Abstract
Activation of proinflammatory cytokines is associated with depressed mood, feelings of fatigue, and changes in cognitive function. This study examined the relationships between cognitive performance and circulating cellular markers of inflammation, interleukin-6 (IL-6) and C-reactive protein (CRP), in moderately depressed and comparison healthy elderly. We conducted a cross-sectional analysis of 87 volunteers (45 nondepressed, 42 depressed) in which participants completed the Structured Clinical Diagnostic Interview and were evaluated by a geriatric psychiatrist for dementia, depression, stroke risk and neurological disorders. Volunteers also completed an EKG, standard battery of laboratory tests and neuropsychological examination that assessed memory functions of Encoding and Recall, Executive Function and Attention/Processing. Mid-morning IL-6 and CRP levels were assessed. The data analysis showed that Encoding and Recall were inversely associated with IL-6 across diagnostic groups after controlling for chronological age, MMSE, body mass index, literacy level, depression severity and sex. CRP was not associated with cognition. Depression status was associated with recall independent of IL-6 levels. In conclusion, IL-6 serum levels among elderly individuals is a significant correlate of memory performance. Women, in particular, appear sensitive to IL-6 fluctuations across diagnostic groups.
Keywords: immune system, cognition, dementia, depression, aging, cytokines, geriatric
OBJECTIVE
Advancing age is associated with disruption of homeostasis in the immune system, or immunosenescence.(1) Although the effects of aging on immune function are poorly understood, the aging brain has been characterized by a shift from the homeostatic balance of inflammatory mediators observed in mid-adulthood to a proinflammatory profile in late-life.(2) The increased neuroinflammation is represented by increased numbers of activated and primed microglia,(3) increased steady state levels of inflammatory cytokines,(4,5) and decreased functionality of humoral and cellular immune responses.(1,6,7) This shift has been associated with increased vulnerability to neurodegenerative and chronic diseases, which increase exponentially during late life.(6,8-11)
The effect of this immunologic shift on cognitive performance is unclear. Some longitudinal studies have indicated a relationship between high cytokine levels, e.g., interleukin-6 (IL-6) and C-reactive protein (CRP), in peripheral plasma and development of multiple forms of dementia through complex mechanisms.(9,11,12) On the other hand, data from the MacArthur Study of Successful Aging indicated that there was no effect of inflammatory status on baseline cognitive function or the rate of longitudinal cognitive change after correcting for potential confounders.(13) However, when the investigators examined subgroups, they found that persons in the top tertile of IL-6 concentrations were at an increased risk of incident declines on a short mental status test. The finding could result from underlying pathology or preclinical cognitive impairment associated with incident dementia, as the MacArthur Study investigators suspected. Another explanation is that the increased inflammatory level could contribute causally to cognitive changes that are independent of an underlying neuropathology, but the change is statistically significant only at the extreme end of a spectrum.
Immune status has also been associated with major depressive disorder (MDD) relative to healthy individuals (14) through the more prolonged adaptive immune response and the transient acute phase response.(14-17) Acute phase response in MDD includes increased serum concentrations of positive acute phase proteins, which includes CRP, and lowered serum concentrations of negative acute phase proteins such as albumin and transferrin.(18) Adaptive changes are based on evidence of in vivo secretion of cytokines, particularly IL-6, interleukin-1 beta (IL-1β) and interleukin-8, (19,20) and the associations are stronger when melancholy is present.(21,22) An early meta-analysis by Herbert (1993) reported that immune alterations include lower proliferative response of lymphocytes to mitogens, lower natural killer cell activity, and changes in levels of several white blood cell populations.(16) The changes were more pronounced in older populations and appeared to be linearly related to the severity of the depressive episode. Studies of experimental sleep loss, a common problem among depressed individuals, report a marked and transient increase in IL-6, increased nuclear factor-kappaB (NF-κB), and a threefold increase in transcription of IL-6 messenger RNA after a single night of sleep loss.(23,24) Despite the differences in the strength of the association between cytokines and sleep loss, IL-6 activity remains characteristic of major depression with or without sleep disorders.(19) Elevated levels of IL-6 occur concurrent with depressive symptoms in patients with renal failure (25), cardiovascular disease, (26) advanced cancer, (27) rheumatoid arthritis (28) and irritable bowel syndrome.(29) Adaptive immunologic changes are thought to represent a chronic neuroinflammation in which responses of microglial cells contribute to and expand the neurodestructive effects of the initial acute neuroinflammation, worsening the disease process.
Although elevated cytokine levels have tentatively been associated with cognitive decline in healthy elderly, the same cognitive-cytokine relationship has not been examined among elderly depressed individuals despite their known cognitive deficits relative to healthy controls. (30,31) One reason for the lack of definition of the relationship is the general nature of the cognitive testing: clinical studies use short screening tests with general memory questions, and experimental studies use animal models. General memory questions cannot clarify whether poor memory performance is a result of poor encoding of material or inability to recall it. Encoding imbues a stimulus with meaning and creates the neural imprint, and recall is the executive search and reactivation of the target memory. Elderly, moderately depressed individuals without mild cognitive impairment show slow encoding ability but comparable ability in recall relative to healthy comparison individuals.(32) Behaviorally, individuals with encoding deficits are not helped functionally with cues or reminders, while those same reminders or cues can be of help to individuals who encoded the material accurately but have difficulty retrieving the information. The question is what kind of memory dysfunction is related to an increased cytokine level. In addition to memory deficits, executive dysfunction and attention/processing deficits are common among depressed older adults and may be related to increased cytokine levels.(30,31,33-35)
The current study examined the type of memory deficit associated with cytokine levels in both healthy and depressed elderly under the hypothesis that IL-6 and CRP would be associated negatively with cognitive performance across diagnostic groups. We also expected that depressed individuals would show a stronger association with cognitive performance than that observed in the comparison group. We also posed a research question as to whether executive dysfunction and attention/processing deficits are related to increased cytokine levels.(34-37) As depression is more common among women, we examined a potential cytokine-by-sex interaction on cognitive performance.
