Abstract
Acute lung injury (ALI) or acute respiratory distress syndrome remains a major cause of morbidity and mortality in hospitalized patients. The pathophysiology of ALI involves complex interactions between the inciting event, such as pneumonia, sepsis or aspiration, and the host immune response resulting in lung protein permeability, impaired resolution of pulmonary edema, an intense inflammatory response in the injured alveolus and hypoxemia. In multiple pre-clinical studies, adult stem cells have been shown to be therapeutic due to both the ability to mitigate injury and inflammation through paracrine mechanisms and perhaps to regenerate tissue by virtue of their multi-potency. These characteristics have stimulated intensive research efforts to explore the possibility of using stem or progenitor cells for the treatment of lung injury. A variety of stem or progenitor cells have been isolated, characterized, and tested experimentally in pre-clinical animal models of ALI. However, questions remain concerning the optimal dose, route and the adult stem or progenitor cell to use. Here, we review current mechanisms underlying the therapeutic effect of stem cells in ALI as well as the questions that will arise as clinical trials for ALI are planned.
Keywords: Acute lung injury, cell-based therapy, endothelial progenitor cells, mesenchymal stem cells, paracrine soluble factors
INTRODUCTION
Acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome (ARDS), are the most common causes of acute respiratory failure in hospitalized patients. Despite extensive research into the pathophysiology, the mortality associated with ALI/ARDS has remained up to 40%, depending on the etiology1,2. Current treatment is primarily supportive with lung-protective ventilation and a fluid conservative strategy3,4. Pharmacological therapies that reduce the severity of lung injury in pre-clinical models have not yet been translated to effective clinical treatment options. Earlier concepts of distinct disease phases underlying the pathophysiology of ALI/ARDS, from an early pro-inflammatory to a later pro-fibrotic phase now appear to be an over-simplification5. Consequently, strategies that targeted one aspect of the disease process have not been successful in limiting overall morbidity and mortality. Innovative therapies are needed.
Stem cells are undifferentiated precursor cells with the capacity for self-renewal and the ability to differentiate into cells of multiple lineages. They can be broadly classified by their potency (pluri-potent vs. multi-potent) and origin (adult vs. embryonic). Adult stem cells are multi-potent post-natal stem cells that remain in body tissues throughout life and have the potential to differentiate into a more limited range of mature cell types. In general, they include hematopoietic stem cells (HSC), mesenchymal stem cells (MSC), endothelial progenitor cells (EPC), and organ specific stem cells such as endogenous lung stem cells. In 1998, Ferrari et al.6 reported in a landmark manuscript that the transplantation of bone marrow derived adult stem cells into injured muscle tissue mitigated damage and regenerated healthy muscle fibers, generating tremendous enthusiasm for stem cell-based therapy.
Much of the initial interest in stem cell-based therapy for lung injury arose initially from the multi-potent properties of the cells. Krause et al.7 reported up to 20% engraftment of bone marrow-derived cells in the lung, including epithelial cells, from a single hematopoietic precursor. However, subsequent studies suggested that the major therapeutic effect of adult stem cells in ALI were primarily from their ability to secrete paracrine factors such as growth factors, factors regulating endothelial and epithelial permeability, anti-inflammatory cytokines and anti-microbial peptides/proteins, not from significant engraftment. To date, due to their ease of isolation and extensive pre-clinical studies, MSCs may offer the best hope for clinical trial. However, other progenitor and adult stem cells5,8–11 has shown some promise as potential therapeutic candidates for ALI/ARDS as well.
Despite these encouraging results, several issues remain which must be addressed12. (1) The isolation and classification of stem cells need to be defined further, particularly concerning the issue of potency; (2) A more precise understanding of the mechanisms underlying the therapeutic effect is needed, such as the role of the constituents of the conditioned medium, of cell-contact-dependent or -independent effects, and whether the phenotype of the cells changes depending on the alveolar milieu; (3) The optimal dose and route of cell delivery remains to be determined; (4) Although most of the current focus is on the use of MSCs, we still need to determine the optimal “stem cells” for cell-based therapy; And (5) despite promising pre-clinical results and public enthusiasm, clinician-scientists involved in the translation of stem cell research into clinical trials must always keep in mind the lessons learned from the field of gene therapy13. Above all, we must do no harm.
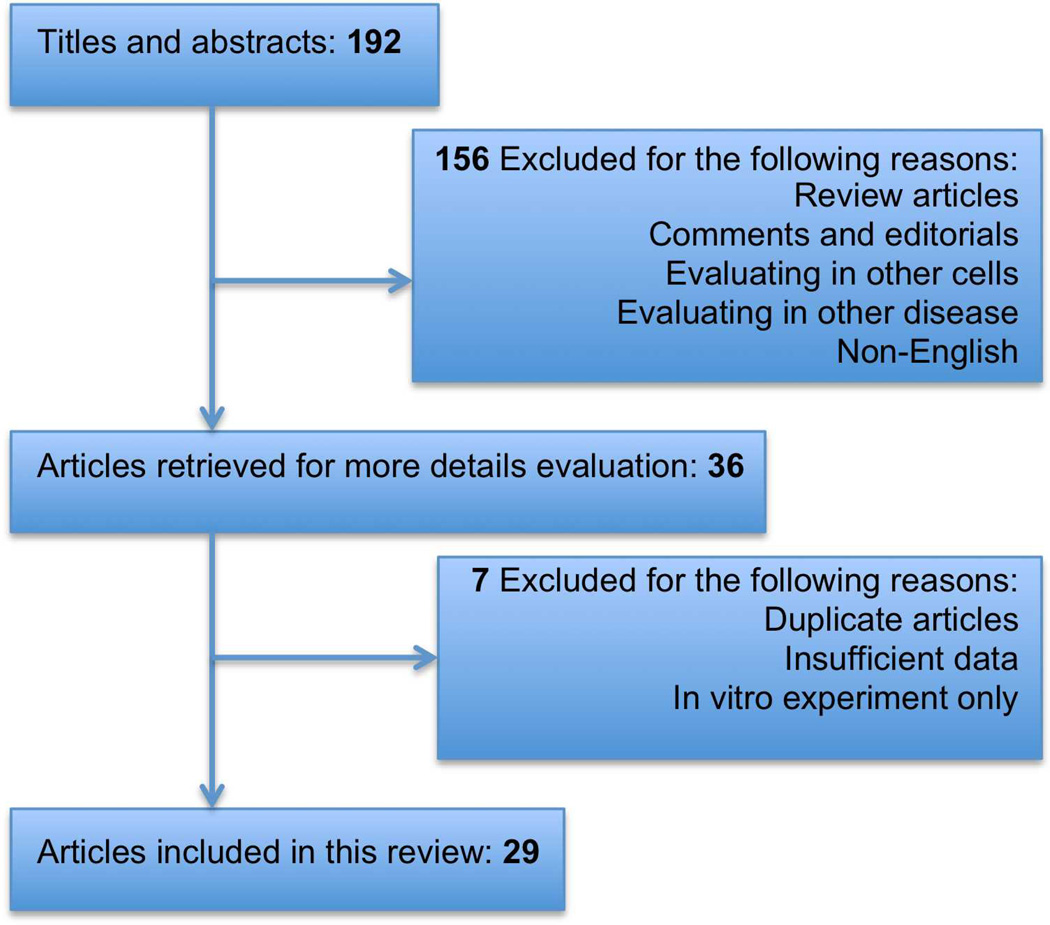
This review will present the most recent advances in the field of adult stem cell therapy for ALI, focusing specifically on MSCs and EPCs, and to issues that will arise in preparation for clinical trial. To accomplish this goal, we searched PubMed for relevant studies published up to Dec 2012. We also searched the proceedings of major relevant conferences, trial databases, the reference lists of identified trials, and major reviews. We identified 192 studies from electronic databases, of which 36 studies were eligible for inclusion, based on the title and abstract. After independent assessment of the full text, 29 articles were finally considered to be eligible for inclusion in the analyses after we contacted authors for additional data. Figure 1 shows the search leading to the selection of the final 29 articles.
Figure 1. Research Strategy for Selection of MSC Pre-clinical Studies.
