -4 (-15, 9) | -2 (-31, 35) | 62 (35, 94) |
Values shown are relative difference (in %); 95% confidence interval given in parentheses. relative difference = Exp(β), and can be interpreted as the relative difference in the biomarker per SD higher increment (or presence) of each covariate, expressed as %. For example, 9 years of advanced age was associated cross-sectionally with 16% higher mean KIM-1 levels. A negative sign refers to a decrease in the biomarker.
Standardized to urinary creatinine.
Each covariate is entered separately into the model.
Abbreviations: kidney injury molecule 1 (KIM-1); neutrophil gelatinase-associated lipocalin (NGAL); low-density lipoprotein (LDL); high-density lipoprotein (HDL); estimated glomerular filtration rate by cystatin C (eGFRcys); SD, standard deviation
Association of Urinary KIM-1 and NGAL with Incident CKD Stage 3 and/or Rapid Decline
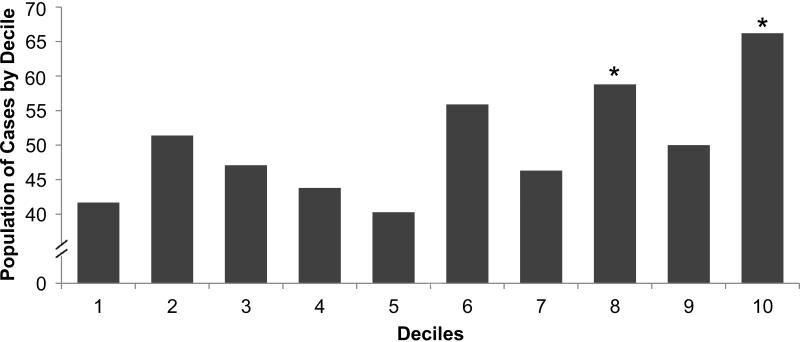
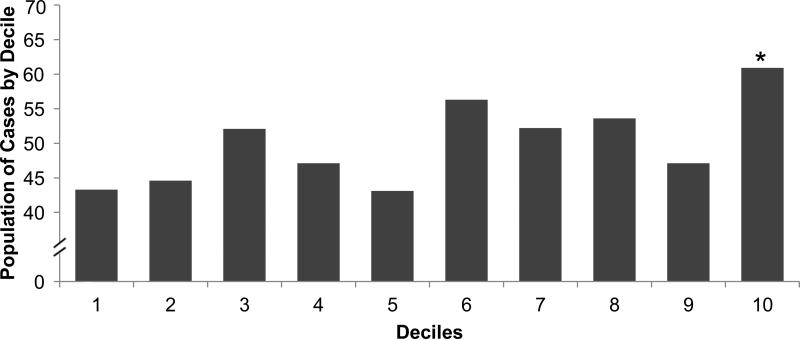
When evaluated as continuous variables, levels of of urinary creatinine–standardized KIM-1 but not NGAL were higher among cases compared with controls (Table 1). When categorized into deciles, persons in the top decile of urinary KIM-1 and NGAL were most likely to develop incident CKD Stage 3 and/or rapid decline. (Figure 1) Over 66% of persons in the highest decile of KIM-1 developed the outcome, compared with 48% among the remainder lower 90th percentile, p-value 0.01. For NGAL, these proportions were 61% vs. 48%, p-value 0.06. In multivariable models, higher levels of KIM-1 were significantly associated with incident CKD Stage 3 and/or rapid decline. (Table 3) Every doubling of KIM-1 was associated with 15% higher odds of the outcome after adjustment for age and hypertension. Adjustment for albuminuria did not attenuate these findings. Findings were similar when KIM-1 was standardized for urinary creatinine. When used as a categorical variable, persons in the highest decile of KIM-1 had 2-fold odds of incident CKD Stage 3 and/or rapid decline compared with the lower 90%. These associations were independent of the presence of albuminuria. The findings did not differ when including baseline eGFRcys in the models (ORs of 1.15 [95% CI, 1.02-1.30] per doubling of KIM-1 and 1.97 [95% CI, 1.12-3.47] for the top decile compared to the lower 90% of KIM-1). Every doubling of NGAL levels was associated with 4% higher odds of the outcome, but this finding was not statistically significant (Table 3), nor was the association of the highest decile of NGAL.
Figure 1.
Proportion of Persons with Incident CKD Stage 3 and/or Rapid Kidney Function Decline by Decile of KIM-1 or NGAL. Proportion of cases in each decile category is represented. By design, the total proportion of cases was 50% in this nested case-control study. Upper panel, KIM-1: *p=0.04 for 8th decile and p=0.004 for 10th decile when compared to the 1st decile. Lower panel, NGAL: *p <0.05 when compared to the 1st decile
Table 3.
