Abstract
Background
Disturbances in sleep associated with chronic cocaine use may underlie abstinence-related cognitive dysfunction. We hypothesized that sleep-related cognitive function would be impaired in chronic cocaine users, and that this impairment would be associated with abstinence-related changes in sleep architecture.
Methods
Twelve chronic cocaine users completed a 23-day in-patient study that included randomized, placebo-controlled, cocaine self-administration sessions. We report polysomnographic measurement of rapid eye-movement (REM) sleep and slow-wave activity, and performance on a visual texture discrimination task.
Findings
Progressive abstinence from cocaine was associated with characteristic changes in REM sleep. REM sleep was shortest on nights following cocaine use and rebounded in the first week of abstinence before diminishing with progressive abstinence, following a pattern opposite that of slow-wave activity. Overnight visual learning was observed over the first night following 3 consecutive days of laboratory cocaine use; however, learning was not observed at 3 days or 17 days of abstinence. Across all points of abstinence, early-night slow-wave activity was associated strongly with non-deterioration of visual performance overnight. Furthermore, overnight enhancement of visual performance was predicted by the co-occurrence of sufficient early-night slow-wave activity and late night REM sleep, similar to results from studies in healthy subjects.
Conclusions
These results suggest that abstinence-associated sleep-dependent learning deficits are related to characteristic changes in sleep architecture, and promote the idea that treatments directed at sleep (`somno-tropic' treatments) could be helpful in offsetting physiological consequences of cocaine abstinence.
Keywords: Cocaine, cognition, procedural learning, REM, sleep, slow-wave sleep
INTRODUCTION
Sleep disturbances are a well-known feature of many psychiatric illnesses [1]. Such disturbances are often regarded as consequences of the primary disease process and hence as symptoms to be managed. There is growing evidence, however, that sleep problems associated with some psychiatric illnesses (e.g. depression [2,3] and alcoholism [4–7]) are integral to the disease process. In other words, the disturbance of sleep is not merely or necessarily a consequence of the underlying illness but, through its own consequences, actually contributes to illness. In such a case, understanding the related sleep problem and its consequences could lead to a better appreciation of disease pathophysiology and promote the development of more effective treatments.
Recent work suggests such a relationship between sleep and cocaine dependence [8]. Cocaine use and acute withdrawal have well-known effects on sleep that are common to psychostimulants (i.e. prolonged wakefulness during use and hypersomnia during early withdrawal). It has become increasingly clear, however, that reproducible sleep disturbances associated with cocaine dependence are not limited to periods of acute intoxication or early withdrawal, but are also present in periods of sustained abstinence [8–12]. Furthermore, these sleep disturbances are associated with parallel deficits in cognitive performance [8,13] that might affect treatment retention and promote relapse [14]. It is not yet clear whether any of the observed cognitive deficits are a direct result of the sleep disturbance or are related more generally to cocaine use and abstinence. Sleep-dependent cognitive tasks such as sleep-dependent procedural skill learning may be useful in determining whether cocaine dependence-related sleep disturbances have direct functional consequences [8].
Procedural skill learning is a form of non-declarative memory [15] that includes sleep-dependent motor [16,17] and visual texture discrimination learning [18]. Notably, sleep promotes enhancement of performance on these tasks. On the visual texture discrimination task (TDT), the degree of enhancement varies with the amount of slow-wave sleep and rapid eye-movement (REM) sleep in the first and last 2 hours, respectively, of an 8-hour night of sleep [19]. Furthermore, performance enhancement does not occur after selective REM-sleep disruption [20] or 30-hour sleep deprivation [18]. Same-day repetition of training, however, leads to deterioration in performance that is reversed by a slow-wave sleep-containing nap [21]. For these reasons, it has been proposed [18,22] that at least two sleep-dependent steps (related to slow-wave sleep and REM sleep) are responsible for stabilization and enhancement of performance.
REM sleep is altered in chronic cocaine users, and appears to vary widely depending on recency of cocaine use, with evidence for an initial suppression followed by a rebound [9–12]. Furthermore, recent work has shown that cocaine has both immediate [23] and abstinence-related [8] effects on slow-wave brain activity. Thus, both REM sleep and slow-wave activity undergo abstinence-related changes—occurring well after cocaine use—that may result in sleep-dependent visual learning changes.
As part of a study of sleep and cognition in cocaine users [8], we examined the relationship between slow-wave activity, REM sleep and TDT learning during abstinence from controlled cocaine administration. We hypothesized that TDT learning would vary with abstinence-related changes in slow-wave activity and REM sleep within cocaine-dependent subjects, similarly to how it varies with differences in these sleep measures between healthy subjects [19]. Such results would support the idea that cognitive deficits associated with cocaine use and abstinence may, in some cases, be caused by the associated disturbance in sleep.