Mesenchymal Stem Cells
Mesenchymal stem or stromal cells are adult non-hematopoietic precursor cells derived from a variety of tissues such as the bone marrow, adipose tissue and placenta which have been used as therapy in multiple diseases and syndromes such as myocardial infarction, renal failure and graft-vs.-host disease (GVDH). The International Society of Cellular Therapy (ISCT) has defined MSCs in 2006 by three criteria: (1) MSCs must be adherent to plastic under standard tissue culture conditions; (2) MSCs must express certain cell surface markers such as CD73, CD90, and CD105, but must not express other markers including CD45, CD34, CD14, or CD11b; and (3) MSCs must have the capacity to differentiate into mesenchymal lineages including osteoblasts, adipocytes, and chrondoblasts under in vitro conditions14.
Mechanisms of Action
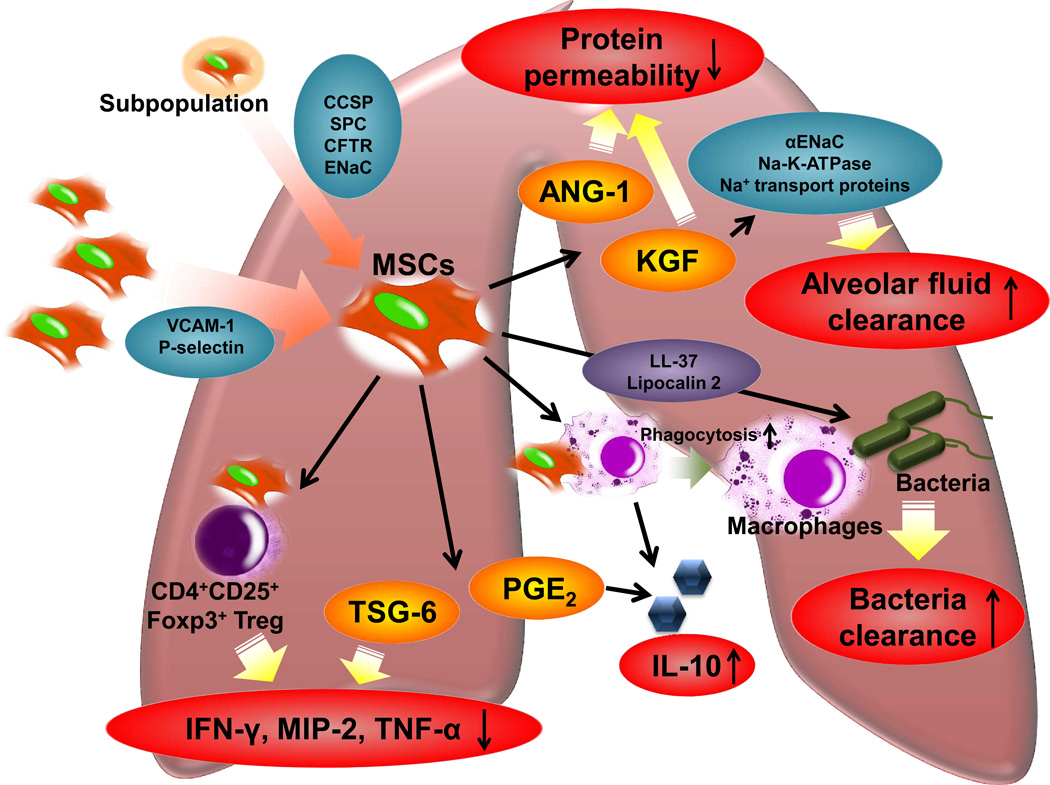
Although the precise mechanisms of action of MSCs remain unclear, a number of important insights from recent preclinical studies have emerged10 (Figure 2).
Figure 2. Potential Therapeutic Mechanisms of MSCs in Lung Injury.
The therapeutic response of MSCs in lung injury appears to be primarily based on immunomodulation of inflammatory cells such as alveolar macrophages or immune cells like CD4+CD25+Foxp3+ regulatory T cells, suppression of lung protein permeability, improvement in alveolar fluid clearance and the secretion of anti-microbial peptides/proteins. Despite the low engraftment rate, however, specific subpopulation of modified MSCs may increase lung engraftment and/or regeneration in vivo. MSCs: Mesenchymal stem cells; VCAM-1:vascular cell adhesion molecule 1; CCSP:club cell secretory protein (Clara cell protein); SP-C:surfactant protein-C; CFTR:cystic fibrosis trans-membrane conductance regulator; ENaC:epithelial sodium channel; KGF:keratinocyte growth factor; ANG-1:angiopoietin-1; PGE2: Prostaglandin E2; TSG-6: tumor necrosis factor-α induced protein 6; MIP-2: macrophage inflammatory protein 2; IL-10: Interleukin-10; CD4+CD25+Foxp3+Treg: CD4+CD25+Forkhead Boxp3+ Regulatory T Cells.
Engraftment
Despite initial interest in MSCs’ multi-potent properties, engraftment in the lung now does not appear to play a major beneficial role15–17. This perception was initially based on earlier studies that demonstrated that intravenous administration of MSCs resulted in high pulmonary engraftment because MSCs expressed adhesion molecules, such as VCAM-1 and P-selectin, which facilitated their retention in the lung18 and which was enhanced by the presence of hyaluronan degradation products in the inflamed tissue19,20. These results were subsequently questioned by multiple groups, who observed only engraftment of leukocyte lineages15 or low engraftment rates in lung injury models with observed rates of <1%16,17. Yet, several new publications21–23 have highlighted the potential of in vitro modification of MSCs, which may increase lung engraftment and/or regeneration. These authors found specific subpopulations of MSCs which expressed functional markers of lung epithelial cells such as club cell secretory protein (Clara cell protein), surfactant protein-C, cystic fibrosis trans-membrane conductance regulator and epithelial sodium channel (ENaC) when cultured in mouse tracheal epithelial cell medium, small airway growth medium or with keratinocyte growth factor (KGF) and retinoic acid respectively. In addition, several groups24–26 have identified adult stem cells within the lung, some with the features of MSCs. Tropea et al.27 demonstrated that systemic treatment with MSCs or MSC condition medium had a direct effect on stimulating bronchioalveolar stem cells to repair and restore injured lung epithelium.
Immunomodulation
Multiple studies have demonstrated that MSCs possess potent immunosuppressive effects by inhibiting the activity of both innate and adaptive immune cells28–31. This immunosuppression has shown to be mediated by cell contact-dependent and independent mechanisms through the release of soluble factors such as tumor necrosis factor α (TNF-α) stimulated gene/protein 6 (TSG-6)32, PGE233, interleukin-10 (IL-10)33,34, and interleukin 1 receptor antagonist (IL-1RN)35 among others. For example, we36 and other investigators37–39 have demonstrated that MSCs may switch the phenotype of alveolar macrophages from a M1 (inflammatory) to a M2 (anti-inflammatory). Also, in an endotoxin-induced ALI model treated with umbilical cord derived MSCs, Sun et al.40 found that MSCs suppressed lung injury by up-regulating CD4+CD25+Foxp3+ Treg cells, reduced the levels of the pro-inflammatory cytokines IFN-γ, macrophage inflammatory protein 2 (MIP-2) and TNF-α and increased the anti-inflammatory cytokine, IL-10. Treg cells play a central role in the prevention of autoimmunity and in the control of immune responses by down-regulating the function of effector CD4+ or CD8+ T cells41–43; these findings are consistent with other reports28,36 that MSCs can decrease T cell responses by shifting from a Th1 to Th2 type response.
Alveolar fluid clearance
We and other investigators have reported that ALI pulmonary edema fluid contained high levels of pro-inflammatory cytokines which down-regulated alveolar fluid clearance (AFC, i.e. the resolution of pulmonary edema)44,45. Interestingly, MSCs are known to produce several epithelial specific growth factors, such as keratinocyte growth factor (KGF), the seventh member of the fibroblast growth factor family, which are known to increase AFC. We46 and other researchers38,47,48 have reported that KGF can reduce lung injury in animal and human models of pulmonary edema whether from direct, indirect or infectious causes. KGF improved AFC in part by up-regulating αENaC gene expression and Na-K-ATPase activity or through increased trafficking of sodium transport proteins to the cell surface46,49–51.
