Abstract
Increasing evidence suggests that the tight junction is a dynamically regulated structure. Cytoskeletal reorganization, particularly myosin light chain phosphorylation–induced actomyosin contraction, has increasingly been recognized as a mediator of physiological and pathophysiological tight junction regulation. However, our understanding of molecular mechanisms of tight junction modulation remains limited. Recent studies using live cell and live animal imaging techniques allowed us to peek into the molecular details of tight junction regulation. At resting conditions, the tight junction is maintained by dynamic protein–protein interactions, which may provide a platform for rapid tight junction regulation. Following stimulation, distinct forms of tight junction protein reorganization were observed. Tumor necrosis factor (TNF-α) causes a myosin light chain kinase (MLCK)–mediated barrier regulation by inducing occludin removal from the tight junction through caveolar endocytosis. In contrast, MLCK- and CK2-inhibition–caused tight junction regulation is mediated by altered zonula occludens (ZO)-1 protein dynamics and requires ZO-1–mediated protein–protein interaction, potentially through regulating claudin function. Although some of the molecular details are missing, studies summarized above point to modulating protein localization and dynamics that are common mechanisms for tight junction regulation.
Keywords: tight junction, protein dynamics, fluorescent recovery after photobleaching, endocytosis, epithelial barrier function
Introduction
Epithelia form boundaries that separate liquid-filled tissue compartments throughout the body. The cell layer is not only critical to forming physically separate spaces, but also is crucial to defining the fluid composition of these spaces. For example, the renal tubular epithelium forms a barrier to maintain the distinct luminal ion and macromolecular content relative to the interstitium, which is critical to supporting transport across the epithelium, and is essential to defining urine composition and normal waste removal and water balance. In the gut, similar requirements exist to allow efficient nutrient absorption and water handling. However, because many microorganisms normally reside in the gut lumen, the intestinal epithelium must also effectively form a barrier to prevent bacteria from entering the lamina propria.
The intestinal epithelial cells are critical to forming such a barrier. Without selective channels and transporters, the plasma membrane would be essentially impermeable to water and solutes. Thus, a continuous epithelial cell layer is critical to maintaining epithelial barrier. In addition, the space between individual epithelial cells also needs to be sealed. This function is carried out by the apical junctional complex, containing the tight junction, adherens junction, and desmosome. While the adherens junction and desmosome do not directly seal the space between epithelial cells, they are critical to providing the adhesive force to ensure the integrity of the cell layer.1,2 Once cell–cell adhesion is established, the tight junction seals the space through its protein components, including the transmembrane proteins claudins and occludin, and the cytoplasmic adaptor proteins such as ZO-1 (Fig. 1A).3–5 Because ZO-1 and related proteins bind to a large number of transmembrane tight junction proteins and the actin filaments through distinct protein domains, it was thought that these proteins may form stable protein complexes.3,5–7 However, such sealing is not absolute. For example, Na+ must be able to pass the tight junction to enter the intestinal lumen to establish a Na+ gradient to drive nutrient absorption, and following digestion, glucose, and other nutrients cross the tight junction from the luminal side to maximize absorption.8–11 Thus, the intestinal tight junction must balance the need to form a barrier and the need to support paracellular transport to ensure the proper function of the gastrointestinal tract.
Figure 1.
Components and dynamics of the tight junction. (A) The tight junction is composed of multiple interacting transmembrane and cytoplasmic proteins that are linked to the actin cytoskeleton. (B) In epithelial monolayers expressing both red fluorescent protein-tagged occludin (red) and EGFP-tagged clauidn-1 (green). Following photobleaching of both fluorescent proteins (shown with arrows), significantly more fluorescent recovery occurred for occludin than claudin-1, making the bleached region appear red. Bar: 5 µm.
Intestinal tight junction regulation occurs in health and disease
The tight junction must adapt to the different needs of providing a seal and allowing paracellular transport under distinct physiological and pathophysiolgical conditions. 12–14 An increasing number of stimuli are found to regulate tight junction function and there is a growing understanding about how defective paracellular sealing or transport may contribute to inherited and acquired human diseases.9,15–19 For example, activation of Na+-nutrient cotransport, such as Na+-glucose cotransport mediated by sodium-glucose cotransporter (SGLT)-1, increases paracellular permeability and allows paracellular water and nutrient absorption.9–11,20–23 Such enhanced paracellular absorption is thought to supplement the saturable transcellular pathways to allow efficient nutrient absorption.