Association of Urinary KIM-1 and Urinary NGAL with Incident CKD and/or Rapid Kidney Function Decline
| Model | KIM-1 | NGAL | ||||
|---|---|---|---|---|---|---|
| Rawa | Cr-standardizeda | Top decile (≥ 927 pg/mL)b | Rawa | Cr-standardizeda | Top decile (≥ 36 ng/mL)b | |
| Combined Outcomee | ||||||
| Age Adjusted | 1.15 (1.02-1.29) | 1.17 (1.02-1.34) | 2.09 (1.21-3.62) | 1.04 (0.99-1.10) | 1.03 (0.98-1.09) | 1.63 (0.96-2.78) |
| Age + HTN Adjusted | 1.15 (1.03-1.29) | 1.16 (1.01-1.33) | 2.13 (1.22-3.70) | 1.04 (0.99-1.10) | 1.03 (0.98-1.09) | 1.58 (0.93-2.71) |
| + Albuminuria | 1.15 (1.02-1.29) | 1.13 (0.98-1.30) | 2.02 (1.15-3.56) | 1.04 (0.99-1.10) | 1.03 (0.97-1.08) | 1.55 (0.89-2.70) |
| Rapid Decline >3 ml/min/1.73 m2 per yearc | ||||||
| Age Adjusted | 1.16 (0.96-1.39) | 1.28 (1.02-1.59) | 1.79 (0.79-4.08) | 1.03 (0.94-1.12) | 1.03 (0.95-1.12) | 1.88 (0.74-4.74) |
| Age + HTN Adjusted | 1.14 (0.95-1.38) | 1.27 (1.01-1.58) | 1.76 (0.77-4.02) | 1.03 (0.95-1.12) | 1.04 (0.95-1.13) | 1.81 (0.71-4.58) |
| + Albuminuria | 1.19 (0.98-1.48) | 1.31 (1.04-1.65) | 1.79 (0.79-4.07) | 1.01 (0.93-1.11) | 1.02 (0.93-1.11) | 1.63 (0.63-4.22) |
| Incident CKD Stage 3d | ||||||
| Age Adjusted | 1.15 (0.99-1.33) | 1.10 (0.93-1.31) | 2.36 (1.12-4.94) | 1.05 (0.99-1.13) | 1.04 (0.97-1.11) | 1.56 (0.81-2.99) |
| Age + HTN Adjusted | 1.18 (1.01-1.37) | 1.11 (0.93-1.32) | 2.54 (1.19-5.41) | 1.05 (0.98-1.13) | 1.03 (0.96-1.10) | 1.53 (0.79-2.98) |
| + Albuminuria | 1.16 (0.99-1.35) | 1.04 (0.86-1.25) | 2.35 (1.08-5.14) | 1.05 (0.98-1.13) | 1.02 (0.96-1.10) | 1.52 (0.76-3.05) |
Note: Values shown are OR (95% CI). Raw KIM-1 measurements were in pg/mL, Cr-standardized as pg/mg. Raw NGAL measurements were in ng/mL, Cr-standardized as ng/mg.
Per doubling of biomarker
Highest decile compared to lower 90%.
Cases defined by rapid kidney function decline; controls were matched on age, gender, race, diabetes status and baseline eGFRcys. Total number is 282 (141 cases; 141 controls). Cases with incident CKD stage 3 in addition to rapid kidney function decline were excluded.
Cases defined by incident CKD Stage 3 (n = 202; these include 57 cases who had incident CKD Stage 3 and rapid kidney function decline); controls (n=202) were matched on age, gender, race, diabetes status and baseline eGFRcys.
Based on 343 cases, 343 controls.
Abbreviations: HTN, hypertension; CKD, chronic kidney disease; kidney injury molecule 1 (KIM-1); neutrophil gelatinase-associated lipocalin (NGAL); creatinine (Cr); estimated glomerular filtration rate by cystatin C (eGFRcys)
To allow comparisons with a clinically established marker of kidney injury, we also evaluated the association of albuminuria (ACR ≥ vs. < 30 mg/g) with the combined outcome. The OR for elevated ACR was 3.25 (95% CI, 1.86-5.69) after adjustment for age and hypertension. Further adjustment for KIM-1 and NGAL minimally attenuated this effect to 3.16 (95% CI, 1.79-5.59).