Lung permeability
The integrity of the lung microvascular endothelium is essential to prevent the influx of protein-rich fluid and inflammatory cells from the plasma, which may aggravate the ability of the lung epithelium to reduce pulmonary edema. Several MSC paracrine soluble factors, such as Angiopoietin-1 (Ang-1) and KGF, are potentially important in these effects. Ang-1, a ligand for the endothelial Tie2 receptor, is a known endothelial survival and vascular stabilization factor that reduces endothelial permeability and inhibits leukocyte-endothelium interactions by modifying cell adhesion molecules and cell junctions52,53. We and other investigators have found that allogeneic human MSCs secreted a significant amount of Ang-1, which was essential to prevent the increase in lung protein permeability54–56.
Anti-microbial properties
MSCs have Toll-like and formyl peptide-like receptors and become activated in response to different bacterial products, suggesting that MSCs may be directly involved in the innate immune response36,57. Recently, we found that human MSCs can inhibit bacterial growth directly in part through the secretion of antimicrobial peptides, such as LL-37, which was up-regulated upon bacterial stimulation58, and antibacterial proteins, such as lipocalin 2, which improved bacterial clearance in a mouse model of E. coli pneumonia59. Mei et al.60 also recently showed that the improvement in bacterial clearance in MSC-treated septic mice following cecal ligation and puncture could be in part explained by enhanced phagocytic activity of host immune cells.
Recent experimental results
To date, MSCs have been studied extensively in preclinical lung injury models (Table 1). Most of the injury32,36–38,40,46,55,56,64–69 (48%, 14 of 29)involved intra-tracheal endotoxin exposure. Rodents were the predominant small animals studied. Over 58% (17 of 29)of studies32,35,36,38–40,47,55,56,58,59,61,63–66,69 used mice while 38% (11 of 29) used rats34,37,48,62,67,68,70–74. Interestingly, Lee et al.46 developed anE. coli endotoxin-induced ALI model in an ex vivo human lung preparation perfused partially with whole blood. Although the preparation was short term (4 h) and did not include the perfusion of other systemic organs such as the liver or spleen, which may mount a significant inflammatory response, this human model replicated most of the early clinical features of patients with ALI.
Table 1.
Descriptive details of MSCs preclinical study
| Study | Lung Injury Model |
Species | Origin | Route | Dosage (×106 cells/kg) | Given Time After Exposure |
Measured Time Points |
Therapeutic Effect |
Possible Mechanism |
|---|---|---|---|---|---|---|---|---|---|
| Rojas et al. 200561 | Bleomycin-induced lung injury | C57BL/6 | Bone marrow | i.v. | 22.2 | 6h | D14 | + | Engraftment Paracrine soluble factor |
| Ortiz et al. 200735 | Bleomycin-induced lung injury | C57BL/6 | Bone marrow | i.v. | 22.2 | 0h | D3 D7 D14 |
+ ++ + |
IL1RN |
| Zhao et al. 200862 | Bleomycin-induced lung injury | SD Rat | Bone marrow | i.v. | 22.2 | 12h | D14 | + | Engraftment |
| Aguilar et al. 200947 | Bleomycin-induced lung injury | C57BL/6 | Bone marrow | i.v. | 20 (40 total) |
8h 72h |
D14 | + | KGF |
| Saito et al. 201163 | Bleomycin-induced lung injury | C57BL/6 | Bone marrow | i.v. | 22.2 | 24h | D2 D3 D7 D14 |
− + + + |
CCL2 |
| Mei et al. 200755 | LPS-induced ALI | C57BL/6 | Bone marrow | i.v. | 12.6 | 0.5h | 15min D3 |
− + |
Ang-1 |
| Gupta et al. 200736 | LPS-induced ALI | C57BL/6 | Bone marrow | i.t. | 33.3 | 4h | 24h 48h |
+ ++ |
Paracrine soluble factor |
| Xu et al. 200856 | LPS-induced ALI | C57BL/6 | Bone marrow | i.v. | 44.4 | 2h | D3 D7 D14 |
− + + |
Ang-1 |
| Lee et al. 200946 | LPS-induced ALI | Human Lung | Allogeneic human BM | Intra-pulmonary | 5×106cells* | 1h | 4h | + | KGF |
| Araujo et al. 201064 | LPS-induced ALI | C57BL/6 | Bone marrow | i.v. | 88.9 | 6h | D1 D7 |
+ + |
Engraftment Paracrine soluble factor |
| Prota et al. 201065 | LPS-induced ALI | C57BL/6 | Bone marrow | i.v. | 888.9 | 1h | D28 | + | Alveolar-capillary membrane repair |
| Sun et al. 201140 | LPS-induced ALI | BALB/C | Human umbilical cord | i.t. | 46.5 | 4h | D1 D2 D3 D7 |
− + + − |
CD4+CD25+ Foxp3+Treg |
| Liang et al. 201137 | LPS-induced ALI | Wistar Rat | Bone marrow | i.v. | 7.1 | 2h | 6h 24h D4 D7 D21 |
− + ++ + − |
Engraftment Paracrine Soluble Factors |
| Danchuk et al. 201132 | LPS-induced ALI | BALB/C | Human BM | i.t. | 11.1 (22.2 total) |
4h 4.5h |
24h 48h |
+ + |
TSG-6 |
| Song et al. 201266 | LPS-induced ALI | BALB/C | Bone marrow | i.v. | 22.2 | 0h | D3 D7 D14 |
+ + − |
HIMF (−) |
| Li et al. 201267 | LPS-induced ALI | SD Rat | Human umbilical cord | i.v. | 1.9 | 1h | 6h 24h 48h |
+ ++ ++ |
MDA HO-1 Oxidative stress |
| Qin et al. 201268 | LPS-induced ALI | SD Rat | Bone marrow | Intra-pleural | 4 | 0h | D1 D3 D7 |
− ++ + |
Paracrine soluble factor |
| Ionescu et al. 201238 | LPS-induced ALI | C57BL/6 | Bone marrow | i.t. | 11.1 | 4h | 48h | + | IGF-I |
| Chien et al. 201269 | LPS-induced ALI | BALB/C | Orbital fat tissue | i.v. | 13.3 | 0.3h | D3 | + | Immunomodulation |
| Manning et al. 201034 | I/R lung injury | Lewis Rat | Bone marrow | i.v. | 75 | 0h | 4h 24h D3 D7 |
+ ++ + + |
IL-10 |
| Sun et al. 201170 | I/R lung injury | SD Rat | Autologous Adipose tissue | i.v. | 4.8 (14.4 total) |
1h 6h 24h |
D3 | + | VCAM-1 ICAM-1 Oxidative stress |
| Chen et al. 201271 |
I/R lung injury | SD Rat | Bone marrow | i.v. | 3.6 | 0h | 24h | + | VEGF SDF-1 IPO |
| Krasnodembskaya et al. 201058 | E. coli Pneumonia | C57BL/6 | Allogeneic human BM | i.t. | 44.4 | 4h | 18h | + | LL-37 |
| Kim et al. 201139 | E. coli Pneumonia | ICR Mouse | Human umbilical veins | i.t. | 3.3 | 3h | D1 D3 D7 |
− ++ + |
Paracrine soluble factor |
| Gupta et al. 201259 | E coli pneumonia | C57BL/6 | Bone marrow | i.t. | 30 | 4h | 4h 8h 24h 48h |
+ + + + |
Lipocalin 2 |
| Curley et al. 201248 | Ventilator-induced lung injury | SD Rat | Allogeneic BM | i.v. | 7.3 (14.5 total) | 0h 24h |
48h | + | KGF Paracrine soluble factor |
| Chimenti et al. 201272 | Ventilator-induced lung injury | SD Rat | Lewis rat BM | i.v./i.t. | 18.2 | −0.5h | 3h | + | VCAM-1 P-selectin |
| Pati et al. 201173 | Hemorrhagic Shock-induced ALI | SD Rat | Human BM | i.v. | 6.4 (12.8 total) | 1h 24h |
D4 | + | VE-cadherin Claudin-1 Occludin-1 |
| Yang et al. 201174 | Paraquat poisoning-induced lung injury | SD Rat | Bone marrow | i.v. | 50 | 6h | D3 D7 D14 |
+ ++ + |
Reducing lung edema and lipid peroxidation Inhibiting the release of inflammatory mediators |
IL1RN, interleukin 1 receptor antagonist; KGF, keratinocyte growth factor; Ang-1, angiopoietin-1; CD4+CD25+Foxp3+Treg, CD4+CD25+Forkhead Boxp3+ Regulatory T Cells; TSG-6, tumor necrosis factor-α induced protein 6; HIMF, hypoxia-induced mitogenic factor; MDA, malondialdehyde; HO-1, heme Oxygenase-1; IGF-1, insulin growth-like growth factor I; IL-10, interleukin-10; VCAM-1, vascular cell adhesion molecule 1; ICAM-1, intercellular adhesion molecule 1; VEGF, vascular endothelial growth factor; SDF-1, stromal cell-derived factor-1; IPO, ischemic post-conditioning; VE, vascular endothelial.
