Abstract
Mucopolysaccharidosis (MPS) VII is a lysosomal storage disease due to deficient activity of β-glucuronidase (GUSB), and results in glycosaminoglycan accumulation. Skeletal manifestations include bone dysplasia, degenerative joint disease, and growth retardation. One gene therapy approach for MPS VII involves neonatal intravenous injection of a gamma retroviral vector expressing GUSB, which results in stable expression in liver and secretion of enzyme into blood at levels predicted to be similar or higher to enzyme replacement therapy. The goal of this study was to evaluate the long-term effect of neonatal gene therapy on skeletal manifestations in MPS VII dogs. Treated MPS VII dogs could walk throughout their lives, while untreated MPS VII dogs could not stand beyond 6 months and were dead by 2 years. Luxation of the coxofemoral joint and the patella, dysplasia of the acetabulum and supracondylar ridge, deep erosions of the distal femur, and synovial hyperplasia were reduced, and the quality of articular bone was improved in treated dogs at 6 to 11 years of age compared with untreated MPS VII dogs at 2 years or less. However, treated dogs continued to have osteophyte formation, cartilage abnormalities, and an abnormal gait. Enzyme activity was found near synovial blood vessels, and there was 2% as much GUSB activity in synovial fluid as in serum. We conclude that neonatal gene therapy reduces skeletal abnormalities in MPS VII dogs, but clinically-relevant abnormalities remain. Enzyme replacement therapy will probably have similar limitations long-term.
Keywords: Mucopolysaccharidosis, gene therapy, retroviral vector, β-glucuronidase, dysostosis multiplex, dog
1. Introduction
The mucopolysaccharidoses (MPS)1 are a subset of lysosomal storage disorders characterized by deficiencies in enzymes that contribute to the degradation of glycosaminoglycans (GAGs), and have an overall incidence of 1:27,000 [1–2]. Disease manifestations can occur in the skeleton, liver, cardiorespiratory system, central nervous system, and other sites, although the severity in different tissues varies with the specific type of MPS. Severe skeletal abnormalities, collectively referred to as dysostosis multiplex [3–4], are commonly associated with MPS I-Hurler syndrome [5–8], MPS IV [9], and MPS VI [10], but skeletal abnormalities have also been reported in MPS II [11], MPS III [12], and attenuated MPS I [13]. Case reports of patients with MPS VII have demonstrated that bone disease can be severe in this rare type of MPS [14–18], while studies in mouse models demonstrate that bone disease is more severe in MPS VII than in MPS I, MPS IIIA, or MPS IVA [19].
Hip disease can manifest as dysplasia of the acetabulum with reduced calcification of the superior lateral region, dysplasia of the head and neck of the femur, coxa valga, subluxation or complete luxation, or degenerative joint disease (DJD) [3–4, 20]. Knee disease can manifest as reduced calcification of the lateral tibial epiphysis that can result in genu valgum, DJD, or patella subluxation [3–4, 21]. Other manifestations of dysostosis multiplex include spine disease and shortening of endochondral bones [3–4].
The pathogenesis of degenerative changes in MPS may involve binding of GAGs to the Toll-like receptor 4 (TLR4=lipopolysaccharide receptor) and/or components of the complement cascade, resulting in induction of inflammatory cytokines such as tumor necrosis factor α (TNFα) [18, 22–30] and upregulation of enzymes such as matrix metalloproteinases (MMP) and cathepsins, which play a destructive role in arthritis. The pathogenesis for the failure to calcify the epiphyses of some bones such as the vertebral bodies [31] is unclear.
Current treatments for some types of MPS in humans include hematopoietic stem cell transplantation (HSCT) and enzyme replacement therapy (ERT) [32–33], both of which involve uptake of enzyme with mannose 6-phosphate (M6P) by the M6P receptor. After HSCT for MPS I, 90% of patients still had hip dysplasia and 46% required corrective surgery, while 70% had genu valgum and 36% required corrective surgery, when HSCT was performed at an average age of 19 months and evaluation was performed a mean of 6 years later [5–8, 34]. Although the effect of ERT on dysostosis multiplex is difficult to gauge as the first drug was only approved in 2003, ERT has clearly improved joint mobility and ambulation for patients with MPS I [35], MPS II [36], and MPS VI [37]. However, orthopedic surgery was still required in some patients. Early initiation of ERT appears to be more effective than later initiation for sibling pairs for which the younger child started ERT sooner [38–39].
Gene therapy, which involves the transfer of a gene into cells of the body, is being tested in animal models [40]. One gene therapy approach for MPS involves the intravenous (IV) injection of a gamma retroviral vector (RV) expressing the appropriate enzyme into newborn animals, which leads to transduction of liver cells, secretion of M6P-modified enzyme into blood, and uptake of enzyme by cells in other organs via the M6P receptor [41–47]. We have previously demonstrated in MPS VII dogs that neonatal IV injection of an RV expressing β-glucuronidase (GUSB) improved long bone lengths of adults [42–43], reduced erosions of femoral heads at 6 months of age [44, 47], improved mobility at 1 year [42], and reduced radiographic evidence of degenerative changes in some joints at 7 years [43]. However, it failed to prevent spine disease at 6 to 11 years of age [45]. This report focuses on the effect of neonatal gene therapy on mobility and on radiographic and histochemical evidence of disease of the coxofemoral (hip) and stifle (knee) joint in MPS VII dogs at 6 to 11 years of age.
2. Materials and methods
2.1. Reagents
Reagents were purchased from Sigma-Aldrich Chemical (St. Louis, MO) unless otherwise stated.
2.2. Animal care and radiographical evaluation
The animals used in this study were raised at the School of Veterinary Medicine at the University of Pennsylvania, under NIH and USDA guidelines for the care and use of animals in research. MPS VII in the dog is due to a missense mutation (R166H) in the GUSB coding sequence [48–50]. Most normal dogs were heterozygous for the GUSB mutation found in MPS VII dogs and were phenotypically normal. Some MPS VII dogs were untreated, while others received an IV injection at 2 to 3 days after birth of the gamma Moloney murine leukemia virus-based LNL6 vector designated hAAT-cGUSB-WPRE which contained an intact long-terminal repeat (LTR) with the canine GUSB cDNA downstream of the human α1-antitrypsin (hAAT) promoter and also contained the Woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) [41–42]. One treated dog (M1287) received hepatocyte growth factor (HGF) in an attempt to promote hepatocyte replication prior to IV injection of 2×1010 transducing units (TU)/kg of RV (HGF/RV-treated), while RV-treated dogs received RV at a dose of 0.3 to 1×1010 TU/kg without administration of HGF. The RV was packaged in the amphotropic GP+AM12 cells and concentrated by ultrafiltration followed by gel filtration to remove low molecular weight contaminants, as reported previously [41]. Radiographs were obtained following anesthetic induction with intramuscular injections of 0.02 mg/kg of Atropine (Phoenix Pharmaceutical, St. Joseph MO) and 0.1 mg/kg of hydromorphone (Elkins-Sinn, Cherry Hill NJ), and an IV injection of 2 mg/kg of Propofol (Abbott, Chicago IL). A board-certified veterinary radiologist (VWK), unaware of the animals’ genotypes or treatments, scored the animals using a scale where 0 represented normal and +3 represented markedly abnormal. Euthanasia was performed for animals with substantial clinical manifestations or for collection of tissues using 80 mg/kg of sodium pentobarbital (Veterinary Laboratories, Lenexa, KS) in accordance with American Veterinary Medical Association guidelines.
2.3. Histochemistry
Bones were fixed for at least several weeks with phosphate buffered saline with 10% formalin and decalcified with 10% EDTA pH 8.0 for 1 to 2 weeks followed by Formical 2000 (Talman, NY) for 1–2 weeks until tissues were pliable and could be cut with a razor blade. Samples were embedded in paraffin and 6-µm sections were stained with Masson’s trichrome. Stained sections were photographed with an Olympus Nanozoomer 2.0-HT system and NDP imaging software. Histology was scored from 0 (normal) to +3 (markedly abnormal). For GUSB staining, 8 µm-thick frozen sections of synovium were stained with naphthol AS-BI-β-D-glucuronide as described [41]. Anti-canine C3 immunohistochemistry used the protocol described previously [22] except that frozen sections were fixed for 30 minutes with 10% formalin at room temperature, incubated with a 1:100 dilution of a goat anti-canine C3 antibody from Bethyl Laboratories, Inc. (Montgomery TX) overnight, incubated with a horse-radish peroxidase-coupled horse anti-goat IgG (Vector Laboratories, Burlingame CA), and then developed.
2.4 Statistical analyses
Scores for radiographs and pathology for different groups were compared statistically using the Kruskal-Wallis one-way analysis of variance on ranks with Dunn’s post-hoc analysis using Sigma Plot12 (Sigma-Aldrich).
3. Results
3.1. Treatment of dogs and their mobility
MPS VII dogs that were injected IV with the gamma RV designated hAAT-cGUSB-WPRE [42] had stable serum GUSB activity throughout their lives, as was shown previously for the same dogs that are being analyzed here [45]. The HGF/RV-treated dog (M1287) lived to age 11 when he was euthanized for an abnormal gait that appeared to be painful; he had a lifetime average serum GUSB activity of 15,935±4263 U/ml (65-fold normal), which was stable over his lifetime as detailed previously [45]. Four RV-treated dogs were euthanized at 6 to 10 years of age for abnormal gaits that appeared to be painful (N=2), for cellulitis of a forelimb that failed to heal (N=1), or for a benign hemangioma in the spleen (N=1). The RV-treated dogs had lifetime average serum GUSB activity that varied considerably from 108±61 U/ml (41% normal) to 4368±1199 U/ml (16-fold normal) in individual dogs, but levels in each individual dog were stable.
As shown Video 1 in the Supplementary data section, an untreated MPS VII dog could not stand up at 1 year of age due to bone and joint abnormalities, while 4 treated dogs ambulated well at 5 years of age, although some had mild gait abnormalities. No obvious relationship was seen between the serum GUSB activity and the ability to walk normally, as the dog with the highest expression (M1287) appeared to have the most abnormal hind leg motion. However, all treated dogs had a substantial reduction in mobility at 6 years of age or older, as shown at 9 years for M1332 and M1287 in Video 2, at 6 years for M2165 in Video 3, and at 11 years for M1287 in Video 4 in the Supplementary Materials section. Although the MPS VII colony is partly derived from a breed that can develop hip dysplasia (German Shepherd), GUSB+/− dogs from the colony could ambulate well for up to 10 years, making it likely that gait abnormalities in the treated MPS VII dogs were caused by their disease.