METHODS
Study Site, Design Overview, Participant Sampling and Procedures
Volunteers (major depressed (N=45) and healthy comparison (N=42) were recruited as part of an ongoing study of late-life depression at UCLA. Prospective subjects who were ≥ 60 years of age with English proficiency were screened with the Structured Clinical Interview for DSM-IV (SCID). Depressed patients were diagnosed by a geriatric psychiatrist according to Diagnostic and Statistical Manual-IV criteria for major depressive disorder and a score ≥ 15 on the 17-item Hamilton Depression Rating Scale (HDRS). Exclusionary criteria were 1) history of substance abuse or other Axis I disorder per the SCID; 2) clinical evidence of dementia or Mini Mental State Examination (MMSE) ≤ 26; 3) history of transient ischemic attack; 4) history of head trauma or lifetime loss of consciousness; 5) current or unstable serious medical illness; 6) chronic neurological disease that could affect cognitive function; or 7) history or evidence of psychotic symptoms or concurrent Axis I psychiatric disorder. Stable chronic conditions such as diabetes mellitus, hypertension or past history of cancer were not exclusionary. Depressed volunteers were drug-free for antipsychotics, anxiolytics and anti-depressant medications for at least 2 weeks prior to inclusion in the study, and many were drug naïve. After describing the study, which was approved by the Institutional Review Board of UCLA, written informed consent was obtained.
After being screened with the SCID, patients were administered Mini Mental State Examination, HDRS, Beck Depression Inventory (BDI), Cerebrovascular Risk Factor Assessment (CVRF) (38) and Cumulative Illness Rating Scale (CIRS).(39). The CVRF quantifies the vascular risk for future stroke according to the American Heart Association guidelines. The CIRS is commonly used to assess medical comorbidity by quantifying medical burden in primary organ systems. Following the neurological evaluation, patients completed an EKG, a standard battery of laboratory tests and neuropsychological assessment.
Assessment of Cognitive Function
The cognitive battery utilized well-established clinical measures to assess multiple domains: Literacy: Wechsler Test for Adult Reading Scale (40); Encoding: California Verbal Learning Test, Version 2, (CVLT) trials 1-5 and trial 5 (41), and immediate recall score from Visual Reproductions (VR) (i.e., reproduction of the stimulus immediately after it has been removed) )(42); Recall: CVLT short (5- minute) and long (20-minute) delayed free recall, VR 20-minute delayed recall, and Rey Osterrieth Complex Design (ROCF) 20-minute delayed recall and percent retention (43); Executive Function: Matrix Reasoning (44) number correct for deductive analysis, Stroop Part 3 time to completion for response inhibition (45), Wisconsin Card Sort Test (WCST)(46) categories correct for abstract conceptualization and generalization, Letter-Number Sequences number correct for working memory manipulation (44); and Attention and Processing: Trailmaking Parts A and B (47) time to completion, Stroop Part 1,(45) time to completion and Digit Symbol Substitution (44) number completed. Cognitive test results were normed using published norms. After examining each variable for normative performance, z-scores were formed from raw scores for each test variable using the mean and standard deviation of the control group with timed tests reversed so that higher scores represented better performance. Composite variables were computed for Encoding, Recall, Executive Function and Attention/Processing. Percent retention of the ROCF was included to avoid penalizing a participant twice for an initial error because no advance warning of a later recall is given in this test.
IL-6 and CRP assays
Blood samples were drawn mid-morning from participants through routine venipuncture after they completed a questionnaire and provided height and weight measurements for a computation of body mass index (BMI). (One subject did not complete the height and weight measurements.) CRP was measured using high-sensitivity immunoassay on a BN-II System (Dade-Behring, Newark, DE). Samples were automatically diluted 1:20 with N Diluent. This technique has a limit of detection of 0.175 mg/L and intra- and inter-assay coefficients of variation of <4%.
Plasma levels of IL-6 were quantified by means of high sensitivity enzyme-linked immunosorbent assay methods (R & D Systems, Minneapolis, MN) with all samples from both groups assayed at the same time, in a single run with a single lot number of reagents and consumables employed by a single operator, with intra-assay coefficients of variation for all variables less than 5%.
Statistical Plan
Sex was a variable of interest, so it was decided a priori to be a covariate due to its association with depression. Age and literacy levels were covariates due to their association with both cytokine level and cognitive performance. Literacy level is a better indicator of intellectual function than years of education, race, economic status or occupation. Variables were examined for distribution. IL-6 and CRP were skewed so natural log transformations were computed, which improved distribution sufficient for analysis. The interrelationships of scores (Cronbach alphas) were computed for each of the composite indices with the following results: Encoding of verbal and nonverbal information (α=.94), Recall of information (α=.83), Executive Function (α = .72), and Attention and Processing (α= .75). Body mass index (BMI) scores were computed from laboratory assessments. Diagnostic groups differed on the Recall and Encoding indices. Analysis of intercorrelations found MMSE was associated with all cognitive measures so it was added as a covariate. Two-tailed partial correlations were computed to determine the associations between IL6 and the four cognitive indices after controlling for age, sex and MMSE. Significant associations were further explored using a multiple hierarchical regression approach. This approach allows the inclusion of multiple covariates and testing for potential interaction effects between different predictors.
RESULTS
The diagnostic groups were comparable on all demographic variables except the BDI and the CIS, on which depressed patients were expected to report more symptoms than controls. See Table 1. The depressed group had both early- and late-onset patients. Diagnostic groups differed on Recall and Encoding indices, but they did not differ on serum cytokine levels.