METHODS
Subjects
Twelve (10 male, two female) non-treatment-seeking, cocaine-dependent subjects, ages 24–49 years, completed this in-patient study. Only non-treatment-seeking subjects were recruited for ethical considerations associated with laboratory cocaine administration. Subjects had an average of 13 years of education (range 9–18 years) and used an average of $500 equivalent of smoked crack cocaine weekly prior to study entry ($200–$1000). Average number of years of cocaine use was 17 years (range 8–27 years). In the week prior to study entry, eight subjects used alcohol (average 3.5 drinks/week, range 1–30) and four subjects used cannabis (average 2.5 joints/week, range 1–6). No subjects used opiates or benzodiazepines in the year prior to study entry. No subject had a chronic medical condition such as diabetes, cardiovascular disease, liver disease, human immunodeficiency virus (HIV)-seropositivity or neurological condition. All subjects met DSM-IV criteria for crack cocaine dependence and had positive urine toxicology for cocaine [and negative for cannabis, opiates, phencyclidine (PCP), amphetamines, benzodiazepines and barbiturates] at the time of screening. Potential subjects were excluded from participation because of a history of dependence on substances other than nicotine and cocaine, or for non-substance-related Axis I diagnoses, as determined by clinical interview. No subject exhibited alcohol withdrawal symptoms during the study, as determined by the Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar, [24]). No subject had been prescribed psycho-active medications in the past year, and no subject reported having been evaluated for, diagnosed with or treated for any sleeping problem. All participants reviewed and signed an informed consent form approved by the institutional review board before participation.
In-patient facility
Subjects were admitted to a 12-bed state psychiatric research facility—a full-service in-patient psychiatric unit with a highly structured daily routine, including individual and group therapy. Subjects did not participate in substance abuse treatment, but did participate in all other unit activities while enrolled in the study. All meals and snacks were provided on the caffeine-free unit and four times per day `fresh-air' breaks allowed smokers to smoke at the same times each day. All subjects were checked by staff at least once every 15 minutes while on the unit, and were monitored constantly if off the unit for protocol related testing or `fresh-air' breaks. Daytime napping was not permitted and was enforced strictly by unit staff. Urine toxicology screens were administered three times per week and (with the exception of positive tests for cocaine temporally consistent with laboratory cocaine administration) each were negative.
Intravenous cocaine self-administration
On study day 0, subjects self-administered cocaine doses of 8, 16 and 32 mg/70 kg intravenously using an infusion pump at 30-minute intervals (beginning at 12 noon), and were then allowed to continue to self-administer the 32 mg/70 kg dose over the next 90 minutes (5-minute lock-out between doses, maximum session use of 384 mg/70 kg). Ability to tolerate each dose of cocaine, as determined by heart rate, blood pressure, cardiac rhythm and subjective response to the cocaine administration, was a requirement for continued participation. In addition to assessing the safety of administration, this session served to standardize the time from last use of cocaine for all subjects.
Subjects were then randomized to receive cocaine on each of days 4–6 (`early binge'; n = 6) or days 18–20 (`late binge'; n = 6) in a placebo-controlled, single-blind manner to control for potential confounding effects of both in-patient hospitalization and potential, subclinical withdrawal from other substances (e.g. alcohol, benzodiazepine or cannabis). Subjects were told that on each of days 4, 5, 6, 18, 19 and 20 they had an even chance of receiving cocaine or placebo in order to minimize effects of anticipation or disinterest. On each cocaine administration day, subjects self-administered cocaine doses of 32 mg/70 kg over a 2-hour binge period from 12.00 p.m. to 2.00 p.m. using the same lock-out period and daily maximum as on day 0; placebo days were identical except for the absence of cocaine in the intravenous fluid (normal saline). Mean daily cocaine use was 58% of maximum available (range 17–100%). Further details of this paradigm have been described previously [8,25,26]. The overall study design is outlined in Table 1.
Table 1.
Outline of study design.*
| Study day | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early binge | Placebo | Placebo | Placebo | |||||||||||||||||||||
| Self-administration | Cocaine | Cocaine | Cocaine | Cocaine | ||||||||||||||||||||
| Abstinence day | Test doses | 1 | 2 | 3 | Binge | Binge | Binge | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| Polysomnography | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| TDT learning | X↔X | X↔X | X↔X | |||||||||||||||||||||
| Late binge | X↔X | |||||||||||||||||||||||
| Self-administration | Cocaine | Placebo | Placebo | Placebo | Cocaine | Cocaine | Cocaine | |||||||||||||||||
| Abstinence day | Test doses | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | Binge | Binge | Binge | 1 | 2 | 3 |
| Polysomnography | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| TDT learning | X↔X | X↔X | X↔X | X↔X |
Shading indicates how sleep dates were grouped into bins. TDT: texture discrimination task.