Increased paracellular permeability is well-recognized in patients with inflammatory bowel disease, particularly Crohn’s disease.24,25 A fraction of patients with quiescent Crohn’s disease has increased intestinal permeability. When stratified by this criteria, patients with increased permeability are more likely to relapse compared to patients with normal intestinal permeability, indicating that intestinal permeability may participate in disease progression.26 Furthermore, such intestinal permeability increases are also present in a subset of healthy first-degree relatives of Crohn’s disease patients, leading to the hypothesis that reduced intestinal barrier function contributes to Crohn’s disease pathogenesis.27,28 This hypothesis is supported by a recent study showing that mice with intrinsic increases in tight junction permeability have subclinical inflammation and accelerated and exacerbated naive CD4 cell adoptive transfer–induced intestinal inflammation.29 Such increased tight junction permeability is at least partially mediated by TNF, as neutralizing antibody for TNF normalizes intestinal permeability defects in Crohn’s disease patients and TNF can directly induce increases in tight junction permeability in both cultured intestinal epithelial cells and mouse epithelium.30–32
MLCK mediates acute physiological and pathophysiolgical tight junction regulation
The mechanisms for tight junction regulation are under close investigation. Studies of humans and mice have demonstrated that tight junction protein expression and targeting are critical to determining tight junction function. For example, IL-13–mediated expression of tight junction protein claudin-2 is associated with increased intestinal permeability in ulcerative colitis patients, but the effects of IL-13 on barrier function do not occur rapidly33,34
In contrast, physiological regulation of the tight junction by SGLT-1 activation and pathophysiolgical tight junction regulation by proinflammatory cytokines, such as TNF, LIGHT (lymphotoxin-like inducible protein that competes with glycoprotein D for herpes virus entry on T cells), and IL-1β, can happen in a matter of hours without changing tight junction protein expression.35–39 Early morphological studies showed that Na+-glucose cotransport activation induces perijunctional actomyosin condensation and is associated with actomyosin contraction, indicated by phosphorylation of myosin light chain (MLC), the regulatory component of actomyosin function.11,38 Further studies demonstrated that MLCK is responsible for MLC phosphorylation and SGLT-1–induced barrier regulation.38 Similarly, TNF also activates MLCK to increase tight junction permeability and remarkably, specific MLCK inhibition either pharmacologically or genetically blocks TNF-induced tight junction regulation in cell culture and animal models.35,40,41 Furthermore, intestinal cell lines or animals with intestinal epithelial specific constitutively active MLCK expression have increased paracellular permeability, confirming MLCK activity alone is sufficient to regulate tight junction function.29,42 Furthermore, increased MLCK activity has been associated with other forms of tight junction regulation, including enteropathogenic E. coli infection.43 Thus, MLCK-mediated actin cytoskeletal reorganization has both physiological and pathophysiological relevance to tight junction barrier regulation.
Caveolar endocytosis of occludin is critical for cytokine-induced, MLCK-dependent TJ reorganization
Despite our understanding of the role of MLCK in regulating the tight junction function, the molecular mechanisms for tight junction regulation downstream of actomyosin contraction remain poorly understood. Although activation of actomyosin contraction by MLCK alters tight junction protein distribution in detergent insoluble glycoprotein rich microdomains, little is known about how such change occurs and the functional significance for these changes.35,42 To understand the molecular basis for cytokine-induced tight junction regulation, we examined the distribution of individual tight junction proteins before and after exposure to T cell–derived cytokines.41 Most strikingly, occludin disappeared from the tight junction in small intestinal epithelial cells and appeared in intracellular vesicles. This occludin removal from the tight junction is MLCK dependent, as both pharmacological and genetic inhibition of MLCK blocked this change.41 Remarkably, this tight junction reorganization closely resembles the acute occludin endocytosis from the tight junction induced by the actin depolymerizing drug latrunculain A in cultured epithelial monolayers.44 In this form of tight junction reorganization, latrunculin A–induced occludin removal is through dynamin II–dependent caveolar endocytosis, and inhibiting such endocytosis blocks latrunculin A–induced tight junction disruption.44
We then thought to test if and how the caveolar-dependent pathway contributes to cytokine-induced tight junction regulation. We first demonstrated that LIGHT, a cytokine closely related to TNF, increases tight junction permeability in an MLCK-dependent mechanism in cultured epithelial monolayers.36 This cytokine also induced occludin redistribution from the tight junction to caveolin-1–containing vesicles, which was blocked by drugs inhibiting caveolar function, but not drugs inhibiting clathrin-mediated endocytosis and macropinocytosis.36 Such studies demonstrated a role for caveolar-mediated processes in cytokine-mediated tight junction regulation, but the direct evidence for occludin endocytosis and if occludin removal itself is critical for cytokine-mediated tight junction regulation remained uncertain.