Result is present in total cell amount because no weight is available.
The bone marrow (BM) remained the most common source of MSCs32,34–38,46–48,55,56,58,59,61–66,68,71–74 (83%, 24 of 29), and most current clinical trials used an allogeneic source from the bone marrow. It was believed that MSC are able to evade clearance by the host immune system through a variety of mechanisms including low expression of the MHC I and II proteins and lack of the T cell co-stimulatory molecules, CD80 and CD86; this is often referred to as being “immunoprivileged.” However, recent studies have shown that MSC can express higher levels of the MHC class proteins than originally thought. In addition, Nauta et al.75 demonstrated that infusion of allogeneic MSC elicited a host response and led to graft rejection. It has now become apparent that MSC have complex interactions with the immune system. However, the alternative approach of harvesting the bone marrow to isolate and culture autologous MSCs may be problematic in acute illnesses such as ALI.
Compared with the bone marrow, human umbilical cord-derived MSCs (UC-MSCs) have a faster population doubling time than BM-MSCs, and such proliferation characteristics do not change even after 30 passages. In addition, UC-MSCs showed lower expression of CD106 and HLA-ABC in comparison with BM-MSCs76. Therefore, UC-MSCs are currently being studied for clinical application due to their accessibility, ease of procurement from donors, and lack of ethical concerns39,40,67. Another adult source is fatty tissue. Human orbital fat-derived stem cells (OFSCs) are advantageous over BM-MSCs because they can be isolated from minimal volume of redundant orbital fat tissue69,77. They also exhibit a higher epithelial differentiation potential than adipose-derived stem cells isolated from subcutaneous fatty tissues70,77. However, the anti-inflammatory ability of OFSCs needs to be evaluated in other clinically relevant models such as pneumonia/sepsis. Clearly, further studies in the optimal source of MSCs are needed.
Optimal dosage and route of cell delivery
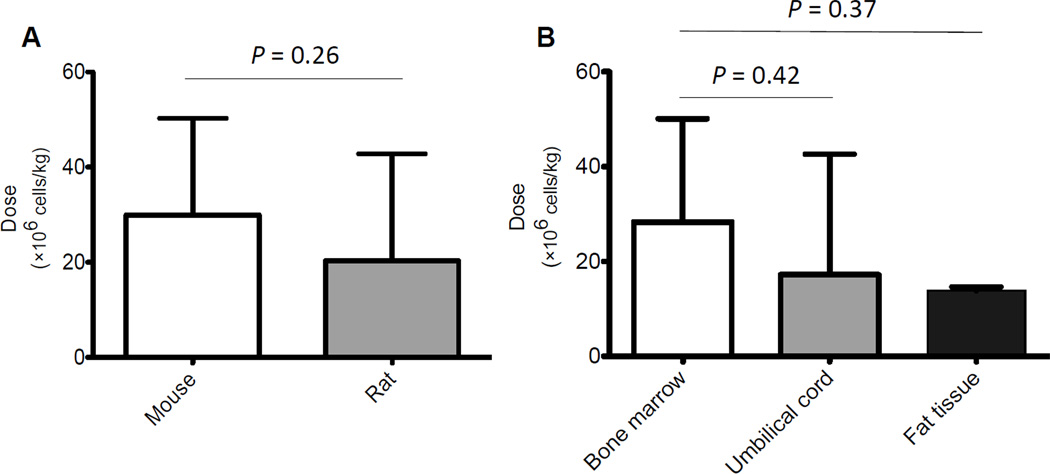
The dose and route of MSCs varies substantially based on different pre-clinical animal studies, and the optimal treatment remains to be determined. The mean dose 32,35,36,38–40,47,55,56,58,59,61,63,64,66,69 instilled during the early phase of lung injury in mice is(29.9 ± 20.4) × 106 cells/kg, which is slightly higher than that of rats, (20.3 ± 22.5)×106 cells/kg (Figure 3A, p=0.26), suggesting that the effective administration dose is about 20~30 × 106 cells/kg. One study65 using an ALI model in C57BL/6 mice gave a particularly large dose (888.9 × 106 cells/kg) of MSCs just 1 hour after exposure. To our knowledge, this is the only study showing the delayed effects of BM-MSCs on day 28 because all others therapies were followed up to day 14. Although the administration of MSCs was able to repair the lung epithelium and endothelium, reduce the amount of alveolar collapse and led to an improvement in lung mechanics, it is still unknown whether the beneficial effects reported at day 28 persist if the MSCs were given later in the course of lung injury. In terms of tissue origin, the average dose of MSCs derived from the bone marrow was (28.3 ± 21.8) × 106 cells/kg. No significant differences were found between the dose of MSCs from the bone marrow or other tissues such as umbilical cord39,40,67 (17.2 ± 25.4) × 106 cells/kg) (Figure 3B, p=0.42) or fat tissue69,70 (13.9 ± 0.8)×106 cells/kg) (Figure 3B, p=0.37). For most clinical trials utilizing MSCs in lung disease such as for idiopathic pulmonary fibrosis (NCT01385644) or bronchopulmonary dysplasia (NCT01632475), the dose administered is approximately 1~20 × 106 cells/kg, which appears to be largely based on earlier clinical trials in GVHD, myocardial infarction, etc. Therefore, a current pilot clinical trial conducted by UCSF (NCT01775774)is underway to assess the safety of intravenous infusion of allogeneic human BM-MSCs in patients with ALI/ARDS by using a dose-escalation protocol from 1 to 10 × 106 cells/kg. What remains unclear is whether there is a dose effect or a therapeutic ceiling in the use of MSCs for lung diseases or whether the dose is limited by the safety concern of the effect of the infusion on pulmonary vascular resistance. And perhaps more importantly, it is unclear whether a second dose of MSCs is needed for the later phase of ALI (the resolution), and whether the functional phenotype of the stem cells is therapeutic at this point. Although many believe that higher doses will give a prolonged response, no actual dose response has been reported in the literature.
Figure 3. Average Dose of MSCs Administered in ALI Models.
(A) No significant difference was found in the average dose of MSCs used between mouse and rat models of ALI. Data are shown as mean ± SD, N = 16 studies in mice and N = 11 studies in rat, P = 0.26. (B) No significant difference was found in the average dose used among bone marrow, umbilical cord or fat tissue derived MSCs for ALI. Data are shown as mean ± SD, N = 22 studies using bone marrow derived, N = 3 studies using umbilical cord derived and N = 2 studies using fat tissue derived. P = 0.42, bone marrow vs. umbilical cord; P = 0.37, bone marrow vs. fat tissue.
Most of the studies39,58,59 using an E. coli endotoxin or bacterial pneumonia models of ALI administrated MSCs intra-tracheally while those using bleomycin-induced35,47,61–63, ischemia reperfusion-induced34,70,71, ventilator-induced 48,72 or other73,74 lung injury models delivered MSCs intravenously. Although for practical reasons it may not be feasible to instill stem cells intra-bronchially in ALI patients who are hypoxemic, the optimal route of delivery is unclear. However, for patients with ALI from pneumonia, it is now known that BM-MSCs possess direct antimicrobial activity through the secretion of anti-microbial peptides/proteins such as LL-37 or lipocalin-2 as well as the ability to enhance macrophage/monocyte phagocytosis of bacteria. Thus, the intrapulmonary delivery may be the most effective route to enhance bacteria clearance. On the other hand, intravenous delivery of MSCs was used by the majority of studies34,35,37,47,48,55,56,61–67,69–74 (69%, 20 of 29) and may be more relevant to clinical practice; it may be more practical to give large amount of MSCs suspension through intravenously.
