Abstract
Background and Aims
The legume clade Lotononis sensu lato (s.l.; tribe Crotalarieae) comprises three genera: Listia, Leobordea and Lotononis sensu stricto (s.s.). Listia species are symbiotically specific and form lupinoid nodules with rhizobial species of Methylobacterium and Microvirga. This work investigated whether these symbiotic traits were confined to Listia by determining the ability of rhizobial strains isolated from species of Lotononis s.l. to nodulate Listia, Leobordea and Lotononis s.s. hosts and by examining the morphology and structure of the resulting nodules.
Methods
Rhizobia were characterized by sequencing their 16S rRNA and nodA genes. Nodulation and N2 fixation on eight taxonomically diverse Lotononis s.l. species were determined in glasshouse trials. Nodules of all hosts, and the process of infection and nodule initiation in Listia angolensis and Listia bainesii, were examined by light microscopy.
Key Results
Rhizobia associated with Lotononis s.l. were phylogenetically diverse. Leobordea and Lotononis s.s. isolates were most closely related to Bradyrhizobium spp., Ensifer meliloti, Mesorhizobium tianshanense and Methylobacterium nodulans. Listia angolensis formed effective nodules only with species of Microvirga. Listia bainesii nodulated only with pigmented Methylobacterium. Five lineages of nodA were found. Listia angolensis and L. bainesii formed lupinoid nodules, whereas nodules of Leobordea and Lotononis s.s. species were indeterminate. All effective nodules contained uniformly infected central tissue. Listia angolensis and L. bainesii nodule initials occurred on the border of the hypocotyl and along the tap root, and nodule primordia developed in the outer cortical layer. Neither root hair curling nor infection threads were seen.
Conclusions
Two specificity groups occur within Lotononis s.l.: Listia species are symbiotically specific, while species of Leobordea and Lotononis s.s. are generally promiscuous and interact with rhizobia of diverse chromosomal and symbiotic lineages. The seasonally waterlogged habitat of Listia species may favour the development of symbiotic specificity.
Keywords: Crotalarieae, Lotononis s.l., Listia, Leobordea, nodulation, nodule structure, symbiotic specificity, rhizobia, Methylobacterium, Microvirga
INTRODUCTION
The basal legume tribe Crotalarieae is the largest tribe of papilionoid legumes within Africa and also the largest tribe within the genistoid alliance, comprising some 51 % of this legume group (Boatwright et al., 2008). Although the rhizobial nodulation database in GRIN (http://www.ars-grin.gov/~sbmljw/cgi-bin/taxnodul.pl) has reported nearly 550 nodulated species within Crotalarieae, very few studies have examined their nodule structure or have characterized the root nodule bacteria (rhizobia) associated with this tribe. Indeterminate nodules with uniformly infected central tissue appear to be a characteristic feature of species in tribe Crotalarieae, and of the genistoid clade within which it is placed (Sprent, 2009). Uniformly infected central tissue is also a feature of the determinate nodules found in the dalbergioid clade (Lavin et al., 2001). In species belonging to these more basal legume clades, rhizobial infection occurs via epidermal entry rather than by root hair curling, and infection threads are not formed (Sprent and James, 2007).
Previous reports suggest that Crotalarieae are nodulated by a wide diversity of rhizobia. Several species of Crotalaria growing in Senegal are specifically nodulated by strains of Methylobacterium nodulans (Sy et al., 2001), whereas some other Crotalaria species are nodulated by bradyrhizobia (Moulin et al., 2004; Renier et al., 2008). More recently, novel rhizobial taxa of Burkholderia have been isolated from root nodules of the crotalarioid legume Lebeckia ambigua collected from the South African Cape fynbos region (Howieson et al., 2013). The acid, infertile soils of the fynbos appear to support a diverse population of Papilionoideae-nodulating Burkholderia rhizobia (Elliott et al., 2007; Garau et al., 2009; Gyaneshwar et al., 2011). Lebeckia has recently been divided into three genera: Lebeckia sensu stricto (s.s.), Calobota and Wiborgiella (Boatwright et al., 2009). While Burkholderia rhizobia nodulate and fix nitrogen with the acicular-leaved fynbos species L. ambigua and L. sepiaria (Howieson et al., 2013), strains of Bradyrhizobium, Mesorhizobium and Sinorhizobium (syn. Ensifer) are reported to nodulate other species within Lebeckia sensu lato (s.l.; Phalane et al., 2008).
The other major Crotalarieae group which has been studied for nitrogen-fixing symbiotic associations is Lotononis s.l. This clade has a centre of origin in South Africa and consists of approx. 150 species, originally divided into 15 taxonomic sections (van Wyk, 1991; Boatwright et al., 2011). In a recent taxonomic revision, the three distinct clades within Lotononis s.l. are now recognized at the generic level as Listia, Leobordea and Lotononis s.s. (Boatwright et al., 2011).