TABLE 1.
Clinical and Demographic Characteristics of Diagnostic Groups
|
Total Sample by Diagnostic Group
N=87 |
Model Values | ||||
|---|---|---|---|---|---|
| Comparison N=45 |
Depressed N=42 |
df | t | P | |
| Age | 69.2 (± 7.1) | 69.7 (± 7.9) | 85 | −0.28 | .78 |
| Education | 16.2(± 2.0) | 15.8 (± 2.8) | 85 | 0.68 | .50 |
| Sex (female / male) | 34/ 11 | 28/ 14 | 1 | χ 2 =0.84 | .36 |
| MMSE | 29.2 (± 0.9) | 29.0 (± 1.2) | 85 | 0.57 | .57 |
| CVRF, age adjusted | 4.9 (± 3.4) | 4.9 (± 2.3) | 84 | −0.01 | .99 |
| CIRS categories | 2.8 (± 2.1) | 4.0 (± 2.1) | 84 | −2.42 | .02 |
| Systolic Blood Pressure | 129.7 (±‘18.2) | 132.0 (±15.8) | 85 | −0.63 | .53 |
| VIQ estimate per WTAR | 114.6 (± 7.3) | 113.1 (± 9.8) | 85 | 0.84 | .40 |
| Body Mass Index (BMI) | 24.9 (±3.7) | 25.6 (±4.4) | 79 | −.69 | .49 |
| Age of Onset | n.a. | 46.4 (±23.6) | |||
| Hamilton Depression Rating | n.a. | 18.7(± 2.9) | |||
| Current Duration (months) | n.a. | 77.7 (±75.41) | |||
| Beck Depression Scale | 1.7 (± 1.9) | 25.3(± 7.6) | 85 | −20.03 | <.01 |
| Ethnicity | 3 | χ2 =3.46 | .32 | ||
| Asian | 3 | 0 | |||
| Caucasian | 35 | 37 | |||
| African-American | 5 | 3 | |||
| Hispanic | 2 | 2 | |||
| Handedness (L/R) | 3 / 39 | 3 / 37 | 2 | χ2 =0.15 | .93 |
| Interleukin 6 | 0.5 (± 0.5) | 0.7 (± 0.6) | 85 | −1.10 | .27 |
| C-Reactive Protein | 0.3 (±0.6) | 0.4 (±0.4) | 85 | −0.75 | .45 |
| Memory Index | .28 (± .84) | −.05 (± .67) | 85 | 2.05 | .04 |
| Attention/Processing Index | .04 (± 1.22) | −.42(± 1.05) | 84 | 1.86 | .07 |
| Executive Index | .34 (± .80) | .12 (± .56) | 83 | 1.45 | .15 |
| Encoding Index | .00 (± .97) | −.51 (± .91) | 85 | 2.55 | .01 |
Notes: CIRS = Cumulative Illness Rating Scale, VIQ = estimated verbal intelligence quotient interpreted from Wechsler Test for Adult Reading results, MMSE = Mini Mental State Examination, CVRF = Cerebrovascular Risk Factor. Standard deviations are in parentheses.
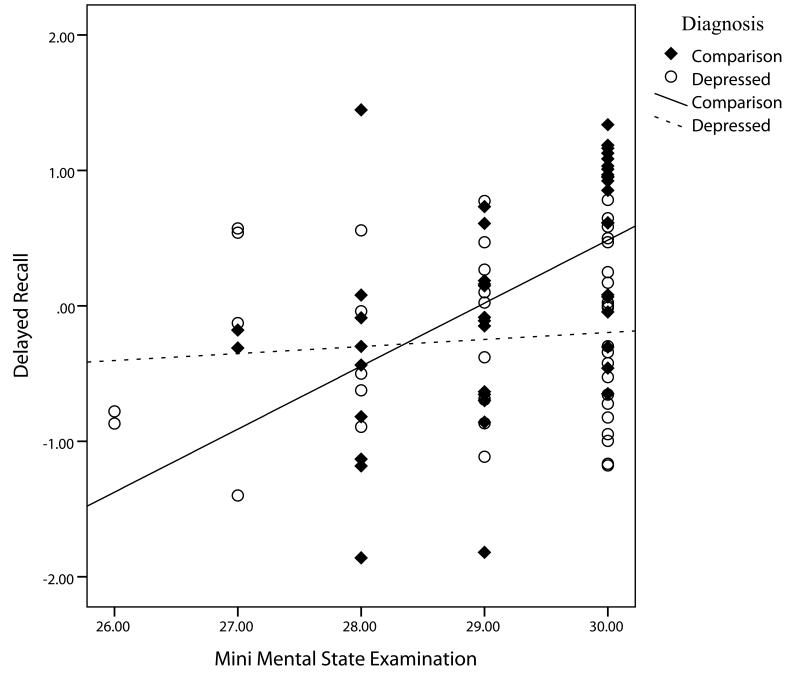
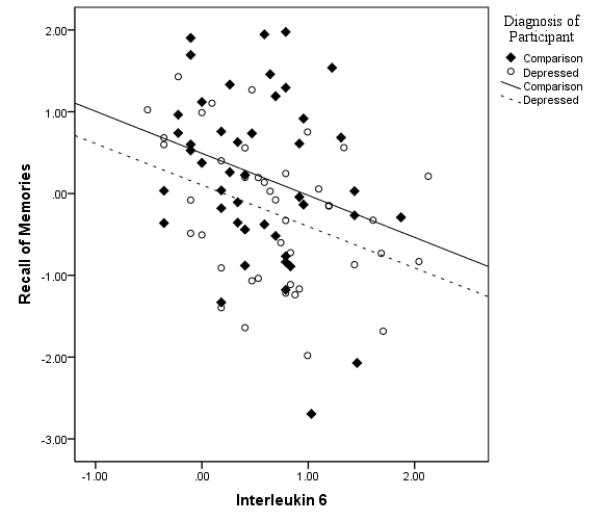
After partialling out the effects of age, sex, literacy level and MMSE, Recall was associated with IL-6 (r79 = −.34, p < .002). It was in the expected inverse direction with increasing IL-6 levels corresponding to lower performance (see Figure 1). Encoding correlated inversely with IL-6 (r79 = −.30, p=.007). IL-6 correlation coefficients with executive function and attention/processing were not significant so analyses were stopped on those variables. CRP was not significantly related to any of the cognitive indices and was dropped from further analysis.