Interestingly, Qin et al.68 developed a novel intra-pleural delivery method. They found that MSCs delivered by this method can survive at least one month in vivo and their distribution was found to be limited to the surface of the pleurae and in the pleural cavity, forming a “MSCs repository” in vivo. The advantage of using the pleural cavity for MSCs delivery is that it is a potential compartment that can receive larger dose of MSCs without restriction. Although promising, the delivery route will need to be investigated further due to the limited number of study.
Timing of MSCs administration
Although pre-clinical animal models cannot replicate the natural course of ALI, MSCs were typically given within 6 h following ALI in these models. In LPS-induced ALI models, the beneficial effects of MSCs were typically found less than 3 days following intra-tracheal delivery but the effects were prolonged when MSCs were given intravenously. What remains to be determined is whether giving MSCs once lung injury is firmly established or during the resolution phase where fibrosis may occur have any therapeutic effect.
For bleomycin induced lung injury, the most optimal time of cell administration and end point needs to be determined. Existing pre-clinical studies35,47,61 demonstrated that MSCs were efficacious in ameliorating the resulting fibrosis, correlating with the early inflammatory steps in the pathogenesis of bleomycin-induced lesions78, only when administered at the time of injury and not at later time points; it may take 2 to 3 days for MSCs to produce soluble factors that modulate inflammation in vivo. For example, Saito et al.63 observed that the highest concentration of 7ND, a dominant-negative inhibitor of CCL2 (an inducer of macrophage recruitment and activation), was obtained on day 2 after MSC, transfected with the 7ND plasmid, administration. However, the serum level of 7ND was undetectable 11 days after MSCs administration, suggesting that the number of MSCs diminished over time in vivo.63. Ortiz et al.35 also showed that MSCs significantly secreted IL-1RN, a competitive inhibitor of IL-1α, only after 72 h of stimulation by IL-1α, which correlated with the development of pulmonary fibrosis exposed to bleomycin. In addition, Aguilar et al.47 demonstrated that the early beneficial of MSCs in bleomycin induced lung injury was lost at a later end point, Day 14. Consequently, MSCs may reach its therapeutic peak 2 to 3 days after administration and decrease over time regardless of the time of cell delivery.
Summary for MSCs in ALI
There are currently more than >300 clinical trials registered with clinicaltrial.gov testing MSCs in a variety of disorders, including GVHD, Crohn’s disease, acute myocardial infarction, and acute kidney failure. More recently, MSCs have been tested in clinical trials for lung diseases such as chronic obstructive pulmonary disease, bronchopulmonary dysplasia, pulmonary emphysema, pulmonary hypertension and silicosis5,79. However, as previously shown, existing preclinical studies to date have used relatively poorly defined MSCs; the potential exists for identifying subpopulations80,81 of MSCs with more efficacy. Although a set of criteria for defining MSCs has been developed by ISCT in 200614, there remains no validated method of measuring MSC bioactivity82, a potency assay, and the duration of benefit in vivo. It may be time to revise the definition of MSCs set forth by ISCT to allow better comparisons between preclinical animal studies and efficacy and the subsequent clinical trials which are underway. How potency will be defined, whether through characterization of secretion of soluble factors or through a functional assay, remains to be determined.
Other Adult Stem Cells for Acute Lung Injury
Endothelial Progenitor Cells
Asahara et al.83 in 1997 purified a population of cells that displayed properties of both endothelial cells (ECs) and progenitor cells, which were capable of trafficking toward ischemic sites and differentiating into mature ECs. These authors termed the cells, endothelial progenitor cells (EPCs). Although a complete phenotypic description of EPCs remains to be determined, the most common surface markers for EPCs include endothelial cell antigens such as CD34 and CD133 as well as endothelial-specific markers such as vascular endothelial growth factor receptor-2 (VEGFR-2) and von Willebrand factor (vWF)83,84.
Mechanisms of action
EPCs appear to exert their therapeutic effects via engraftment and differentiation into the endothelium of the damaged vascular site, via secretion of growth factors and cytokines inducing neovascularization and via an immunomodulatory effect. Although most previous studies indicated that the level of engraftment of EPCs in lung injury was low, with observed rates of less than 5%, these authors85–87 studied the trafficking of the EPCs to the lung with immunohistochemistry or fluorescence-conjugated cell tracers for up to 14 days. Because of the short time period of injury studied and the fact that the intravenous infusion of bone marrow-derived EPCs typically are trapped in the pulmonary microcirculation, additional studies are needed to determine the contribution of engraftment in the therapeutic response of EPCs. Interestingly, similar to MSCs, Cao et al.88 found that EPCs modulated immune cell response following lung injury by reducing pro-inflammatory cytokines and increasing anti-inflammatory cytokine, IL-10, and inhibiting the influx of inflammatory cells, specifically neutrophils.
Recent experimental results
Based on a pilot study89 in 2004, which showed that the suppression of bone marrow-derived progenitor cells by irradiation before intrapulmonary LPS led to disruption of tissue structure and emphysema-like changes that was prevented by the reconstitution of the bone marrow, a number of pre-clinical animal studies85–88,90–92 have been conducted to evaluate the effect of EPCs mobilization or administration in reducing lung injury or in regenerating the lung following the initial insult. Most studies85,86,88,90,91 (71%, 5 of 7) used an endotoxin-induced ALI model. Rabbit (57%, 4 of 7)was the predominant animal model studied86–88,91, and autologous EPCs isolated from peripheral blood was the primary source of the progenitor cells (Table 2). The advantages of auto-transplantation with EPCs included no need to use an allogeneic source, completely avoiding immunological rejection, potential use in clinical applications and a simple isolation technique using a blood cell separator93. The disadvantage was isolating progenitors cells in patients with on-going inflammation and/or infection, possibly changing the phenotype of the EPCs.
Table 2.
Descriptive details of EPCs preclinical study
| Study | Lung Injury Model | Species | Origin | Route | Dosage (×105cells/kg) |
Given Time After Exposure | Measured Time Points |
Thera-peutic Effect | Possible Mechanism |
|---|---|---|---|---|---|---|---|---|---|
| Wary et al. 200990 | LPS-induced ALI | C57BL/6 | Bone marrow | i.v. | 145 | 4h | 12h 24h D2 D3 |
+ + + ++ |
α4β1 α5β1 intergin |
| Mao et al. 201085 | LPS-induced ALI | SD Rat | Bone marrow | i.v. | 182 | 0.5h | D3 D7 D14 |
− + + |
Engraftment Immunomodulation |
| Lam et al. 201186 | LPS-induced ALI | New Zealand Rabbit | Peripheral blood | i.v. | 0.36 | 0.5h | D2 | + | Alveolar-capillary membrane repair Restore gas exchange |
| Gao et al. 201191 | LPS-induced ALI | New Zealand Rabbit | Peripheral blood | i.v. | 0.36 | 0.5h | D1 D3 D5 |
+ ++ + |
ICAM-1 P-selectin |
| Cao et al. 201288 | LPS-induced ALI | New Zealand Rabbit | Peripheral blood | i.v. | 5 | 4h | D2 | + | iNOS VEGF |
| Kähler et al. 200792 | Left sided lung transplantation-induced ALI | SD Rat | Bone marrow | i.v. | 41 | 1–2h | D1 D3 D9 |
+ | Homing Establish endothelial integrity |
| Lam et al. 200887 | Oleic acid-induced lung injury | New Zealand Rabbit | Peripheral blood | i.v. | 0.36 | 0.5h | D2 | + | HO-1 MnSOD |
ICAM-1, intercellular adhesion molecule 1; iNOS, inducible nitric oxide synthase; VEGF, vascular endothelial growth factor; HO-1, heme Oxygenase-1; MnSOD, manganese superoxide dismutase.
Optimal dosage and route of cell delivery
Based on animal models, the primary route of cell delivery is intravenously because EPCs are thought to originate primarily from the bone marrow and circulate in the peripheral blood94,95. Similar to MSCs, intravenous delivery of exogenous EPCs is an effective route for a pulmonary endothelial-targeted therapeutic because EPCs have been shown to be effectively retained in the pulmonary micro-circulation96. For example, Mao et al.85 studied the effect of EPCs on the lung capillary endothelium following intravenous administration of LPS in order to explore the effect of EPCs in vascular repair more directly. The choice of dosage, however, varied from different studies. The average dose in rabbit models was (1.52 ± 2.32) × 105 cell/kg while that in rodent models was (122.7 ± 73.1) × 105 cell/kg. It is unclear why the dosages differ among these species. In addition, what remains to be determined is whether there is a dose effect and whether the paracrine mechanisms discussed is clinically relevant.