Sleep measurement
Polysomnographic measurement of sleep (PSG; Grass Instruments, Colleague System, West Warwick, RI, USA) was obtained on study nights 1, 3–8, 10, 12, 15 and 17–22, and except for REM sleep measurements has been reported previously [8]. The first 2 nights of PSG data collected were considered to be accommodation nights, as it was anticipated that subjects would need to adjust to the more rigid sleep–wake cycle of the in-patient unit and to sleeping with the PSG equipment in a novel environment. PSG measurement was performed in a dedicated sleep room, and included four electroencephalogram (EEG) channels (C3/A2, C4/A1, O1/A2, O2/A1), right and left electro-oculogram (EOG), and chin electromyo-gram (EMG). PSG records were scored using standard criteria [27] by a sleep technician unaware of the study hypotheses. Sleep variables were defined in the typical fashion and largely reported previously [8]: sleep onset latency was defined as the time from lights out until the appearance of the first sleep stage; time awake after sleep onset was defined as the amount of time spent awake after the onset of sleep and before final awakening; total sleep time was defined as the time from sleep onset until final awakening minus the time awake after sleep onset; sleep efficiency was defined as total sleep time divided by time in bed, where time in bed is time between lights off and getting out of bed, and is also the sum of total sleep time, sleep latency, time awake after sleep onset and any time spent lying in bed after final awakening. Time in slow-wave sleep is the sum of the time spent in stage 3 sleep and stage 4 sleep. REM latency is the time from initial sleep onset until the first epoch of REM sleep. REM sleep time and REM sleep time in each quarter of the sleep period were measured. REM sleep occurring after 6 hours of total sleep time was also measured. Similar to the previously reported sleep measures [8], there were no statistically significant differences between the early and late binge groups on any sleep measurement reported here, and the changes observed across binge and abstinence were qualitatively similar in both groups.
Because several subjects had little to no slow-wave sleep on at least some nights [8], slow-wave sleep defined by standard criteria was not used to assess the relationship between slow-wave activity and TDT performance. Sleep EEG spectral analysis was performed on the C4 channel using a privately developed software package. Fast-Fourier transforms were performed on 5-second epochs using a Hamming window; epochs with movement or electrode artifacts were excluded from analysis. Absolute spectral power was collated and binned according to frequency as follows: delta (0.5–3 Hz), theta (3–8 Hz), alpha (8–12 Hz), sigma (12–16 Hz) and beta (16–25 Hz). Absolute delta power was correlated strongly with slow-wave sleep time [8], and was used as a marker of slow-wave activity. For each subject, the Z-score of slow-wave activity on each of the TDT test nights was also used as a relative measure (i.e. for each subject, the difference between the absolute delta power recorded on that night and the average delta power for all recorded nights divided by the standard deviation). Slow-wave activity occurring in each quarter of the sleep period was defined and measured similarly.
Frequent measurement of sleep allowed for the study of the naturalistic response of sleep to cocaine use and abstinence. As such, subjects were allowed to dictate their bed-time and waking time, except that the open period for sleep throughout the study was restricted to the period between 9.30 p.m. and 7.45 a.m., and subjects were required to be in bed by 12.30 a.m. There were no significant differences in the mean time of awakening across the phases of the study (range 6.40 a.m.–6.52 a.m.) and mean time to bed differed only between 4–6 and 14–17 days abstinent (range 11.53 p.m.–12.20 a.m.) [8].
The visual TDT
The TDT was administered as described previously [19]. In this computer-based task, a target screen is displayed for 17 ms, followed by a blank screen for a variable inter-stimulus interval (ISI) and then a mask, also displayed for 17 ms. The target screen consists of three diagonal bars in one quadrant of the screen, in either a vertical or horizontal array, displayed against a background of horizontal bars, with a rotated letter `T' or `L' displayed centrally at the visual fixation point. After presentation of the mask, subjects must indicate whether the fixation letter was a `T' or `L' and whether the array of diagonal bars was oriented vertically or horizontally. Subjects are tested over a range of ISIs, and the minimum ISI required to reach a threshold accuracy on the horizontal–vertical discrimination of 80% is determined. Improvement on the task is defined as the difference (decrease) in threshold ISI at retest compared to training. Training and retest sessions each contained 1250 trials in 25 blocks. The interval between trials was determined by the subjects themselves, who initiated each trial with a keyboard press. All subjects were screened for sufficient visual acuity during initial training by having them identify correctly the `T' or `L' and the orientation of the array of diagonal bars in 10 consecutive trials with no mask screen (i.e. the target screen was presented for 17 ms followed by a blank screen but no mask screen). In healthy subjects, overnight, sleep-dependent improvements in performance averages just over 10 ms [18,19], and for this reason 10 ms was chosen as a cut-off for robust overnight improvement in some of the analyses here. Decreases (improvement) in threshold ISI are reported below as positive changes in ISI and increases (worsening) in threshold ISI are reported below as negative changes in ISI.