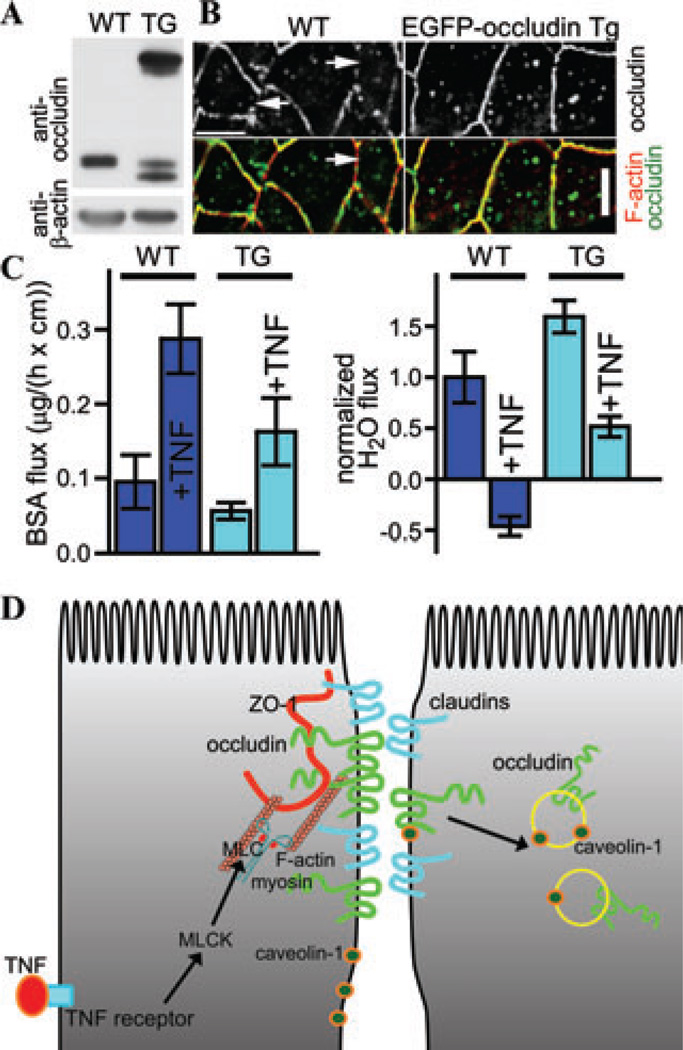
To address this question, we took advantage of the in vivo model for cytokine-induced barrier regulation. When live animal imaging of the small intestinal epithelium was performed on mice with intestinal epithelial-specific transgenic expression of enhanced green fluorescent protein (EGFP)–tagged occludin, we found TNF-induced focal accumulation of occludin and evidence of endocytosis, indicated by vesicle budding from sites of accumulation.45 Using inhibitors for endocytosis, we demonstrated that this internalization is through the caveolar pathway.45 Furthermore, pharmacological inhibitors for caveolar endocytosis and caveolin-1 deficiency both block TNF-induced occludin endocytosis and loss of tight junction function.45 Studies using the occludin transgenic animal showed EGFP-occludin overexpression can maintain large amounts of occludin at the tight junction, which inhibited TNF-induced increase in tight junction permeability, showing occludin itself is a critical component for TNF-induced tight junction regulation (Fig. 2).45 This finding is consistent with the report that occludin participates in TNF-induced tight junction regulation in canine kidney epithelial cells, although the transepithelial resistance (TER) is increased rather than decreased in this cell type following TNF treatment.46
Figure 2.
Occludin overexpression limits TNF-induced tight junction regulation. (A) Overexpression of EGFP-occludin in intestinal epithelium determined by SDS-PAGE immunoblot. Jejunal epithelial cells were isolated from wild-type and EGFP-occludin transgenic mice and were subjected to immunoblot. (B) EGFP-occludin expression preserves occludin localization at the tight junction following TNF treatment. Jejunal tissues from wild-type and EGFP-occluidn transgenic mice treated with 5 µg TNF for 120 min were sectioned and stained for occludin (top, and green in merge), and F-actin (red in merge). Regions of the tight junction lacking occludin developed in wild-type, but not EGFP-occludin transgenic mice. Bar: 10 µm. (C) Intestinal epithelial EGFP-occludin overexpression limits TNF-induced barrier loss and water secretion in small intestine. In vivo perfusion assays show increased paracellular BSA flux (left) and water secretion (right) in wild-type mice. TNF-induced paracellular BSA flux (left) was attenuated, while TNF-induced water secretion did not occur in EGFP-occludin transgenic mice. (D) Schematic presentation of the pathways for TNF to regulate tight junction in intestinal epithelial cells. TNF activates MLCK in intestinal epithelial cells to phosphorylate MLC and cause actomyosin contraction (left side). These events lead to tight junction reorganization to cause occludin to internalize in a caveolin-1–dependent fashion (right side). The original data are adapted from Ref. 45.
MLCK induces TJ regulation through changing ZO-1 protein dynamics
Although occludin endocytosis occurs prominently in cytokine-induced tight junction regulation, it is not seen in all forms of MLCK-mediated tight junction regulation. For example, no occludin internalization can be observed in cells with activated SGLT-1, and direct inhibition of MLCK failed to demonstrate changes in occludin internalization, although both stimuli affect tight junction function. These findings suggest the tight junction organizational changes, if any, are subtle following these stimuli. Understanding the underlying mechanisms has been hampered by the lack of experimental tools to study protein distribution and protein–protein interactions in situ.
The first breakthrough came when it was discovered that distinct tight junction proteins have unique dynamic behaviors when assessed by fluorescent recovery after photobleaching (FRAP) experiments. By using epithelial cell lines expressing well-validated tight junction proteins with N-terminally fused EGFP, we demonstrated that transmembrane proteins claudin-1 and occludin have very different FRAP behaviors at the tight junction: while ~60% of claudin-1 molecules are immobile at the tight junction, only about ~30% of occludin molecules are immobile (Fig. 1B).47 Furthermore, a large fraction of the cytoplasmic protein ZO-1 undergoes constant exchange between the cytoplasmic and tight junction pools.47 Thus, despite the ability of ZO-1 to directly bind to claudin-1, occludin, other tight junction proteins, and the actin cytoskeleton, these proteins do not always form a stable protein complex. Furthermore, this study suggested that tight junction protein dynamics can be altered by a range of stimuli. For example, cholesterol depletion blocked occludin, but not ZO-1, fluorescent recovery while ATP depletion selectively inhibited ZO-1 fluorescent recovery.47 These findings show that tight junction protein dynamic behavior, which reflects protein– protein interactions and protein–lipid interactions, can be modulated. Such modulations may be critical for functional regulation of the tight junction, as both cholesterol depletion and ATP depletion also decrease paracellular barrier function. Thus, studying the biochemical–functional relationships of the tight junction by combining measurements of protein dynamics and paracellular barrier function may provide us mechanistic insights toward tight junction regulation.