Timing of EPCs administration
A recent clinical investigation97 provided evidence that EPCs could be released from the bone marrow in inflammatory condition, and the number of EPCs at an early phase correlated with the recovery process. In pre-clinical animal studies, EPCs are usually administered within 4 hours of injury. In these studies85,90, 80–95% of injected EPCs are trapped in lung tissue within 20 minutes, and a significant population of these cells remained in the lungs during the first 12 hours. The number of cells retained in the lungs declined to 40–50% by the end of 24 hours, but a significant number of cells remained sequestered in lungs even for up to 8 weeks. The findings suggested that EPCs homed to the pulmonary endothelium and subsequently engrafted into the injured endothelium. However, the number of EPCs was very low in the pulmonary circulation and lung tissue after intravenous administration in experimental model of oleic acid induced87 and LPS induced ALI86. These findings may have been due to the extremely large surface area of the pulmonary circulation, leading to an artificially low count in the thin histological sections. In addition, low homing efficacy of progenitors in the target organs has been reported after systemic or regional delivery, as a result of a washout effect98. Further investigation into the level of engraftment may offer a more effective way to measure the efficacy of EPCs.
Summary of EPCs in ALI
To date, several descriptive studies have been conducted to quantify circulating EPCs in the peripheral blood of patients with ALI/ARDS. Two groups97,99 found a higher number of colony-forming units of EPCs from patients with ALI compared with healthy control subjects, and in patients with ALI, an increased number of circulating EPCs were associated with improved survival, suggesting that circulating EPCs were mobilized from the bone marrow to replenish the injured endothelium. While the above-mentioned clinical studies only quantified EPCs and correlated the count to disease outcome, two small pilot clinical trials100,101 (NCT00257413, NCT00641836) investigated the intravenous infusion of autologous EPCs at ~2 × 105 cell/kg in idiopathic pulmon aryarterial hypertension (IPAH), showing an increase in 6-min walk test by 18% and 11% in adult and children respectively after 12 weeks of follow-up with no immunologic reactions or adverse effects noted from EPCs infusion.
However, barriers to clinical application of EPCs for ALI remain. (1) It would be difficult to harvest and culture enough EPCs for autologous transplantation because their phenotype and function and cell number may be impaired during a systemic inflammatory state; (2) The number of EPCs needed to target the large surface area of the pulmonary circulation is unknown. No dose response experiment has been performed; And (3) in addition, an allogeneic source of EPCs may cause an immune reaction in the host; it is unclear if EPCs are “immunoprivileged” in a manner similar to that of MSCs. Further preclinical studies are warranted prior to any clinical trial for ALI.
Hematopoietic stem cells (HSCs)
Hematopoietic CD34+ cells are rare stem cell-derived progenitors, representing only 1 in 104 to 105 of total blood cells in the bone marrow, with combined myeloid and lymphoid differentiation and self-renewal potential79,102. Since cultured HSCs rapidly lose their ability to engraft and self-renew in vivo, which limits the options to maintain, expand or manipulate HSCs in vitro for therapeutic purposes, very little is known about the potential of HSCs in lung injury models. Aguilar et al.47 demonstrated a successful HSCs-based KGF gene therapy by using an inducible lentiviral vector (Tet-On) in bleomycin-induced lung fibrosis. They demonstrated that transplantation of lentivirus-transduced HSCs-KGF showed a significant reduction in lung fibrosis, specifically a reduction in collagen 1α1 mRNA and collagen content, and lung damage using a histological score. However, the beneficial effects of HSCs have to be demonstrated with further preclinical studies.
Endogenous lung stem cells
The ideal cell type to regenerate the injured lung would be the lung’s own endogenous stem cell population. The ability of the lung to regenerate following injury provides clear evidence for the existence of one or more native lung stem cell populations. Kajstura et al.103 reported a human c-KIT-positive adult lung stem cell that was clonogenic and able to regenerate the architecture of the lung bronchiole, alveoli, and arteriole after cryoablation injury in mice. Chapman et al.104 also identified a novel subpopulation of mouse alveolar epithelial cells expressing the laminin receptor α6β4 that can repair the lung following ALI. Taken together, these studies offer considerable promise for a therapeutic role for endogenous lung stem/progenitor cells in lung diseases. However, this putative adult lung-derived stem cell population remains poorly characterized and needs to be replicated to determine the translational potential of these cells for ALI.
CONCLUSIONS
The major findings of this review of adult stem cells for ALI can be summarized as follows: 1) For MSCs, the effective administration dose from pre-clinical studies in animals is approximately 20~30 × 106 cells/kg, regardless of the origin of the cells. Whereas, from current on-going clinical trials (>300 for a variety of diseases/syndromes), the average dose of MSC is approximately 5~10 × 106 cells/kg; 2)Intrapulmonary delivery of MSCs may be a more effective route for direct anti-microbial effect, while the intravenous route may be more practical for clinical application where the patients are hypoxic; 3)In most pre-clinical studies, MSCs were delivered in lung injury models early usually within 6 hours following injury. Whereas, in bleomycin-induced ALI, the therapeutic effect of MSCs reached its peak 2 to 3 days after administration; 4) The beneficial effects of MSCs in LPS-induced ALI model were typically found less than 3 days after intra-tracheal delivery but the effects were prolonged when MSCs were given intravenously; 5) For EPCs, intravenous delivery of exogenous EPCs is an effective route for pulmonary endothelial-targeted therapeutic strategy, but research into the optimal dose is needed; 6) EPCs were delivered usually within 4 hours following injury; And 7) although the engraftment rate was low, multiple studies suggest that EPCs appear to home to the injured pulmonary endothelium and re-populate the injured tissue.
Despite some limitations in the characterization of MSCs or EPCs, especially concerning the issue of potency, a significant amount of pre-clinical data in both animal models and clinical trials suggest that stem or progenitor cell-based therapies may be beneficial in lung repair and remodeling after ALI. Although questions and concerns still remain, a novel and safe therapy for acute lung diseases might eventually emerge.
ACKNOWLEDGEMENTS
This work was supported by NHLBI Grant HL-093026 & HL-113022 and the UCSF Hamilton Endowment Funds.
Footnotes
Ying-gang Zhu, MD, is an attending physician and PhD candidate in the Department of Pulmonary Medicine at Huadong Hospital, Fudan University in Shanghai, China. He is currently a visiting scholar at UCSF (USA) studying inflammatory lung diseases such as acute lung injury. Qi Hao, PhD, is a senior researcher in the Department of Anesthesiology at UCSF (USA) with primary research interest in understanding the potential therapeutic role of adult and embryonic stem cells in acute lung injury. Antoine Monsel, MD, finished his critical care fellowship at La Pitié-Salpétrière Hospital (Paris, France) and has research interests in acute lung injury and pneumonia. He is currently a visiting scholar in the Department of Anesthesiology at UCSF (USA) and his research focuses on new treatments for acute lung injury. Xiao-mei Feng, MD, PhD, is an attending anesthesiologist at Ruijin Hospital, School of Medicine, Jiaotong University in Shanghai, China, with primary research interest in the treatment of sepsis. Dr Feng is currently performing research at UCSF (USA) looking at the role of inflammation in postcognitive dysfunction. Jae-Woo Lee, MD, is an Associate Professor in the Department of Anesthesiology at UCSF (USA) and his research focuses on the mechanisms by which mesenchymal stem cells may normalize alveolar epithelial permeability and fluid transport in animal and human models of acute lung injury.
