Abstract
This study aimed to examine sex differences in cocaine self-administration and cocaine-induced subjective and cardiovascular measures. The research was based on secondary analysis of data collected in our human laboratory in which subjects self-administered cocaine infusions (8, 16 and 32 mg/70 kg) over a 2-hour period under a fixed ratio 1, 5 minute time out schedule in three test sessions. Subjects were 10 women and 21 men with a history of either cocaine abuse or dependence who were not currently seeking treatment. Women and men self-administered similar amounts of cocaine. None of the subjective effects measures showed a significant main effect of sex during the cocaine self-administration session. Significant interactions were observed for subjective ratings of ‘high’ (sex × time) and ‘stimulated’ (sex × time × dose), with women reporting lower ratings over time/doses than men. Relative to men, cocaine produced dose- and time-dependent increases in feelings of hunger (i.e., reduced appetite suppression) in women. Systolic and diastolic blood pressures showed different patterns of change in men and women, with women showing less robust cocaine-induced increases than men. Taken together, these findings suggest that women and men may differ in their subjective and cardiovascular responses to self-administered cocaine. Further research that prospectively controls for hormonal influences upon these measures is needed.
Keywords: Cardiovascular effects, cocaine, human, patient-controlled analgesia (PCA), regulation, subjective effects
INTRODUCTION
Despite the large number of women that abuse cocaine, few investigations have examined the question of sex differences in cocaine abuse research. Evidence from studies in laboratory animals indicates that males and females differ in their behavioral response to cocaine and in their sensitivity to the reinforcing effects of the drug as measured using self-administration and conditioned place-preference paradigms (for reviews, see Lynch, Roth & Carroll 2002; Carroll et al. 2004). For example, female rats show a greater locomotor response to acute and repeated cocaine than do males (for review, see Becker, Molenda & Hummer 2001), and they generally acquire cocaine self-administration more rapidly and after less drug exposure (Lynch & Carroll 1999; Hu et al. 2004). When intake is compared, the results have generally shown that male and female rats do not differ in levels of daily cocaine intake (Lynch et al. 2002), although females will work harder to obtain cocaine infusions (e.g., as assessed by responding under a progressive ratio schedule; Roberts, Bennett & Vickers 1989). Taken together, these pre-clinical findings suggest that there are sex differences in response to aspects of the behavioral and reinforcing effects of cocaine.
Potential differences in response to cocaine in women and men are less clear due to the relatively few clinical studies conducted under controlled laboratory conditions. Three studies have compared the acute effects of cocaine between men and women. Lukas and colleagues (Lukas et al. 1996) found that following a single dose of intranasal cocaine (0.9 mg/kg), men and women had a similar cardiovascular response to intranasal cocaine, but that men detected the effects of cocaine faster and had a greater number of intense good and bad effects from cocaine compared with women. Kosten and colleagues (Kosten et al. 1996) found that a single intra-nasal dose of cocaine (2.0 mg/kg) produced similar cardiovascular and euphoric (‘high’) responses in women and men, but that women reported higher levels of ‘nervousness’ compared with men. Singha and colleagues (Singha et al. 2000) reported that following an oral delivery of cocaine (80 mg/70 kg), men had a greater baseline increase in systolic blood pressure (SBP). Additionally, they found that women reported higher levels of ‘bad drug effects’ and ‘anxious/nervous’ effects, though similar observations were reported following placebo.
To our knowledge, only two studies have examined sex differences following repeated administration. Evans and colleagues (Evans et al. 1999) compared the effects of repeated smoked cocaine (up to six doses of 50 mg) on cocaine choice (i.e., cocaine versus nothing) and cardiovascular and subjective effects. They found that men and women obtained a similar number of cocaine doses, though both men and women obtained most, if not all, of the cocaine that was available. They also reported that men and women showed similar changes in subjective ratings and cardiovascular effects, although the dose of cocaine tested was not adjusted for sex differences in body weights. Using a similar choice procedure, Sofuoglu and colleagues (Sofuoglu et al. 1999) found that women and men self-administered a similar number of cocaine doses, with men and women obtaining most, if not all, of the cocaine available. Here, with a similar g/kg dose of cocaine, they found that the cardiovascular response to multiple doses of cocaine was similar between men and women, but that women had lower ratings on ‘high’, ‘stimulated’ and ‘heart racing/pounding’. Hence, these human cocaine studies suggest that men and women may show a differential response to some of cocaine’s effects.