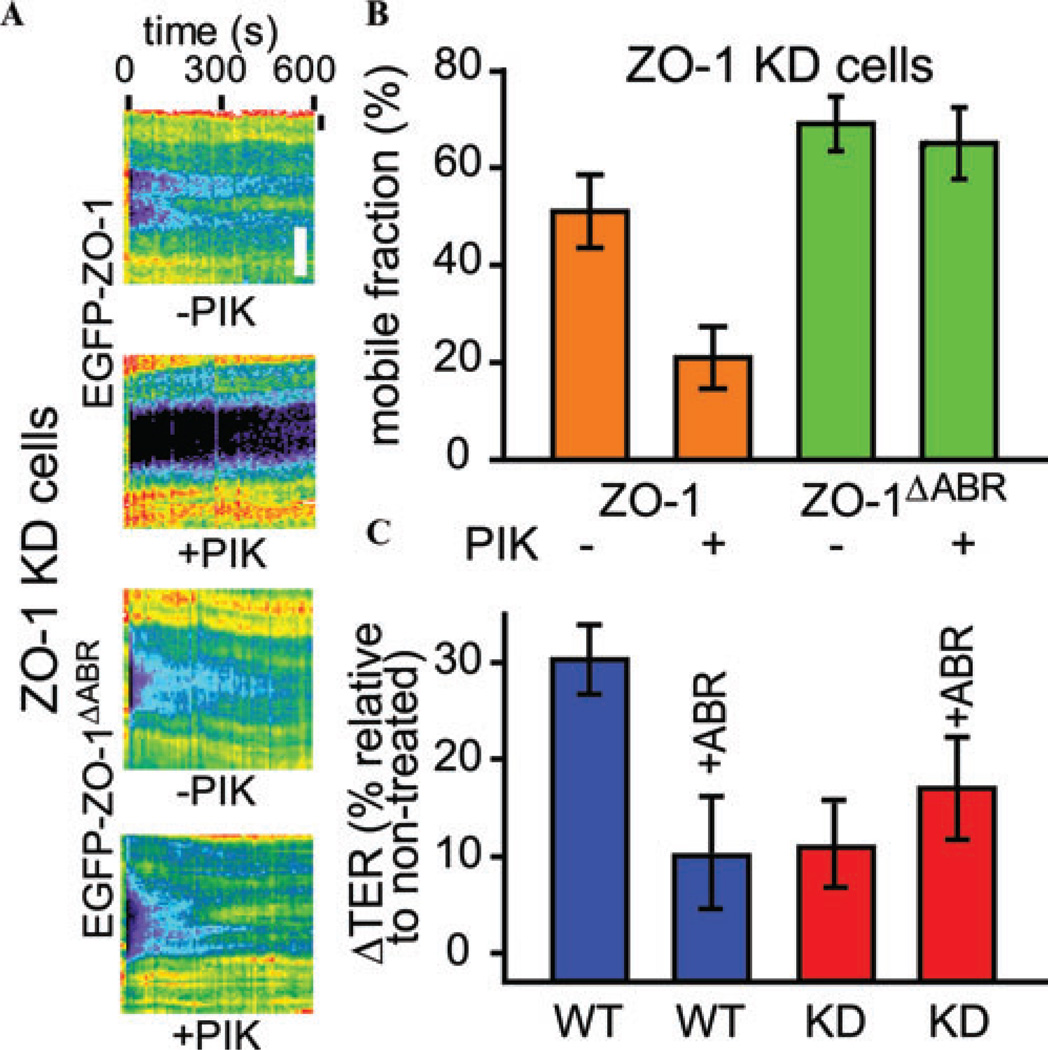
We then applied such an approach to understand how MLCK may affect tight junction protein dynamics and function.48 When the dynamic behaviors for representative tight junction proteins were measured in cells with active or inhibited MLCK by FRAP experiments, altered exchange of ZO-1 to and from the tight junction was observed, but the dynamic behaviors of occludin, and claudin-1 were unchanged.48 Following MLCK inhibition, ZO-1 had decreased fluorescent recovery, indicating it had increased anchoring at the tight junction (Fig. 3A and B).48 The same results were seen in vivo, after MLCK inhibition, in mice with transgenic expression of fluorescently tagged ZO-1.48 Furthermore, in knockout animals lacking the epithelial-specific isoform of MLCK, ZO-1 anchoring are insensitive to MLCK inhibition.48 In addition, in mice with constitutively active MLCK expression in intestinal epithelial cells, ZO-1 recovery is faster.48 These results demonstrate a critical role for MLCK to regulate ZO-1 dynamics. Using the model epithelium, we further showed that ZO-1 anchoring depends on its ability to directly bind to the actin cytoskeleton, as the ZO-1 mutant lacking its actin-binding region (ABR) failed to alter its dynamic behavior following MLCK inhibition (Fig. 3A and B).48,49 In addition, ZO-1 ABR expression blocks MLCK inhibition caused ZO-1 anchoring at the tight junction.48 With the results described above, we were able to test the contribution of ZO-1 and its ABR to tight junction permeability regulation. MLCK inhibition caused a rapid decrease in tight junction permeability in ZO-1–sufficient cells, however, in cells lacking ZO-1, MLCK inhibition failed to regulate tight junction function, indicating a critical role for ZO-1 in MLCK-dependent tight junction regulation (Fig. 3C).48 While expression of the ZO-1 ABR domain was able to block the MLCK inhibition-induced tight junction regulation in ZO-1–sufficient cells, it failed to affect tight junction function in ZO-1-knockdown cells following MLCK inhibition (Fig. 3C),48 demonstrating that the ability of ZO-1 ABR to affect tight junction function depends on endogenous ZO-1 expression. Taken together, the FRAP and barrier function studies demonstrate that ABR-mediated ZO-1 anchoring is responsible for decreased tight junction permeability following MLCK inhibition.
