Abstract
Astrocytes might function as brain interoceptors capable of detecting different (chemo)sensory modalities and transmitting sensory information to the relevant neural networks controlling vital functions. For example, astrocytes that reside near the ventral surface of the brainstem (central respiratory chemosensitive area) respond to physiological decreases in pH with vigorous elevations in intracellular Ca2+ and release of ATP. ATP transmits astroglial excitation to the brainstem respiratory network and contributes to adaptive changes in lung ventilation. Here we show that in terms of pH-sensitivity, ventral brainstem astrocytes are clearly distinct from astrocytes residing in the cerebral cortex. We monitored vesicular fusion in cultured rat brainstem astrocytes using total internal reflection fluorescence microscopy and found that ∼35% of them respond to acidification with an increased rate of exocytosis of ATP-containing vesicular compartments. These fusion events require intracellular Ca2+ signaling and are independent of autocrine ATP actions. In contrast, the rate of vesicular fusion in cultured cortical astrocytes is not affected by changes in pH. Compared to cortical astrocytes, ventral brainstem astrocytes display higher levels of expression of genes encoding proteins associated with ATP vesicular transport and fusion, including vesicle-associated membrane protein-3 and vesicular nucleotide transporter. These results suggest that astrocytes residing in different parts of the rat brain are functionally specialized. In contrast to cortical astrocytes, astrocytes of the brainstem chemosensitive area(s) possess signaling properties that are functionally relevant—they are able to sense changes in pH and respond to acidification with enhanced vesicular release of ATP.
Introduction
Astrocytes—a subtype of a group of brain cells known as glia—enwrap all penetrating and intracerebral arterioles and capillaries, control the ionic and metabolic environment of the neuropil (Tsacopoulos and Magistretti, 1996; Magistretti, 2006), provide synapses with a renewable source of transmitters, and play a role in neurovascular coupling (Haydon and Carmignoto, 2006; Takano et al., 2006; Iadecola and Nedergaard, 2007; Gordon et al., 2008). Astrocytes are electrically nonexcitable but display Ca2+ excitability and are capable of releasing signaling molecules—ATP/adenosine, glutamate, d- serine, and possibly many others (Haydon and Carmignoto, 2006; Halassa and Haydon, 2010). Via release of gliotransmitters, astrocytes are implicated in the regulation of neuronal excitability, synaptic transmission, plasticity, and information processing (Kang et al., 1998; Pascual et al., 2005; Volterra and Meldolesi, 2005; Haydon and Carmignoto, 2006; Wang et al., 2006; Jourdain et al., 2007; Schummers et al., 2008; Filosa et al., 2009; Henneberger et al., 2010; Ortinski et al., 2010). Since astrocytes are functionally interconnected via gap junctions and other mechanisms, including gliotransmitter release, they can potentially modulate the activities of extensive neural networks (Angulo et al., 2004; Fellin et al., 2009). However, the functional significance of [Ca2+]i-dependent (glio) transmitter release by astrocytes was questioned by the work of Ken D. McCarthy (University of North Carolina, Chapel Hill, NC) and his group (Fiacco et al., 2007; Agulhon et al., 2010) and the very ability of astrocytes to release physiologically significant quantities of (glio)transmitters in a regulated manner was recently debated (Hamilton and Attwell, 2010).
We reported that astrocytes which reside at and near the ventral surface of the brainstem are exquisitely sensitive to changes in pH and are capable of transmitting changes in PCO2/pH of brain parenchyma on patterns of the respiratory activity (Gourine, 2005; Gourine et al., 2005; Gourine et al., 2010). Activation of the brainstem respiratory network that follows Ca2+ excitation of local astrocytes (induced by either decreases in pH or optogenetic stimulation) is mediated by ATP release, which in response to acidification of the external milieu may involve an exocytotic mechanism (Gourine et al., 2010).
Here we used total internal reflection fluorescence (TIRF) microscopy to determine whether changes in pH indeed trigger vesicular exocytosis in astrocytes residing in a distinct PCO2/pH-sensitive area of the brainstem, the retrotrapezoid nucleus (RTN). To test the hypothesis that ventral brainstem astroglia are functionally specialized, we determined whether these astrocytes are different from cortical astrocytes in terms of their high pH sensitivity and differential expression of genes encoding proteins associated with vesicular transport and exocytosis.
Materials and Methods
All experiments were performed on cell culture preparations from Sprague Dawley rats used in accordance with the United Kingdom Animals (Scientific Procedures) Act of 1986 and associated guidelines.
Cell culture.
Primary astroglial cell cultures were prepared from the brainstems and cerebral cortices of rat pups [postnatal days (P)2–3] of either sex as described in detail previously (Marriott et al., 1995). In rats, respiratory CO2 sensitivity is fully developed at birth. Although it decreases during the first week of life, the slope of the CO2-evoked ventilatory response in 2- to 3-day-old rat pups is similar to that of the adults (Stunden et al., 2001).