In a sensitivity analysis, we also studied the association of biomarkers categorized into quartiles with kidney function decline. The age and hypertension adjusted OR for the highest quartile of KIM-1 (≥558.13) compared to the lowest was 1.59 (95% CI, 1.02-2.48). Adjustment for albuminuria only minimally attenuated the OR to 1.53 (95% CI, 0.97-2.41). The OR for the highest NGAL quartile compared to the lowest was 1.35 (95% CI, 0.85-2.15) in fully adjusted models
We also evaluated the two case definitions (incident CKD Stage 3 and rapid decline) separately as secondary outcomes. Higher levels of of urinary creatinine–standardized KIM-1 were associated with higher odds of rapid decline when used as a continuous variable, but not when dichotomized at the top decile. In contrast, persons with urinary KIM-1 levels in the highest decile had a significantly higher odds of incident CKD Stage 3 compared with the lower 90%, but not of rapid decline. Higher levels of NGAL were not significantly associated with rapid decline or incident CKD Stage 3 (Table 3).
Discussion
Identification of persons with mild kidney injury who may be at risk for development of chronic kidney disease (CKD) has been hindered by our inability to noninvasively capture injury within the kidney while it may still be reversible. Available clinical markers of kidney function (i.e. creatinine, cystatin C) become elevated only after extensive damage has already accrued and the glomerular filtration rate (GFR) has decreased. Albuminuria can detect earlier forms of kidney disease, but its presence primarily reflects glomerular damage. Tubulointerstitial damage is a common pathway for progression of most forms of kidney disease, including glomerular diseases.(12, 23) We hypothesized that urinary levels of proteins known to be expressed by injured renal tubular cells would be associated with future risk of CKD. We found elevated urinary levels of KIM-1 to be significantly associated with incident CKD Stage 3 and rapid kidney function decline, and that this association was independent of albuminuria. We also found that traditional kidney disease risk factors such as diabetes and high blood pressure were not associated with KIM-1 levels. In contrast to findings with KIM-1, levels of urinary NGAL were not significantly associated with kidney disease risk in this study.
To our knowledge, the study of associations between urine biomarkers of kidney injury with incident CKD Stage 3 or rapid kidney function decline in persons without CKD has only recently begun. In a case-control study nested within the ARIC (Atherosclerosis Risk in Communities) Carotid MRI Study (143 cases), higher levels of trefoil factor 3 (TFF3) were associated with incident CKD Stage 3.(24) A recent case-control study from the Framingham cohort (100 cases) found that higher levels of urinary connective tissue growth factor (CTGF), a fibrosis marker, were associated with a lower risk for incident CKD Stage 3, but this finding was not replicated in ARIC.(25) Each of these studies used the MDRD (Modification of Diet in Renal Disease) Study equation to define CKD.(20)
Urinary KIM-1, which is among the most well-studied urinary biomarkers is expressed in the apical membrane of proximal tubular cells in response to injury.(26) KIM-1 levels rise in multiple types of injury including glomerular, tubular, or interstitial, and its levels have been shown to correlate with degree of injury.(27, 28) KIM-1 is also a well-established predictor of AKI after cardiac surgery and in the critical care setting,(11) it can predict allograft rejection after kidney transplant;(29) and adverse outcomes among persons with heart failure.(10) Our findings suggest that KIM-1 may also reflect ongoing, chronic kidney damage and it may potentially identify persons at high risk for developing CKD. Our findings also suggest that the mechanisms may differ from those associated with albuminuria.
Our results must be replicated in future studies in other large population-based cohorts. Since urinary KIM-1 levels have been shown to decrease in parallel with reduction of proteinuria among persons with non diabetic CKD,(30) future studies should determine whether KIM-1 levels measured longitudinally are indicative of the success of prevention strategies such as blood pressure control. Another potential use of KIM-1 that should be studied could be to detect renal toxicity of drugs before damage is irreversible. In fact, the US Food and Drug Administration and the European Medicines Agency have recommended the use of KIM-1 as part of a panel of urinary markers to detect drug toxicity in preclinical settings.(31)
Our findings that urinary levels of NGAL were not associated with future kidney disease risk were somewhat surprising. NGAL has also been shown to predict AKI,(11, 32) and urine NGAL predicts kidney disease progression in persons with established CKD.(33, 34) One possibility is that urinary NGAL may be more useful as a marker of progression in existing disease.(35) Moreover, limited power may have biased our results toward the null. Future studies are required to confirm these negative findings.
Our study strengths include the use of two novel urinary biomarkers well studied in humans, the relatively number of cases, the multiethnic sample, and the use of eGFRcys. Several important limitations should be noted. The use of a case-control design limits our ability to account for persons who died or were not available for follow-up. We do not have direct measures of GFR or kidney biopsies, but this approach is not feasible for large population studies. Our length of follow up is relatively short; however cases of rapid decline are likely to capture persons with clinically important risk. The strength of the association between KIM-1 and incident CKD Stage 3 or rapid decline was smaller than that reported for ACR.(22) However, the knowledge that levels of KIM-1 rise in the setting of many forms of kidney injury makes it a particularly promising biomarker.