3.2 Evaluation of the coxofemoral joint
3.2.1 Radiographic evaluation of the coxofemoral joint
Radiographs were obtained at a mean of 8.2±1.9 [standard deviation (SD)] years of age in treated MPS VII dogs (N=5) and compared with those of normal dogs at 8.1±0.7 years (N=5) as well as with MPS VII dogs at 1.5±0.4 years (N=8) [42, 45]. Older untreated MPS VII dogs could not be evaluated as they do not live past 2 years of age. The treated animals included 1 HGF/RV-treated dog (M1287) and 4 RV-treated dogs (M1328, M1332, M1337, and M2165).
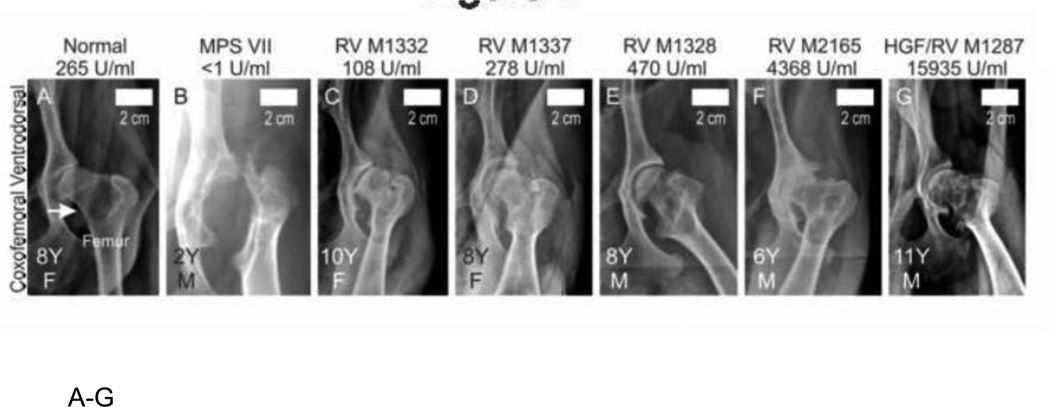
The proximal femur was luxated from the acetabulum in the untreated MPS VII dog shown in Fig. 1B. In contrast, the coxofemoral joint was properly aligned or at most moderately subluxated for the normal (Fig. 1A) and treated (Fig. 1C–1G) dogs. Luxation was scored from 0 (normal), +1 (mild subluxation), +2 (moderate subluxation), or +3 (complete luxation), as shown in Fig. 1H. Untreated MPS VII dogs (N=16 hips from 8 dogs) had a luxation score of 1.9±1.2, which was higher than the value in treated MPS VII dogs with a score of 0.6±1.1 (N=10 hips from 5 dogs; p<0.05) and in normal dogs with 0.8±0.9 (N=10 hips from 5 dogs), although the latter was not significant.
Fig. 1. Radiographs of coxofemoral joints in dogs.
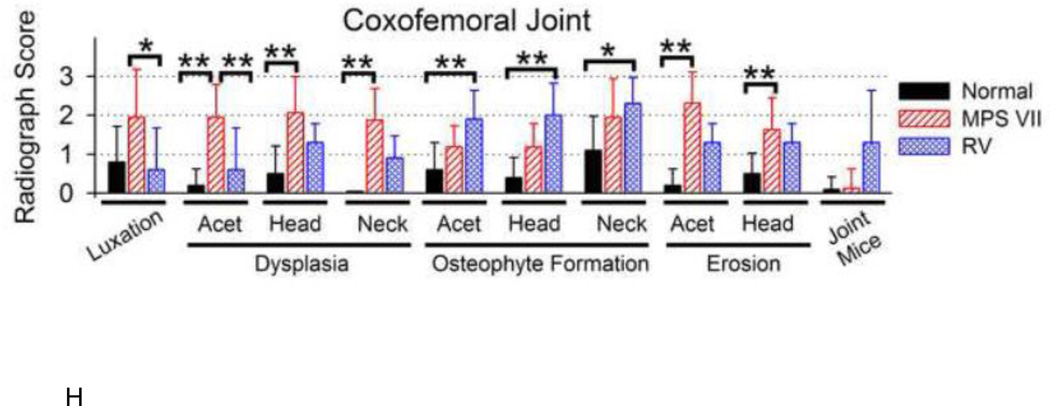
A–G. Representative radiographs. Representative craniocaudal radiographs of the coxofemoral joint are presented from 1 normal, 1 untreated MPS VII (MPS VII), and 5 gene therapy-treated MPS VII dogs as indicated above the panel. Treated MPS VII dogs received a neonatal IV injection of the retroviral vector hAAT-cGUSB-WPRE either with preceding hepatocyte growth (HGF/RV) or without preceding HGF (RV). The average lifetime serum GUSB activity for dogs is indicated, and treated dogs are arranged from lowest (left) to highest (right) serum GUSB activity. Radiographs were obtained at the indicated age in years (Y) for females (F) or males (M), as indicated in the lower left corner. The white arrow in panel A indicates the neck of the femur for a normal dog. For the MPS VII dog in panel B, the femur is luxated from the acetabulum and the head and neck are dysplastic. Size bars indicate 2 cm. H. Radiographic scores of coxofemoral joints. Radiographs were scored from 0 (normal) to +3 (severely abnormal) for luxation of the femur from the acetabulum, dysplasia, osteophyte formation, and/or surface erosion of the acetabulum (Acet), femoral head (Head) and femoral neck (Neck), and the average number of joint mice (calcified stones) per joint. Scores are shown for 10 coxofemoral joints from 5 normal dogs (age 8.1±0.7 years), 16 joints from 8 untreated MPS VII dogs (age 1.6 ±0.4 years), and 10 joints from 5 RV-treated MPS VII dogs (age 8.2±1.9 years). The mean ± standard deviation (SD) is shown; (*) indicates p<0.05, and (**) indicates p<0.01 for comparison of the groups connected by a bracket using the Kruskal-Wallis one-way analysis of variance on ranks with Dunn’s post-hoc analysis. Untreated and treated MPS VII dogs had approximately equal numbers of males and females, while normal dogs were all females from the colony that were used for breeding purposes. Three coxofemoral joints from untreated MPS VII dogs could not be evaluated for osteophyte formation or articular erosion as the dysplasia was so severe.
Regions of the coxofemoral joint were evaluated radiographically for dysplasia, which indicates an abnormal shape, using a graded score from 0 (normal) to +3 (severely abnormal). Dysplasia was substantial in untreated MPS VII dogs in the acetabulum (score 1.9±0.8), the femoral head (2.1±0.9), and the femoral neck (1.9±0.8); these scores were higher than in normal dogs where scores were all <0.5 (p<0.01 in all cases). Dysplasia was significantly reduced in the acetabulum in the treated MPS VII dogs (score 0.6±1.1) compared with the untreated MPS VII dogs (p<0.01). Femoral head and neck dysplasia scores were lower in treated dogs than in untreated dogs; however, these differences were not significant.
Different regions of the coxofemoral joint were scored for osteophyte formation, which refers to abnormal bone proliferation and is a manifestation of DJD, using a score from 0 (normal) to +3 (severely abnormal). Osteophyte formation was not significantly different between untreated MPS VII and normal dogs at any of these regions, which may reflect the younger mean age of the MPS VII dogs (1.5 years) when compared with the normal dogs (8.1 years). Osteophyte formation scores were high in the treated MPS VII dogs in the acetabulum (1.9±0.7), the head of the femur (2.0±0.8), and the neck of the femur (2.3±0.8); all of these scores were significantly higher (p<0.05) than scores for normal dogs of ≤1.1 at all sites. Osteophyte scores were higher in the treated MPS VII dogs than in untreated MPS VII dogs, which might reflect the older age of the treated dogs (8.2 years), although none of these differences were statistically significant.
Another manifestation of DJD is surface erosion, which was scored radiographically from 0 (none) to +3 (severe) in Fig. 1H. A high score for surface erosion could be due to either loss of cartilage at the surface, which narrows the joint space between the acetabulum and the proximal femur, or to irregularities of the surface. Surface erosion scores were high in untreated MPS VII dogs in the acetabulum at 2.3±0.8 and in the head of the femur at 1.6±0.8, which were both higher than the values of ≤0.5 in normal dogs (p<0.01). Scores were slightly lower in the treated MPS VII dogs at 1.3±0.5 in both sites compared with values in untreated MPS VII dogs, although these differences were not significant.
Calcified bodies that can be found within a joint in DJD and are referred to as joint mice. A lateral radiograph depicts a large joint mouse in M1287 that measured 0.8 cm in diameter, as shown in Supplementary Fig. 1A. Radiographically-identified joint mice were found in 3 of 5 treated dogs including the HGF/RV-treated dog M1287 and the RV-treated dogs M1332 and M2165, but were present in only 1 of 8 untreated MPS VII dogs, and 1 of 5 normal dogs. Fig. 1H demonstrates that the average number identified radiographically was 1.3±1.3 joint mice per treated dog, although this was not statistically different from the values in normal or untreated MPS VII dogs.
3.2.2. Gross evaluation of the proximal femur
All treated dogs received a post-mortem examination at the time of euthanasia and dissected bones were evaluated grossly. For the treated MPS VII dogs, the total number of femurs evaluated was only 7, which was fewer than the number of coxofemoral joints evaluated with radiographs due to the collection of only 1 side for some animals. For normal and untreated MPS VII dogs, the total number of proximal femurs evaluated was 3 and 9, respectively. The average age of bones that were evaluated for the normal and treated groups was 8.6±0.6 and 8.7±2.1 years, respectively, which was similar to the average age evaluated for radiographs. However, the average age of the untreated dogs whose bones were evaluated histochemically was only 0.6±0.1 years, which was much younger than the age of 1.6 years at which radiographs were evaluated, as bones were not collected from those older untreated dogs.
For the HGF/RV-treated dog M1287, several joint mice were identified within the coxofemoral joint, as shown in Supplementary Fig. 1B and 1C, with the largest stone 0.8 cm in diameter. Smaller stones were identified in M2165 at post-mortem (not shown). The other 3 treated MPS VII dogs, the untreated MPS VII dogs, and the normal dogs were not evaluated carefully for stones at post-mortem, as they were sacrificed before we were aware of this issue, but large stones probably would have been detected. The coxofemoral joint of an additional RV-treated female dog who was 3 years old and had poor mobility was opened carefully at the time of post-mortem to try to identify the location of joint mice. Supplementary Fig. 1D and 1E show the acetabulum and femur, respectively, of the left side of this dog, which demonstrates joint mice in the rim around the acetabulum and under the lip of the proximal femur.