In brief, for cultures of the ventral brainstem astrocytes, 500-μm-thick coronal brainstem slices were cut. Slices were inspected under a low-magnification dissecting microscope, and sections containing the facial nucleus were selected. A microblade was used to dissect the most ventral brainstem surface layer (300 μm thick) ∼0.5–2 mm lateral from the midline. Tissue cuts from 2–3 animals were used for cell culture preparation. After isolation, the cells were plated on poly-d-lysine-coated coverslips and adenoviral vector AVV-sGFAP-Case12 or AVV-sGFAP-DsRed was added to the medium at 5 × 108–5 × 1010 transducing units ml−1. AVV-sGFAP-Case12 drives the expression of a Ca2+-sensitive cyclically permuted green fluorescent protein, Case12, under the control of an enhanced GFAP promoter for subsequent identification of astrocytes and monitoring of their Ca2+ responses (Guo et al., 2010). AVV-sGFAP-DsRed drives the expression of DsRed and allows identification of astrocytes on the basis of red fluorescence (Figs. 1Aiii, 3A). In this study astroglial cultures transduced to express Case12 were used in the experiments aimed at confirming that brainstem astrocytes in culture conditions respond to changes in pH with elevations in [Ca2+]i and to identify pH-responding cells for comparison of the gene expression between pH-sensitive and pH-insensitive astrocytes. The rest of the experiments were conducted in astroglial cultures transduced to express DsRed. Experiments were performed after 7–10 days of incubation.
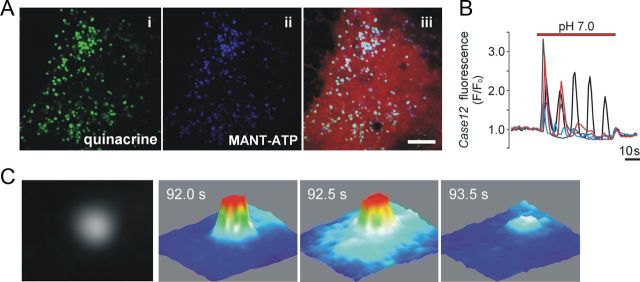
Figure 1.
Vesicular fusion in cultured astrocytes detected using total internal reflection fluorescence microscopy. A, Incubation of cultured astrocytes with quinacrine (i) and MANT-ATP (ii) labels the same intracellular compartments. Astrocytes were transduced with an adenoviral vector to express DsRed (iii) under the control of a GFAP promoter. Scale bar, 10 μm. B, Cultured ventral brainstem astrocytes display [Ca2+]i oscillations in response to a decrease in pH from 7.4 to 7.0. To monitor Ca2+ responses, astrocytes were transduced to express a Ca2+-sensitive protein, Case12, under the control of GFAP promoter. C, Three-dimensional surface plots illustrating detection of a single vesicular fusion event as evident from a progressive lateral spread and decrease of fluorescence intensity visualized using TIRF microscopy (cultured brainstem astrocyte loaded with MANT-ATP). Images were acquired every 0.5 s.
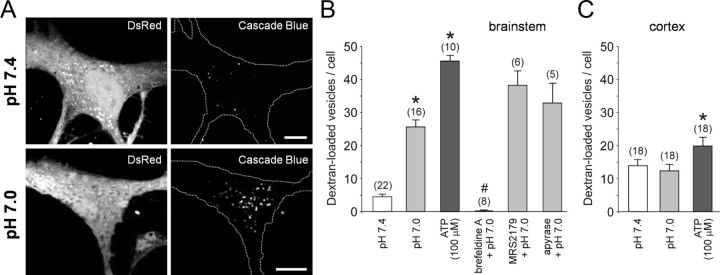
Figure 3.
Ventral brainstem astrocytes respond to acidification with an enhanced endocytotic recovery of the granules. A, Confocal images of cultured DsRed-expressing brainstem astrocytes in control conditions (pH 7.4) and after stimulation with pH 7.0 (10 min) in the presence of 3 kDa dextran conjugated to Cascade Blue. B, Summary data showing the number of fluorescent puncta in the cytosol of ventral brainstem astrocytes stimulated with ATP or exposed to a decreased pH (7.0) in the absence and presence of brefeldin A (50 μm), MRS2179 (30 μm), or ATP-degrading enzyme apyrase (25 U ml−1). *p < 0.01 compared to the number of fluorescent puncta in control conditions at pH 7.4; #p < 0.01 compared to the number of fluorescent puncta at pH 7.4 and pH 7.0. C, Summary data showing the number of fluorescent puncta in the cytosol of cortical astrocytes exposed to a decreased pH (7.0) or stimulated with ATP. *p < 0.01 compared to the number of fluorescent puncta in control conditions at pH 7.4.