Figure 3.
MLCK inhibition regulates ZO-1 dynamics and tight junction permeability. (A and B) MLCK inhibition by peptide inhibitorPIK decreaseswild-type ZO-1 protein, but not ZO-1 mutant lacking the ABRexchangeatthe tightjunction. ZO-1 knockdown of Caco-2 monolayers expressing either the EGFP-ZO-1 or EGFP-ZO-1 mutant lacking the ABR were treated with 300 µM MLCK inhibitor PIK and then subjected to FRAP experiments. Representative kymographs of ZO-1 protein fluorescent recovery in FRAP experiments are shown in A. Bar: 5 µm. Mobile fractions of the wild-type ZO-1 and ZO-1 mutants lacking the ABR with or without MLCK inhibition were determined on the basis of these experiments (B). (C) The ZO-1 ABR domain overexpression affects the MLCK inhibition–induced TER increase in ZO-1–sufficient, but not ZO-1 knockdown, cells. Treating wild-type Caco-2 monolayers with 300 µM PIK increased TER, which was blocked by ZO-1 ABR expression. In contrast, ZO-1 knockdown cells had minimal TER increases following PIK treatment, which could not be blocked by ZO-1 ABR expression. The original data are adapted from Ref. 48.
CK2 regulates tight junction through affecting tight junction protein complex formation
The study discussed above demonstrated that ZO-1 anchoring is critical for MLCK inhibition-caused decrease in tight junction permeability. However, it did not identify how ZO-1, a cytoplasmic protein, may affect permeability across the tight junction. One can hypothesize that ZO-1 could do so by regulating the function of transmembrane proteins at the tight junction. Such evidence comes from studies of how CK2 inhibition regulates tight junction function. CK2 is a kinase that directly phosphorylates occludin,50–52 and CK2 inhibition results in decreased tight junction permeability to small, but not large ions.53 Knocking down occludin blocked CK2 inhibition– induced barrier regulation, indicating a critical role for occludin in CK2-mediated tight junction regulation.53 In occludin knockdown cells, exogenous expression of wild-type occludin, but not occludin with nonphosphorylable or phosphomimetic mutations at S408, a major phosphorylation site for CK2-mediated occludin phosphorylation, were able to reverse the phenotype, indicating a critical role of occludin S408 phosphorylation in CK2-mediated tight junction regulation.53 When the dynamic behavior of tight junction proteins were measured, occludin, ZO-1, claudin-1, and claudin-2 FRAP behavior were altered following CK2 inhibition.53 Although these molecules have dramatically different FRAP behaviors at steady state, CK2 inhibition caused decreased occludin dynamics with increased claudin-1, claudin-2, and ZO-1 dynamics, making the FRAP behavior for these molecules to converge.53 Such results can be explained by formation of a large protein complex containing occludin, ZO-1, claudin-1, and claudin-2 following CK2 inhibition.53 As occludin does not directly bind to claudins, we tested the idea that ZO-1, which binds to claudins through its first PDZ domain and occludin through its U5-GuK region,54–58 is responsible for complex formation.53 Indeed, ZO-1 knockdown blocked CK2 inhibition-caused alterations in claudin-2 dynamics, and expression of wild-type ZO-1, but not ZO-1 mutants that cannot bind to occludin or claudins, were able to reverse the phenotype.53 Such findings suggest that ZO-1 may serve as an adaptor molecule to transduce the CK2-mediated occludin phosphorylation signal to claudins.