Figure 1.
A hierarchical multiple linear regression that examined Recall’s association with IL-6 used Recall as the dependent variable with covariates of chronological age, literacy level, sex, diagnosis, MMSE and BMI as the independent variables in the first step. The second step added IL-6, and the third step added the interaction terms for diagnosis-by-MMSE, sex-by-IL-6 and IL-6-by-diagnosis. Cumulatively, the interactions explored if cognitive scores varied across diagnostic groups as a function of MMSE scores or IL-6 levels, or across men and women based on IL-6 levels.
As can be seen in Table 2, the original model explained the Recall scores well, but the model was significantly improved with the addition of IL-6. Adding interaction terms did not add to the explanatory power of the model (ΔR2 = .04, F(3,73) = 1.80, p=.15),(not shown in table) although the MMSE-by-diagnosis interaction was individually significant and was retained (t73=−2.26, p=.03). In the final model (last step in Table 2), IL-6 continued to be significant after accounting for age, sex, literacy level, MMSE, diagnosis, and the MMSE-by-diagnosis interaction. The interaction, shown in Figure 2, improved the model again (ΔR2 = .04, F(3,73) = 5.82, p=.02). Analysis of the interaction indicated that comparison subjects increased more in Recall per unit increase in MMSE than did depressed patients. The final model explained 40% of the variance in recall performance.
TABLE 2.
Hierarchical regression coefficients of IL-6 and other variables predicting recall performance.
| Variable values | Model Values | Change parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | F | p | df | Δ R2 | Significance of F change |
|||||
| B | β | t Value | p | |||||||
| 1st STEP Age |
−.03 | −.29 | −2.93 | .004 | ||||||
| WTAR | .01 | .11 | 1.10 | .28 | ||||||
| Sex | −.33 | −.20 | −2.02 | <.05 | ||||||
| Diagnosis | −.33 | −.21 | −2.17 | .03 | ||||||
| MMSE | .18 | .25 | 2.43 | .02 | ||||||
| BMI | −.03 | −.02 | −.16 | .88 | ||||||
| .28 | 5.08 | <.001 | 6,77 | |||||||
| 2nd STEP Age |
−.03 | −.25 | −2.59 | .01 | ||||||
| WTAR | .01 | .12 | 1.20 | .23 | ||||||
| Sex | −.25 | −.15 | −1.54 | .13 | ||||||
| Diagnosis | −.26 | −.18 | −1.81 | .08 | ||||||
| MMSE | .22 | .30 | 3.07 | <.01 | ||||||
| BMI | <.01 | .02 | .20 | .84 | ||||||
| IL-6 | −.39 | −.30 | −3.08 | <.01 | ||||||
| .36 | 6.19 | <.001 | 7,76 | .08 | .003 | |||||
| FINAL MODEL Age |
−.02 | −.24 | −2.62 | .01 | ||||||
| WTAR | .01 | .11 | 1.23 | .22 | ||||||
| Sex | −.33 | −.20 | −2.17 | .03 | ||||||
| Diagnosis | 9.20 | 6.14 | 2.35 | .02 | ||||||
| MMSE | .41 | .58 | 3.92 | <.01 | ||||||
| IL-6 | −.30 | −.23 | −2.43 | .02 | ||||||
| MMSE x Dx | −.33 | −6.31 | −2.41 | .02 | ||||||
| .40 | 7.58 | <.001 | 7,79 | .04 | .02 | |||||
Notes: WTAR = Wechsler Test of Adult Reading, MMSE = Mini Mental State Examination, BMI = Body Mass Index Df for the t-tests correspond to the model’s denominator df given in the table. B = unstandardized betas, and β = standardized betas
Figure 2.
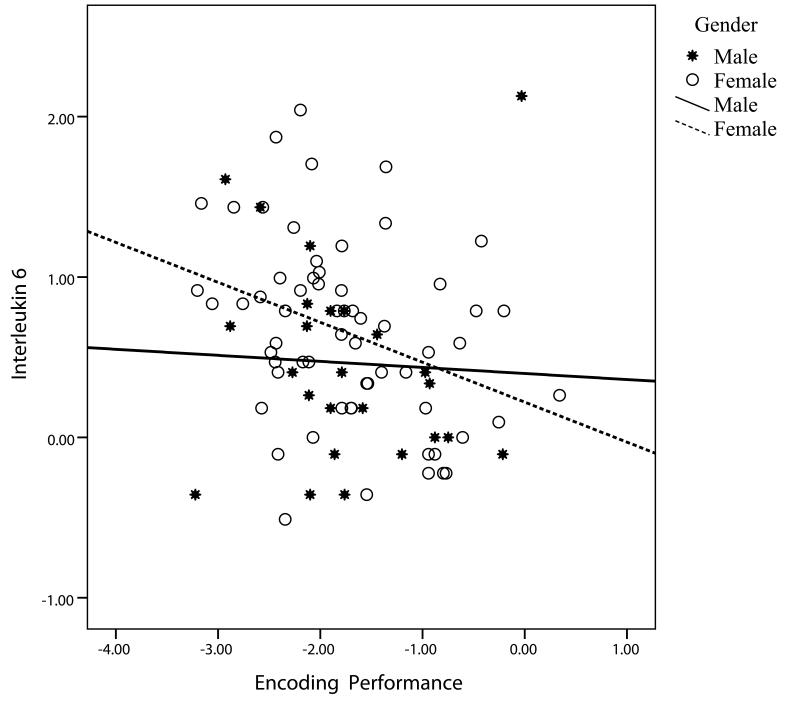
The original model was recomputed with Encoding as the dependent variable (Table 3). The first step explained the variation in Encoding satisfactorily, but the inclusion of IL-6 again improved the model significantly. In the third step (not shown), adding the interaction terms improved the model (ΔR2 = .10, F(3,73) = 4.50, p=.006) but only the sex-by-IL-6 interaction was individually significant (beta = −.95, t73 = −2.53, p=.01) and was retained. The final model showed age, literacy level, MMSE, diagnosis, and the sex-by-IL-6 interaction as significant predictors. IL-6 and sex were marginal independent predictors. Examination of the sex-by-IL-6 interaction (Figure 3) showed that women declined more in Encoding of memories per unit increase in IL-6 than did men.