TDT training was conducted at 8 p.m. on study day 2 (familiarization), on the third of 3 consecutive cocaine administration days (post-drug phase; ≥ 6 hours after last cocaine administration), and following 2 (early phase) and 16 or 17 days (late phase) of experimental cocaine abstinence. Visual quadrant was randomized to training phase, and retesting in the same quadrant was performed the next morning (9 a.m.). Because half the subjects had cocaine early in the stay and half late in the stay, there were two different orders for testing following the familiarization testing. Half the subjects had post-drug testing on night 6 of the protocol and the other half on night 20 (i.e. the night following the last of 3 consecutive cocaine administration days). Similarly, early and late abstinence testing occurred on nights 8 or 22 and nights 22 or 17 of the protocol, respectively.
The two different orders potentially minimized non-cocaine-related effects (e.g. hospitalization time and sub-clinical withdrawal from non-revealed substance use if any) and allowed for determination that repeated exposure to the task did not affect overnight learning. One compromise of this study design is that late abstinence testing in the two groups of subjects was not exactly matched for laboratory cocaine use—for those who received laboratory cocaine on days 4–6, late abstinence testing occurred following those 3 days of laboratory cocaine use; for those who received laboratory cocaine on days 18–20, late abstinence testing occurred following 1 day (study day 0) of laboratory cocaine use that was preceded by ongoing street use of cocaine.
Data analysis
Individual subject sleep data were averaged into seven bins: one bin for the first 3 days of the study (accommodation phase), one bin for the 3 days of consecutive cocaine use (binge) and five bins for the period of abstinence (1–3, 4–6, 7–9, 10–13 and 14–17 days abstinent). Such binning allowed data from early and late binge subjects to be combined and reduced the effects of day-to-day variation [8].
Except where otherwise indicated in the text, repeated measures analysis of variance (rmANOVA) was used to determine statistically significant changes across experimental phases at the P < 0.05 level (as reported in Table 2 and Fig. 1). There was no evidence of non-normality in the TDT or sleep data; however, the small sample size precludes ruling out this possibility. Post-hoc paired t-tests were used with partial Bonferroni correction for multiple comparisons applied to control for Type I errors at the 0.05 level. Partial Bonferroni correction provides a more liberal adjustment than full Bonferroni correction when there are correlations among the compared outcomes (particularly relevant for the sleep measurements), but is equivalent to full Bonferroni correction in the absence of correlations [28].
Table 2.
Rapid eye movement (REM) sleep measurements.
| REM sleep measure (min ± SE) | Post-drug days | Days abstinent |
ANOVA P-value | ||||
|---|---|---|---|---|---|---|---|
| 1–3 | 4–6 | 7–9 | 10–13 | 14–17 | |||
| Total REM time | 89 ± 8⇓ | 95 ± 8 | 100 ± 9↑ | 98 ± 10⇓↑ | 88 ± 9↓ | 84 ± 8↓ | 0.018 |
| First quarter | 7 ± 2⇓ | 15 ± 2⇑ | 15 ± 3⇑ | 17±3⇑↑ | 10 ± 3↓ | 10 ± 3↓ | 0.007 |
| Second quarter | 19 ± 2 | 18 ± 2 | 22 ± 2 | 23 ± 3 | 20 ± 2 | 20 ± 2 | NS |
| Third quarter | 27 ± 3 | 33 ± 3 | 27 ± 3 | 26 ± 3 | 26 ± 3 | 26 ± 3 | NS |
| Fourth quarter | 32 ± 3 | 26 ± 4 | 31 ± 3 | 30 ± 4 | 29 ± 3 | 26 ± 3 | NS |
| REM latency | 66 ± 6⇑ | 42 ± 5⇓↓ | 47 ± 8⇓ | 35 ± 11⇓▼ | 55 ± 6↑ | 61 ± 8↑▲ | 0.001 |
Across each row, a downward arrow indicates a measurement that is significantly lower than any measurement marked with an upward arrow of the same style (post-hoc Pcorr < 0.05 or better) and vice-versa. NS: non-significant; SE: standard error; ANOVA: analysis of variance.
Figure 1.