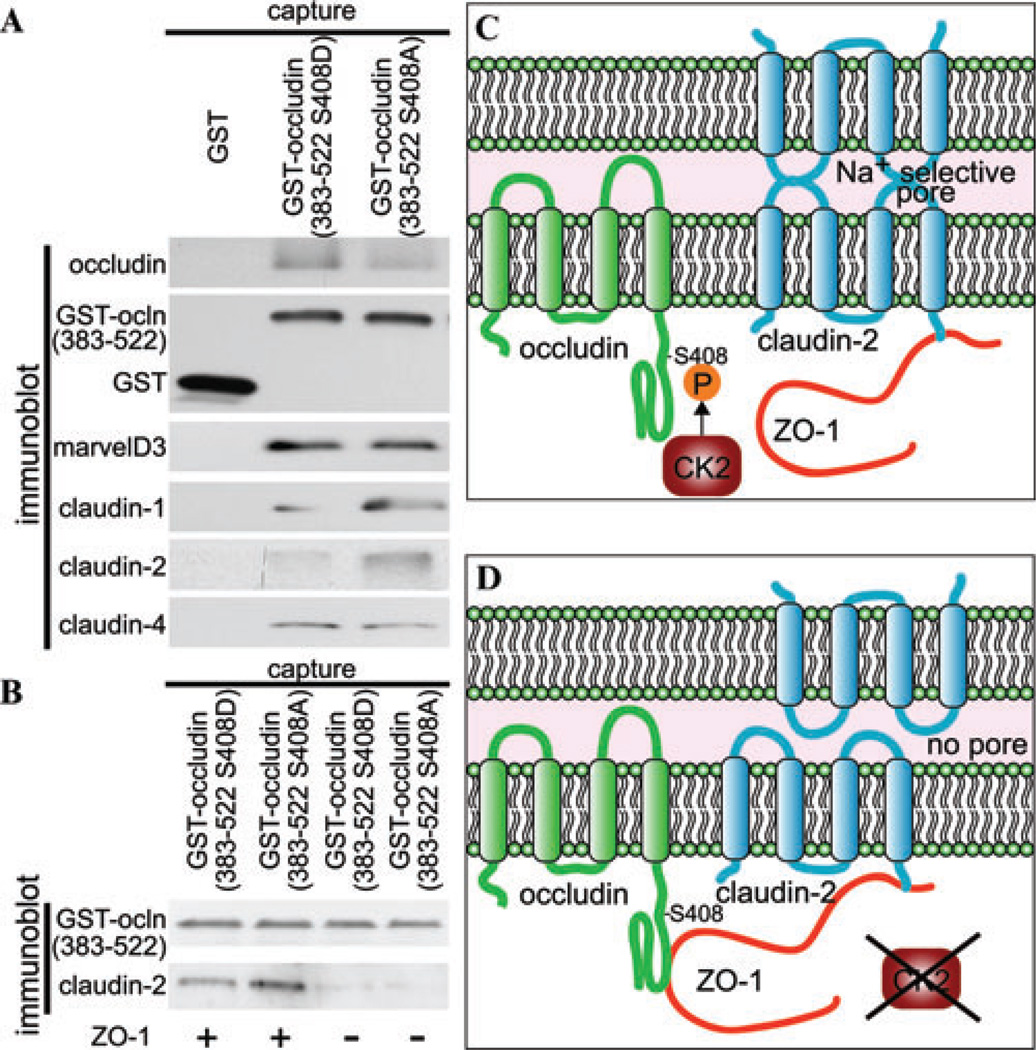
Guided by these FRAP findings, functional studies were performed. Knocking down occludin or ZO-1 individually blocked CK2 inhibition–induced tight junction regulation.53 While separate knockdown of claudin-1 or −2 attenuated CK2-mediated tight junction regulation, double knockdown of claudin-1 and −2 abolished this effect, indicating a cooperative role for these claudins.53 Furthermore, occludin C-terminal tail with S408A mutation, a protein mimics the dephosphorylated occludin generated by CK2 inhibition, has increased association with ZO-1, claudin-1, and claudin-2 than wild-type occludin tail and occludin tail with S408D mutation, suggesting a multiple protein complex formation among occludin, ZO-1, claudin-1, and claudin-2 following CK2 inhibition (Fig. 4A).53 Such a complex formation depends on ZO-1, as claudin-2 cannot be recruited to occludin in the absence of ZO-1 when the occludin C-terminal tail S408A mutant in protein lysates without ZO-1 expression (Fig. 4B).53 Together with FRAP studies, these findings suggest tight junction protein complex formation is responsible for CK2 inhibition– induced tight junction regulation.
Figure 4.
CK2 inhibition alters tight junction protein interaction and barrier function. (A) GST-occludin C-terminal tails (383– 522) immobilizedon glutathione-agarose beads were used tocapture proteins from Caco-2 lysates. Recovered proteins were assessed by SDS-PAGE immunoblot. (B) GST-occludin C-terminal tails (383–522) were used to capture control and ZO-1 knockdown Caco-2 lysates. Recovered proteins were assessed as in A. (C) In the presence of CK2 activity, claudin-2 forms Na+ permeable channels and does not form a complex with occludin. (D) When CK2 activity is blocked, occludin is not phosphorylated at S408, which allows it to bind to ZO-1. Occludin, ZO-1, and claudin-2 form a multimolecular complex to disrupt claudin-2 function, which leads to decreased paracelllar ion permeability. The original data are adapted from Ref. 53.
Modulating tight junction protein interactions as a general mechanism for tight junction regulation
MLCK inhibition and CK2 inhibition are very different stimuli, and they regulate tight junction protein dynamics in distinct manners. MLCK inhibition selectively anchors ZO-1 at the tight junction, without changing the FRAP behavior of claudin-1 and occludin.48 However, how ZO-1 is anchored at the tight junction and the transmembrane component for thisformof tight junctionregulation still needs to be defined. In contrast, CK2 inhibition modulates occludin, ZO-1, claudin-1, and claudin-2 dynamics to allow formation of a large protein complex.53 In this form of barrier regulation, the direct contribution of occludin in forming the barrier remains to be addressed. As claudin-1 and −2 double knockdown made CK2 inhibition unable to regulate barrier function even in cells expressing occludin and ZO-1, it is likely occludin is primarily a signaling intermediate rather than the molecule that directly seals the paracellular space for CK2 to regulate paracellular permeability.