In the current study, we examined women’s and men’s responses to the reinforcing, behavioral and physiological effects of cocaine under less restrictive, ad libitum cocaine self-administration procedures (e.g., in contrast to prior choice methods). We analyzed data from existing studies carried out in our human laboratory in which subjects self-administered cocaine infusions (8, 16 and 32 mg/70 kg) over a 2-hour period under a fixed ratio 1, 5 minute time out schedule in three test sessions (Sughondhabirom et al. 2005; Kalayasiri et al. 2006; Lynch et al. 2006). Although these studies differed with respect to the study design, in each of these studies subjects were given access to self-regulated intravenous cocaine infusions (8, 16 and 32 mg/70 kg body weight; one condition per day) under a fixed ratio 1: 5 minutes time out schedule during daily 2-hour sessions. Two studies were model validation experiments that used either an escalating-dose paradigm (Sughondhabirom et al. 2005) or a fully randomized design (Lynch et al. 2006). A third is an ongoing study examining the effects of 250 mg disulfiram or placebo pre-treatment on cocaine self-administration. Only the placebo pretreatment sessions were included in the present analysis (three sessions total; 8, 16, 32 mg/70 kg cocaine doses; see Kalayasiri et al. 2006). Subject data from all three studies were pooled in the current manuscript in order to obtain a sufficient sample size to address the new question of sex differences in the regulation of cocaine self-administration and response to cocaine.
MATERIALS AND METHODS
Participants
Twenty-one men (9 Caucasian, 12 African American) and ten women (3 Caucasian, 7 African American) between the ages of 23 and 46 years (37 ± 1 versus 40 ± 2, respectively) were studied as part of several ongoing in-patient studies (as described later; also see Kalayasiri et al. 2006). Recruitment focused on current regular and heavy intravenous and/or smoked cocaine users. All subjects had a history of either cocaine abuse or dependence of at least 2 years’ duration and were not seeking treatment at the time of enrollment. Exclusion criteria included non-substance-related Axis I disorders, cardiac conditions, seizures, diabetes, any current condition requiring medication, sedative hypnotic or opiate dependence, and for women, a positive serum β-human chorionic gonadotropin (i.e., pregnancy) test. All participants were determined to be in good health by unstructured psychiatric interview, physical and neurological examinations, ECG, and routine laboratory testing (i.e., blood chemistries, hematology and urinalysis). Demographic information (e.g., age, race) and retrospective reports of cocaine use history (e.g., age of onset of cocaine use, days per week of cocaine use and average money spent for cocaine per day) were obtained at the time of screening by unstructured interview. The weight of each subject was also recorded at intake, and because of large individual differences in body weights, including differences between men and women (i.e., the average weight in kg was 87 ± 2 for men and 74 ± 9 for women), the unit dose of cocaine was adjusted for differences in body weight (mg/70 kg). The studies were approved by the Yale Department of Psychiatry Research Committee, the Yale University Human Investigation Committee and the Yale General Clinical Research Center General Advisory Committee; voluntary, written, informed consent was obtained from each participant prior to study initiation. All study participants were compensated for their involvement.
Cocaine self-administration studies
Participants resided in a 13-bed, closed, research-dedicated, in-patient psychiatric unit (Clinical Neuroscience Research Unit, CNRU) for at least 5 days while participating in this study. Participants were maintained on a caffeine-free diet and they had access to television, video games, radio, reading material and recreational equipment. Participants were not allowed to leave the unit unless accompanied by a staff member. Subjects who smoked cigarettes were allowed supervised ‘off-unit’ smoke-breaks but were not allowed to smoke during experimental sessions, which lasted approximately 3.5 hours each. Urine specimens were collected and screened three times per week to monitor for the use of illicit drugs.
Experimental sessions
Experimental sessions were conducted in a private room in the Yale General Clinical Research Center between the hours of 10 am and 5 pm. All subjects first completed a safety/training session as described previously (Sughondhabirom et al. 2005). Subsequently, subjects participated in an identical ‘binge’ paradigm of self-regulated intravenous cocaine self-administration wherein cocaine infusions were available under a fixed ratio 1: 5 minutes time out schedule. Sessions consisted of a 2-hour self-administration period during which participants were allowed to self-administer cocaine boluses (8, 16 or 32 mg/70 kg) via manual presses of a corded patient-controlled analgesia (PCA) pump button. The 2-hour self-administration period was preceded by a 30-minute baseline period and followed by a 60-minute period of monitoring post-cocaine.