Loading cells with quinacrine, MANT-ATP, and LysoTracker.
Visualization of putative ATP-containing vesicles using quinacrine staining was performed as described (Bodin and Burnstock, 2001). Astroglial cell cultures were incubated with quinacrine (5 μm) for 5 min at 37°C. ATP-containing vesicles were also visualized by loading astrocytes with MANT-ATP, 2′,3′-O-(N′-methylanthraniloyl)-ATP. MANT-ATP is an ATP analog in which either the ribose 2′-hydroxyl group or the 3′-hydroxyl group is esterified by the fluorescent methylisatoic acid and used for studying ATP stores and nucleotide-binding proteins (Sorensen and Novak, 2001). Cell cultures were incubated with MANT-ATP (50 μm) for 4 h in DMEM supplemented with 10% fetal calf serum. The cultures were then washed and incubated for a further 3.5 h to allow compartmentalization of MANT-ATP. In a preliminary study using [Ca2+]i imaging of astrocytes transduced to express Case12, we found that MANT-ATP in this concentration had no effect on [Ca2+]i of either cortical or brainstem astrocytes. After loading, cultures were washed 5 times with HBSS (in mm: 137 NaCl, 5.4 KCl, 0.25 Na2HPO4, 0.44 KH2PO4, 1.3 CaCl2, 1.0 MgSO4, 4.2 NaHCO3, 10 HEPES, pH 7.4) and used for TIRF imaging experiments as described in the next paragraph. For identification of acidic organelles, including lysosomes, cells were incubated with LysoTracker Red (100 nm) for 15 min (37°C).
TIRF microscopy.
An objective-type Olympus TIRF microscope was used to detect vesicular fusion events in astrocytes transduced to express DsRed and grown on high-refractive index glass coverslips coated with poly-l-lysine. The imaging setup included a high-numerical aperture oil-immersion objective (60×, 1.65 NA), an inverted microscope (IX71; Olympus) and a cool-charge-coupled-device camera (Hamamatsu). Images were acquired and analyzed using cell tool software (Olympus). To assess changes in fluorescence intensity, a region of interest was drawn around the fluorescent granule and the average intensity was measured in this region. Fluorescence was excited at 365 nm or 488 nm and collected at 430–480 nm or 500–530 nm for MANT-ATP-loaded and quinacrine-loaded cells, respectively. The same settings were used in experiments with quinacrine and MANT-ATP costaining to avoid spectral overlapping (quinacrine does not absorb at 365 nm, while MANT-ATP does not absorb at 488 nm). All experiments were conducted at 37°C.
Dextran loading.
Exocytotic vesicles typically undergo recycling, and the first step in this process is endocytosis. To assess the degree of endocytotic recovery of the granules, astrocytes transduced to express DsRed were incubated with 3 kDa dextran conjugated with a fluorescent marker. This approach may also detect putative partial fusion events since the hydrodynamic diameter of the 3 kDa dextran molecule is ∼2.3 nm (Choi et al., 2010), which is smaller than the diameter of the fusion pore in the neuronal “kiss-and-run” mode estimated to be ∼3.5 nm (Staal et al., 2004). After 10 min of incubation with 3 kDa dextran conjugated with either Cascade Blue or FITC (1 mg ml−1) at pH 7.4 or 7.0, cell cultures were washed 3 times with PBS (0.1 m, pH 7.4) and fixed with 4% paraformaldehyde for 30 min at 4°C. Fixed cells were then mounted on microscope slides using Prolong Antifade Kit (Invitrogen) and imaged using Zeiss LSM 510 confocal laser scanning microscope with a 63× objective.
Immunostaining.
Astroglial cultures were fixed with 4% paraformaldehyde for 30 min at 4°C, permeabilized by incubation with Triton X-100 (0.01% in PBS) for 10 min and treated with bovine serum albumin (10%) for 30 min at room temperature. Cultures were then incubated with rabbit anti-vesicular nucleotide transporter (VNUT) antibodies (1:500; Sawada et al., 2008) for 12 h at 4°C. After 3 washes with PBS, the cultures were subsequently incubated for 1 h with anti-rabbit IgG-Alexa-488 and imaged using Zeiss LSM 510 confocal microscope.
Single-cell RT-PCR.