Despite these differences, these two studies presented a unified theme in the mechanisms for tight junction regulation. Both MLCK inhibition and CK2 inhibition (1) selectively decrease paracellular permeability of small, but not large ions, (2) affect ZO-1 dynamic behavior, and (3) require ZO-1 to regulate barrier. Furthermore, in contrast to wild-type ZO-1, ZO-1 mutants lacking the protein domains that are responsible for interacting with its upstream binding partners, that is, actin cytoskeleton or occludin, do not alter their FRAP behaviors following stimulation, and these protein domains are responsible for tight junction barrier regulation. Although some of the molecular details are still missing, such studies demonstrate a common modality for barrier regulation: ZO-1 functions as an adaptor to receive signals from the actin cytoskeleton or other tight junction proteins, which in turn regulates the ability of its transmembrane binding partners, such as claudins to regulate epithelial barrier.
These studies demonstrate that we are on the verge of understanding the molecular mechanisms for acute tight junction regulation. Using similar approaches, the molecular mechanisms for tight junctions to be affected by a variety of stimuli can be dissected. Detailed understanding of tight junction protein–protein interactions at the resting state and their regulation following stimulation at amino acid residues or even at the atomic level will not only provide us with scientific insight, but also will provide targets for specific modulation of tight junction functions for disease treatment and prevention.
Acknowledgments
The author is grateful for continued support of his mentor, Jerrold R. Turner and acknowledges Christopher R. Weber, David Raleigh, Amanda Marchiando, and Dan Yu for contributing graphs and helpful discussions. Work from the group was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK61931, R01DK68271, and P01DK67887 (JRT). The author is supported by a Crohn’s Colitis Foundation of America (CCFA) Career Development Award and was supported by a CCFA Research Fellowship Award.
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Hermiston ML, Gordon JI. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J. Cell Biol. 1995;129:489–506. doi: 10.1083/jcb.129.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- 3.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 4.Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu. Rev. Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- 6.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am. J. Physiol.—Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 7.Guillemot L, Paschoud S, Pulimeno P, et al. The cytoplasmic plaque of tight junctions: a scaffolding and signalling center. Biochim. Biophys. Acta. 2008;1778:601–613. doi: 10.1016/j.bbamem.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Tamura A, Hayashi H, Imasato M, et al. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology. 2011;140:913–923. doi: 10.1053/j.gastro.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Pappenheimer JR. Physiological regulation of transepithelial impedance in the intestinal mucosa of rats and hamsters. J. Membr. Biol. 1987;100:137–148. doi: 10.1007/BF02209146. [DOI] [PubMed] [Google Scholar]
- 10.Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J. Membr. Biol. 1987;100:123–136. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- 11.Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J. Membr. Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- 12.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am. J. Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu D, Turner JR. Stimulus-induced reorganization of tight junction structure: the role of membrane traffic. Biochim. Biophys. Acta. 2007;1778:709–716. doi: 10.1016/j.bbamem.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner JR. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 15.Shen L, Turner JR. Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: tight junction dynamics exposed. Am. J. Physiol.—Gastrointest. Liver Physiol. 2006;290:G577–G582. doi: 10.1152/ajpgi.00439.2005. [DOI] [PubMed] [Google Scholar]
- 16.Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions: IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am. J. Physiol.—Gastrointest. Liver Physiol. 2000;279:G851–G857. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- 17.Simon DB, Lu Y, Choate KA, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2 +resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 18.Carlton VE, Harris BZ, Puffenberger EG, et al. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat. Genet. 2003;34:91–96. doi: 10.1038/ng1147. [DOI] [PubMed] [Google Scholar]
- 19.Pappenheimer JR, Volpp K. Transmucosal impedance of small intestine: correlation with transport of sugars and amino acids. Am. J. Physiol. 1992;263:C480–C493. doi: 10.1152/ajpcell.1992.263.2.C480. [DOI] [PubMed] [Google Scholar]
- 20.Pappenheimer JR. On the coupling of membrane digestion with intestinal absorption of sugars and amino acids. Am. J. Physiol. 1993;265:G409–G417. doi: 10.1152/ajpgi.1993.265.3.G409. [DOI] [PubMed] [Google Scholar]
- 21.Turner JR, Madara JL. Physiological regulation of intestinal epithelial tight junctions as a consequence of Na+-coupled nutrient transport. Gastroenterology. 1995;109:1391–1396. doi: 10.1016/0016-5085(95)90605-3. [DOI] [PubMed] [Google Scholar]
- 22.Atisook K, Carlson S, Madara JL. Effects of phlorizin and sodium on glucose-elicited alterations of cell junctions in intestinal epithelia. Am. J. Physiol. 1990;258:C77–C85. doi: 10.1152/ajpcell.1990.258.1.C77. [DOI] [PubMed] [Google Scholar]