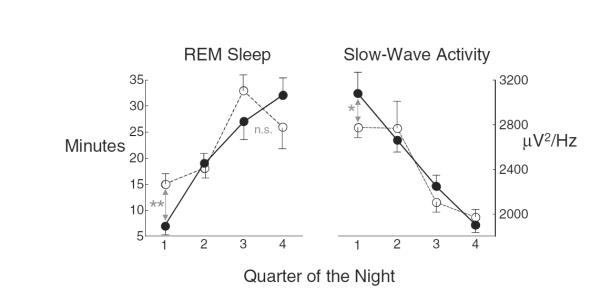
Rapid eye movement (REM) sleep and slow-wave activity showed reciprocal changes in the first part of the night from post-drug nights (filled circles) to nights after 1–3 full days of abstinence (open circles). Left panel: REM sleep in the first quarter of the night is significantly greater on nights after 1–3 full days of abstinence than on post-drug nights (**Pcorr < 0.001). Right panel: slow-wave activity is significantly lower in the first quarter on nights after 1–3 full days of abstinence than on post-drug nights (*Pcorr < 0.01)
RESULTS
REM sleep
Changes in REM sleep with cocaine use and abstinence were observed and are summarized in Table 2. Modest but statistically significant increases in total REM time occurred in the first 9 days of abstinence relative to post-drug nights and/or later in abstinence. These increases were accounted for by increases in REM sleep occurring in the first quarter of each period of sleep, with first-quarter REM time more than doubling from post-drug nights to the first 9 days of abstinence (Table 1). The changes in first-quarter REM occurred with complementary changes in REM latency, with REM latency significantly shorter in the first 9 days of abstinence than post-drug nights or later in abstinence.
TDT performance
Mean overnight improvement in TDT performance was observed only in the post-drug phase (+12 ± 4 ms; Pcorr < 0.05), and this improvement was significantly greater than the worsening seen at early (−11 ± 9 ms; Pcorr < 0.05) and late (−17 ± 11 ms; Pcorr < 0.05) abstinence (rmANOVA, F3,33 = 3.14, P < 0.05; Table 3). There were no significant differences in mean overnight improvement when data were arranged by temporal order of the administrations (F3,33 = 0.11, P = 0.95), as performance gains with repeated testing affected test and retest similarly (Table 3). Although poor average improvement at late abstinence was not unexpected, the lack of average improvement at early abstinence could not be explained so easily, as total REM time (Table 2) was higher than at post-drug testing.
Table 3.
Visual texture discrimination task performance by order of administration and phase.
| Threshold (ms ± SE) | Test | Retest | Improvement |
|---|---|---|---|
| By order of administration | |||
| First (familiarization) | 93 ± 12 | 99 ± 14 | −6 ± 9 |
| Second | 74 ± 10 | 78 ± 14 | −4 ± 12 |
| Third | 71 ± 10 | 74 ± 12 | −3 ± 5 |
| Fourth | 60 ± 8 | 69 ± 13 | −9 ± 8 |
| By phase* | |||
| Post-drug (2nd/3rd) | 75 ± 11 | 63 ± 9 | +12 ± 4† |
| Early abstinence (3rd/4th) | 66 ± 10 | 77 ± 14 | −11 ± 9 |
| Late abstinence (2nd/4th) | 64 ± 7 | 81 ± 15 | −17 ± 11 |
Parenthetical ordinals indicate which test sessions (by order) contributed to each phase;
Pcorrected < 0.05 for difference from zero, and Pcorrected < 0.05 for being significantly different than the change at early or late abstinence (see text). SE: standard error.
Slow-wave sleep activity, sleep architecture and TDT performance
Although total REM time increased at post-drug testing, only REM sleep from the last quarter of an 8-hour night of sleep is thought to contribute to TDT consolidation [19], and the increase during early-abstinence in REM (compared to post-drug) was significant only in the first quarter of the night (Pcorr < 0.001; Fig. 1, left panel), when slow-wave sleep is thought to be important for consolidation [19,22]. In fact, slow-wave activity, the critical component of slow-wave sleep, decreased during this period (Fig. 1, right panel). We therefore speculated that decreased first-quarter slow-wave activity disrupted overnight TDT improvement, regardless of how much REM sleep occurred near the end of sleep.
To examine this hypothesis, we considered both first-quarter slow-wave activity (SWA1) and REM sleep occurring after 6 hours (REM6+) in relationship to TDT improvement. The presence of both REM6+ > 0 and relatively high levels of SWA1 (i.e. Z-score > 0) was associated with robust overnight improvement on the TDT (> 10 ms faster, consistent with mean overnight improvement in healthy subjects [18,19] (Fig. 2). However, sleeping more than 6 hours was not sufficient, as none of 3 nights with more than 6 hours of sleep but REM6+ equal to zero showed performance enhancement (despite two having high levels of SWA1). For nights with REM6+ > 0 (n = 13), the product of SWA1 and REM6+ (transformed similarly into unit-less, positive quantities to allow multiplication) was correlated strongly with overnightTDT improvement (R = 0.80, P < 0.001). In contrast, for the several nights with REM6+ = 0 and below-average SWA1, large overnight deterioration (>10 ms) was seen, consistent with drops in performance seen with repeated, same-day testing in normal subjects [21].
Figure 2.