TABLE 3.
Hierarchical regression coefficients of IL-6 and other variables predicting encoding performance.
| Variable values | Model Values | Change parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | F | p | df | Δ R2 | Significance of F change |
|||||
| B | β | t Value | p Value | |||||||
| 1st STEP Age |
−.04 | −.33 | −3.36 | <.01 | ||||||
| WTAR | .03 | .26 | 2.57 | .01 | ||||||
| Sex | −.08 | −.05 | −.51 | .61 | ||||||
| Diagnosis | −.22 | −.14 | −1.43 | .16 | ||||||
| MMSE | .15 | .20 | 2.00 | <.05 | ||||||
| BMI | −.03 | −.15 | −1.54 | .13 | ||||||
| .3 | 5.60 | <.001 | 6,77 | |||||||
| 2nd STEP Age |
−.03 | −.30 | −3.06 | <.01 | ||||||
| WTAR | .03 | .26 | 2.70 | <.01 | ||||||
| Sex | −.01 | −.01 | −.05 | .96 | ||||||
| Diagnosis | −.16 | −.10 | −1.09 | .28 | ||||||
| MMSE | .19 | .25 | 2.50 | .02 | ||||||
| BMI | −.02 | −.12 | −1.28 | .21 | ||||||
| IL-6 | −.33 | −.25 | −2.55 | .01 | ||||||
| .36 | 6.07 | <.001 | 7,76 | .06 | .01 | |||||
| FINAL MODEL Age |
−.03 | −.32 | −3.53 | <.01 | ||||||
| WTAR | .03 | .24 | 2.60 | .01 | ||||||
| Sex | .34 | −.20 | 1.71 | .09 | ||||||
| Diagnosis | −.29 | −.18 | 2.02 | <.05 | ||||||
| MMSE | .19 | .26 | 2.76 | <.01 | ||||||
| IL-6 | .78 | −.58 | 1.74 | .09 | ||||||
| Sex x IL-6 | −.67 | −.92 | −2.62 | .01 | ||||||
| .41 | 7.77 | <.001 | 7,79 | .05 | .01 | |||||
Notes: WTR = Wechsler Test for Adult Reading, MMSE = Mini Mental State Examination, BMI = Body Mass Index Df for the t-tests correspond to the model’s denominator df given in the table. B = unstandardized beats and β = standardized betas
Figure 3.
To determine if depression severity rather than the categorical depression diagnosis added to the model’s explanatory power for Recall, the linear regression was recomputed with BDI scores entered at the first step instead of depression diagnosis. The results were essentially the same for the full model at all steps, most likely attributable to the strong differences between the groups in BDI mean scores. Adding the BDI instead of depression diagnosis to the model predicting Encoding also produced very similar results to the original model including the marginally significant interaction between BDI and IL-6 (beta=.33, t79=1.86, p=.07).
To clarify which memory scores were most closely associated with IL-6, the individual test scores within the Recall and Encoding indices were correlated with IL-6 values after controlling for age, sex and MMSE. No differences emerged between diagnostic groups in the partial correlation coefficients based on Fisher’s Z testing, so scores were combined. Six of the eight tests of both delayed recall and encoding of information were correlated with IL-6 levels, and the two remaining tests (CVLT short-delayed recall and VR immediate recall) trended toward significance.
DISCUSSION
The aging process is defined by a slow disruption of immunologic homeostasis with a shift toward a proinflammatory state. This neuroinflammation is thought to compromise the brain’s functioning by increasing its vulnerability to neurotoxicity and impairing neurogenerativity. Our results support this theory in that both encoding and recall of memories were negatively associated with serum IL-6 levels in depressed and healthy elderly adults. As IL-6 levels increased, recall ability decreased for information learned from 5 to 20 minutes earlier as did the ability to encode new information. In addition, the encoding association with IL-6 was modulated by sex, with women showing a greater sensitivity to higher levels of IL-6 than men. Contrary to our hypothesis, patients and controls did not differ in the strength of the relationship between IL-6 and encoding or recall performance. Also contrary to our hypothesis, IL-6 levels did not correlate negatively with executive function or attention/processing, and CRP was not associated with any cognitive function assessed. The relationship between IL-6 and both memory functions currently assessed is in addition to significant contributions from age, MMSE scores, literacy level and depression diagnosis.
The effect sizes (standardized betas) from the multiple regression equation for Recall indicate that MMSE and diagnosis are the strongest predictors of recall performance, yet IL-6 makes approximately the same contribution as age or sex. Thus, it is an important and potentially modifiable condition for patients who complain of changes in recall ability. Elderly patients recovering from illness may also experience transient problems with memory with elevated IL-6 levels.