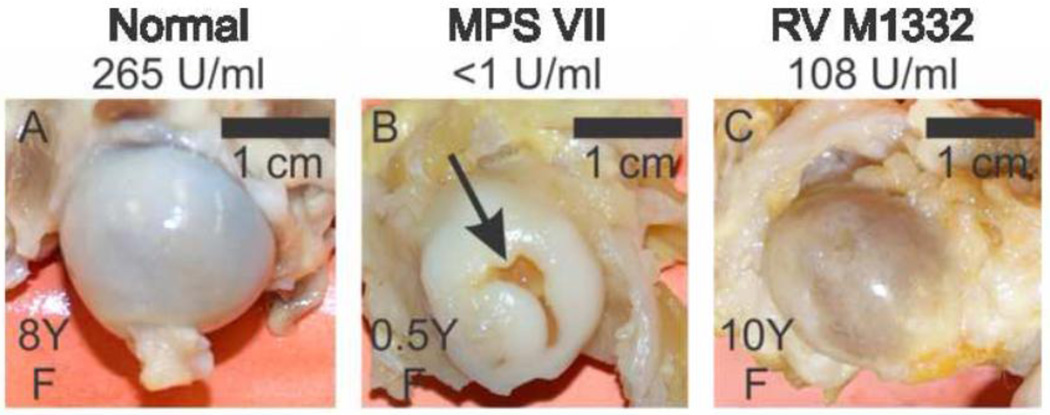
A representative photograph of the proximal head of the femur shown in Fig. 2B demonstrates that an untreated MPS VII dog had a 3 mm-deep erosion on the articular surface what was close to a cm long, which was representative of deep erosions found in 4 of 9 total proximal femurs that were evaluated from untreated MPS VII dogs. In contrast, no deep erosions were seen in any of 7 femoral heads from treated dogs (Fig. 2C), or any of 3 femoral heads from normal dogs (Fig. 3A). However, the cartilage layer of all 5 treated dogs appeared thinner, as the heads were less shiny and the underlying bone was more visible than for the normal dogs. In contrast, the cartilage layer appeared to be whiter and thicker for the untreated MPS VII dog than for the normal dog.
Fig. 2. Gross evaluation of the head of the proximal femur.
Post-mortem examination was performed for animals of the indicated genotype and treatment group (top) at the indicated age in years (Y) for animals of the indicated gender (M or F) (lower left corner), and photographs were obtained. The normal dog had a bright white shiny smooth surface of the head of the femur. An untreated MPS VII dog maintained a bright white shiny color over most of the head of the femur, but had a deep fissure that was 3 mm deep. The HGF/RV-treated dog did not have any erosions, but the color of the surface was less white and shiny than in a normal dog, suggesting that the amount of cartilage on the surface was reduced.
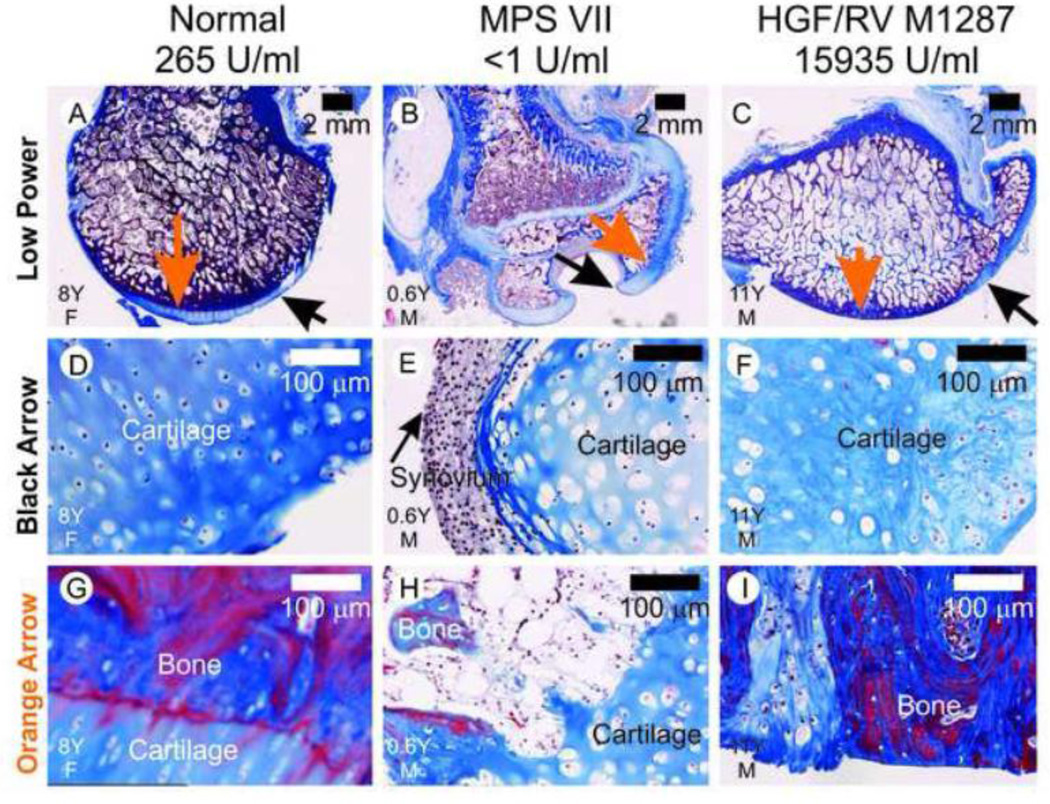
Fig. 3. Histology of the proximal femurs.
Formalin-fixed samples were embedded in paraffin and 6 µm-thick sections were stained with Masson’s trichrome, which stains collagen blue. The treatment group is indicated above the panel, and the age in years (Y) and the gender (M or F) are indicated in the lower left hand corner. The black arrows in panels A–C identify a region of cartilage in each dog that is shown at higher power in panels D–F. The black arrow in panel E identifies synovium on the surface of cartilage in an untreated MPS VII dog. The orange arrows in panels A–C indicate the areas at the bone:cartilage interface that are shown at higher magnification in panels G–I. For the MPS VII dog in panel H, there are regions where the bone is discontinuous under the cartilage layer. For the treated MPS VII dog in panel I, there is a loss of cartilage over most of the articular surface.
3.2.3. Histochemical evaluation of the proximal femur
Sections of formalin-fixed paraffin-embedded proximal femurs were stained with Masson’s trichrome, which stains cartilage and ligaments blue, and bone blue to purple. The head of the femur from an untreated MPS VII dog had 2 large erosions that were >3 mm deep, as shown in Fig. 3B. The region identified with a black arrow in Fig. 3B was lined with synovial membrane and lysosomal storage material was visible in the cytoplasm of synovial membrane and cartilage, as shown in Fig. 3E. The bone underlying the cartilage was poorly formed and discontinuous in the untreated MPS VII dog (Fig. 3H). In contrast, the HGF/RV-treated dog M1287 did not have any visible erosion of the femur head (Fig. 3C). Cartilage was present at the periphery of the head of the femur (Fig. 3F), although the cells contained lysosomal storage. However, although the bone at the surface of the head of the femur was continuous, there was little cartilage on most of the surface (Fig. 3I). For the normal dog, the bone underlying the cartilage was dense and had a smooth surface that was covered with cartilage on most of its surface (Fig. 3A, 3D, and 3G).
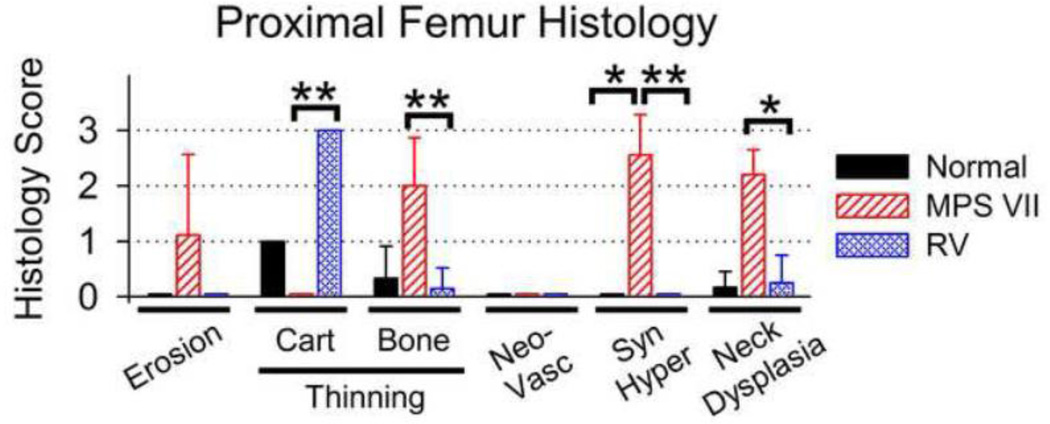
Histology of the proximal femurs was scored in Fig. 4, where 0 represents normal and +3 represents markedly abnormal. The average articular erosion score for the femoral head for untreated MPS VII dogs of 1.1±1.5 was higher than the value of 0 in normal and treated dogs, although this was not significant. The score for diffuse thinning of the cartilage of the head was markedly elevated in treated dogs at 3.0±0, which was significantly higher than the value of 0±0 in untreated MPS VII dogs (p<0.01); the lower value in the untreated MPS VII dogs was likely due to their younger age. Cartilage thinning in normal dogs (score 1±0) was lower than for treated dogs, although this was not significant. The score for diffuse thinning of the bone underlying the articular surface was elevated in untreated MPS VII dogs at 2.0±0.9, which was higher than the value of 0.3±0.6 in normal dogs (not significant) and the value of 0.1±0.4 in treated dogs (p<0.01). No dogs had neovascularization of the articular cartilage. Synovial hyperplasia is shown for an untreated dog in Fig. 3E, and for a different region of the same dog in Supplemental Fig. 2. Untreated MPS VII dogs had a synovial hyperplasia score of 2.7±0.7, which was higher than the value of 0 in both normal and treated MPS VII dogs (p<0.05). The neck of the femur was also evaluated with histochemistry, as this was a region with substantial radiographic abnormalities. Supplemental Fig. 3 demonstrates that the bone in the neck of an untreated MPS VII dog had surface irregularities, while the surface of the bones of normal and treated dogs were smooth. Fig. 4 shows that the femoral neck dysplasia score of 2.2±0.4 in untreated MPS VII dogs was significantly higher than in treated dogs (0.3±0.5; p<0.05) and in normal dogs (0.2±0.3), although the latter was not significant.
Fig. 4. Scoring of histology of proximal femurs.
Sections of bones that were stained with Masson’s trichrome as shown in Fig. 3 were scored for erosion of the articular surface, diffuse thinning of the cartilage (Cart) or the underlying bone (Bone) on the articular surface, neovascularization (Neovasc), synovial hyperplasia (Syn Hyper), and for dysplasia of the neck of the femur using a score from 0 (normal) to +3 (severely abnormal). The mean ± SD is shown, and statistics were performed as in Fig. 1H. The mean age for collection of samples for normal, untreated MPS VII, and treated MPS VII dogs was 8.6±0.6, 0.6±0.1, and 8.7±2.1 years, respectively.