Astrocytes in cortical and brainstem cultures transduced with AVV-sGFAP-Case12 were selected for RT-PCR on the basis of green fluorescence (e.g., expression of Case12 driven by astrocyte-specific promoter) and individually collected using patch pipettes (∼5 μm tip) made of borosilicate glass. Thirty cortical and thirty ventral brainstem astrocytes were collected. In a separate experiment, ventral brainstem astrocytes were selected on the basis of a [Ca2+]i response (n = 24 astrocytes) or lack of a response (n = 38 astrocytes) to decreases in pH. RNA was isolated with the SV Total RNA Isolation System (Promega) and used as a template for cDNA synthesis with oligo-dT primers to reverse transcribe poly(A+) mRNA (Superscript III System; Invitrogen). The product of this reaction was then treated with RNase H (Invitrogen). A negative control included omission of reverse transcriptase from the reaction tube. RT-PCR was performed using the CFX96TM Real-Time System (Bio-Rad) with TaqMan probes. RT-PCR s were carried out in a buffer containing 10 mm Tris-HCl (pH 8.3), 50 mm KCl, 0.25 mm dNTPs, 0.3 μm each primer, 0.1 μm probe, and 1 unit of Taq polymerase. The primers used were obtained from Syntol and were complementary to the exons of the following rat genes: VAMP2 (vesicle-associated membrane protein 2; also known as synaptobrevin 2; GI:50054292), VAMP3 (vesicle-associated membrane protein 3; GI:158631236), VAMP8 (vesicle-associated membrane protein 8; GI:13929181), SLC1A2 (excitatory amino-acid transporter 2, EAAT2; GI:78126166), SLC17A7 (vesicular glutamate transporter 1; VGLUT1; GI:16758725), and SLC17A9 (vesicular nucleotide transporter, VNUT; GI:157820386).
Relative mRNA quantification was carried out as described (Pfaffl, 2001). In brief, to determine the dynamic range and RT-PCR efficiency, a method of multiple (serial) dilutions of cDNA derived from isolated brain mRNA was used. RT-PCR efficiency was estimated using the formula E = 10[−1/slope] (where E is efficiency of the reaction and slope is the slope of the regression line obtained with serial dilutions of sample cDNA). Relative differences in gene expression were assessed using formula R = E−ΔΔCt [where R is relative gene expression and ΔΔCt = ΔCt(brainstem) − ΔCt(cortex)]. Ct value of β-actin was used as a reference for normalization.
Data analysis.
Olympus or Zeiss built-in analysis software tools were used to analyze the results of the imaging experiments. Group data were compared using one-way ANOVA followed by the Tukey-Kramer post hoc test (for comparisons among three or more experimental groups) or Student's t test, as appropriate. Wilcoxon test was used for quality control of the RT-PCR data. Data are presented as means ± SEM. Differences at p < 0.05 were considered to be significant.
Results
The acridine derivative quinacrine is a weak base that binds ATP with high affinity and can be used to visualize putative ATP-containing vesicles in living cells (Bodin and Burnstock, 2001), including astrocytes (Coco et al., 2003). Cultured ventral brainstem astrocytes incubated with quinacrine displayed abundant punctate fluorescent staining in the cytosol (Fig. 1Ai). A similar pattern of intracellular labeling was observed in both cortical and ventral brainstem astrocytes loaded with the fluorescent ATP analog MANT-ATP (Fig. 1Aii). Incubation of astrocytes with both markers revealed that quinacrine and MANT-ATP label the same intracellular compartments (Fig. 1Aiii). Quinacrine- or MANT-ATP-labeled vesicular compartments were estimated to have an average size of 0.5 ± 0.01 μm (50 vesicles; n = 5 astrocytes). Preincubation of astrocytes with bafilomycin A1 (an inhibitor of vacuolar type H+-ATPase; Bowman et al., 1988) prevented MANT-ATP staining (data not shown), indicating that vesicular accumulation of the marker is H+-dependent.
In agreement with the results obtained in organotypic brainstem slices, acute brainstem slices, and in vivo (Gourine et al., 2010), cultured ventral brainstem astrocytes displayed [Ca2+]i oscillations in response to decreases in pH (Fig. 1B). Time-lapse TIRF imaging revealed that lowering external pH from 7.4 to 7.0 causes progressive decrease in quinacrine (Fig. 2 A) or MANT-ATP (Figs. 1C, 2B) fluorescence intensity in the evanescent field in 35% (49 of 140 cells) of examined brainstem astrocytes. This indicates loss of a fluorescent marker from the labeled organelles as a result of their fusion with plasma membrane. Approximately 50% of labeled vesicles underwent fusion in response to acidification (total number of MANT-ATP-containing puncta under epifluorescence illumination was 240 ± 69; of that pool, 109 ± 26 granules lost fluorescence in response to a decrease in external pH from 7.4 to 7.0; n = 6 pH-sensitive astrocytes).
Figure 2.