On the other hand, the multiple regression equation for Encoding indicates that IL-6 and the IL-6-by-sex interaction are the strongest predictors of performance and are stronger than age, literacy level, diagnosis or MMSE score. Unfortunately, poor encoding performance can be mistaken for mild cognitive impairment or prodromal dementia because recall does not improve with the use of cues. When being tested for memory function, cues facilitate recall performance if encoding occurred successfully. When encoding does not occur, cues are not helpful and suggest possible damage in the hippocampal and entorhinal complexes and indicate need of further investigation. Thus, immunologic imbalance could lead to a misrepresentation of an elderly person’s, particularly a woman’s, memory problems.
Moderately depressed patients and controls did not differ in their levels of IL-6, yet depressed individuals showed a deficit in memory performance relative to controls. This difference may result from an enhanced sensitivity to the presence of IL-6 among depressed patients if they have experienced earlier episodes or have subtle increases in IL-6 that do not reach statistical significance but which are physiologically important. Trzonkowski and colleagues (2004) reported that older depressed individuals showed elevated levels of TNF-α and IL-6 after receiving an anti-influenza vaccination together with insufficient production of the anti-inflammatory IL-10 and natural killer cell cytotoxicity compared to age and sex-matched controls.(48) Although the differences disappeared when they corrected for anti-CMV antibodies, Trznokowski and colleagues concluded that the depressed patients demonstrated a proinflammatory profile suspected of resulting from prior exposure to elevated levels of IL-6 and deficiency of suppressive IL-10+ cells.
Our results are consistent with Trznokowski and colleagues’ findings. Although depressed and healthy individuals were comparable in IL-6 levels, the difference in cognitive performance may indicate that moderately depressed patients develop a sensitivity based on prior periods of increases in IL-6 that is independent of absolute IL-6 levels. In our model, depression status could potentially represent this enhanced sensitivity to IL-6 as it was an independent predictor of recall and encoding ability after accounting for IL-6 levels. A recent review by Miller et al (2009) outlines how depressed individuals exhibit features of inflammation including elevations in relevant inflammatory cytokines and their soluble receptors in peripheral blood and cerebrospinal fluid.(49) Peripheral blood concentrations of acute phase proteins, chemokines, adhesion molecules, and inflammatory mediators such as prostaglandins show concurrent elevations during depressed episodes.(14) Thus, depression status may be associated with a broad immunologic disruption of which IL-6 is one factor. Subgroups of depressed patients may experience one or multiple changes while healthy elderly have a more limited response.
The link between memory ability and cytokines may occur at the molecular level via cytokines’ role in neurogenesis, memory consolidation and synaptic plasticity. (50) Transgenic mice that over-express IL-6 have a 63% reduction in neurogenesis in the dentate gyrus of the hippocampus, a medial temporal structure critical for memory consolidation.(51) Inflammation resulting from cranial radiation therapy among humans causes cognitive loss that appears associated with impaired neurogenesis in the dentate gyrus via stem cell dysfunction.(52) Additionally, the hippocampus and the hypothalamus have the highest expression of inflammatory cytokine receptors for IL-6 and IL-1β in the brain.(53) Synaptic plasticity may be modulated when peripheral cytokine IL-1 elevation induces nuclear factor κB (NF-κB) in the brain.(54) This induction in the brain is thought to contribute to changes in neuronal growth and survival, especially through the further induction of nitric oxide and, ultimately, oxidative stress, which has been shown to alter promoter function for several genes central to synaptic plasticity. (55,56)
Among elderly depressed patients, hippocampal surface contractions have been observed by our research team among elderly depressed patients, particularly among late-onset patients, and the contractions correlate with decreased performance in nonverbal memory (encoding and recall were not tested separately).(57) A postmortem study of brains from depressed individuals reported condensation of neuronal architecture in the hippocampus, which is consistent with neuronal dearborization rather than apoptosis in hippocampal tissue suggestive of a pathodegenerative process (58) such as developing Alzheimer’s disease. Furthermore, increased levels of IL-1β disrupt long-term potentiation,(59,60) a cellular mechanism believed to be important for recall, one type of memory assessed in this study. In mice, treatment with lipopolysaccharide (LPS), which causes an inflammatory response, produces increased IL=1β, IL-6 and TNF in brain areas including the hippocampus.(61) Pugh et al (62) demonstrated that injections of LPS or IL-1β directly into the hippocampus disrupted hippocampal-dependent memory consolidation. In mice, the Morris water maze, a hippocampal-dependent test of spatial learning, has been shown to be sensitive to disruption by infection or peripheral immune activation and subsequent upregulation of proinflammatory cytokines.(63) Data from the Longitudinal Aging Study Amsterdam indicates that in persons with a high level of IL-6, there is a negative association between higher homocysteine at baseline and immediate recall.(64) Consequently, people with higher steady state levels of IL-6 appear more vulnerable to current and future cognitive deficits. Future research is needed that explores the links between hippocampal neuronal dearborization, pro-inflammatory response patterns, decreased cognitive performance and the onset and severity of mood disorders.
Future research also needs to examine the relationship between IL-6 and IL-1β in depressed and healthy elderly because the relationship of IL-1β to memory functions is variable. At basal levels, IL-1β appears to support memory mechanisms, but changes in the basal level, whether increase or decrease, appear to disrupt memory consolidation and may result in cognitive impairment.(63) Baune et al (65) reported an association between IL-1β, IL-6 and TNF and cognitive performance among healthy elderly humans in the general population, but the TNF was thought to be protective of processing speed.(66) Finally, longitudinal research is needed to address the question of whether elevated IL-6 predisposes one to developing depressive syndrome, or does the onset of the depressive episode from another, as-yet-unknown cause, precipitate either an increased immune response or the failure of the suppression response that causes a cascade of brain changes that have functional implications.