3.3. Evaluation of the stifle joint
3.3.1 Radiographic evaluation of the stifle joint
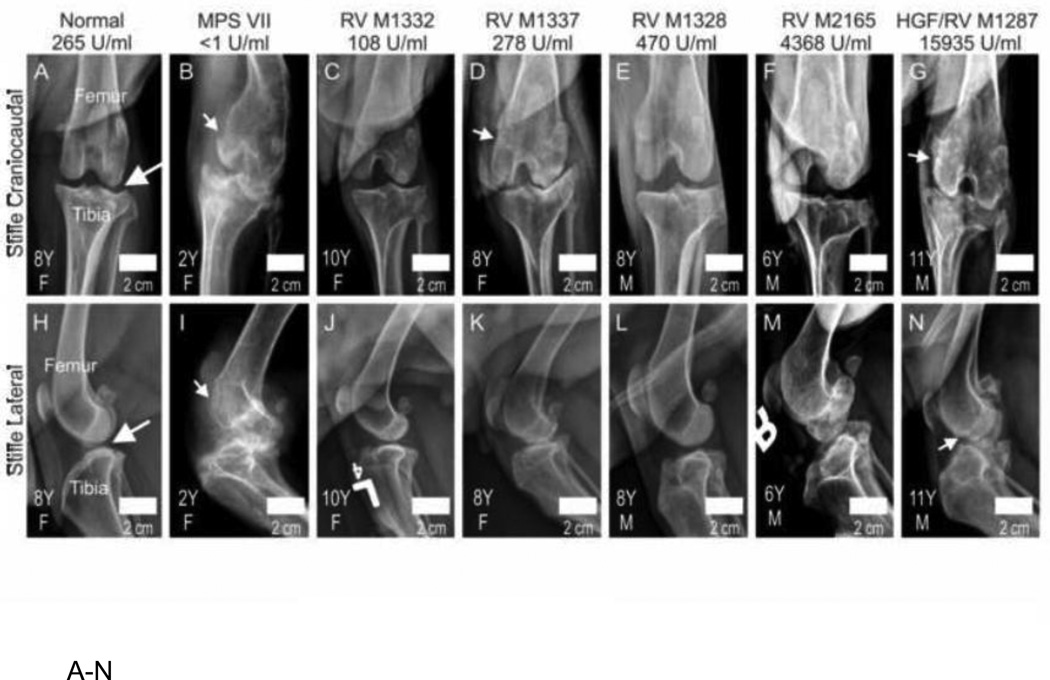
The stifle joint was evaluated radiographically for the same dogs that were evaluated above in Fig. 1. Representative examples of radiographs are shown in Fig. 5A–5N. The untreated MPS VII dog shown in Fig. 5B and 5I had an effusion in the stifle joint, luxation of the patella, dysplasia of the bones, and irregular articular surfaces. The treated MPS VII dogs shown in Fig. 5C–5G and Fig. 5J–5N had improvements in many of these parameters, but reduction of joint space indicative of loss of articular cartilage was visible (see Fig. 5D and 5K for one example) and some irregularities of the surface were seen even in the HGF/RV-treated dog M1287 (Fig. 5G and 5N).
Fig. 5. Radiographs of the stifle joints in dogs.
A–N. Representative radiographs. Craniocaudal (top) or lateral (bottom) radiographs of the stifle joint of dogs of the indicated genotype and treatment, age in years (Y), and gender (M or F; see lower left), were obtained as described in Fig. 1. The average lifetime serum GUSB activity for treated dogs is indicated, and average serum GUSB activity in individual dogs increases from left to right. The long white arrows in panels A and H indicate the non-calcified joint space between the femur and the tibia of a normal dog. The short white arrows in some of the other panels identify erosions of the bone. O. Scoring of radiographs. The stifle joint was scored on a scale of 0 (normal) to +3 (severely abnormal) for effusion, patellar luxation, dysplasia, osteophyte formation, and surface erosion of the condyle (Condyle), the supracondylar ridge (Ridge), and the tibia, and for joint mice. The mean score ± SD was calculated and statistical comparisons performed as in Fig. 1. The mean age for obtaining radiographs was 8.1±0.7, 1.6 ±0.4, and 8.2±1.9 years for normal, untreated MPS VII, and treated MPS VII dogs, respectively.
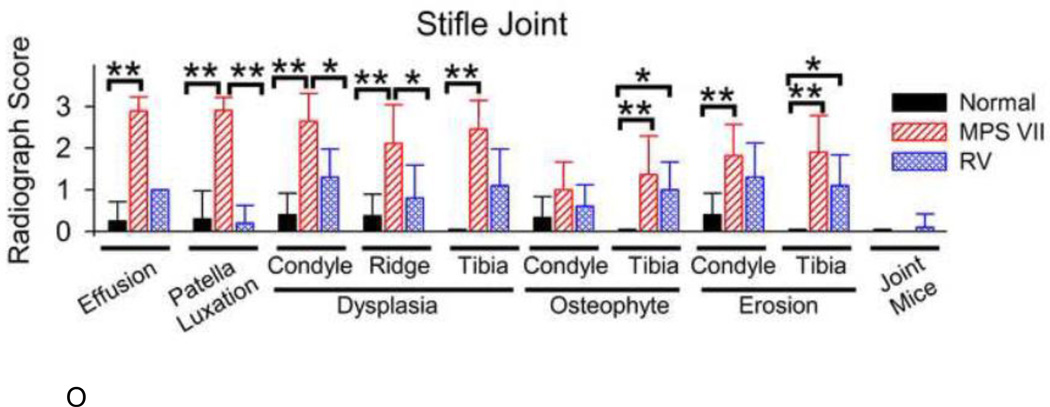
Fig. 5O shows a graphical analysis of scores of various parameters. The presence of a stifle joint effusion was scored from 0 (not observed, which is normal) to +3 (large). The score for the untreated dogs of 2.9±0.3 was higher than in normal dogs (0.3±0.5; p<0.01) and in treated MPS VII dogs (1.0±0.0), although the latter was not significant. Scores for patellar luxation were elevated in untreated MPS VII dogs at 2.9±0.3, which was significantly higher (p<0.01) than scores in normal (0.3±0.7) and in treated MPS VII dogs (0.2±0.4).
Dysplasia scores were elevated in untreated MPS VII dogs in the condyle at 2.6±0.7, the supracondylar ridge at 2.1±0.9, and in the tibia at 2.5±0.7, all of which were higher than in the normal dogs with scores ≤0.4 (p<0.01). Treated dogs had dysplasia scores that were reduced at 1.3±0.7 in the condyle (p<0.01) and at 0.8±0.8 in the supracondylar ridge (p<0.05); the dysplasia score in the tibia of treated dogs was reduced at 1.1±0.9, but this was not significant.
Osteophyte scores were relatively low in the condyle and were not significantly different between the different groups. Osteophyte scores were elevated in untreated MPS VII dogs in the tibia at 1.4±0.9 compared with a score of 0±0 in normal dogs (p<0.01), and remained elevated in treated dogs at 1.0±0.7 (p<0.05 vs. normal, not significant vs. MPS VII).
Surface erosion was present in untreated MPS VII dogs with scores of 1.8±0.8 in the condyle and 1.9±0.9 in the proximal tibia, both of which were higher than the values in normal dogs of ≤0.4 (p<0.01). The surface erosion score of 1.1±0.7 in the tibia of treated dogs was significantly higher than in the normal dogs (p<0.05), while the score in the condyle was elevated at 1.3±0.8, although this was not significantly different from the values in normal or untreated MPS VII dogs. The number of joint mice was low in all groups.
3.3.2. Gross evaluation of the distal femur
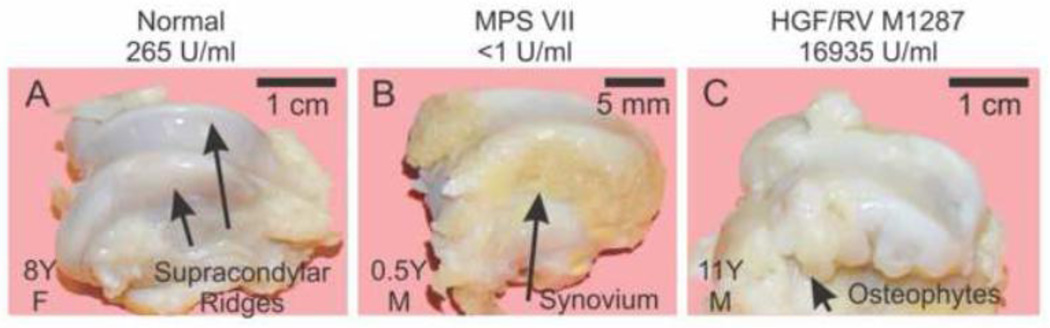
The distal femurs from the same dogs that were evaluated in Section 3.2.2 were dissected and photographed, as shown for representative examples in Fig. 6. The supracondylar ridge, which is the region proximal to the condyle that articulates with the patella, had brownish material on the sides for an untreated MPS VII dog (Fig. 6B), which was demonstrated histologically to be synovial membrane (not shown). The HGF/RV-treated dog M1287 (Fig. 6C) had numerous irregular projections arising from the supracondylar ridge which felt hard with palpation that were found to be calcified with radiographs, which were present to a similar extent in M1332 but were not as severe in the other 3 treated dogs.
