Abstract
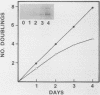
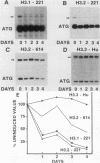
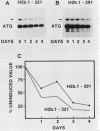
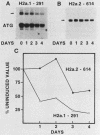
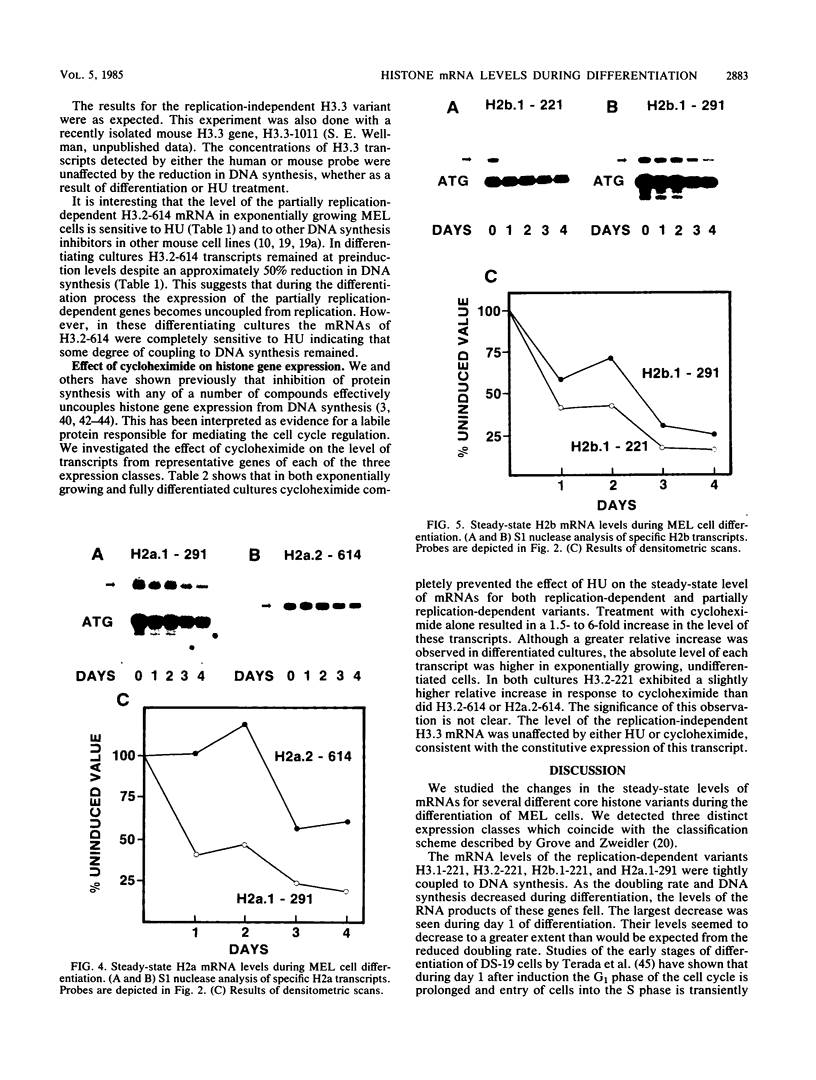
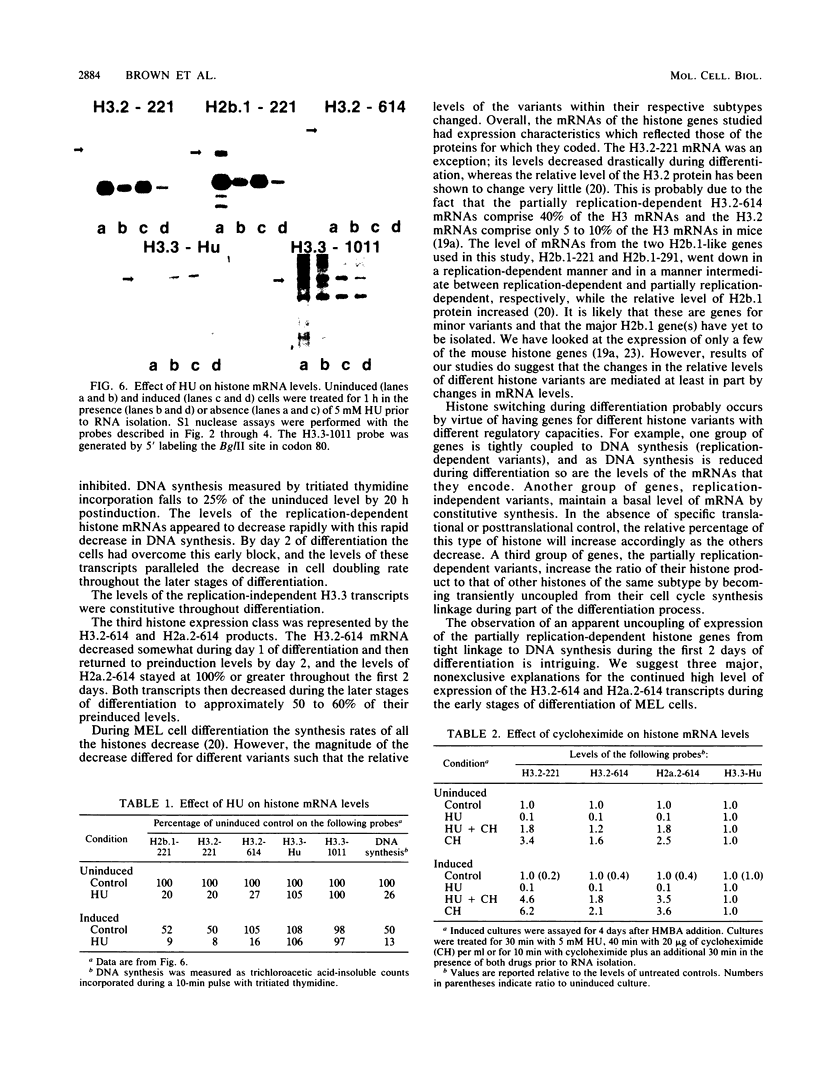
We used a gene-specific S1 nuclease assay to study the changes in steady-state mRNA levels of several core histone variants during the differentiation of murine erythroleukemia cells. These studies allowed us to distinguish three distinct expression classes of histone genes. The expression of the major replication-dependent class of histone genes was tightly linked to DNA synthesis. The concentrations of these transcripts decreased rapidly as cell division slowed during the process of differentiation. In contrast, the replication-independent H3.3 transcript levels were constitutively maintained throughout differentiation and were unaffected by inhibitors of DNA or protein synthesis. We also identified among the cloned histone genes used as probes a third expression class, the partially replication-dependent variants. Expression of these transcripts became transiently uncoupled from the reduced rate of DNA synthesis accompanying the early stages of differentiation. We show that their synthesis is sensitive to the DNA synthesis inhibitor hydroxyurea but that selective uncoupling from DNA synthesis of these histone mRNAs occurs at a specific stage of differentiation. We present several hypotheses to explain how this might be accomplished. The expression characteristics of the mRNAs studied coincided with those of the proteins for which they code, indicating that changes in the relative levels of the different variants is mediated at least in part by changes in mRNA levels.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alterman R. B., Ganguly S., Schulze D. H., Marzluff W. F., Schildkraut C. L., Skoultchi A. I. Cell cycle regulation of mouse H3 histone mRNA metabolism. Mol Cell Biol. 1984 Jan;4(1):123–132. doi: 10.1128/mcb.4.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artishevsky A., Delegeane A. M., Lee A. S. Use of a cell cycle mutant to delineate the critical period for the control of histone mRNA levels in the mammalian cell cycle. Mol Cell Biol. 1984 Nov;4(11):2364–2369. doi: 10.1128/mcb.4.11.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach L. L., Marashi F., Plumb M., Stein G., Stein J. Inhibition of DNA replication coordinately reduces cellular levels of core and H1 histone mRNAs: requirement for protein synthesis. Biochemistry. 1984 Apr 10;23(8):1618–1625. doi: 10.1021/bi00303a006. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Blankstein L. A., Levy S. B. Changes in histone f2a2 associated with proliferation of Friend leukaemic cells. Nature. 1976 Apr 15;260(5552):638–640. doi: 10.1038/260638a0. [DOI] [PubMed] [Google Scholar]
- Blankstein L. A., Stollar B. D., Franklin S. G., Zweidler A., Levy S. B. Biochemical and immunological characterization of two distinct variants of histone H2A in Friend leukemia. Biochemistry. 1977 Oct 18;16(21):4557–4562. doi: 10.1021/bi00640a003. [DOI] [PubMed] [Google Scholar]