In conclusion, results suggest that influences on memory performance for elderly individuals and depressed patients are multifactorial, but one factor is the serum level of IL-6. Elevated levels of IL-6 are associated with some of the variation in recall performance for all elderly individuals, but encoding ability may be singularly sensitive to fluctuations in IL-6 levels for older women.
Acknowledgments
Research grant support provided by National Institute of Mental Health RO1 MH 63764 (Anand Kumar, PI); 2RO1 MH063764-05A2 (Anand Kumar, PI); (General Clinical Research Center at UCLA) MO1 RR00865; HL 079955, AG 026364, CA 10014152, CA116778, (Michael R. Irwin, PI), the UCLA Cousins Center at the Semel Institute for Neurosciences, and the UCLA Older Americans Independence Center Inflammatory Biology Core P30-AG028748.
Footnotes
No author has a conflict of interest to report.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Panda A, Arjona A, Sapey E, et al. Human innate immunosenescence: causes and consequences for immunity in old age. Trends in Immunology. 2009;30:325–333. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godbout JP, Johnson RW. Interleukin-6 in the aging brain. J Neuroimmunology. 2004;147:141–144. doi: 10.1016/j.jneuroim.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant entral inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forsey RJ, Thompson JM, Ernerudh J, et al. Plasma cytokine profiles in elderly humans. Mech Ageing Dev. 2003;124:487–493. doi: 10.1016/s0047-6374(03)00025-3. [DOI] [PubMed] [Google Scholar]
- 5.Licastro F, Candore G, Lio D, et al. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun Ageing. 2005;18:2–8. doi: 10.1186/1742-4933-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad A, Banerjee S, Wang Z, Kong D, Majumdar APN, Sarkar FH. Aging and inflammation: etiological culprits of cancer. Curr Aging Sci. 2009;2:174–186. doi: 10.2174/1874609810902030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garnder EM, Gonzale EW, Nagusa S, Mursko DM. Age-related changes in the immune response to influenza vaccination in a racially diverse, healthy elderly population. Vaccine. 2006;24:1609–1614. doi: 10.1016/j.vaccine.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 8.Codarri L, Fontana A, Becher B. Cytokine networks in multiple sclerosis: lost in translation. Curr Opin Neurol. 2010;23:205–211. doi: 10.1097/WCO.0b013e3283391feb. [DOI] [PubMed] [Google Scholar]
- 9.Engelhart MJ, Geerlligs MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: The Rotterdam Study. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 10.Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer IJ. Early inflammation and dementia: a 25-year follow-up study of the Honolulu-Asia Study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 12.Rainero I, Rubino E, Cappa G, et al. Pro-inflammatory cytokine genes influence the clinical features of frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2009;27:543–547. doi: 10.1159/000225962. [DOI] [PubMed] [Google Scholar]
- 13.Alley DE, Crimmins EM, Karlamangla A, Hu P, Seeman TE. Inflammation and rate of cognitive change in high-functioning older adults. J Gerontol A Biol Sci, Med Sci. 2008;63A:50–55. doi: 10.1093/gerona/63.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol. 2008;85:1–74. doi: 10.1016/j.pneurobio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Herbert TB, Cohen S. Depression and immunity: a meta-analytic review. Psychol Bull. 1993;113:472–486. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- 17.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Maes M. In: Major depression and activation of the imflammatory response system, in Advances in Experimental Medicine and Biology. Dantzer R, Wollman EE, Yirmiya R, editors. Springer US; New York, NY: 1999. pp. 25–46. [DOI] [PubMed] [Google Scholar]
- 19.Song C, Lin A, Bonaccorso S, et al. The inflammatory response system and the availability of plasma tryptophan in patients with primary sleep disorders and major depression. J Affect Disord. 1998;49:211–219. doi: 10.1016/s0165-0327(98)00025-1. [DOI] [PubMed] [Google Scholar]
- 20.Seidel A, Arolt V, Hunstiger M, Rink L, Behnisch A, Kirchner H. Cytokine production and serum proteins in depression. Scand J Immunol. 1995;41:534–538. doi: 10.1111/j.1365-3083.1995.tb03604.x. [DOI] [PubMed] [Google Scholar]
- 21.Maes M, Scharpe S, Meltzer HY, et al. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res. 1993;49:11–27. doi: 10.1016/0165-1781(93)90027-e. [DOI] [PubMed] [Google Scholar]
- 22.Maes M, Lambrechts J, Bosmans E, et al. Evidence for a systemic immune activation during depression: results of leukocyte enumeration by flow cytometry in conjunction with monoclonal antibody staining. Psychol Med. 1992;22:45–53. doi: 10.1017/s0033291700032712. [DOI] [PubMed] [Google Scholar]
- 23.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activationn of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 24.Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogrizovic S, Jovanovic D, Dopsaj V, et al. Could depression be a new branch of MIA syndrome? Clin Nephrology. 2009;71:164–172. doi: 10.5414/cnp71164. [DOI] [PubMed] [Google Scholar]
- 26.Rugulies R. Depression as a predictor for coronary heart disease. a review and meta-analysis. Am J Prev Med. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson CM, Rosenfeld B, Pessin H, Breitbart W. Depression and IL-6 blood plasma concentrations in advanced cancer patients. Psychosomatics. 2008;49:64–66. doi: 10.1176/appi.psy.49.1.64. [DOI] [PubMed] [Google Scholar]
- 28.Zautra AJ, Yocum DC, Villanueva I, et al. Immune activation and depression in women with rheumatoid arthritis. J Rheumatol. 2004;31:457–463. [PubMed] [Google Scholar]