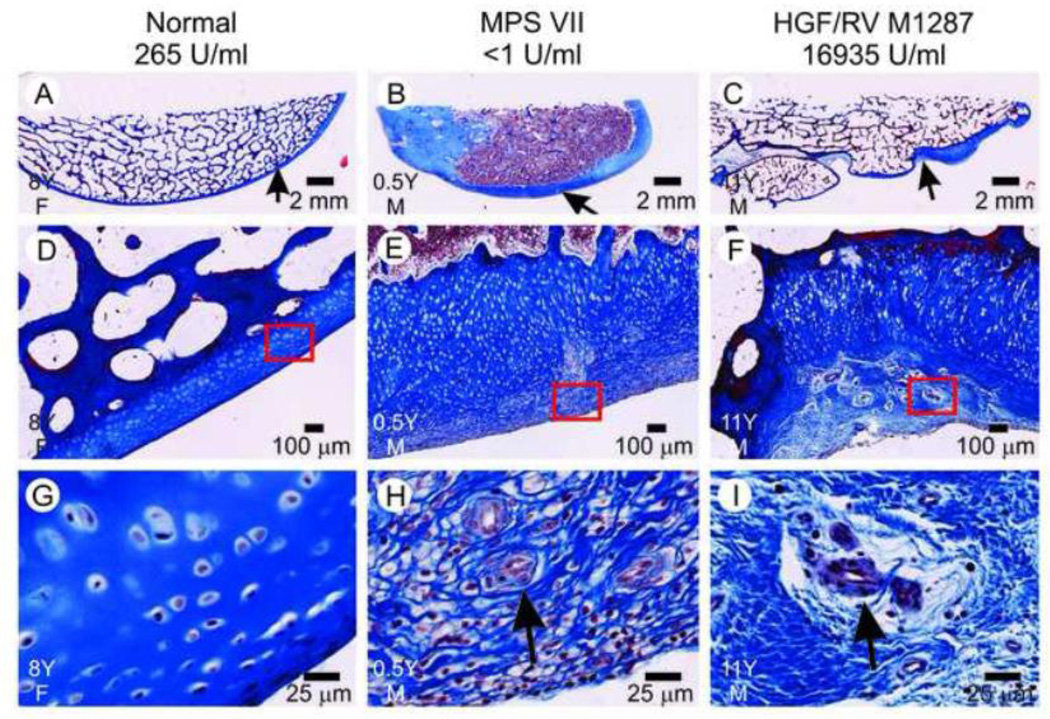
Fig. 6. Gross photographs of the distal femur.
Gross photographs were taken of the distal femur after dissection of the stifle joint of dogs of the indicated group at the indicated years (Y) of age and gender (M or F). The supracondylar ridges are identified with black arrows for a normal dog in panel A. Synovial hyperplasia resulted in the tan tissue at the side of the supracondylar ridge in the MPS VII dog in panel B (long arrow). Osteophytes are identified in the HGF/RV-treated dog in panel C.
Seven of 11 condyles from untreated MPS VII dogs that were evaluated had grossly visible erosions, which tended to be on the caudal region (data not shown). None of 4 distal femurs from normal dogs or 7 distal femurs from treated dogs had grossly visible erosions. The percentage of dogs with deep erosions (>2 mm) of the femur condyle was statistically higher in untreated MPS VII dogs than for treated MPS VII dogs (p=0.01 with Fisher’s exact test).
3.3.3. Histological evaluation of the distal femur
Sections of the supracondylar ridge of the femur that were stained with Masson’s trichrome are shown in Fig. 7. This demonstrated that the irregular projections in the HGF/RV-treated dog M1287 were osteophytes (Fig. 7C), and that there were regions on the surface of the ridge with thickened cartilage with superficial blood vessels (Fig. 7F and 7I), which will be referred to below as neovascularization. The untreated MPS VII dog had thickening of the cartilage in a more diffuse pattern (Fig. 7B) with surface neovascularization (Fig. 7E and 7H). Supplemental Fig. 4 demonstrates that the condyles of two different untreated MPS VII dogs had markedly reduced quality of the bone underlying the articular surface, and that one of these condyles had synovial membrane on the surface and blood vessel invasion into the cartilage. In contrast, two treated dogs had good quality bone at the articular region, although cartilage on the surface was completely absent. Supplemental Fig. 5 demonstrates blood vessel invasion into the cartilage of the condyle as well as synovial hyperplasia in untreated MPS VII dogs, while Supplemental Figs. 6–7 demonstrate erosions of the condyles of two different untreated MPS VII dogs.
Fig. 7. Histopathology of the supracondylar ridge.
The supracondylar ridge of dogs of the indicated genotype and treatment group that were of the indicated age in years (Y) and gender (M or F) was processed and stained with Masson’s trichrome. The short arrows in panels A–C indicate the area at the surface of the supracondylar ridge that is shown at higher magnification in panels D–F; the red box in the latter panels indicates a region that is shown at still higher magnification in panels G–I. The black arrows in panels H and I indicate blood vessels.
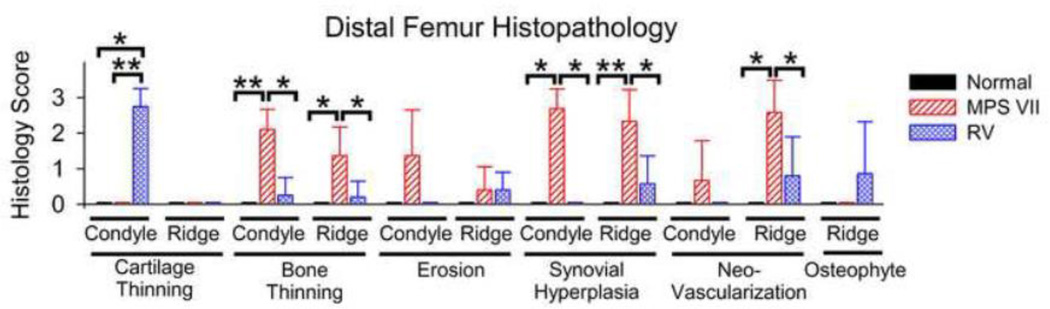
Fig. 8 demonstrates scores of the histochemical analysis for the distal femur, where 0 represents normal, and +3 markedly abnormal. Treated MPS VII dogs had marked thinning of the cartilage over the condyle, with a score of 2.8±0.5, which was higher than the value of 0 in untreated MPS VII (p<0.01) and normal (p<0.01) dogs; the higher score in the treated dogs likely reflects their older age. However, the cartilage was not thin in the treated dogs in the supracondylar ridge region. Untreated MPS VII dogs had marked thinning of the bone underlying the articular surface of the condyle, with a score of 2.1±0.6, which was significantly higher than in the normal (p<0.01) or the treated (p<0.05) dogs. There was also thinning of bone in the supracondylar ridge in the untreated MPS VII dogs where the score was 1.4±0.8, which was significantly higher than the values in normal or treated dogs (p<0.05). Condyle and supracondylar ridge erosion scores were not significantly different between the groups. Synovial hyperplasia scores were high in untreated MPS VII dogs at 2.6±0.6 and 2.3±0.9 for the condyle and the supracondylar ridge, respectively, which were significantly higher than the values of <0.6 in normal and treated dogs (p<0.05). Finally, the neovascularization score in the supracondylar ridge of untreated MPS VII dogs was high at 2.6±0.9, which was significantly higher than the values of ≤0.8 in both other groups (p<0.05).
Fig. 8. Scoring of histology of distal femurs.
Sections of bones that were stained with Masson’s trichrome as shown in Fig. 7 were scored for diffuse thinning of the cartilage or the underlying bone, erosion of the articular surface, synovial hyperplasia, neovascularization, and for osteophyte formation of the condyles or the supracondylar ridge (Ridge) of the distal femur using a score from 0 (normal) to +3 (severely abnormal). The mean ± SD is shown, and statistics were performed as in Fig. 1H. The mean age for collection of samples for normal, untreated MPS VII, and treated MPS VII dogs was 8.6±0.6, 0.6±0.1, and 8.7±2.1 years, respectively.
3.4. GUSB and complement activity in synovium and synovial fluid
Synovial membrane was stained for GUSB activity to determine if enzyme activity was detectable. Supplemental Fig. 8 demonstrates that GUSB activity was diffusely present in the synovium of a normal dog, but was absent in an untreated MPS VII dog. GUSB activity was present near the blood vessels in the synovium of an RV-treated dog at 6 months of age, but was not readily visible in other regions, although the assay has limited sensitivity and it is likely that small amounts of enzyme were present.
Synovial membrane was also stained for the presence of factor C3 of the complement pathway on the surface of membranes, which indicated activation and covalent attachment to surfaces, as a previous study demonstrated that complement was activated in the media of the aorta [22]. Immunostaining for canine C3 results in a dark brown stain in positive regions. Supplemental Fig. 8F demonstrates that the complement signal was very strong near a lymphoid aggregate in the synovium of an untreated MPS VII dog, suggesting that the complement cascade was activated. In contrast, there was little C3 in the synovium in a normal or an RV-treated dog.
4. Discussion
The goal of this study was to evaluate the long-term efficacy on bone and joint disease of neonatal gene therapy in MPS VII dogs. The coxofemoral joint and stifle joint were examined because they frequently exhibit abnormalities in human patients with various types of MPS, and often require surgical interventions to stabilize the joints or reduce pain, as discussed in Section 1. Although skeletal disease also affects bone lengths and the spine, these aspects were evaluated previously [43, 45] and they will not be discussed here.
4.1. Age of evaluation
Although both the treated MPS VII and the normal dogs were evaluated radiographically and histochemically at a mean age of ~8 years, the untreated MPS VII dogs were evaluated radiographically at a mean age of only 1.5 years, as untreated MPS VII dogs have a limited lifespan because of sudden death or humane concerns regarding their inability to ambulate. Untreated MPS VII dogs were evaluated histochemically at an even lower age at 0.5 years, as bone samples from the older untreated MPS VII dogs that were evaluated radiographically were not collected. Thus, the level of improvement in treated dogs compared with untreated dogs could be underestimated, as bone disease often progresses with age, and treated dogs were evaluated at a much older age than untreated dogs. We cannot rule out the possibility that the younger age of the untreated dogs could affect some radiographic or histochemical parameters.
4.2 Mobility
The ability to walk is a significant lifestyle factor that can be compromised in patients affected with MPS. The videos demonstrate that treated dogs have excellent mobility for up to 5 years of age, albeit with mild gait abnormalities in some, while untreated dogs cannot stand after 6 months of age. Although all treated animals had moderate to severe gait abnormalities at 6 years or older, they maintained some level of mobility throughout their lifetime, which was a mean of 8.2 years.
There are at least two likely causes for reduced quality of gait in the treated dogs. M1287 had several joint mice within the coxofemoral joint capsule, the largest of which was approximately 1/3 of the diameter of the femoral head:acetabulum interface (Supplemental Fig. 1) at 0.8 cm, while at least two of the other four treated dogs also had joint mice. It is possible that such joint mice contributed to pain, and could be removed surgically with arthroscopy if found in patients. It is unclear why joint mice are more numerous in the coxofemoral than in the stifle joint, but the marked degenerative changes seen in most treated dogs just under the lip of the head of the femur could be a source for pieces of tissue that serve as a nidus for joint mouse formation. A second possible cause for an abnormal gait in treated dogs is the DJD that developed in all 5 treated dogs, which included osteophyte formation and loss of cartilage on the surface of the articulating bone of both the head and condyle of the femur (Fig. 1 and Fig. 5).