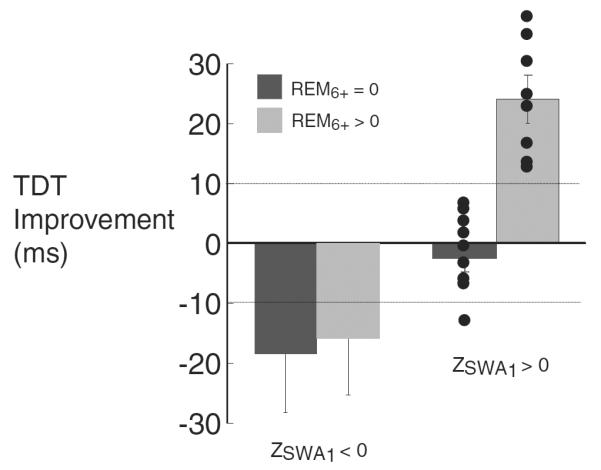
Mean overnight texture discrimination task (TDT) improvement arranged by first-quarter slow-wave activity (SWA)1 and rapid eye movement after 6 hours (REM)6+. Overnight improvements greater than 10 ms were present if and only if ZSWA1 and REM6+ > 0. Dotted lines at +10 and −10 ms indicate lower cut-offs for enhancement and non-deterioration of performance used for further analysis and supported by work in healthy subjects [18,19,21]
To describe further the relevance of SWA1 and REM6+ to TDT learning, we tested the associations between these sleep measures and both deterioration and enhancement of performance, defined as a slowing or speeding, respectively, of greater than 10 ms. These levels are supported by previous work in healthy subjects [18,19,21] that established the phenomena of deterioration and enhancement of performance. A lack of overnight deterioration was associated with ZSWA1 > 0 (P < 0.01, two-sided Fisher test for same or stronger association; positive/negative predictive values = 94%/47%) but not with REM6+ > 0 (P = 0.27). In contrast, performance enhancements were associated with both ZSWA1 > 0 (P = 0.001; 47%/100%) and REM6+ > 0 (P < 0.0001; 62%/100%), and the co-occurrence of ZSWA1 and REM6+ > 0 predicted performance enhancement exactly (P < 10−7; 100%/100%).
DISCUSSION
Cognitive deficits associated with cocaine dependence are well recognized [13,29–34], and may be important in treatment retention and outcomes [14,35,36]. We have suggested previously that the poor sleep associated with abstinence from cocaine in chronic users may be responsible for some of these cognitive consequences and so could be a target for treatment [8]. The principal finding of the current work is that a disruption in one type of sleep-dependent memory consolidation appears to be explained by abstinence-related alterations in sleep architecture.
Similar both to sleep-dependent motor learning reported previously in this sample [8] and to results in healthy subjects [19], robust TDT learning occurred only across nights with more than about 6 hours of sleep. In contrast, more than 6 hours of sleep alone was not sufficient for overnight improvement on the TDT, unlike in healthy subjects with normal sleep [18,19]. One difference is that normal sleep in healthy subjects is characterized by robust slow-wave activity in the first quarter of the night and substantial REM sleep after the 6th hour [19] (i.e. normal sleep architecture). After 1–3 days of abstinence, however, sleep in the cocaine-dependent sample was characterized by diminished slow-wave activity and increased REM sleep in the first quarter of the night.
The decrease in REM latency that led to the increase in first-quarter REM sleep is not unprecedented, but rather a consistent finding in the largely male cohorts of cocaine users examined in the first week of abstinence [9–11]. More recent evidence suggests that cocaine use increases slow-wave activity acutely [23] and that total slow-wave activity during sleep decreases in the first week of abstinence relative to post-drug nights [8]. As REM sleep contains little to no slow-wave activity, it is altogether possible that the reduction in first-quarter slow-wave activity observed here is due to the intrusion of REM sleep early in the night. The possibility that this intrusion has a functional consequence—the impairment of sleep-dependent procedural learning—would add substantially to the correlational arguments that have been proposed that associate sleep-architectural differences with differences in TDT learning.
Stickgold and colleagues have proposed, based on correlational findings, that the neuronal processes that underlie overnight improvement on the TDT occur during two distinct stages following training: early-night slow-wave sleep and late-night REM sleep [19]. Here we show that early-night slow-wave activity and late-night REM sleep predicted overnight TDT performance enhancement exactly, suggesting that the relevant underlying neuronal processes are each necessary and together sufficient for such enhancement. Furthermore, the association between early-night slow-wave activity and lack of deterioration suggests that processes underlying early-night slow-wave sleep serve two not necessarily distinct roles in TDT performance—one preventing the performance deterioration seen with repeated testing and another priming the brain for the late-night, REM-dependent enhancement of learning. This possibility is supported by earlier findings that a slow-wave sleep containing nap can reverse same-day deterioration in TDT performance [21], and that early-night (predominantly slow-wave) sleep is necessary for the late-night (predominantly REM) sleep enhancement of TDT performance in healthy subjects [22].