- 29.Dinan TG, Clarke G, Quigley EM, et al. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol. 2008;103:2570–2576. doi: 10.1111/j.1572-0241.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- 30.Elderkin-Thompson V, Kumar A, Bilker WB, et al. Neuropsychological deficits among patients with late-onset minor and major depression. Arch Clin Neuropsychol. 2003;18:529–549. doi: 10.1016/s0887-6177(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 31.Elderkin-Thompson V, Mintz J, Haroon E, Lavretsky H, Kumar A. Executive dysfunction and memory in older patients with major and minor depression. Arch Clin Neuropsychol. 2007;22:261–270. doi: 10.1016/j.acn.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Elderkin-Thompson V, Moody T, Knowlton B, Hellemann GKA. Explicit and implicit memory in late-life depression. Am J Geriatr Psychiatry. 2011;19:249–255. doi: 10.1097/JGP.0b013e3181e89a5b. [DOI] [PubMed] [Google Scholar]
- 33.Alexopoulos GS. Frontostriatal and limbic dysfunction in late-life depression. Am J Geriatr Psychiatry. 2002;10:687–695. [PubMed] [Google Scholar]
- 34.Boone KB, Lesser I, Miller B, et al. Cognitive functioning in a mildly to moderately depressed geritric sample: relationship to chronological age. The Journal of Neuropsychiatry and Clinical Neurosciences. 1994;6:267–272. doi: 10.1176/jnp.6.3.267. [DOI] [PubMed] [Google Scholar]
- 35.Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. American of Journal Psychiatry. 2000;157:1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 36.Alexopoulos GS. Frontostriatal and limbic dysfunction in late-life depression ALEXOPOULOS2002. Am J Geriatr Psychiatry. 2002;10:687–695. [PubMed] [Google Scholar]
- 37.Elderkin-Thompson V, Ballmaier M, Hellemann G, Pham D, Lavretsky H, Kumar A. Daily functioning and prefrontal brain morphology in healthy and depressed community-dwelling elderly. Am J Geriatr Psychiatry. 2008:633–642. doi: 10.1097/JGP.0b013e3181794629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 39.Linn BJ, Linn BW, Gurel L. Cumulative Illness Rating Scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 40.Wechsler D, editor. WTAR: Wechsler Test for Adult Reading. Pearson Assessment; San Antonio, TX: 2001. [Google Scholar]
- 41.Delis DC, Kramer JH, Kaplan E, Ober BA, editors. California Verbal Learning Test. 2nd edition Harcourt Assessment, Inc; San Antonio, TX: 2000. [Google Scholar]
- 42.Wechsler D, editor. WMS-III: Wechsler Memory Scale. 3rd ed The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 43.Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Archives de Psychologie. 1941;28:286–340. [Google Scholar]
- 44.Wechsler D, editor. WAIS-III: Administration and scoring manual. 3rd ed The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- 45.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 46.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G, editors. Wisconsin Card Sorting Test Manual. 2nd ed Psychological Assessment Resources, Inc; Odessa, FL: 1993. [Google Scholar]
- 47.Lezak MD, editor. Neuropsychological assessment. Third Edition Oxford University Press; New York: 1995. [Google Scholar]
- 48.Trzonkowski P, Mysliwska J, Godlewska B, et al. Immune consequences of the spontaneous pro-inflammatory status in depressed elderly patients. Brain Behav Immun. 2004;18:135–148. doi: 10.1016/S0889-1591(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 49.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 53.Godbout JP, Johnson RW. Age and neuroinflammation: A lifetime of psychoneuroimmune consequences. Immuno Allergy Clin N Am. 2009;29:321–337. doi: 10.1016/j.iac.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 54.Nadjar A, Bluthe R-M, May MJ, Dantzer R, Parnet P. Inactivation of the cerebral NF kappaB pathway inhibits interleukin-1beta-induced sickness behavior and c-Fos expression in various brain nuclei. Neurospchopharmacology. 2005;30:1492–1499. doi: 10.1038/sj.npp.1300755. [DOI] [PubMed] [Google Scholar]
- 55.Lu T, Pa Y, Kao SY, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 56.Madrigal JL, Hurtado O, Moro MA, et al. The increase in TNF-alpha lebels is implicted in NF-kappa BB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology. 2002;26:155–163. doi: 10.1016/S0893-133X(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 57.Ballmaier M, Narr KL, Toga AW, et al. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am J Psychiatry. 2008;165:229–237. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stockmeier CA, Mahajan GJ, Konick LC, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628:227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- 60.Parnet P, Kelley KW, Bluthe RM, Dantzer R. Expression and regulation of interleukin-1 receptors in the brain. Role in cytokines-induced sickness behavior. J Neuroimmunol. 2002;125:5–14. doi: 10.1016/s0165-5728(02)00022-x. [DOI] [PubMed] [Google Scholar]
- 61.Richwine AF, Sparkman NL, Dilger RN, Buchanan JB, Johnson RW. Cognitive deficits in interleukin-10-deficient mice after peripheral injection of lipopolysaccharide. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rachal PC, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1beta. Neurosci Biobehav Rev. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- 63.Goshen I, Kreisel T, Ounallah-Saad H, et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 64.van den Kommer TN, Dik MG, Comijs HC, Jonker C, Deeg DJ. Homocysteine and inflammation: Predictors of cognitive decline in older persons? Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 65.Baune BT, Ponath G, Rothermundt M, Riess O, Funke H, Berger K. Association between genetic variants of IL-1beta, IL-6 and TNF-alpha cytokines and cognitive performance in the elderly general population of the MEMO-study. Psychoneuroendocrinology. 2008;33:68–76. doi: 10.1016/j.psyneuen.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Baune BT, Ponath G, Golledge J, et al. Association between IL-8 cytokine and cognitive performance in an elderly general population--the MEMO-Study. Neurobiol Aging. 2008;29:937–944. doi: 10.1016/j.neurobiolaging.2006.12.003. [DOI] [PubMed] [Google Scholar]