4.3 Bone dysplasia
Bone dysplasia can compromise the articulation of bones, resulting in pain, loss of normal movement, or dislocation. MPS VII bones were dysplastic radiographically compared to normal bones in all of the locations that were evaluated, including the acetabulum, head and neck of the proximal femur, the condyle and supracondylar ridge of the distal femur, and the proximal tibia. Dysplasia was significantly reduced in the treated dogs in the acetabulum, the femoral head, the condyle, and the supracondylar ridge compared to those of untreated dogs.
Dysplasia could be due in part to the inability to form bone correctly. Indeed, untreated MPS VII dogs had a marked reduction in the thickness of bone under the cartilage surface in the femur head, the condyle, and the supracondylar ridge, which suggests that they have difficulties producing bone near articulating surfaces. In contrast, treated MPS VII dogs had near-normal bone thickness in all three areas. This improved bone formation in treated dogs may relate to the diffusion of some GUSB enzyme to bone, as GAG accumulation was reduced in osteocytes of treated dogs compared to those of untreated MPS VII dogs seen in this study (Supplemental Fig. 2) and in our previous study of younger animals [44]. Furthermore, although it is difficult to perform GUSB staining on bones from large animals due to difficulties in obtaining frozen sections from large calcified bones, we have previously shown that GUSB can diffuse to the inside surface and trabeculae of the rib of gene therapy-treated MPS VII mice [44]. Therefore, we believe that bones are improved in treated MPS VII dogs because enzyme can diffuse to some extent into bone.
Acetabular dysplasia occurs in many patients with skeletal manifestations of MPS, and surgery is often performed in human patients to enlarge the acetabulum to better contain the head of the femur. Thus, the reduced acetabular dysplasia in treated MPS VII dogs is encouraging and could translate to improvement in this aspect of disease with gene therapy or ERT in patients. Another common manifestation of dysostosis multiplex in patients is the failure to calcify the lateral tibial plateau. This was difficult to assess here as this abnormality was not overtly present in either untreated or treated dogs.
4.4. Synovial hyperplasia and deep bone erosions
The synovial membrane lines the capsule of synovial joints and produces synovial fluid, which lubricates the joints. However, synovium can proliferate and produce degradative enzymes such as MMPs or cathepsins in MPS [23] or in rheumatoid arthritis [51–52]. This study demonstrated striking synovial hyperplasia in untreated MPS dogs, which was generally found near the edges of articular surfaces, within erosions of the articular surface, and on the medial or lateral surface of the supracondylar ridges (Fig. 3, Fig. 4, Fig. 6, Fig. 8, Supplemental Fig. 3–7). Synovial hyperplasia was reduced at all sites that were evaluated in treated MPS VII dogs, which may reduce the occurrence or severity of erosions. Reduced synovial hyperplasia in treated dogs was probably due to diffusion of GUSB enzyme into the vascularized synovium (Supplemental Fig. 8). Previously [42], synovial fluid for the coxofemoral and stifle joints in the HGF/RV-treated dog M1287 was found to have 103±26 U/ml of GUSB activity (10-fold normal, 1181-fold MPS VII), while synovial fluid GUSB activity for RV-treated dogs was 7±5 U/ml (67% of normal, 78-fold MPS VII). Individual treated dogs had 2±2% as much GUSB activity in synovial fluid as in blood, suggesting that diffusion of GUSB into synovial fluid occurs, but is not very efficient.
Deep cartilage erosions (>2 mm deep) were found in 55% (N=20) of the heads or condyles of the femur of the untreated MPS VII dogs, and were absent in bones from the treated (N=14; p<0.001 with Fisher’s exact test) or the normal (N=7; p=0.02) dogs. This improvement in treated dogs may reflect the presence of GUSB enzyme activity in the synovial fluid. We are in the process of testing if inflammatory markers in synovial fluid are upregulated in untreated MPS VII relative to normal dogs, and if gene therapy can normalize any abnormalities. We doubt that gene therapy itself with an RV with an amphotropic envelope protein induces inflammation, as we have not seen inflammatory infiltrates near transduced cells in the liver.
4.5 Cartilage disease
The major limitation of this gene therapy approach was the inability of gene therapy to prevent osteophyte formation and loss of articular cartilage. The inability to prevent the loss of cartilage at the surface of articulating bone (Fig. 3, Fig. 4, Fig. 8, and Supplemental Fig. 4) likely reflects the limited diffusion of GUSB to the avascular cartilage in the treated dogs. DJD in patients with MPS can progress to arthrosis, which is due to delamination of cartilage from the surface of the articulating bone, and is similar to the histochemical findings seen in the treated dogs. It seems unlikely that articular cartilage disease will be effectively treated with higher serum GUSB activity after gene therapy or with extremely high doses of ERT, as even the dog with 65-fold normal serum GUSB activity (M1287) still had loss of articular cartilage. This failure is likely due to the known avascular nature of cartilage, and will probably be a major limitation of gene therapy or ERT for MPS IV, MPS VI, or MPS VII, as the enzymes that are deficient in these disorders contribute to enzymatic reactions that are important for degrading chondroitin sulfate A, chondroitin sulfate C, and keratan sulfate, the major GAGs of cartilage. However, poor diffusion of enzyme to cartilage may be less of a problem for MPS I and MPS II, as iduronic acid is not a component of chondroitin sulfate A or C or of keratan sulfate, although there are low levels of the iduronic acid-containing dermatan and heparan sulfates in cartilage. It is also possible that other disorders will be treated more effectively with gene therapy or ERT than MPS VII, as the enzyme deficient in MPS VII is a tetramer with a molecular weight of 360 kD that should have more difficulty diffusing to cartilage than the enzymes deficient in other types of MPS with a smaller molecular weight.
The prevailing hypothesis is that GAGs that accumulate in MPS bind to a receptor that induces signaling transduction pathways, which results in upregulation of cytokines and proteolytic enzymes. It is possible that a drug that blocks this signal transduction process could reduce disease in cartilage, a site to which GUSB enzyme probably does not readily diffuse. Therefore, future studies will attempt to identify signal transduction pathways and cytokines that are affected, which might enable us to prevent this process. Indeed, anti-TNFα therapy has improved some parameters of bone disease in animals with MPS VI [53], although the relatively large molecular weight of available drugs might also pose a problem for diffusion into cartilage.
4.6. Similarity of bone abnormalities in MPS VII with other cartilage disorders
The destructive changes that occur in the bones and joints of patients or animals with MPS are likely due to the upregulation of MMPs, cathepsins, and possibly other enzymes that degrade extracellular matrix components. Indeed, we have data that cathepsin enzyme activity is >100-fold normal in the synovium and the articular cartilage of untreated MPS VII dogs, and that the activity in extracts is inhibited with a cathepsin B inhibitor (LX and KPP, unpublished data). Proteolytic enzymes could degrade collagen itself, or proteins that are important for collagen to assemble into a higher order structure. Interestingly, cathepsin B can degrade aggrecan (ACAN) [54–55], a proteoglycan that is very important for collagen assembly, and mutations in the C-type lectin domain of the aggrecan G3 domain (V2303M) that reduce association with the extracellular matrix proteins tenascin-R (TNR), fibulin-1 (FBLN1), and fibulin-2 (FBLN2) are associated with autosomal dominant osteochondritis dissecans [56–56], a disease where the cartilage separates from bone. Genetic diseases associated with DJD include multiple epiphyseal dysplasia that can be caused by mutations in collagen 9 genes (COL9A1, COL9A2, and COL9A3), cartilage oligomeric matrix protein (COMP), matrilin-3 (MATN3), diastrophic dysplasia sulfate transporter (DTDST, renamed SCL26A2), and Strickler syndrome that can be caused by mutations in collagen 2 (COL2A1) and collagen 11 (COL11A1 and COL11A2), as reviewed in reference [56]. Future studies will evaluate the expression of these genes in cartilage for untreated and treated MPS VII dogs.
4.7. Relationship of serum GUSB in gene therapy-treated dogs to dose of ERT
This neonatal gene therapy approach resulted in stable serum GUSB activity for life at levels that vary from close to normal to as high as 65-fold normal [45]. We estimate that the amount of enzyme secreted from transduced cells (primarily liver, but spleen and blood cells also contribute) for the HGF/RV-treated dog M1287 exceeds that delivered with ERT, and the average amount secreted in the RV-treated dogs with near-normal serum levels is comparable to that given with ERT, as was discussed previously [45]. Thus, we believe that our results will be predictive of what will occur long-term with ERT initiated shortly after birth. There was no obvious relationship between the serum GUSB activity in individual dogs and their radiographic and histochemical scores, although variation in the age of sacrifice, gender, other genetic differences in these outbred dogs, and the small numbers of animals analyzed confounds analysis. Expression of GUSB after systemic administration of adenoviral [57], adeno-associated virus (AAV) [58], and lentiviral [59] vectors has been lower than for RV in mouse models, making it unlikely that other existing vectors would achieve a better clinical effect.
4.8 Implications
This study demonstrates that neonatal gene therapy markedly, but not completely, reduces bone and joint disease in MPS VII dogs, and leads us to predict that ERT will be similarly effective. However, the gamma retroviral vector used here included a complete LTR that has contributed to leukemia after gene therapy for X-linked severe combined immunodeficiency or Wiskott-Aldrich syndrome [60], which raises an issue regarding the safety of this vector. Indeed, we have observed one benign hemangioma that expressed retroviral vector sequences in a treated dog at 8 years of age, although this tumor was not malignant and this was the only adverse event that we have noted to date (KPP, MEH, unpublished data). Future studies will further evaluate the safety of our vector, and attempt to identify vectors without a complete LTR that maintain efficacy. A major focus will be to identify signal transduction pathways and proteolytic enzymes that are activated in synovium and cartilage of MPS VII dogs, and to test small molecule inhibitors of inflammation or proteases that might affect this process.
Supplementary Material
Video 1. Video of an untreated MPS VII dog at 1 year of age and 4 treated dogs at 5 years of age. The untreated dog is sitting on the blanket at the start of the video, and cannot stand up throughout the video due to bone and joint disease. Four treated MPS VII dogs (M1328, M1332, M1337, and M1287) can all walk at 5 years of age. The specific dogs and their level of serum GUSB activity are indicated on the video.
Video 2. Video of an untreated MPS VII dog at 6 months of age, the RV-treated dog M1332 at 9 years of age with a lifetime serum GUSB activity of 108 U/ml (41% normal), and the HGF/RV-treated dog M1287 at 9 years of age with a lifetime serum GUSB activity of 15,935 U/ml (65-fold normal). The untreated MPS VII dog cannot stand, while the treated dogs move readily, albeit with gait abnormalities.
Video 3. Video of the mobility of RV-treated dog M2165 at 6 years of age. M2165 had an average lifetime serum GUSB activity of 4368 U/ml (16-fold normal). M2165 walks slowly with short stops, and appears to be in pain when he walks.