- Borun T. W., Gabrielli F., Ajiro K., Zweidler A., Baglioni C. Further evidence of transcriptional and translational control of histone messenger RNA during the HeLa S3 cycle. Cell. 1975 Jan;4(1):59–67. doi: 10.1016/0092-8674(75)90134-8. [DOI] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Rapidly labeled, polyribosome-associated RNA having the properties of histone messenger. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1977–1983. doi: 10.1073/pnas.58.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. B., Mueller G. C. Control of histone synthesis in HeLa cells. Biochim Biophys Acta. 1973 Feb 4;294(1):481–496. doi: 10.1016/0005-2787(73)90104-4. [DOI] [PubMed] [Google Scholar]
- DeLisle A. J., Graves R. A., Marzluff W. F., Johnson L. F. Regulation of histone mRNA production and stability in serum-stimulated mouse 3T6 fibroblasts. Mol Cell Biol. 1983 Nov;3(11):1920–1929. doi: 10.1128/mcb.3.11.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke S., Lichtler A., Phillips I., Stein J., Stein G. Reassessment of histone gene expression during cell cycle in human cells by using homologous H4 histone cDNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4995–4999. doi: 10.1073/pnas.76.10.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. D., Sugarman B. J., Dodgson J. B. A chicken histone H3 gene contains intervening sequences. Nature. 1982 Jun 3;297(5865):434–436. doi: 10.1038/297434a0. [DOI] [PubMed] [Google Scholar]
- Franklin S. G., Zweidler A. Non-allelic variants of histones 2a, 2b and 3 in mammals. Nature. 1977 Mar 17;266(5599):273–275. doi: 10.1038/266273a0. [DOI] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz D., Mueller G. C. Histone synthesis in vitro on HeLa cell microsomes. The nature of the coupling to deoxyribonucleic acid synthesis. J Biol Chem. 1969 Nov 10;244(21):5947–5952. [PubMed] [Google Scholar]
- Garrett C., Kredich N. M. Induction of hemoglobin synthesis by xylosyladenine in murine erythroleukemia cells. Metabolism of xylosyladenine and effects on transmethylation. J Biol Chem. 1981 Dec 25;256(24):12705–12709. [PubMed] [Google Scholar]
- Geller R., Levenson R., Housman D. Significance of the cell cycle in commitment of murine erythroleukemia cells to erythroid differentiation. J Cell Physiol. 1978 May;95(2):213–222. doi: 10.1002/jcp.1040950211. [DOI] [PubMed] [Google Scholar]
- Graves R. A., Marzluff W. F. Rapid reversible changes in the rate of histone gene transcription and histone mRNA levels in mouse myeloma cells. Mol Cell Biol. 1984 Feb;4(2):351–357. doi: 10.1128/mcb.4.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves R. A., Wellman S. E., Chiu I. M., Marzluff W. F. Differential expression of two clusters of mouse histone genes. J Mol Biol. 1985 May 25;183(2):179–194. doi: 10.1016/0022-2836(85)90211-6. [DOI] [PubMed] [Google Scholar]