Subjective effects measures
A computerized questionnaire was completed every 5 minutes during each 3.5-hour session (including the 30-minute baseline period and the 60-minute period of monitoring post-cocaine). The questionnaire consisted of a series of 12 visual analog scale (VAS) ratings that were anchored by not at all (0) at one end and most ever (10) at the other. Ten of these VAS measures were labeled as ‘I feel …’ ‘high’, ‘stimulated’, ‘talkative’, ‘good’, ‘bad’, ‘anxious’, ‘paranoia’, ‘hungry’, ‘restless’, and ‘tongue-tied’, and two questions were labeled as ‘I want …’ ‘cocaine’ and ‘nicotine’. Six of the 31 subjects (five men, one woman) completed only three of the 12 VAS ratings (i.e., ‘high’, ‘stimulated’, ‘paranoia’; see Sughondhabirom et al. 2005).
Vital signs measures
Vital signs [heart rate, SBP, diastolic blood pressure (DBP)] were monitored every 5 minutes throughout each 3.5-hour session. If cardiovascular safety parameters were exceeded during the course of a session, the response button was either temporarily taken away from the participant (i.e., heart rate ≥ 75% of age-adjusted maximum, SBP ≥ 170 mmHg, DBP ≥ 100 mmHg) or permanently suspended (i.e., heart rate ≥ 160 bpm, SBP ≥ 180 mmHg, DBP ≥ 100 mm). In the former case, PCA controls were returned to the subject after vital sign checks revealed a return to levels below these same parameters. Out of 93 cocaine self-administration sessions, there were six sessions in which the safety parameters were exceeded that resulted in pump withholding (i.e., five sessions with the 32 mg/70 kg dose, four men and one woman; one session with the 8 mg/70 kg dose, one man). A basic life support-trained research assistant and an advanced cardiac life support-trained research nurse were present in the study room for the duration of all sessions.
Drugs
Cocaine hydrochloride (Mallinkrodt, St. Louis, MO) was dissolved in sterile saline using aseptic technique by a trained research pharmacist in accordance with Good Manufacturing Practices. Cocaine infusions were delivered via a PCA pump (Abbott Pain Manager II or APM II; Abbott Laboratories, Abbott Park, IL) that had been loaded and programmed by a trained research pharmacist to ensure that other research personnel remained blind to cocaine dose. The PCA pump was programmed to deliver cocaine as a bolus injection of 1 ml over a 35-second interval followed by a 5-minute lockout period. Given this lockout period, it was possible to obtain up to a total of 12 cocaine infusions each hour; however, there was a preprogrammed total dose limit of either 384 mg/70 kg/session (Kalayasiri et al. 2006; Lynch et al. 2006) or 288 mg/70 kg (Sughondhabirom et al. 2005). Once the sum of all doses equaled this limit (seven out of 93 sessions; all at 32 mg/70 kg dose, five cases were men, two cases were women), subsequent button presses did not lead to pump activation. Participants were not informed about either the 5-minute lockout period or the total dose limit.
Data analysis
Self-administration data were checked for normality prior to analysis using Kolmogorov–Smirnov statistics and normal probability plots. Normally distributed data (i.e., infusions of cocaine) were analyzed using mixed effects models, and non-normally distributed data (i.e., responses for cocaine, inter-dose intervals) were subjected to a normalizing transformation. Baseline data on subjective effects and vital signs were analyzed in 5-minute bins and compared between men and women using repeated measures ANOVA. The average values observed at baseline (i.e., during the 30-minute period that preceded the self-administration session) were then subtracted from each value (in 5-minute bins) observed during the self-administration session and the 60-minute post-session monitoring period. This was done regardless of whether or not a baseline sex difference was observed in an attempt to equate men and women prior to the cocaine self-administration session. Because previous work has noted differential effects of sex during the self-administration session as compared with during the post-session monitoring period (Evans et al. 1999), data from the 2-hour self-administration session and the 60-minute post-self-administration monitoring period in the present study were analyzed using separate repeated measures ANOVAs. The results presented focus on differences between men and women because our previous studies have already detailed the effects of cocaine on subjective effects and cardiovascular measures using our validated methods.