Video 4. Video of the mobility of HGF/RV-treated dog M1287 at 11 years of age. M1287 had an lifetime average serum GUSB activity of 15,935 U/ml (65-fold normal), and survived the longest of any MPS VII dog throughout the history of the colony. M1287 has a markedly abnormal hind leg gait.
Highlights.
The effect of neonatal gene therapy at 6 to 11 years after birth is evaluated
Mobility was maintained in treated dogs and many but not all radiographic and histochemical parameters were improved
These results should predict the effect of enzyme replacement therapy if started at birth
Acknowledgments
This study was funded by grants from the NIH (DK054481, HD061879, RR02512) and the National MPS Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: mucopolysaccharidosis (MPS); glycosaminoglycans (GAGs); degenerative joint disease (DJD); Toll-like receptor 4 (TLR4); tumor necrosis factor ± (TNF±); matrix metalloproteinase (MMP); hematopoietic stem cell transplantation (HSCT); enzyme replacement therapy (ERT); mannose 6-phosphate (M6P); intravenous (IV); retroviral vector (RV); ²-glucuronidase (GUSB); hepatocyte growth factor (HGF); long-terminal repeat (LTR), human ±1-antitrypsin (hAAT), woodchuck hepatitis virus post-transcriptional regulatory element (WPRE), transducing unit (TU), and standard deviation (SD).
References
- 1.Neufeld EF, Muenzer J. The Mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. p. 3421. [Google Scholar]
- 2.Moore D, Connock MJ, Wraith E, Lavery C. The prevalence of and survival in Mucopolysaccharidosis I: Hurler, Hurler-Scheie and Scheie syndromes in the UK. Orphanet J Rare Dis. 2008 Sep;16(3):24. doi: 10.1186/1750-1172-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White KK, Harmatz P. Orthopedic management of mucopolysaccharide disease. J Pediatr Rehabil Med. 2010;3:47–56. doi: 10.3233/PRM-2010-0102. [DOI] [PubMed] [Google Scholar]
- 4.White KK. Orthopaedic aspects of mucopolysaccharidoses. Rheumatology (Oxford) 2011;50(Suppl 5):v26–V33. doi: 10.1093/rheumatology/ker393. [DOI] [PubMed] [Google Scholar]
- 5.Masterson EL, Murphy PG, O'Meara A, Moore DP, Dowling FE, Forgarty EE. Hip dysplasia in Hurler's syndrome: orthopaedic management after bone marrow transplantation. J Pediatr Orthop. 1996;16:731–733. doi: 10.1097/00004694-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Field RE, Buchanan JA, Copplemans MG, Aichroth RW. Bone-marrow transplantation in Hurler's syndrome. Effect on skeletal development. J Bone Joint Surg Br. 1994;76:975–981. [PubMed] [Google Scholar]
- 7.Taylor C, Brady P, O’Meara A, Moore D, Dowling F, Fogarty E. Mobility in Hurler syndrome. J Pediatr Orthop. 2008;28:163–168. doi: 10.1097/BPO.0b013e3181649e25. [DOI] [PubMed] [Google Scholar]
- 8.Weisstein JS, Delgado E, Steinbach LS, Hart K, Packman S. Musculoskeletal manifestations of Hurler syndrome: long-term follow-up after bone marrow transplantation. Pediatr Orthop. 2004;24:97–101. doi: 10.1097/00004694-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Montano AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30:165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- 10.Valayannopoulos V, Nicely H, Harmatz P, Turbeville S. Mucopolysaccharidosis VI. Orphanet J Rare Dis. 2010;5(5):1–20. doi: 10.1186/1750-1172-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muenzer J, Beck M, Eng CM, Escolar ML, Giugliani R, Guffon NH, Harmatz P, Kamin W, Kampmann C, Koseoglu ST, Link B, Martin RA, Molter DW, Muñoz Rojas MV, Ogilvie JW, Parini R, Ramaswami U, Scarpa M, Schwartz IV, Wood RE, Wraith E. Multidisciplinary management of Hunter syndrome. Pediatrics. 2009;124:e1228–e1239. doi: 10.1542/peds.2008-0999. [DOI] [PubMed] [Google Scholar]
- 12.White KK, Karol LA, White DR, Hale S. Musculoskeletal manifestations of Sanfilippo Syndrome (mucopolysaccharidosis type III) J Pediatr Orthop. 2011;31:594–598. doi: 10.1097/BPO.0b013e31821f5ee9. [DOI] [PubMed] [Google Scholar]
- 13.Arn P, Wraith JE, Underhill L. Characterization of surgical procedures in patients with mucopolysaccharidosis type I: findings from the MPS I Registry. J Pediatr. 2009;154:859–864. doi: 10.1016/j.jpeds.2008.12.024. e853. [DOI] [PubMed] [Google Scholar]
- 14.Sly WS, Quinton BA, McAlister WH, Rimoin DL. Beta glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr. 1973;82:249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- 15.Beaudet AL, DiFerrante NM, Ferry GD, Nichols BL, Jr., Mullins CE. Variation in the phenotypic expression of beta-glucuronidase deficiency. J Pediatr. 1975;86:388–394. doi: 10.1016/s0022-3476(75)80968-1. [DOI] [PubMed] [Google Scholar]
- 16.Vogler C, Levy B, Kyle JW, Sly WS, Williamson J, Whyte MP. Mucopolysaccharidosis VII: postmortem biochemical and pathological findings in a young adult with beta-glucuronidase deficiency. Mod Pathol. 1994;7:132–137. [PubMed] [Google Scholar]
- 17.de Kremer RD, Givogri I, Argarana CE, Hliba E, Conci R, Boldini CD, Capra AP. Mucopolysaccharidosis type VII (beta-glucuronidase deficiency): a chronic variant with an oligosymptomatic severe skeletal dysplasia. Am J Med Genet. 1992;44:145–152. doi: 10.1002/ajmg.1320440206. [DOI] [PubMed] [Google Scholar]
- 18.Smith LJ, Baldo G, Wu S, Liu Y, Whyte MP, Giugliani R, Elliott DM, Haskins ME, Ponder KP. Pathogenesis of lumbar spine disease in mucopolysaccharidosis VII. Mol Genet Metab. 2012;107:153–160. doi: 10.1016/j.ymgme.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowan DJ, Tomatsu S, Grubb JH, Montaño AM, Sly WS. Assessment of bone dysplasia by micro-CT and glycosaminoglycan levels in mouse models for mucopolysaccharidosis type I, IIIA, IVA, and VII. J Inherit Metab Dis. 36(2013):235–246. doi: 10.1007/s10545-012-9522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guiral J, Sanchez JM, Gonzalez MA. Stress fracture of the femoral neck in a young adult with Maroteaux-Lamy syndrome. Acta Orthop Belg. 1992;58:91–92. [PubMed] [Google Scholar]
- 21.Baz AB, Akalin S, Arik H, Ergün A. Proximal realignment surgery for unilateral chronic patella dislocation in Morquio syndrome: a case report. Acta Orthop Traumatol Turc. 2011;45:466–469. doi: 10.3944/AOTT.2011.2446. [DOI] [PubMed] [Google Scholar]
- 22.Baldo G, Wu S, Howe RA, Ramamoothy M, Knutsen RH, Fang J, Mecham RP, Liu Y, Wu X, Atkinson JP, Ponder KP. Pathogenesis of aortic dilatation in mucopolysaccharidosis VII mice may involve complement activation. Mol Genet Metab. 2011;104:608–619. doi: 10.1016/j.ymgme.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simonaro CM. Cartilage and chondrocyte pathology in the mucopolysaccharidoses: The role of glycosaminoglycan-mediated inflammation. J Pediatr Rehabil Med. 2010;3:85–88. doi: 10.3233/PRM-2010-0120. [DOI] [PubMed] [Google Scholar]
- 24.Simonaro CM, D'Angelo M, Haskins ME, Schuchman EH. Joint and bone disease in mucopolysaccharidoses VI and VII: identification of new therapeutic targets and biomarkers using animal models. Pediatr Res. 2005;57:701–707. doi: 10.1203/01.PDR.0000156510.96253.5A. [DOI] [PubMed] [Google Scholar]
- 25.Ausseil J, Desmaris N, Bigou S, Attali R, Corbineau S, Vitry S, Parent M, Cheillan D, Fuller M, Maire I, Vanier MT, Heard JM. Early neurodegeneration progresses independently of microglial activation by heparan sulfate in the brain of mucopolysaccharidosis IIIB mice. PLoS One. 2008;3:e2296. doi: 10.1371/journal.pone.0002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonaro CM, D'Angelo M, He X, Eliyahu E, Shtraizent N, Haskins ME, Schuchman EH. Mechanism of glycosaminoglycan-mediated bone and joint disease: implications for the mucopolysaccharidoses and other connective tissue diseases. Am J Pathol. 2008;172:112–122. doi: 10.2353/ajpath.2008.070564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 28.Lai A, Simonaro CM, Schuchman EH, Ge Y, Laudier DM, Iatridis JC. Structural, compositional, and biomechanical alterations of the lumbar spine in rats with mucopolysaccharidosis type VI (Maroteaux-Lamy syndrome) J Orthop Res. 2013;31:621–631. doi: 10.1002/jor.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metcalf JA, Linders B, Wu S, Bigg P, O'Donnell P, Sleeper MM, Whyte MP, Haskins M, Ponder KP. Upregulation of elastase activity in aorta in mucopolysaccharidosis I and VII dogs may be due to increased cytokine expression. Mol Genet Metab. 2010;99:396–407. doi: 10.1016/j.ymgme.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simonaro CM, Ge Y, Eliyahu E, He X, Jepsen KJ, Schuchman EH. Involvement of the Toll-like receptor 4 pathway and use of TNF-alpha antagonists for treatment of the mucopolysaccharidoses. Proc Natl Acad Sci U S A. 2010;107:222–227. doi: 10.1073/pnas.0912937107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith LJ, Martin JT, Szczesny SE, Ponder KP, Haskins ME, Elliott DM. Altered lumbar spine structure, biochemistry, and biomechanical properties in a canine model of mucopolysaccharidosis type VII. J Orthop Res. 2010;28:616–622. doi: 10.1002/jor.21030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valayannopoulos V, Wijburg FA. Therapy for the mucopolysaccharidoses. Rheumatology (Oxford) 2011;50(Suppl 5):v49–v59. doi: 10.1093/rheumatology/ker396. [DOI] [PubMed] [Google Scholar]
- 33.Beck M. Therapy for lysosomal storage disorders. IUBMB Life. 2010;62:33–40. doi: 10.1002/iub.284. [DOI] [PubMed] [Google Scholar]