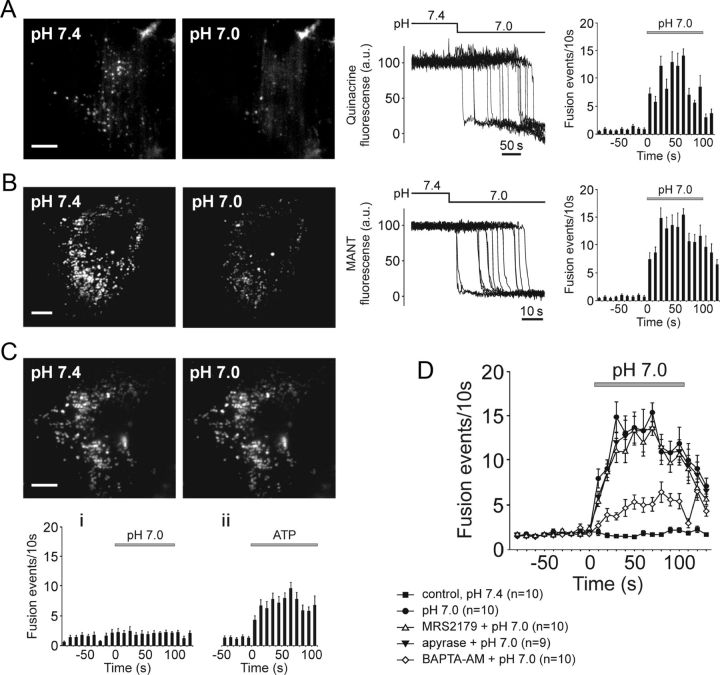
Ventral brainstem astrocytes respond to acidification with an increased rate of exocytosis of putative ATP-containing vesicular compartments. A, B, TIRF images (left) and plots of TIRF intensity changes (middle) showing loss of quinacrine (A) or MANT-ATP (B) fluorescence from a proportion of labeled organelles in cultured ventral brainstem astrocytes in response to a decrease in external pH from 7.4 to 7.0. Plots on the right depict averaged temporal distribution of acidification-evoked fusion events detected in 10 quinacrine loaded (A) and 10 MANT-ATP loaded (B) pH-sensitive brainstem astrocytes. Scale bars, 10 μm; C, Higher basal (at pH 7.4) rate of vesicular fusion events in cultured cortical astrocytes is not affected by acidification. Plots on the bottom depict averaged temporal distribution of fusion events detected in cortical astrocytes exposed to (i) acidification of the external milieu (n = 10 cells) or (ii) application of ATP (10 μm; n = 8 cells). Scale bar, 10 μm. D, Summary data illustrating averaged temporal distributions of acidification-evoked fusion events detected in MANT-ATP-loaded brainstem astrocytes in the absence and presence of MRS2179 (30 μm), ATP degrading enzyme apyrase (25 U ml−1), or after 1 h incubation with a Ca2+-chelator BAPTA-AM (30 μm).
Compared to brainstem astrocytes, cultured cortical astrocytes displayed a higher rate of vesicular fusion events in resting conditions at pH 7.4 (p = 0.0009; Fig. 2C). However, the ongoing fusion rate in cortical astrocytes was not affected by acidification (Fig. 2C). Significant increases in fusion rate in response to application of exogenous ATP (10 μm; Fig. 2Cii) suggest that cortical astrocytes possess purinergic receptors but lack the ability to sense changes in pH.
To determine whether pH-evoked exocytosis of MANT-ATP-containing vesicles in brainstem astrocytes occurs in a Ca2+-dependent manner, the cells were preincubated with a cell-permeable Ca2+-chelator BAPTA-AM (30 μm) for 1 h and then exposed to low pH. The number of fusion events triggered by acidification in brainstem astrocytes treated with BAPTA was significantly (p = 0.001) reduced (Fig. 2 D) indicating that pH-evoked vesicular exocytosis depends (at least in part) upon intracellular Ca2+ signaling.
Previously we found that acidification-evoked [Ca2+]i responses in ventral brainstem astrocytes are reduced or abolished by blockade of ATP-mediated signaling with either a P2 receptor antagonist MRS2179 or an ATP-degrading enzyme apyrase (Gourine et al., 2010). This suggested that propagation of Ca2+ excitation among brainstem astrocytes is mediated by released ATP. Therefore, we next determined whether vesicular exocytosis evoked by lowering external pH depends upon autocrine or paracrine ATP-mediated signaling. Neither MRS2179 (30 μm) nor apyrase (25 U ml−1) affected the time course and magnitude of acidification-evoked vesicular fusion responses in ventral brainstem astrocytes (Fig. 2D). Efficacy of apyrase in blocking ATP actions was confirmed in a separate experiment. In response to mechanical stimulation, astrocytes in culture are known to generate “waves” of Ca2+ excitation propagating mainly via vesicular release of ATP (Bowser and Khakh, 2007). In a confluent culture of cortical astrocytes the wave of Ca2+ excitation traveled 138 ± 27 μm (n = 10 cultures) from the stimulated cell in 20 s, while in the presence of apyrase (25 U ml−1) this distance was reduced to 39 ± 5 μm (n = 3 cultures; p = 0.024).