- 23.Atisook K, Madara JL. An oligopeptide permeates intestinal tight junctions at glucose-elicited dilatations. Implications for oligopeptide absorption. Gastroenterology. 1991;100:719–724. doi: 10.1016/0016-5085(91)80016-3. [DOI] [PubMed] [Google Scholar]
- 24.Hollander D, Vadheim CM, Brettholz E, et al. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann. Intern. Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 25.May GR, Sutherland LR, Meddings JB. Is small intestinal permeability really increased in relatives of patients with Crohn’s disease? Gastroenterology. 1993;104:1627–1632. doi: 10.1016/0016-5085(93)90638-s. [DOI] [PubMed] [Google Scholar]
- 26.Wyatt J, Vogelsang H, Hubl W, et al. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- 27.Katz KD, Hollander D, Vadheim CM, et al. Intestinal permeability in patients with Crohn’s disease and their healthy relatives. Gastroenterology. 1989;97:927–931. doi: 10.1016/0016-5085(89)91499-6. [DOI] [PubMed] [Google Scholar]
- 28.Buhner S, Buning C, Genschel J, et al. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut. 2006;55:342–347. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su L, Shen L, Clayburgh DR, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–563. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marini M, Bamias G, Rivera-Nieves J, et al. TNF-alpha neutralization ameliorates the severity of murine Crohn’s-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc. Natl. Acad. Sci. USA. 2003;100:8366–8371. doi: 10.1073/pnas.1432897100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suenaert P, Bulteel V, Lemmens L, et al. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am. J. Gastroenterol. 2002;97:2000–2004. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- 32.Zeissig S, Bojarski C, Buergel N, et al. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–1302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber CR, Raleigh DR, Su L, et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J. Biol. Chem. 2010;285:12037–12046. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Wang F, Graham WV, Wang Y, et al. Interferongamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarz BT, Wang F, Shen L, et al. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007;132:2383–2394. doi: 10.1053/j.gastro.2007.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J. Immunol. 2007;178:4641–4649. doi: 10.4049/jimmunol.178.7.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner JR, Rill BK, Carlson SL, et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 39.Mullin JM, Laughlin KV, Marano CW, et al. Modulation of tumor necrosis factor-induced increase in renal (LLC-PK1) transepithelial permeability. Am. J. Physiol. 1992;263:F915–F924. doi: 10.1152/ajprenal.1992.263.5.F915. [DOI] [PubMed] [Google Scholar]
- 40.Zolotarevsky Y, Hecht G, Koutsouris A, et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 41.Clayburgh DR, Barrett TA, Tang Y, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J. Clin. Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen L, Black ED, Witkowski ED, et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell Sci. 2006;119:2095–2106. doi: 10.1242/jcs.02915. [DOI] [PubMed] [Google Scholar]
- 43.Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterol. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
- 44.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol. Biol. Cell. 2005;16:3919–3936. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchiando AM, Shen L, Graham WV, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J. Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J. Cell Sci. 2010;123:2844–2852. doi: 10.1242/jcs.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell Biol. 2008;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu D, Marchiando AM, Weber CR, et al. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc. Natl. Acad. Sci. USA. 2010;107:8237–8241. doi: 10.1073/pnas.0908869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fanning AS, Ma TY, Anderson JM. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J. 2002;16:1835–1837. doi: 10.1096/fj.02-0121fje. [DOI] [PubMed] [Google Scholar]
- 50.Smales C, Ellis M, Baumber R, et al. Occludin phosphorylation: identification of an occludin kinase in brain and cell extracts as CK2. FEBS Lett. 2003;545:161–166. doi: 10.1016/s0014-5793(03)00525-8. [DOI] [PubMed] [Google Scholar]
- 51.Cordenonsi M, Turco F, D’Atri F, et al. Xenopus laevis occludin. Identification of in vitro phosphorylation sites by protein kinase CK2 and association with cingulin. Eur. J. Biochem. 1999;264:374–384. doi: 10.1046/j.1432-1327.1999.00616.x. [DOI] [PubMed] [Google Scholar]
- 52.Cordenonsi M, Mazzon E, De Rigo L, et al. Occludin dephosphorylation in early development of Xenopus laevis. J. Cell Sci. 1997;110:3131–3139. doi: 10.1242/jcs.110.24.3131. [DOI] [PubMed] [Google Scholar]
- 53.Raleigh DR, Boe DM, Yu D, et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J. Cell Biol. 2011;193:565–582. doi: 10.1083/jcb.201010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt A, Utepbergenov DI, Krause G, Blasig IE. Use of surface plasmon resonance for real-time analysis of the interaction of ZO-1 and occludin. Biochem. Biophys. Res. Commun. 2001;288:1194–1199. doi: 10.1006/bbrc.2001.5914. [DOI] [PubMed] [Google Scholar]
- 55.Muller SL, Portwich M, Schmidt A, et al. The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1. J. Biol. Chem. 2005;280:3747–3756. doi: 10.1074/jbc.M411365200. [DOI] [PubMed] [Google Scholar]
- 56.Fanning AS, Little BP, Rahner C, et al. The Unique-5 and −6 Motifs of ZO-1 Regulate Tight Junction Strand Localization and Scaffolding Properties. Mol. Biol. Cell. 2007;18:721–731. doi: 10.1091/mbc.E06-08-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 58.Itoh M, Furuse M, Morita K, et al. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]