- Grove G. W., Zweidler A. Regulation of nucleosomal core histone variant levels in differentiating murine erythroleukemia cells. Biochemistry. 1984 Sep 11;23(19):4436–4443. doi: 10.1021/bi00314a030. [DOI] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo-Kemenes T., Hörz W., Zachau H. G. Chromatin. Annu Rev Biochem. 1982;51:89–121. doi: 10.1146/annurev.bi.51.070182.000513. [DOI] [PubMed] [Google Scholar]
- Jacob E. Histone-gene reiteration in the genome of mouse. Eur J Biochem. 1976 May 17;65(1):275–284. doi: 10.1111/j.1432-1033.1976.tb10415.x. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Marzluff W. F., Jr, Murphy E. C., Jr, Huang R. C. Transcription of ribonucleic acid in isolated mouse myeloma nuclei. Biochemistry. 1973 Aug 28;12(18):3440–3446. doi: 10.1021/bi00742a013. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- Nudel U., Salmon J., Fibach E., Terada M., Rifkind R., Marks P. A., Bank A. Accumulation of alpha- and beta-globin messenger RNAs in mouse erythroleukemia cells. Cell. 1977 Oct;12(2):463–469. doi: 10.1016/0092-8674(77)90122-2. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Tanaka M., Terada M., Miller O. J., Bank A., Marks P., Rifkind R. A. Erythroid cell differentiation: murine erythroleukemia cell variant with unique pattern of induction by polar compounds. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1232–1236. doi: 10.1073/pnas.73.4.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old R. W., Woodland H. R. Histone genes: not so simple after all. Cell. 1984 Oct;38(3):624–626. doi: 10.1016/0092-8674(84)90256-3. [DOI] [PubMed] [Google Scholar]
- Reuben R. C., Wife R. L., Breslow R., Rifkind R. A., Marks P. A. A new group of potent inducers of differentiation in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):862–866. doi: 10.1073/pnas.73.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond T. J., Finch J. T., Rushton B., Rhodes D., Klug A. Structure of the nucleosome core particle at 7 A resolution. Nature. 1984 Oct 11;311(5986):532–537. doi: 10.1038/311532a0. [DOI] [PubMed] [Google Scholar]
- Rickles R., Marashi F., Sierra F., Clark S., Wells J., Stein J., Stein G. Analysis of histone gene expression during the cell cycle in HeLa cells by using cloned human histone genes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):749–753. doi: 10.1073/pnas.79.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche J., Gorka C., Goeltz P., Lawrence J. J. Association of histone H1(0) with a gene repressed during liver development. Nature. 1985 Mar 14;314(6007):197–198. doi: 10.1038/314197a0. [DOI] [PubMed] [Google Scholar]
- Rougeon F., Mach B. Cloning and amplification of alpha and beta mouse globin gene sequences synthesised in vitro. Gene. 1977 May;1(3-4):229–239. doi: 10.1016/0378-1119(77)90047-6. [DOI] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Sheffery M., Krauter K., Darnell J. E., Jr, Rifkind R., Marks P. A. Induced transcription of the mouse beta-globin transcription unit in erythroleukemia cells. Time-course of induction and of changes in chromatin structure. J Mol Biol. 1984 Feb 5;172(4):437–450. doi: 10.1016/s0022-2836(84)80016-9. [DOI] [PubMed] [Google Scholar]