Three kilodaltons (3 kDa) dextran is thought to be impermeable to unbroken cell membrane, and its appearance inside of a cell should primarily occur via endocytosis or partial fusion (Turvey and Thorn, 2004). Exposure of ventral brainstem astrocytes to low pH (7.0) in the presence of 3 kDa dextran conjugated to FITC or Cascade Blue significantly (p = 0.005) increased the number of fluorescent puncta in the cytosol (Fig. 3A,B). Application of exogenous ATP (100 μm) had a significantly stronger effect (Fig. 3B). The number of fluorescent vesicles identified in ventral brainstem astrocytes upon exposure to low pH was not reduced in the presence of P2 receptor antagonist MRS2179 or an ATP-degrading enzyme apyrase (Fig. 3B), indicating that it was not a result of prior release of ATP. Acidification-induced appearance of florescent puncta was abolished when astrocytes were preincubated for 1 h with a vesicular trafficking inhibitor, brefeldin A (50 μm; Fig. 3B).
The number of fluorescent puncta detected in individual cortical astrocytes at pH 7.4 was higher (p = 0.001) in comparison to that of brainstem astrocytes (Fig. 3C). Although application of exogenous ATP (100 μm) slightly increased the number of dextran-labeled fluorescent granules in cortical astrocytes, lowering external pH had no effect (Fig. 3C).
Immunostaining for vesicular nucleotide transporter (Sawada et al., 2008) revealed abundant VNUT-positive puncta in the cytosols of both ventral brainstem (Fig. 4A) and cortical (not shown) astrocytes. In cultured brainstem astrocytes the average size of VNUT-positive puncta was 0.3 ± 0.05 μm (25 granules; n = 7 cells), similar to the estimated sizes of vesicles visualized after loading with quinacrine or MANT-ATP. However, intracellular compartments labeled with quinacrine/MANT-ATP- and VNUT-positive vesicles were clearly distinct from the organelles stained with a lysosomal marker LysoTracker (1.4 ± 0.05 μm, 25 granules; n = 3 cells).
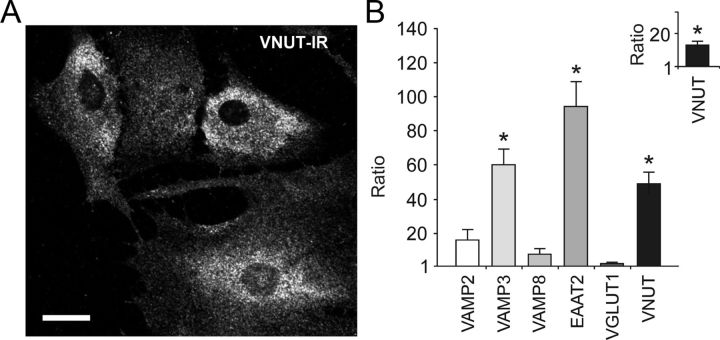
Figure 4.
Differential expression of vesicular transport- and vesicular fusion-associated proteins in brainstem and cortical astrocytes. A, Immunohistochemical detection of VNUT in cultured ventral brainstem astrocytes. Scale bar, 10 μm. B, Relative transcription level of selected vesicular transport- and vesicular fusion-associated proteins in cultured brainstem versus cortical astrocytes. Inset, Relative mRNA level of VNUT in pH-sensitive versus pH-insensitive ventral brainstem astrocytes. *p < 0.01.
Quantitative real time RT-PCR analysis of genes encoding proteins associated with vesicular fusion and transmitter transport revealed that mRNA levels of VAMP3, EAAT2, and VNUT are significantly higher in ventral brainstem astrocytes as compared to cortical astrocytes (2ΔCt = −4.9; 2ΔCt = −6.88; 2ΔCt = −2.61, respectively; all differences are significant with p < 0.01; Fig. 4B). Brainstem astrocytes demonstrated similar to cortical astroglia level of expression of VAMP2, VAMP8, and VGLUT1 (Fig. 4B). Ventral brainstem astrocytes that demonstrated [Ca2+]i responses to acidification also displayed higher level of VNUT expression when compared to brainstem astrocytes not sensitive to changes in pH within the range used in this study (2ΔCt = −3.085; p < 0.01; Fig. 4B, inset).
Discussion
The present study directly demonstrates that brainstem astrocytes respond to acidification with increased exocytosis of putative ATP-containing vesicular compartments. In contrast, cortical astrocytes at resting conditions (pH 7.4) have a higher rate of vesicular fusion events that is not affected by extracellular acidification. Thus, in terms of sensitivity to changes in pH, astrocytes that reside in the chemosensory area of the brainstem appear to be clearly distinct from cortical astrocytes.