The genus Listia consists of seven species of herbaceous perennials: L. angolensis, L. bainesii, L. heterophylla (previously Lotononis listii), L. marlothii, L. minima, L. solitudinis and L. subulata (Boatwright et al., 2011). Listia angolensis has a tropical distribution (in the uplands encircling the Zaire basin), while L. heterophylla extends from South Africa into southern Central Africa, L. bainesii is native to Botswana, Mozambique (south), Namibia and South Africa, and the remaining species are endemic to South Africa (van Wyk, 1991). Listia species are distinguished by a stoloniferous habit, which is thought to be associated with their seasonally wet habitats (van Wyk, 1991; Boatwright et al., 2011) (although they are also drought tolerant), and the formation of lupinoid root nodules (Yates et al., 2007). In contrast, field observations have indicated that Leobordea and Lotononis s.s. species have indeterminate nodules (J. Howieson and R. Yates, unpubl. data). Species of these two genera are annuals or perennials and are mostly endemic to South Africa, although a few species have a wider distribution in Africa, or extend into the Mediterranean and to Asia (van Wyk, 1991).
Renewed interest in the potential of Listia (and other Lotononis s.l. species) as perennial pasture plants in southern Australian agricultural systems has prompted recent research into their associated rhizobia (Yates et al., 2007; Howieson et al., 2008). Most previous studies have been conducted on L. bainesii, which is recognized as a model of strict symbiotic specificity (Pueppke and Broughton, 1999), able to nodulate only with red-pigmented, narrow host range rhizobia (Norris, 1958), although Norris did not test L. bainesii with any rhizobial strains derived from other Lotononis s.l. hosts. The pigmented rhizobia isolated from L. bainesii and other Listia hosts have since been identified as strains of Methylobacterium sp. (Jaftha et al., 2002; Yates et al., 2007) that are unable to utilize methanol (Ardley et al., 2009).
These pigmented methylobacteria have now been isolated from L. bainesii, L. heterophylla, L. marlothii, L. solitudinis and L. subulata, and are effective for nitrogen fixation on all studied Listia species except L. angolensis, which forms ineffective (i.e. non-fixing) nodules with these rhizobia (Yates et al., 2007; R. Yates, unpubl. data). Instead, L. angolensis is effectively nodulated by species of Microvirga (Ardley et al., 2012). There has as yet been no detailed characterization of the rhizobia associated with Leobordea and Lotononis s.s., although studies conducted on various Lotononis s.l. hosts by the CSIRO in Queensland, Australia indicate that their rhizobial isolates were phenotypically diverse (Eagles and Date, 1999).
Rhizobial Methylobacterium and Microvirga species are uncommon microsymbionts, as the majority of legumes nodulate with strains of Bradyrhizobium, Mesorhizobium, Rhizobium and Sinorhizobium (Lindström et al., 2010). Do Listia species form symbioses only with these unusual rhizobia or can they be nodulated by the rhizobial species that are associated with the other genera that comprise Lotononis s.l.? Has the evolution and diversification of Lotononis s.l. species within their centre of origin resulted in their recruitment of different microsymbiont lineages and, if so, what factors might influence the patterns of symbiotic association found in this legume group?
As part of the development of novel perennial pasture legumes and associated rhizobia that are adapted to the arid climate and acid, infertile soils found in Western Australian agricultural systems, the Centre for Rhizobium Studies (CRS) undertook collections of nodules and seeds from a range of Lotononis s.l. species growing in diverse sites in South Africa. This collection of legume germplasm and rhizobia, together with Microvirga strains isolated from L. angolensis hosts, was used to examine symbiotic relationships and specificity within Lotononis s.l. In addition, we present details of nodule morphology and structure in Listia, Leobordea and Lotononis s.s. species, and the processes of infection and nodule initiation in L. angolensis and L. bainesii.
MATERIALS AND METHODS
Rhizobial strains and plant material
The Lotononis s.l. rhizobia used in this study are listed in Table 1, together with details of their host plant and the collection site. The host species are described according to their position within the Lotononis s.l. taxonomic groupings given by van Wyk (1991) and Boatwright et al. (2011), where this was known. The bioregion of the South African collection sites was described according to Mucina and Rutherford (2006). As the important research question for us was to determine symbiotic relationships and specificities within Lotononis s.l., rather than to perform a comprehensive survey of the rhizobia that nodulate this group of legumes, all strains used were authenticated sub-sets of a wider collection of nodule isolates obtained from South African or Zambian Lotononis s.l. species. Strain WSM2598 was selected from 67 pink-pigmented methylobacterial isolates obtained from nodules of South African Listia bainesii, L. heterophylla and L. solitudinis, which were all authenticated as effective for N2 fixation on L. bainesii (R. Yates, unpubl. data). Strain WSM3557T is the type strain of the novel rhizobial species Microvirga lotononidis (Ardley et al., 2012). It was selected for this study from seven L. angolensis strains, derived from strains housed in the CB Strain Collection (Eagles and Date, 1999), due to its high symbiotic effectiveness on L. angolensis (Ardley, 2012). The remaining seven strains were isolated from diverse South African Leobordea and Lotononis s.s. species. All had been authenticated as root nodule bacteria, but did not form nodules on L. bainesii or L. heterophylla (Ardley, 2012; R. Yates, unpubl. data).