REFERENCES
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 4.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N. Engl. J. Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 5.Mairead Hayes, Gerard Curley, Bilal Ansari, et al. Clinical review: Stem cell therapies for acute lung injury/acute respiratory distress syndrome-hope or hype? Crit. Care. 2012;16:205. doi: 10.1186/cc10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 7.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 8.Cribbs SK, Matthay MA, Martin GS. Stem cells in sepsis and acute lung injury. Crit. Care Med. 2010;38:2379–2385. doi: 10.1097/CCM.0b013e3181f96f5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cribbs SK, Martin GS. Stem cells in sepsis and acute lung injury. Am. J. Med. Sci. 2011;341:325–332. doi: 10.1097/MAJ.0b013e3181f30dee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JW, Fang X, Krasnodembskaya A, et al. Concise review: Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29:913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes M, Curley G, Laffey JG. Mesenchymal stem cells-a promising therapy for Acute Respiratory Distress Syndrome. F1000 Med. Rep. 2012;4:2. doi: 10.3410/M4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JW, Gupta N, Serikov V, et al. Potential application of mesenchymal stem cells in acute lung injury. Expert Opin. Biol. Ther. 2009;9:1259–1270. doi: 10.1517/14712590903213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson JM. A history lesson for stem cells. Science. 2009;324:727–728. doi: 10.1126/science.1174935. [DOI] [PubMed] [Google Scholar]
- 14.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 15.Wagers AJ, Sherwood RI, Christensen JL, et al. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 16.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am. J. Respir. Cell Mol. Biol. 2005;33:328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loi R, Beckett T, Goncz KK, et al. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am. J. Respir. Crit. Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teder P, Vandivier RW, Jiang D, et al. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 20.Zhu H, Mitsuhashi N, Klein A, et al. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 21.Wong AP, Dutly AE, Sacher A, et al. Targeted cell replacement with bone marrow cells for airway epithelial regeneration. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293:L740–L752. doi: 10.1152/ajplung.00050.2007. [DOI] [PubMed] [Google Scholar]
- 22.Sueblinvong V, Loi R, Eisenhauer PL, et al. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am. J. Respir. Crit. Care Med. 2008;177:701–711. doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong AP, Keating A, Lu WY, et al. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J. Clin. Invest. 2009;119:336–348. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 25.Hennrick KT, Keeton AG, Nanua S, et al. Lung cells from neonates show a mesenchymal stem cell phenotype. Am. J. Respir. Crit. Care Med. 2007;175:1158–1164. doi: 10.1164/rccm.200607-941OC. [DOI] [PubMed] [Google Scholar]
- 26.Jarvinen L, Badri L, Wettlaufer S, et al. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J. Immunol. 2008;181:4389–4396. doi: 10.4049/jimmunol.181.6.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tropea KA, Leder E, Aslam M, et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 2012;302:L829–L837. doi: 10.1152/ajplung.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 29.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 30.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 31.Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 32.Danchuk S, Ylostalo JH, Hossain F, et al. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-α-induced protein 6. Stem Cell Res. Ther. 2011;2:27. doi: 10.1186/scrt68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manning E, Pham S, Li S, et al. Interleukin-10 delivery via mesenchymal stem cells: a novel gene therapy approach to prevent lung ischemia-reperfusion injury. Hum. Gene Ther. 2010;21:713–727. doi: 10.1089/hum.2009.147. [DOI] [PubMed] [Google Scholar]
- 35.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc. Natl. Acad. Sci. U S A. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta N, Su X, Popov B, et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J. Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 37.Liang ZX, Sun JP, Wang P, et al. Bone marrow-derived mesenchymal stem cells protect rats from endotoxin-induced acute lung injury. Chin. Med. J. (Engl) 2011;124:2715–2722. [PubMed] [Google Scholar]
- 38.Ionescu L, Byrne RN, van Haaften T, et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am. J. Physiol. Lung Cell Mol. Physiol. 2012;303:L967–L977. doi: 10.1152/ajplung.00144.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim ES, Chang YS, Choi SJ, et al. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice. Respir. Res. 2011;12:108. doi: 10.1186/1465-9921-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun J, Han ZB, Liao W, et al. Intrapulmonary delivery of human umbilical cord mesenchymal stem cells attenuates acute lung injury by expanding CD4+CD25+ Forkhead Boxp3 (FOXP3)+ regulatory T cells and balancing anti- and pro-inflammatory factors. Cell Physiol. Biochem. 2011;27:587–596. doi: 10.1159/000329980. [DOI] [PubMed] [Google Scholar]
- 41.D’Alessio FR, Tsushima K, Aggarwal NR, et al. Cd4+cd25+foxp3+ tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J. Clin. Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cambos M, Belanger B, Jacques A, et al. Natural regulatory (cd4+cd25+foxp+) t cells control the production of pro-inflammatory cytokines during plasmodium chabaudi adami infection and do not contribute to immune evasion. Int. J. Parasitol. 2008;38:229–238. doi: 10.1016/j.ijpara.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Liesz A, Suri-Payer E, Veltkamp C, et al. Regulatory t cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 44.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol. Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 45.Folkesson HG, Matthay MA. Alveolar epithelial ion and fluid transport: Recent progress. Am. J. Respir. Cell Mol. Biol. 2006;35:10–19. doi: 10.1165/rcmb.2006-0080SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JW, Fang X, Gupta N, et al. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. U S A. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aguilar S, Scotton CJ, McNulty K, et al. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS One. 2009;4:e8013. doi: 10.1371/journal.pone.0008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curley GF, Hayes M, Ansari B, et al. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 2012;67:496–501. doi: 10.1136/thoraxjnl-2011-201059. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Folkesson HG, Jayr C, et al. Alveolar epithelial fluid transport can be simultaneously upregulated by both KGF and beta-agonist therapy. J. Appl. Physiol. 1999;87:1852–1860. doi: 10.1152/jappl.1999.87.5.1852. [DOI] [PubMed] [Google Scholar]
- 50.Guery BP, Mason CM, Dobard EP, et al. Keratinocyte growth factor increases transalveolar sodium reabsorption in normal and injured rat lungs. Am. J. Respir. Crit. Care Med. 1997;155:1777–1784. doi: 10.1164/ajrccm.155.5.9154891. [DOI] [PubMed] [Google Scholar]
- 51.Planes C, Blot-Chabaud M, Matthay MA, et al. Hypoxiaandbeta2-agonists regulate cell surface expression of the epithelial sodium channel in native alveolar epithelial cells. J. Biol. Chem. 2002;277:47318–47324. doi: 10.1074/jbc.M209158200. [DOI] [PubMed] [Google Scholar]
- 52.Gamble JR, Drew J, Trezise L, et al. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ. Res. 2000;87:603–607. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- 53.Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 54.Fang X, Neyrinck AP, Matthay MA, et al. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type ii cells by secretion of angiopoietin-1. J. Biol. Chem. 2010;285:26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mei SH, McCarter SD, Deng Y, et al. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu J, Qu J, Cao L, et al. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J. Pathol. 2008;214:472–481. doi: 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- 57.Nemeth K, Mayer B, Mezey E. Modulation of bone marrow stromal cell functions in infectious diseases by toll-like receptor ligands. J. Mol. Med. 2010;88:5–10. doi: 10.1007/s00109-009-0523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta N, Krasnodembskaya A, Kapetanaki M, et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67:533–539. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mei S, Haitsma J, Dos Santos C, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am. J. Respir. Crit. Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 61.Rojas M, Xu J, Woods CR, et al. Bonemarrow-derived mesenchymal stem cells in repair of the injured lung. Am. J. Respir. Cell Mol. Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao F, Zhang YF, Liu YG, et al. Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplant. Proc. 2008;40:1700–1705. doi: 10.1016/j.transproceed.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 63.Saito S, Nakayama T, Hashimoto N, et al. Mesenchymal stem cells stably transduced with a dominant-negative inhibitor of CCL2 greatly attenuate bleomycin-induced lung damage. Am. J. Pathol. 2011;179:1088–1094. doi: 10.1016/j.ajpath.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Araújo IM, Abreu SC, Maron-Gutierrez T, et al. Bone marrow-derived mononuclear cell therapy in experimental pulmonary and extrapulmonary acute lung injury. Crit. Care Med. 2010;38:1733–1741. doi: 10.1097/CCM.0b013e3181e796d2. [DOI] [PubMed] [Google Scholar]