Previously using in vivo animal models, we demonstrated that ATP is released from the ventral surface of the brainstem in response to an increase in inspired CO2 (Gourine et al., 2005). ATP release precedes adaptive augmentation of the respiratory activity and contributes to it, since blockade of ATP receptors following application of P2 antagonists on the ventral surface of the brainstem reduces ventilatory response to CO2 (Gourine et al., 2005). In the same experimental model (urethane-anesthetized rat with denervated peripheral chemoreceptors), blockade of ATP receptors within the RTN (Wenker et al., 2012) or acute silencing of the chemosensitive RTN neurons (Marina et al., 2010) results in a quantitatively similar inhibition of the CO2-evoked respiratory response, suggesting that ATP-mediated signaling plays a significant role in the mechanisms of RTN chemosensitivity. Subsequently, we found that in response to decreases in pH (which follow increases in PCO2), astrocytes that reside at and near the ventral surface of the brainstem display profound increases in [Ca2+]i (Gourine et al., 2010).
Propagation of Ca2+ excitation among ventral brainstem astrocytes could be abolished by pharmacological agents that interfere with either ATP-mediated signaling or exocytotic machinery (Gourine et al., 2010)— suggesting exocytotic release of ATP in response to acidification. There is also evidence that CO2 can directly open certain connexin hemichannels allowing egress of ATP, while blockade of connexin hemichannels on the ventral brainstem surface reduces CO2-evoked respiratory response (Huckstepp et al., 2010). Therefore, it remained unclear whether hypercapnia/acidification-induced propagation of Ca2+ excitation among ventral brainstem astrocytes is initiated by exocytotic or hemichannel-mediated release of ATP or both.
TIRF microscopy was used in this study to visualize putative ATP-containing vesicular compartments in cultured ventral brainstem astrocytes and to determine whether the rate of exocytosis of these compartments is increased by acidification. In ∼35% of ventral brainstem astrocytes, quinacrine- or MANT-ATP-labeled vesicles underwent fusion when external pH was lowered from its normal value of 7.4 to 7.0. This pH-evoked exocytosis required intracellular Ca2+ signaling because it could be reduced by a Ca2+ chelator, BAPTA, but was not affected by P2 receptor antagonist MRS2179 or an ATP-degrading enzyme, apyrase. These data suggest that vesicular fusion induced by acidification in brainstem astrocytes is an intrinsic cellular process that is not triggered by prior release of ATP via other mechanism(s), e.g., connexin hemichannels.
Exposure of cultured ventral brainstem astrocytes to pH 7.0 in the presence of fluorescent 3 kDa dextran in the media significantly increased the number of labeled puncta in the cytosol. The number of fluorescent puncta detected in individual cortical astrocytes did not change upon acidification, although it was higher at resting conditions (pH 7.4) compared to the brainstem astrocytes. Although this approach can be used to identify vesicular compartments undergoing partial fusion we cannot delineate between kiss-and-run and full fusion mode based on our observations. Nevertheless, the data obtained in this experiment are in full agreement with the data obtained using TIRF microscopy and indicate enhanced vesicular fusion and/or recovery in brainstem astrocytes in response to acidification, higher rate of vesicular events in cortical astrocytes at normal pH, and insensitivity of fusion events in cortical astrocytes to lowering external pH.
It was reported previously that in response to increases in PCO2/[H+], ATP is only released from highly restricted areas near the ventral brainstem surface, while no release was detected from the structures located deeper in the brainstem or from its dorsal surface (Gourine et al., 2005; Huckstepp et al., 2010). Moreover, in contrast to ventral brainstem astrocytes, cortical astrocytes did not display [Ca2+]i elevations in response to acidification (Gourine et al., 2010). The data reported here are consistent with these previous observations and also demonstrate that differential sensitivity of cortical and brainstem astrocytes to changes in pH is retained in a culture model, convenient for detailed interrogation of possible cellular and molecular mechanisms.
Indeed, these differences indicate functional specialization of astrocytes residing at and near the ventral surface of the brainstem. They are clearly distinct from cortical astrocytes in terms of their ability to respond to acidification with increased fusion of putative ATP-containing vesicles. We next hypothesized that expression profile of genes encoding proteins associated with vesicular transport and fusion might be different between cortical and brainstem astrocytes. Indeed, brainstem astrocytes had higher levels of VAMP3 and VNUT expression. VNUT is a member of the SLC17 family of anion transporters and requires energy generated by the vacuolar-type ATPase to actively accumulate nucleotides into the vesicular compartments (Sawada et al., 2008; Larsson et al., 2012). VNUT is active in cultured brainstem astrocytes, since accumulation of MANT-ATP does not occur when vacuolar type H+-ATPase is inhibited by bafilomycin A1. Heterogeneity within the population of brainstem astrocytes was revealed when VNUT expression levels in pH-sensitive astrocytes was found to be higher in comparison to pH-insensitive astrocytes.