- Sheffery M., Marks P. A., Rifkind R. A. Gene expression in murine erythroleukemia cells. Transcriptional control and chromatin structure of the alpha 1-globin gene. J Mol Biol. 1984 Feb 5;172(4):417–436. doi: 10.1016/s0022-2836(84)80015-7. [DOI] [PubMed] [Google Scholar]
- Sittman D. B., Chiu I. M., Pan C. J., Cohn R. H., Kedes L. H., Marzluff W. F. Isolation of two clusters of mouse histone genes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4078–4082. doi: 10.1073/pnas.78.7.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Structure of a cluster of mouse histone genes. Nucleic Acids Res. 1983 Oct 11;11(19):6679–6697. doi: 10.1093/nar/11.19.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive H. L., Heintz N., Roeder R. G. Regulation of human histone gene expression during the HeLa cell cycle requires protein synthesis. Mol Cell Biol. 1984 Dec;4(12):2723–2734. doi: 10.1128/mcb.4.12.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Gallwitz D. Fate of histone messenger RNA in synchronized HeLa cells in the absence of initiation of protein synthesis. Eur J Biochem. 1977 Jan;72(2):385–392. doi: 10.1111/j.1432-1033.1977.tb11263.x. [DOI] [PubMed] [Google Scholar]
- Stimac E., Groppi V. E., Jr, Coffino P. Inhibition of protein synthesis stabilizes histone mRNA. Mol Cell Biol. 1984 Oct;4(10):2082–2090. doi: 10.1128/mcb.4.10.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada M., Fried J., Nudel U., Rifkind R. A., Marks P. A. Transient inhibition of initiation of S-phase associated with dimethyl sulfoxide induction of murine erythroleukemia cells to erythroid differentiation. Proc Natl Acad Sci U S A. 1977 Jan;74(1):248–252. doi: 10.1073/pnas.74.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. Histone-H1-dependent chromatin superstructures and the suppression of gene activity. Cell. 1984 Aug;38(1):17–27. doi: 10.1016/0092-8674(84)90522-1. [DOI] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]
- Wells D., Kedes L. Structure of a human histone cDNA: evidence that basally expressed histone genes have intervening sequences and encode polyadenylylated mRNAs. Proc Natl Acad Sci U S A. 1985 May;82(9):2834–2838. doi: 10.1073/pnas.82.9.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. S., Bonner W. M. Separation of basal histone synthesis from S-phase histone synthesis in dividing cells. Cell. 1981 Dec;27(2 Pt 1):321–330. doi: 10.1016/0092-8674(81)90415-3. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Tsai S., Bonner W. M. Changes in histone H3 composition and synthesis pattern during lymphocyte activation. Biochemistry. 1983 Aug 2;22(16):3868–3873. doi: 10.1021/bi00285a023. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Tsai S., Bonner W. M. Patterns of histone variant synthesis can distinguish G0 from G1 cells. Cell. 1982 Dec;31(2 Pt 1):367–374. doi: 10.1016/0092-8674(82)90130-1. [DOI] [PubMed] [Google Scholar]