RESULTS
Baseline
There was a significant sex difference on baseline ratings of ‘talkative’, with women reporting significantly lower ratings (F1,23 = 4.5, P < 0.05). No other subjective effects measure or cardiovascular measure differed between men and women at baseline. Additionally, as shown in Table 1, women and men did not differ on demographic information or retrospective reports of cocaine use history.
Table 1.
Mean (± SE) demographic and cocaine use data in men and women.
| Women | Men | P-value | |
|---|---|---|---|
| Age | 40.1 ± 2.2 | 37.2 ± 1.2 | P > 0.10 |
| Race | 7 AA, 3 EA | 12 AA, 9 EA | P > 0.10 |
| Age of first cocaine use (years) | 20.2 ± 0.8 | 19.4 ± 1.0 | P > 0.10 |
| Days per week of cocaine use | 5.4 ± 0.6 | 5.8 ± 0.4 | P > 0.10 |
| Amount spent on cocaine per day | 151 ± 36 | 132 ± 32 | P > 0.10 |
AA = African American; EA = European American.
Self-administration
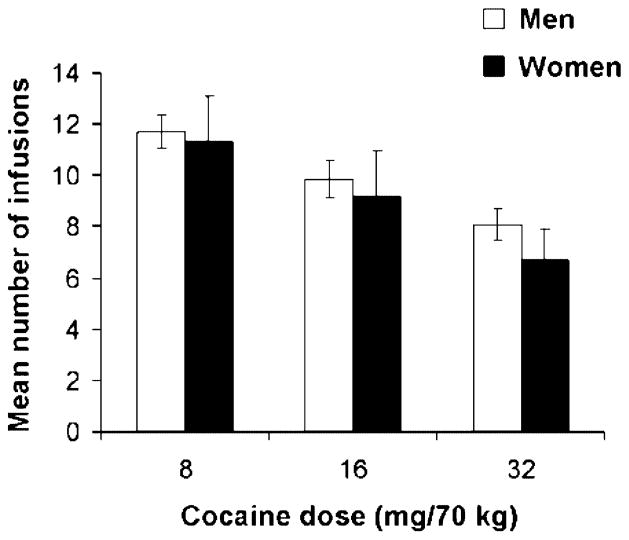
Figure 1 shows the mean number of infusions as a function of cocaine dose for men and women. For both men and women, the number of infusions obtained varied with cocaine dose, with the highest mean values observed for the 8 mg/70 kg dose, intermediate values observed for the 16 mg/70 kg dose and the lowest values observed with the 32 mg/70 kg dose of cocaine. Analysis of infusions as a function of cocaine dose (8, 16 and 32) revealed a significant overall effect for dose (F2,58 = 33.0, P < 0.0001) but non-significant effects of sex and the interaction of sex by dose (Ps > 0.10). Men and women also did not differ on total levels of cocaine self-administered or on patterns of self-administration as assessed by total number of responses and the average cocaine inter-dose intervals (Ps > 0.10; data not shown).
Figure 1.
Cocaine self-administration data depicted as mean number of infusions for men and women for 8, 16 and 32 mg/70 kg test sessions
Subjective effects
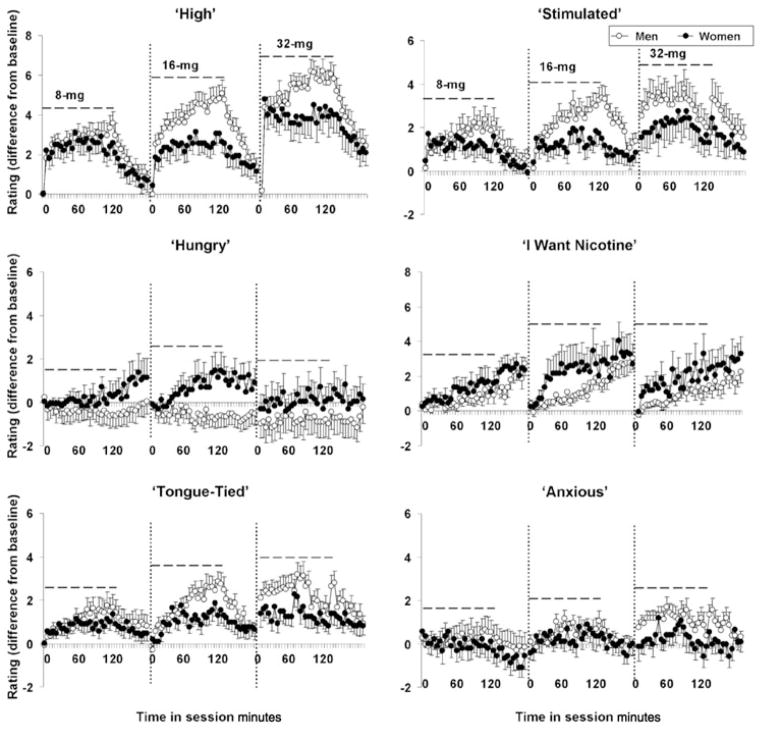
Although none of the subjective effects measures showed a significant main effect of sex during the cocaine self-administration session, significant interactions of sex and time and/or cocaine dose were observed for several of the subjective effects measures (see Fig. 2). Specifically, a significant sex-by-time interaction was observed for ratings of ‘high’, with women reporting lower levels over the course of a session (F24,696 = 2.2, P < 0.001; Fig. 2, upper left panel). Similarly, a significant sex-by-time-by-dose interaction was observed for ratings of ‘stimulated’, with women reporting lower ratings of ‘stimulated’ over the course of a session, particularly at the higher cocaine doses (sex by time by dose; F2,58 = 21.2, P < 0.0001; Fig. 2, upper right panel). Ratings of ‘hungry’ increased throughout the session for women, particularly at the two lower cocaine doses; whereas in men, ratings of ‘hungry’ were dose-dependently decreased throughout the self-administration sessions (Fig. 2, middle left panel). A