High levels of VAMP3 and VNUT gene expression in brainstem astrocytes does not, however, reveal the mechanism(s) underlying their high sensitivity to changes in pH. This would require further investigation of the possible mechanisms linking changes in extracellular and/or intracellular pH to facilitated exocytosis of ATP-containing vesicles. However, results of the present study provide the most direct currently available evidence that astrocytes residing in different parts of the brain are functionally specialized. In contrast to cortical astrocytes, brainstem astrocytes possess signaling properties that are functionally relevant—they are able to sense physiological decreases in pH and respond to acidification with vesicular release of ATP. ATP actions in this part of the brain play an important role in the control of breathing (Gourine, 2005).
The present results also contribute to an ongoing debate (Hamilton and Attwell, 2010): “Do astrocytes really exocytose neurotransmitters?” The data directly show that in response to increases in PCO2/[H+], brainstem astrocytes do release ATP by exocytotic mechanism. From other studies we know that this release plays a role in chemosensory transduction, contributes to the adaptive changes in ventilation, and therefore is physiologically significant. Other mechanisms of ATP release, such as release via connexin hemichannels (Huckstepp et al., 2010), are hypothesized to operate in parallel or at the levels of PCO2 when pH-dependent vesicular release is not activated.
Footnotes
This study was supported by The Wellcome Trust and British Heart Foundation. A.V.G. is a Wellcome Trust Senior Research Fellow. We thank Dr. Yoshinori Moriyama (Okayama University, Okayama, Japan) for providing anti-VNUT antibody.
The authors declare no competing financial interests.
References
- Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science. 2010;327:1250–1254. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24:6920–6927. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J Gen Physiol. 2007;129:485–491. doi: 10.1085/jgp.200709780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JJ, Wang S, Tung YS, Morrison B, 3rd, Konofagou EE. Molecules of various pharmacologically-relevant sizes can cross the ultrasound-induced blood-brain barrier opening in vivo. Ultrasound Med Biol. 2010;36:58–67. doi: 10.1016/j.ultrasmedbio.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- Fellin T, Halassa MM, Terunuma M, Succol F, Takano H, Frank M, Moss SJ, Haydon PG. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc Natl Acad Sci U S A. 2009;106:15037–15042. doi: 10.1073/pnas.0906419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X, Chen J, McCarthy KD. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron. 2007;54:611–626. doi: 10.1016/j.neuron.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Filosa A, Paixão S, Honsek SD, Carmona MA, Becker L, Feddersen B, Gaitanos L, Rudhard Y, Schoepfer R, Klopstock T, Kullander K, Rose CR, Pasquale EB, Klein R. Neuron-glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nat Neurosci. 2009;12:1285–1292. doi: 10.1038/nn.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV. On the peripheral and central chemoreception and control of breathing: an emerging role of ATP. J Physiol. 2005;568:715–724. doi: 10.1113/jphysiol.2005.095968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Liu B, Tang F, Lane S, Souslova EA, Chudakov DM, Paton JF, Kasparov S. Astroglia are a possible cellular substrate of angiotensin(1–7) effects in the rostral ventrolateral medulla. Cardiovasc Res. 2010;87:578–584. doi: 10.1093/cvr/cvq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11:227–238. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of d-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol. 2010;588:3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Larsson M, Sawada K, Morland C, Hiasa M, Ormel L, Moriyama Y, Gundersen V. Functional and anatomical identification of a vesicular transporter mediating neuronal ATP release. Cereb Cortex. 2012;22:1203–1214. doi: 10.1093/cercor/bhr203. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF, Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci. 2010;30:12466–12473. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott DR, Hirst WD, Ljungberg MC. Astrocytes. In: Cohen J, Wilkin GP, editors. Neural cell culture - a practical approach. Oxford: Oxford UP; 1995. pp. 85–96. [Google Scholar]
- Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13:584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Sorensen CE, Novak I. Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem. 2001;276:32925–32932. doi: 10.1074/jbc.M103313200. [DOI] [PubMed] [Google Scholar]
- Staal RG, Mosharov EV, Sulzer D. Dopamine neurons release transmitter via a flickering fusion pore. Nat Neurosci. 2004;7:341–346. doi: 10.1038/nn1205. [DOI] [PubMed] [Google Scholar]
- Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW. Development of in vivo ventilatory and single chemosensitive neuron responses to hypercapnia in rats. Respir Physiol. 2001;127:135–155. doi: 10.1016/s0034-5687(01)00242-0. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvey MR, Thorn P. Lysine-fixable dye tracing of exocytosis shows F-actin coating is a step that follows granule fusion in pancreatic acinar cells. Pflugers Arch. 2004;448:552–555. doi: 10.1007/s00424-004-1288-z. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- Wenker IC, Sobrinho CR, Takakura AC, Moreira TS, Mulkey DK. Regulation of ventral surface CO2/H+-sensitive neurons by purinergic signaling. J Physiol. 2012;590:2137–2150. doi: 10.1113/jphysiol.2012.229666. [DOI] [PMC free article] [PubMed] [Google Scholar]