- 65.Prota LF, Lassance RM, Maron-Gutierrez T, et al. Bone marrow mononuclear cell therapy led to alveolar-capillary membrane repair, improving lung mechanics in endotoxin-induced acute lung injury. Cell Transplant. 2010;19:965–971. doi: 10.3727/096368910X506845. [DOI] [PubMed] [Google Scholar]
- 66.Song L, Xu J, Qu J, et al. A therapeutic role for mesenchymal stem cells in acute lung injury independent of hypoxia-induced mitogenic factor. J. Cell Mol. Med. 2012;16:376–385. doi: 10.1111/j.1582-4934.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Li D, Liu X, et al. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J. Inflamm. (Lond) 2012;9:33. doi: 10.1186/1476-9255-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qin ZH, Xu JF, Qu JM, et al. Intrapleural delivery of MSCs attenuates acute lung injury by paracrine/endocrine mechanism. J. Cell Mol. Med. 2012;16:2745–2753. doi: 10.1111/j.1582-4934.2012.01597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chien MH, Bien MY, Ku CC, et al. Systemic human orbital fat-derived stem/stromal cell transplantation ameliorates acute inflammation in lipopolysaccharide-induced acute lung injury. Crit. CareMed. 2012;40:1245–1253. doi: 10.1097/CCM.0b013e31823bc89a. [DOI] [PubMed] [Google Scholar]
- 70.Sun CK, Yen CH, Lin YC, et al. Autologous transplantation of adipose-derived mesenchymal stem cells markedly reduced acute ischemia-reperfusion lung injury in a rodent model. J. Transl. Med. 2011;9:118. doi: 10.1186/1479-5876-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen S, Chen L, Wu X, et al. Ischemia postconditioning and mesenchymal stem cells engraftment synergistically attenuate ischemia reperfusion-induced lung injury in rats. J. Surg. Res. 2012;178:81–91. doi: 10.1016/j.jss.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 72.Chimenti L, Luque T, Bonsignore MR, et al. Pre-treatment with mesenchymal stem cells reduces ventilator-induced lung injury. Eur. Respir. J. 2012;40:939–948. doi: 10.1183/09031936.00153211. [DOI] [PubMed] [Google Scholar]
- 73.Pati S, Gerber MH, Menge TD, et al. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One. 2011;6:e25171. doi: 10.1371/journal.pone.0025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang H, Wen Y, Bin J, et al. Protection of bone marrow mesenchymal stem cells from acute lung injury induced by paraquat poisoning. Clin. Toxicol. (Phila) 2011;49:298–302. doi: 10.3109/15563650.2011.566882. [DOI] [PubMed] [Google Scholar]
- 75.Nauta AJ, Westerhuis G, Kruisselbrink AB, et al. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017–1026. [PubMed] [Google Scholar]
- 77.Ho JH, Ma WH, Tseng TC, et al. Isolation and characterization of multi-potent stem cells from human orbital fat tissues. Tissue Eng. Part A. 2011;17:255–266. doi: 10.1089/ten.TEA.2010.0106. [DOI] [PubMed] [Google Scholar]
- 78.Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur. Respir. J. 2007;30:835–839. doi: 10.1183/09031936.00069307. [DOI] [PubMed] [Google Scholar]
- 79.Abreu SC, Antunes MA, Pelosi P, et al. Mechanisms of cellular therapy in respiratory diseases. Intensive Care Med. 2011;37:1421–1431. doi: 10.1007/s00134-011-2268-3. [DOI] [PubMed] [Google Scholar]
- 80.Smith JR, Pochampally R, Perry A, et al. Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem cells. 2004;22:823–831. doi: 10.1634/stemcells.22-5-823. [DOI] [PubMed] [Google Scholar]
- 81.Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc. Natl. Acad. Sci. U S A. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahrlund-Richter L, De Luca M, Marshak DR, et al. Isolation and production of cells suitable for human therapy: challenges ahead. Cell Stem Cell. 2009;4:20–26. doi: 10.1016/j.stem.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 83.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 84.Mao M, Xu X, Zhang Y, et al. Endothelial progenitor cells: the promise of cell-based therapies for acute lung injury. Inflamm. Res. 2013;62:3–8. doi: 10.1007/s00011-012-0570-3. [DOI] [PubMed] [Google Scholar]
- 85.Mao M, Wang SN, Lv XJ, et al. Intravenous delivery of bone marrow-derived endothelial progenitor cells improves survival and attenuates lipopolysaccharide-induced lung injury in rats. Shock. 2010;34:196–204. doi: 10.1097/SHK.0b013e3181d49457. [DOI] [PubMed] [Google Scholar]
- 86.Lam CF, Roan JN, Lee CH, et al. Transplantation of endothelial progenitor cells improves pulmonary endothelial function and gas exchange in rabbits with endotoxin-induced acute lung injury. Anesth. Analg. 2011;112:620–627. doi: 10.1213/ANE.0b013e3182075da4. [DOI] [PubMed] [Google Scholar]
- 87.Lam CF, Liu YC, Hsu JK, et al. Autologous transplantation of endothelial progenitor cells attenuates acute lung injury in rabbits. Anesthesiology. 2008;108:392–401. doi: 10.1097/ALN.0b013e318164ca64. [DOI] [PubMed] [Google Scholar]
- 88.Cao JP, He XY, Xu HT, et al. Autologous transplantation of peripheral blood-derived circulating endothelial progenitor cells attenuates endotoxin-induced acute lung injury in rabbits by direct endothelial repair and indirect immunomodulation. Anesthesiology. 2012;116:1278–1287. doi: 10.1097/ALN.0b013e3182567f84. [DOI] [PubMed] [Google Scholar]
- 89.Yamada M, Kubo H, Kobayashi S, et al. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J. Immunol. 2004;172:1266–1272. doi: 10.4049/jimmunol.172.2.1266. [DOI] [PubMed] [Google Scholar]
- 90.Wary KK, Vogel SM, Garrean S, et al. Requirement of alpha(4)beta(1) and alpha(5)beta(1) integrin expression in bone-marrow-derived progenitor cells in preventing endotoxin-induced lung vascular injury and edema in mice. Stem Cells. 2009;27:3112–3120. doi: 10.1002/stem.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao X, Chen W, Liang Z, et al. Autotransplantation of circulating endothelial progenitor cells protects against lipopolysaccharide-induced acute lung injury in rabbit. Int. Immunopharmacol. 2011;11:1584–1590. doi: 10.1016/j.intimp.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 92.Kähler CM, Wechselberger J, Hilbe W, et al. Peripheral infusion of rat bone marrow derived endothelial progenitor cells leads to homing in acute lung injury. Respir. Res. 2007;8:50. doi: 10.1186/1465-9921-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim SR, Choung HK, Kim DW, et al. Evaluation of a new cell separator for collection of peripheral blood CD34+ progenitor cells in pediatric patients. Transfusion. 2011;51:306–312. doi: 10.1111/j.1537-2995.2010.02864.x. [DOI] [PubMed] [Google Scholar]
- 94.Hristov M, Erl W, Weber PC. Endothelial Progenitor Cells: Mobilization, Differentiation, and Homing. Arterioscler. Thromb. Vasc. Biol. 2003;23:1185–1189. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 95.Rafat N, Tönshoff B, Bierhaus A, et al. Endothelial progenitor cells in regeneration after acute lung injury - Do they play a role? Am. J. Respir. Cell Mol. Biol. 2012 Oct 25; doi: 10.1165/rcmb.2011-0132TR. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 96.Warburton D, Perin L, Defilippo R, et al. Stem/progenitor cells in lung development, injury repair, and regeneration. Proc. Am. Thorac. Soc. 2008;5:703–706. doi: 10.1513/pats.200801-012AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamada M, Kubo H, Ishizawa K, et al. Increased circulating endothelial progenitor cells in patients with bacterial pneumonia: evidence that bone marrow derived cells contribute to lung repair. Thorax. 2005;60:410–413. doi: 10.1136/thx.2004.034058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oettgen P, Boyle AJ, Schulman SP, et al. Cardiac stem cell therapy: need for optimization of efficacy and safety monitoring. Circulation. 2006;114:353–358. doi: 10.1161/CIRCULATIONAHA.106.639385. [DOI] [PubMed] [Google Scholar]
- 99.Burnham EL, Taylor WR, Quyyumi AA, et al. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am. J. Respir. Crit. Care Med. 2005;172:854–860. doi: 10.1164/rccm.200410-1325OC. [DOI] [PubMed] [Google Scholar]
- 100.Zhu JH, Wang XX, Zhang FR, et al. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Pediatr. Transplant. 2008;12:650–655. doi: 10.1111/j.1399-3046.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- 101.Wang XX, Zhang FR, Shang YP, et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J. Am. Coll. Cardiol. 2007;49:1566–1571. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 102.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat. Rev. Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 103.Kajstura J, Rota M, Hall SR, et al. Evidence for human lung stem cells. N. Engl. J. Med. 2011;364:1795–1806. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Chapman HA, Li X, Alexander JP, et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J. Clin. Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]