repeated measures ANOVA of change scores on ratings of ‘hungry’ during the self-administration session revealed significant interactions of sex by time (F24,552 = 2.3, P < 0.001) and sex by time by dose (F48,1104 = 1.5, P < 0.05). Even though a similar number of men and women smoked cigarettes, ratings of ‘want nicotine’ tended to change differently between men and women, with women tending to report higher ratings of ‘want nicotine’, particularly at later time-points in the session and at the intermediate cocaine dose (sex by dose by time; F48,1104 = 1.3, P = 0.07; Fig. 2, middle right panel). Ratings of ‘tongue-tied’ also changed differently between men and women during the cocaine self-administration session, with women tending to report lower effects, particularly at later time-points in the self-administration session (F24,552 = 1.5, P = 0.06; Fig. 2, lower left panel). Although ratings of ‘anxious’ did not differ significantly between men and women during the 2-hour self-administration session, a trend for a significant interaction of sex by dose by time was observed following cocaine self-administration (F22,506 = 1.5, P = 0.07), with ratings tending to be lower in women as compared with men (Fig. 2, lower right panel). Ratings of ‘restless’ also showed a tendency to interact with sex, dose and time (F48,1104 = 1.3, P = 0.08), with women tending to report lower ratings of ‘restless’ at higher cocaine dose and during the later parts of the session (data not shown). No significant differences/meaningful trends were observed between men and women for cocaine-induced changes in ratings of ‘talkative’, ‘good’, ‘bad’, ‘paranoia’ or ‘want cocaine’ (Ps > 0.10).
Figure 2.
Sex differences in the subjective effects of cocaine over time and as a function of cocaine dose. Mean (± SE) minus baseline visual analog scale (VAS; 0 = not at all, 10 = most ever) self-ratings (every 5 minutes) of ‘high’, ‘stimulated’, ‘hungry’, ‘want nicotine’, ‘tongue-tied’ and ‘anxious’ for men (open symbols) and women (closed symbols) subjects during (0–120 minutes) and post- (120–180 minutes) cocaine self-administration for the 8, 16 and 32 mg/70 kg test sessions. The dashed lines indicate ratings that occurred during the cocaine self-administration session (0–120 minutes)
Vital signs measures
Although men and women did not differ on any of the cardiovascular measures during the self-administration session, significant interactions of sex and time and/or dose were observed following cocaine self-administration (Fig. 3). Specifically, men and women showed a different pattern of changes in SBP over the 60-minute post-session monitoring period (sex by dose by time; F11,308 = 1.9, P < 0.05), with men decreasing more slowly than women, particularly at the highest cocaine dose. Similarly, a different pattern of changes in DBP was observed between men and women as a function of time (F22,616 = 1.7, P < 0.05). No sex differences were observed for HR.
Figure 3.