An important limitation in this study is the small sample size. Although this is ameliorated somewhat by the within-subjects design, it may limit the ability to generalize these results in the absence of confirmatory findings. Another possible limitation, or trade-off, in the study design is the largely ad libitum sleep period time used versus a fixed time-in-bed design. Although this design was useful in evaluating the naturalistic changes in sleep-architecture with abstinence, and the relationship between sleep architecture differences and TDT performance differences are still valid, most subjects spent substantially less than 8 hours in bed. Hence, it is possible that some subjects may have had increased total sleep time, and in particular REM time, after 6 hours of sleep if they had been required to spend 8 hours in bed. However, this concern is perhaps less relevant, given the long duration of the study with multiple nights of sleep assessment.
It has been known for some time that abstinence from cocaine in chronic users leads to both cognitive impairment [33] and altered sleep [8–12]. However, these have been assumed to be independent consequences of drug withdrawal. It is striking that, in this case, an impairment in visual learning seen during cocaine abstinence can be predicted completely, on an individual-by-individual basis, by alterations in sleep quality, suggesting strongly that it is, in fact, the alterations in sleep quality that lead to the learning deficit. As these alterations persist for at least 2–3 weeks [8–10,12], and there is no evidence as to when (or if) they return to normal, it is possible that their functional consequences may have a significant and deleterious [14,35,36] effect on attempted recovery from cocaine dependence. Interestingly, medications that may treat cognitive impairment (e.g. modafinil [37]) or affect sleep (e.g. tiagabine [38]) have shown promise in the treatment of cocaine dependence. The present work opens the possibility of a pharmacotherapeutic approach to the treatment of cocaine dependence: a `somno-tropic' pharmacotherapy that aims simultaneously to ameliorate deficits in both sleep and cognitive function not merely through the prolongation of sleep, but through the specific targeting of abstinence-related changes in sleep architecture.
Acknowledgements
This work was supported by NIH grants R01DA11744 and R01MH48832 (R. A. S.), NCRR grant K12RR17594 (P. T. M.), R01 DA15857 and K02 DA00397 (R. T. M.), the Yale General Clinical Research Center (funded by NIH/NCRR/GCRC Program Grant M01-RR00125) and the Connecticut Department of Mental Health and Addiction Services (DMHAS).
References
- 1.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–18. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 2.Fava M, McCall WV, Krystal A, Wessel T, Rubens R, Caron J, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59:1052–60. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Le Bon O. Contribution of sleep research to the development of new antidepressants. Dialogues Clin Neurosci. 2005;7:305–13. doi: 10.31887/DCNS.2005.7.4/olebon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landolt HP, Gillin JC. Sleep abnormalities during abstinence in alcohol-dependent patients. Aetiology and management. CNS Drugs. 2001;15:413–25. doi: 10.2165/00023210-200115050-00006. [DOI] [PubMed] [Google Scholar]
- 5.Monnelly EP, Ciraulo DA, Knapp C, LoCastro J, Sepulveda I. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24:532–5. doi: 10.1097/01.jcp.0000138763.23482.2a. [DOI] [PubMed] [Google Scholar]
- 6.Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcohol Clin Exp Res. 1998;22:1864–71. [PubMed] [Google Scholar]
- 7.Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–39. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- 8.Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: evidence for occult insomnia. Drug Alcohol Depend. 2006;82:238–49. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Johanson CE, Roehrs T, Schuh K, Warbasse L. The effects of cocaine on mood and sleep in cocaine-dependent males. Exp Clin Psychopharmacol. 1999;7:338–46. doi: 10.1037//1064-1297.7.4.338. [DOI] [PubMed] [Google Scholar]
- 10.Kowatch RA, Schnoll SS, Knisely JS, Green D, Elswick RK. Electroencephalographic sleep and mood during cocaine withdrawal. J Addict Dis. 1992;11:21–45. doi: 10.1300/J069v11n04_03. [DOI] [PubMed] [Google Scholar]
- 11.Watson R, Bakos L, Compton P, Gawin F. Cocaine use and withdrawal: the effect on sleep and mood. Am J Drug Alcohol Abuse. 1992;18:21–8. doi: 10.3109/00952999209001608. [DOI] [PubMed] [Google Scholar]
- 12.Pace-Schott E, Stickgold R, Muzur A, Wigren P, Ward AS, Hart C, et al. Sleep quality deteriorates over a binge–abstinence cycle in chronic smoked cocaine users. Psychopharmacology. 2005;179:873–83. doi: 10.1007/s00213-004-2088-z. [DOI] [PubMed] [Google Scholar]