In summary, we found that higher levels of urinary KIM-1, but not NGAL, may predict future CKD risk and kidney function decline, and these findings were independent of the presence of albuminuria. Traditional CKD risk factors were not associated with KIM-1 levels. These findings suggest that KIM-1 may be capturing kidney injury in mechanisms that differ from albuminuria. Future studies are needed to evaluate whether KIM-1 may be useful in detecting persons at high risk for CKD.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at www.mesa-nhlbi.org.
Support: This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute, by the NIDDK (1K23DK082793-01, CP), and by the Robert Wood Johnson Foundation (Harold J. Amos Award, CP). These funding sources had no involvement in the design or execution of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
N SECTION: Because the Editor-in-Chief and Deputy Editor recused themselves from consideration of this manuscript, the peer-review and decision-making processes were handled entirely by a Co-Editor (Laura M. Dember, MD) who served as Acting Editor-in-Chief. Details of the journal's procedures for potential editor conflicts are given in the Editorial Policies section of the AJKD website.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 4.Guasch A, Deen WM, Myers BD. Charge selectivity of the glomerular filtration barrier in healthy and nephrotic humans. J Clin Invest. 1993;92:2274–2282. doi: 10.1172/JCI116831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nickolas TL, O'Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 8.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 9.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4:873–882. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damman K, Van Veldhuisen DJ, Navis G, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart. 2010;96:1297–1302. doi: 10.1136/hrt.2010.194878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parikh CR, Devarajan P, Zappitelli M, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuncio GS, Neilson EG, Haverty T. Mechanisms of tubulointerstitial fibrosis. Kidney Int. 1991;39:550–556. doi: 10.1038/ki.1991.63. [DOI] [PubMed] [Google Scholar]
- 13.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 14.Bild D, Bluemke DA, Burke G, et al. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 15.Inker LA, Eckfeldt J, Levey AS, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58:682–684. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49:S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Shlipak MG, Katz R, Kestenbaum B, et al. Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol. 2009;20:2625–2630. doi: 10.1681/ASN.2009050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168:2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peralta CA, Katz R, Sarnak MJ, et al. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol. 2011;22:147–155. doi: 10.1681/ASN.2010050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaidya VS, Waikar SS, Ferguson MA, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1:200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shastri S, Katz R, Shlipak MG, et al. Cystatin C and albuminuria as risk factors for development of CKD stage 3: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2011;57:832–840. doi: 10.1053/j.ajkd.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 24.Astor BC, Kottgen A, Hwang SJ, Bhavsar N, Fox CS, Coresh J. Trefoil Factor 3 Predicts Incident Chronic Kidney Disease: A Case-Control Study Nested within the Atherosclerosis Risk in Communities (ARIC) Study. Am J Nephrol. 2011;34:291–297. doi: 10.1159/000330699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Seaghdha CM, Hwang SJ, Bhavsar NA, et al. Lower urinary connective tissue growth factor levels and incident CKD stage 3 in the general population. Am J Kidney Dis. 2011;57:841–849. doi: 10.1053/j.ajkd.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonventre JV, Yang L. Kidney injury molecule-1. Curr Opin Crit Care. 2010;16:556–561. doi: 10.1097/MCC.0b013e32834008d3. [DOI] [PubMed] [Google Scholar]
- 27.Chiusolo A, Defazio R, Zanetti E, et al. Kidney injury molecule-1 expression in rat proximal tubule after treatment with segment-specific nephrotoxicants: a tool for early screening of potential kidney toxicity. Toxicol Pathol. 2010;38:338–345. doi: 10.1177/0192623310362244. [DOI] [PubMed] [Google Scholar]
- 28.Tonomura Y, Tsuchiya N, Torii M, Uehara T. Evaluation of the usefulness of urinary biomarkers for nephrotoxicity in rats. Toxicology. 2010;273:53–59. doi: 10.1016/j.tox.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Szeto CC, Kwan BC, Lai KB, et al. Urinary expression of kidney injury markers in renal transplant recipients. Clin J Am Soc Nephrol. 2010;5:2329–2337. doi: 10.2215/CJN.01910310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waanders F, Vaidya VS, van Goor H, et al. Effect of renin-angiotensin-aldosterone system inhibition, dietary sodium restriction, and/or diuretics on urinary kidney injury molecule 1 excretion in nondiabetic proteinuric kidney disease: a post hoc analysis of a randomized controlled trial. Am J Kidney Dis. 2009;53:16–25. doi: 10.1053/j.ajkd.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dieterle F, Sistare F, Goodsaid F, et al. Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol. 2010;28:455–462. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- 32.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 33.Bolignano D, Coppolino G, Campo S, et al. Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol. 2007;27:373–378. doi: 10.1159/000103912. [DOI] [PubMed] [Google Scholar]
- 34.Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80:806–821. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]