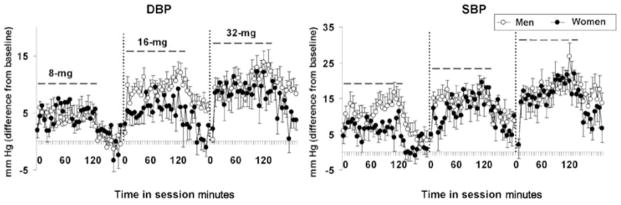
Sex differences in the effects of cocaine on cardiovascular changes following cocaine self-administration. Average (mean ± SE) values minus baseline for diastolic blood pressure (DBP) (mmHg, left panel) and systolic blood pressure (SBP) (mmHg, right panel) for men (open symbols) and women (closed symbols) subjects during (0–120 minutes) and post- (120–180 minutes) cocaine self-administration for the 8, 16 and 32 mg/70 kg test sessions. The dashed lines indicate ratings that occurred during the cocaine self-administration session (0–120 minutes)
DISCUSSION
In this study, we compared men and women on cocaine self-administration under relatively unrestricted access conditions across a broad range of cocaine doses. We found that despite similarities in levels and patterns of use, men rated some of the subjective effects from cocaine higher than did women and showed a different course of cardiovascular changes. These findings will be discussed later.
The sex differences in subjective ratings of cocaine effects in the present study are similar to those reported previously, where men reported more intense good and bad effects from cocaine than did women (Lukas et al. 1996; Kosten et al. 1996; Sofuoglu et al. 1999; Singha et al. 2000). While these data support the idea that men are more sensitive to the subjective effects of cocaine than women, they are in contrast to a large body of work in laboratory animals showing that females are more sensitive than males to many of cocaine’s effects, including the behavioral activating and reinforcing effects (Lynch et al. 2002; Carroll et al. 2004). One possible explanation for this discrepancy is that levels of drug exposure differ greatly between pre-clinical and clinical studies. Specifically, studies with laboratory animals typically use either drug-naïve animals or animals exposed to cocaine for only short periods of time (1–2 weeks); this is in contrast to the years of exposure that cocaine-dependent subjects have typically had at the time of laboratory study. It is interesting to note that in non-addicted populations, studies have consistently shown that women are more sensitive than men to the positive subjective effects of psychostimulant drugs (Terner & de Wit 2006). Another related question is whether subjective reports reflect sensitivity to the reinforcing effects of the drug that would correlate with vulnerability to abuse or some correlate with mechanisms that serve to maintain drug-taking behaviors. Further research is needed to determine how the subjective effects of cocaine change over the course of addiction and whether such measures correlate with vulnerability to cocaine dependence.
In the present study women, but not men, reported that craving for nicotine increased over time. Interestingly, reports of ‘feel hungry’ also increased over time in women compared with men (perhaps a reduced anorectic effect of cocaine in women compared with men). In contrast to differences in desire for food, however, our findings do not suggest differences in craving for cocaine or nicotine.
The findings regarding sex differences in cocaine’s cardiovascular effects have been somewhat controversial. For example, Lukas et al. (1996) reported that following a single dose of cocaine, men and women did not differ on their cardiovascular response to cocaine, but because women had lower plasma cocaine levels, they suggested that women may be more sensitive to the cardiovascular effects of cocaine. Following repeated exposure to cocaine, Sofuoglu et al. (1999) reported no sex differences in the cardiovascular response, whereas Evans et al. (1999) found that the women had a prolonged course of cardiovascular changes. However, in this latter study, the cocaine dose was not adjusted for sex differences in body weight, and women received a higher unit dose of cocaine and achieved higher plasma cocaine levels (i.e, plasma levels were 60% higher in women compared with men by the end of the self-administration session). Indeed, the authors speculate that if the dose had been adjusted for body weight difference (as was done in the current study), then perhaps women may actually be less sensitive to the cardiovascular effects of cocaine. Our present findings showed that despite similar levels of intake, SBP and DBP showed a different pattern of changes following cocaine self-administration, with men showing a prolonged course of cocaine-induced increases. Although in the present study we were unable to compare blood levels of cocaine between men and women, our data support the idea that following the same mg/kg dose, women are less sensitive than men to the cardiovascular effects of cocaine, an idea that is well supported by studies in laboratory animals (i.e., Morishima et al. 1993).