- 13.Pace-Schott EF, Stickgold R, Muzur A, Walker MP, Hobson JA, Wigren PE, et al. Cognitive performance by humans during a smoked cocaine binge–abstinence cycle. Am J Drug Alcohol Abuse. 2005;31:571–91. doi: 10.1081/ada-200068120. [DOI] [PubMed] [Google Scholar]
- 14.Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive–behavioral treatment. Drug Alcohol Depend. 2003;71:207–11. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 16.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 17.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep dependent motor skill learning. Neuron. 2002;35:205–11. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 18.Stickgold R, James L, Hobson JA. Visual discrimination learning requires post-training sleep. Nat Neurosci. 2000;2:1237–8. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- 19.Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: a multi-step process occurring during sleep. J Cogn Neurosci. 2000;12:246–54. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- 20.Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–82. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 21.Mednick SC, Nakayama K, Cantero JL, Atienza M, Levin AA, Pathak N, et al. The restorative effect of naps on perceptual deterioration. Nat Neurosci. 2002;5:677–81. doi: 10.1038/nn864. [DOI] [PubMed] [Google Scholar]
- 22.Gais S, Plihal W, Wagner U, Born J. Early sleep triggers memory for early visual discrimination skills. Nat Neurosci. 2000;3:1335–9. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- 23.Reid MS, Flammino F, Howard B, Nilsen D, Prichep LS. Topographic imaging of quantitative EEG in response to smoked cocaine self-administration in humans. Neuropsychopharmacology. 2006;31:872–84. doi: 10.1038/sj.npp.1300888. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–7. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 25.Lynch WJ, Sughondhabirom A, Pittman B, Gueorguieva R, Kalayasiri R, Joshua D, et al. A paradigm to investigate the regulation of cocaine self-administration in human cocaine users: a randomized trial. Psychopharmacology (Berl) 2006;185:306–14. doi: 10.1007/s00213-006-0323-5. [DOI] [PubMed] [Google Scholar]
- 26.Sughondhabirom A, Jain D, Gueorguieva R, Coric V, Berman R, Lynch WJ, et al. A paradigm to investigate the self-regulation of cocaine administration in humans. Psychopharmacology (Berl) 2005;180:436–46. doi: 10.1007/s00213-005-2192-8. [DOI] [PubMed] [Google Scholar]
- 27.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Humans. United States Department of Health; Bethesda, MD: 1968. [Google Scholar]
- 28.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16:2529–42. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 29.Bauer LO. Psychomotor and electroencephalographic sequelae of cocaine dependence. NIDA Res Monogr. 1996;163:66–93. [PubMed] [Google Scholar]
- 30.Di Sclafani V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend. 2002;66:161–71. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoff AL, Riordan H, Morris L, Cestaro V, Wieneke M, Alpert R, et al. Effects of crack cocaine on neurocognitive function. Psychiatry Res. 1996;60:167–76. doi: 10.1016/0165-1781(96)02758-8. [DOI] [PubMed] [Google Scholar]
- 32.Horner MD. Attentional functioning in abstinent cocaine abusers. Drug Alcohol Depend. 1999;54:19–33. doi: 10.1016/s0376-8716(98)00141-0. [DOI] [PubMed] [Google Scholar]
- 33.O'Malley S, Adamse M, Heaton RK, Gawin FH. Neuropsychological impairment in chronic cocaine abusers. Am J Drug Alcohol Abuse. 1992;18:131–44. doi: 10.3109/00952999208992826. [DOI] [PubMed] [Google Scholar]
- 34.van Gorp WG, Wilkins JN, Hinkin CH, Moore LH, Hull J, Horner MD, et al. Declarative and procedural memory functioning in abstinent cocaine abusers. Arch Gen Psychiatry. 1999;56:85–9. doi: 10.1001/archpsyc.56.1.85. [DOI] [PubMed] [Google Scholar]
- 35.Teichner G, Horner MD, Harvey RT. Neuropsychological predictors of the attainment of treatment objectives in substance abuse patients. Int J Neurosci. 2001;106:253–63. doi: 10.3109/00207450109149753. [DOI] [PubMed] [Google Scholar]
- 36.Teichner G, Horner MD, Roitzsch JC, Herron J, Thevos A. Substance abuse treatment outcomes for cognitively impaired and intact outpatients. Addict Behav. 2002;27:751–63. doi: 10.1016/s0306-4603(01)00207-6. [DOI] [PubMed] [Google Scholar]
- 37.Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–11. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez G, Sevarino K, Sofuoglu M, Poling J, Oliveto A, Gonsai K, et al. Tiagabine increases cocaine-free urines in cocaine-dependent methadone-treated patients: results of a randomized pilot study. Addiction. 2003;98:1625–32. doi: 10.1046/j.1360-0443.2003.00544.x. [DOI] [PubMed] [Google Scholar]