- 34.van der Linden MH, Kruyt MC, Sakkers RJ, de Koning TJ, Oner FC, Castelein RM. Orthopaedic management of Hurler's disease after hematopoietic stem cell transplantation: a systematic review. J Inherit Metab Dis. 2011;34:657–669. doi: 10.1007/s10545-011-9304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sifuentes M, Doroshow R, Hoft R, Mason G, Walot I, Diament M, Okazaki S, Huff K, Cox GF, Swiedler SJ, Kakkis ED. A follow-up study of MPS I patients treated with laronidase enzyme replacement therapy for 6 years. Mol Genet Metab. 2007;90:171–180. doi: 10.1016/j.ymgme.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 36.White KK, Hale S, Goldberg MJ. Musculoskeletal health in Hunter disease (MPS II): ERT improves functional outcomes. J Pediatr Rehabil Med. 2010;3:101–107. doi: 10.3233/PRM-2010-0112. [DOI] [PubMed] [Google Scholar]
- 37.Harmatz P, Giugliani R, Schwartz IV, Guffon N, Teles EL, Miranda MC, Wraith JE, Beck M, Arash L, Scarpa M, Ketteridge D, Hopwood JJ, Plecko B, Steiner R, Whitley CB, Kaplan P, Yu ZF, Swiedler SJ, Decker C. MPS VI Study Group, Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: Final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase. Mol Genet Metab. 2008;94:469–475. doi: 10.1016/j.ymgme.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Furujo M, Kubo T, Kosuga M, Okuyama T. Enzyme replacement therapy attenuates disease progression in two Japanese siblings with mucopolysaccharidosis type VI. Mol Genet Metab. 2011;104:597–602. doi: 10.1016/j.ymgme.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 39.McGill JJ, Inwood AC, Coman DJ, Lipke ML, de Lore D, Swiedler SJ, Hopwood JJ. Enzyme replacement therapy for mucopolysaccharidosis VI from 8 weeks of age--a sibling control study. Clin Genet. 2010;77:492–498. doi: 10.1111/j.1399-0004.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- 40.Ponder KP, Haskins ME. Gene therapy for mucopolysaccharidosis. Expert Opin Biol Ther. 2007;7:1333–1345. doi: 10.1517/14712598.7.9.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu L, Haskins ME, Melniczek JR, Gao C, Weil MA, O'Malley TM, O'Donnell PA, Mazrier H, Ellinwood NM, Zweigle J, Wolfe JH, Ponder KP. Transduction of hepatocytes after neonatal delivery of a Moloney murine leukemia virus based retroviral vector results in long-term expression of beta-glucuronidase in mucopolysaccharidosis VII dogs. Mol Ther. 2002;5:141–153. doi: 10.1006/mthe.2002.0527. [DOI] [PubMed] [Google Scholar]
- 42.Ponder KP, Melniczek JR, Xu L, Weil MA, O'Malley TM, O'Donnell PA, Knox VW, Aguirre GD, Mazrier H, Ellinwood NM, Sleeper M, Maguire AM, Volk SW, Mango RL, Zweigle J, Wolfe JH, Haskins ME. Therapeutic neonatal hepatic gene therapy in mucopolysaccharidosis VII dogs. Proc Natl Acad Sci U S A. 2002;99:13102–13107. doi: 10.1073/pnas.192353499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herati RS, Knox VW, O'Donnell P, D'Angelo M, Haskins ME, Ponder KP. Radiographic evaluation of bones and joints in mucopolysaccharidosis I and VII dogs after neonatal gene therapy. Mol Genet Metab. 2008;95:142–151. doi: 10.1016/j.ymgme.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mango RL, Xu L, Sands MS, Vogler C, Seiler G, Schwarz T, Haskins ME, Ponder KP. Neonatal retroviral vector-mediated hepatic gene therapy reduces bone, joint, and cartilage disease in mucopolysaccharidosis VII mice and dogs. Mol Genet Metab. 2004;82:4–19. doi: 10.1016/j.ymgme.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Smith LJ, Martin JT, O'Donnell P, Wang P, Elliott DM, Haskins ME, Ponder KP. KP, Effect of neonatal gene therapy on lumbar spine disease in mucopolysaccharidosis VII dogs. Mol Genet Metab. 2012;107:145–152. doi: 10.1016/j.ymgme.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Traas AM, Wang P, Ma X, Tittiger M, Schaller L, O'donnell P, Sleeper MM, Vite C, Herati R, Aguirre GD, Haskins M, Ponder KP. Correction of clinical manifestations of canine mucopolysaccharidosis I with neonatal retroviral vector gene therapy. Mol Ther. 2007;15:1423–1431. doi: 10.1038/sj.mt.6300201. [DOI] [PubMed] [Google Scholar]
- 47.Ponder KP, O'Malley TM, Wang P, O'Donnell PA, Traas AM, Knox VW, Aguirre GA, Ellinwood NM, Metcalf JA, Wang B, Parkinson-Lawrence EJ, Sleeper MM, Brooks DA, Hopwood JJ, Haskins ME. Neonatal gene therapy with a gamma retroviral vector in mucopolysaccharidosis VI cats. Mol Ther. 2012;20:898–907. doi: 10.1038/mt.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haskins M, Casal M, Ellinwood NM, Melniczek J, Mazrier H, Giger U. Animal models for mucopolysaccharidoses and their clinical relevance. Acta Paediatr Suppl. 2002;91:88–97. doi: 10.1111/j.1651-2227.2002.tb03117.x. [DOI] [PubMed] [Google Scholar]
- 49.Haskins ME, Desnick RJ, DiFerrante N, Jezyk PF, Patterson DF. Beta-glucuronidase deficiency in a dog: a model of human mucopolysaccharidosis VII. Pediatr Res. 1984;18:980–984. doi: 10.1203/00006450-198410000-00014. [DOI] [PubMed] [Google Scholar]
- 50.Ray J, Scarpino V, Laing C, Haskins ME. Biochemical basis of the beta-glucuronidase gene defect causing canine mucopolysaccharidosis VII. J Hered. 1999;90:119–123. doi: 10.1093/jhered/90.1.119. [DOI] [PubMed] [Google Scholar]
- 51.Solau-Gervais E, Zerimech F, Lemaire R, Fontaine C, Huet G, Flipo RM. Cysteine and serine proteases of synovial tissue in rheumatoid arthritis and osteoarthritis. Scand J Rheumatol. 2007;36:373–377. doi: 10.1080/03009740701340172. [DOI] [PubMed] [Google Scholar]
- 52.Jorgensen I, Kos J, Krasovec M, Troelsen L, Klarlund M, Jensen TW, Hansen MS, Jacobsen S. Serum cysteine proteases and their inhibitors in rheumatoid arthritis: relation to disease activity and radiographic progression. Clin Rheumatol. 2011;30:633–638. doi: 10.1007/s10067-010-1585-1. [DOI] [PubMed] [Google Scholar]
- 53.Eliyahu E, Wolfson T, Ge Y, Jepsen HJ, Schuchman EH, Simonaro CM. Anti-TNF-alpha therapy enhances the effects of enzyme replacement therapy in rats with mucopolysaccharidosis type VI. PLoS One. 2011;6:e22447. doi: 10.1371/journal.pone.0022447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mort JS, Magny MC, Lee ER. Cathepsin B: an alternative protease for the generation of an aggrecan 'metalloproteinase' cleavage neoepitope. Biochem J. 1998;335:491–494. doi: 10.1042/bj3350491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fosang AJ, Neame PJ, Last K, Hardingham TE, Murphy G, Hamilton JA. The interglobular domain of cartilage aggrecan is cleaved by PUMP, gelatinases, and cathepsin B. J Biol Chem. 1992;267:19470–19474. [PubMed] [Google Scholar]
- 56.Stattin EL, Wiklund F, Lindblom K, Onnerfjord P, Jonsson BA, Tegner Y, Sasaki T, Struglics A, Lohmander S, Dahl N, Heinegård D, Aspberg AA. A missense mutation in the aggrecan C-type lectin domain disrupts extracellular matrix interactions and causes dominant familial osteochondritis dissecans. Am J Hum Genet. 2010;86:126–137. doi: 10.1016/j.ajhg.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamata Y, Tanabe A, Kanaji A, Kosuga M, Fukuhara Y, Li XK, Suzuki S, Yamada M, Azuma N, Okuyama T. Long-term normalization in the central nervous system, ocular manifestations, and skeletal deformities by a single systemic adenovirus injection into neonatal mice with mucopolysaccharidosis VII. Gene Ther. 2003;10:406–414. doi: 10.1038/sj.gt.3301869. [DOI] [PubMed] [Google Scholar]
- 58.Daly TM, Ohlemiller KK, Roberts MS, Vogler CA, Sands MS. Prevention of systemic clinical disease in MPS VII mice following AAV-mediated neonatal gene transfer. Gene Ther. 2001;8:1291–1298. doi: 10.1038/sj.gt.3301420. [DOI] [PubMed] [Google Scholar]
- 59.Macsai CE, Derrick-Roberts AL, Ding X, Zarrinkalam KH, McIntyre C, Anderson PH, Anson SD, Byers S. Skeletal response to lentiviral mediated gene therapy in a mouse model of MPS VII. Mol Genet Metab. 2012;106:202–213. doi: 10.1016/j.ymgme.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 60.Wu C, Dunbar CE. Stem cell gene therapy: the risks of insertional mutagenesis and approaches to minimize genotoxicity. Front Med. 2011;5:356–371. doi: 10.1007/s11684-011-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Video of an untreated MPS VII dog at 1 year of age and 4 treated dogs at 5 years of age. The untreated dog is sitting on the blanket at the start of the video, and cannot stand up throughout the video due to bone and joint disease. Four treated MPS VII dogs (M1328, M1332, M1337, and M1287) can all walk at 5 years of age. The specific dogs and their level of serum GUSB activity are indicated on the video.
Video 2. Video of an untreated MPS VII dog at 6 months of age, the RV-treated dog M1332 at 9 years of age with a lifetime serum GUSB activity of 108 U/ml (41% normal), and the HGF/RV-treated dog M1287 at 9 years of age with a lifetime serum GUSB activity of 15,935 U/ml (65-fold normal). The untreated MPS VII dog cannot stand, while the treated dogs move readily, albeit with gait abnormalities.
Video 3. Video of the mobility of RV-treated dog M2165 at 6 years of age. M2165 had an average lifetime serum GUSB activity of 4368 U/ml (16-fold normal). M2165 walks slowly with short stops, and appears to be in pain when he walks.
Video 4. Video of the mobility of HGF/RV-treated dog M1287 at 11 years of age. M1287 had an lifetime average serum GUSB activity of 15,935 U/ml (65-fold normal), and survived the longest of any MPS VII dog throughout the history of the colony. M1287 has a markedly abnormal hind leg gait.