A limitation of the present study is that the hormonal status of the women was not controlled for. This may be important in light of previous work showing that while intake and the physiological response to cocaine generally do not vary with menstrual cycle phase, the subjective response to psychostimulants may (for review see Terner & de Wit 2006). However, it should be noted that majority of the studies in this area have been conducted in non-dependent populations, and because chronic exposure to cocaine is known to profoundly affect menstrual cycle functioning (Mello & Mendelson 1997), the effects are likely to differ between dependent and a non-dependent populations. Indeed, in a recent study of cocaine-dependent women, the subjective and cardiovascular response to intranasal cocaine did not differ between phases of the menstrual cycle (Collins et al. 2007). Despite this limitation, this report is important because it adds to the literature on sex differences by providing information on regulation of cocaine self-administration in men and women and on the effects of self-administered cocaine on subjective and cardiovascular measures in men and women using a broad dose range. The present study shows that under relatively unrestricted access conditions, men and women self-administer a similar amount of cocaine. While men appear to be more sensitive to some of the subjective and cardiovascular effects of cocaine, future research is needed to understand whether these differences may translate to behavioral differences outside the laboratory and to determine the influence of the menstrual cycle on the subjective effects of self-regulated cocaine intake.
Acknowledgments
We would like to acknowledge the Yale Interdisciplinary Women’s Health Research Scholar Program on Women and Drug Abuse (BIRCWH, Carolyn Mazure, P.I.), the Specialized Centers of Research on Women’s Health (Rajita Sinha, P.I.), and the Yale General Medical Clinical Research Center (funded by NIH/NCRR/GCRC Program Grant M01-RR00125), the National Center for Research Resources (K12RR17594, PTM) and the Department of Mental Health and Addiction Services of the State of Connecticut. This work was supported by grants from the NIH Office of Research on Women’s Health (AR049469) and NIDA (DA00397, DA15857, DA12283, RTM; and DA018978, WJL).
References
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann NY Acad Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Collins SL, Evans SM, Foltin RW, Haney M. Intranasal cocaine in humans: effects of sex and menstrual cycle. Pharmacol Biochem Behav. 2007;86:117–124. doi: 10.1016/j.pbb.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, Fischman MW, Foltin RW. Limited sex differences in response to ‘binge’ smoked cocaine use in humans. Neuropsychopharmacology. 1999;21:445–454. doi: 10.1016/S0893-133X(98)00120-1. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Kalayasiri R, Sughondhabirom A, Gueorguieva R, Coric V, Lynch WJ, Morgan PT, Cubells JF, Malison RT. Self-reported paranoia during laboratory ‘binge’ cocaine self-administration in humans. Pharmacol Biochem Behav. 2006;83:249–256. doi: 10.1016/j.pbb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, Oliveto AH, Price LH. Gender differences in response to intranasal cocaine administration to humans. Biol Psychiatry. 1996;39:147–148. doi: 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology. 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Sughondhabirom A, Pittman B, Gueorguieva R, Kalayasiri R, Joshua D, Morgan P, Coric V, Malison RT. A paradigm to investigate the regulation of cocaine self-administration in human cocaine users: a randomized trial. Psychopharmacology. 2006;185:306–314. doi: 10.1007/s00213-006-0323-5. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Cocaine’s effects on neuroendocrine systems: clinical and preclinical studies. Pharmacol Biochem Behav. 1997;57:571–599. doi: 10.1016/s0091-3057(96)00433-9. [DOI] [PubMed] [Google Scholar]
- Morishima HO, Abe Y, Matsuo M, Akiba K, Masaoka T, Cooper TB. Gender-related differences in cocaine toxicity in the rat. J Lab Clin Med. 1993;122:157–163. [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Singha AK, McCance-Katz EF, Petrakis I, Kosten TR, Oliveto A. Sex differences in self-reported and physiological response to oral cocaine and placebo in humans. Am J Drug Alcohol Abuse. 2000;26:643–657. doi: 10.1081/ada-100101900. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sughondhabirom A, Jain D, Gueorguieva R, Coric V, Berman R, Lynch WJ, Self D, Jatlow P, Malison RT. A paradigm to investigate the self-regulation of cocaine administration in humans. Psychopharmacology. 2005;180:436–446. doi: 10.1007/s00213-005-2192-8. [DOI] [PubMed] [Google Scholar]
- Terner JM, de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006;84:1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]