Table 1.
Rhizobial strains used in this study
| Strain (synonym) | Identification from 16S rRNA gene sequence | Host* (Lotononis s.l. section) | Geographical location, site and bioregion† | Site pH‡ | Latitude | Longitude | Reference (collector) |
|---|---|---|---|---|---|---|---|
| WSM2596 | Bradyrhizobium sp. | Leobordea pulchra (Lipozygis) | South Africa SA34 Drakensberg Grassland | 5·5 | S29 04 414 | E29 36 491 | This study (Yates, Real and Law) |
| WSM2598 | Methylobacterium sp. | Listia bainesii (Listia) | South Africa SA35 Drakensberg Grassland | 6·0 | S29 01 540 | E29 52 165 | Yates et al. (2007) (Yates, Real and Law) |
| WSM2624 | Mesorhizobium tianshanense | Lotononis s.l. sp. (ND) | South Africa SA20 Upper Karoo | 7·0 | S32 14 082 | E22 55 081 | This study (Yates, Real and Law) |
| WSM2632 | Bradyrhizobium sp. | Lotononis s.l. sp. (ND) | South Africa SA41 Sub-escarpment Savannah | 5·5 | S28 38 902 | E31 55 793 | This study (Yates, Real and Law) |
| WSM2653 | Ensifer meliloti | Lotononis sparsiflora (Oxydium) | South Africa SA22 Upper Karoo | 8·0 | S32 21 109 | E22 36 127 | This study (Yates, Real and Law) |
| WSM2667 | Methylobacterium nodulans | Leobordea calycina (Leptis) | South Africa SA53 Central Bushveld Savannah | 6·0 | S25 35 432 | E28 22 665 | This study (Yates, Real and Law) |
| WSM2783 | Bradyrhizobium sp. | Leobordea carinata (Leptis) | South Africa SA49 Mesic Highveld Grassland | 6·5 | S27 12 979 | E31 10 635 | This study (Yates, Real and Law) |
| WSM3040 | Ensifer meliloti | Lotononis laxa (Oxydium) | South Africa SA14 Upper Karoo | 6·5 | S32 11 075 | E23 55 631 | This study (Yates, Real and Law) |
| WSM3557T (CB1322) | Microvirga lotononidis | Listia angolensis (Listia) | Chipata§, Zambia ND¶ | ND | S 13·65 | E 32·63 | Eagles and Date (1999), Ardley et al. (2012) (Verboom) |
*According to the taxonomy of van Wyk (1991) and Boatwright et al. (2011).
†Bioregion according to Mucina and Rutherford (2006).
‡pH measured with a kit.
§Formerly known as Fort Jameson.
¶Likely to have been woodland savannah, consisting of a grass cover 1–2 m high and hardwood trees and/or shrubs, interspersed with grassy wetlands, according to vegetation maps of the area.
ND, not determined.
Additional rhizobial strains inoculated onto L. angolensis, L. bainesii and L. heterophylla are listed in Supplementary Data Table S1.
Root nodules were collected from field sites in South Africa and desiccated in situ for transport back to the CRS laboratories in Australia (Yates et al., 2004). The desiccated nodules were then re-imbibed in distilled water, surface sterilized and rhizobia were subsequently isolated (Yates et al., 2004). Strains were routinely sub-cultured on modified ½ lupin agar (½ LA) (Yates et al., 2007), or on ½ LA with succinate replacing glucose and mannitol as a carbon source, or on TY agar (Beringer, 1974). All strains were stored at –80 °C in media supplemented with 12 % (v/v) glycerol.
The Lotononis s.l. species used in this study are listed in Table 2, along with details of their taxonomy and distribution. Seeds of L. angolensis, L. bainesii and L. heterophylla were obtained from the Department of Agriculture and Food Western Australia and the CRS. Cultivar names or line numbers for these species are also given in Table 2. Seed for all other Lotononis s.l. species was collected from wild plants growing at various sites in South Africa.
Table 2.
List of host plants used in this study and their distribution in southern Africa
| Glasshouse experiment |
||||||
|---|---|---|---|---|---|---|
| Genus (Lotononis s.l. section) | Species (accession no./cultivar) | Distribution† | 1 | 2 | 3 | 4 |
| Listia (Listia) | Listia angolensis (8363) | Tropical central Africa | * | * | * | |
| Listia bainesii (Miles) | Eastern and central southern Africa | * | * | * | ||
| Listia heterophylla (2004 CRSL69) | Eastern and central southern Africa | * | ||||
| Leobordea (Digitata) | Leobordea longiflora | Dry mountainous regions of the north-west Cape | * | |||
| Leobordea (Leptis) | Leobordea mollis | Western Cape of South Africa | * | |||
| Leobordea (Leobordea) | Leobordea platycarpa | Drier areas of southern Africa | * | |||
| Leobordea stipulosa | North-eastern interior of southern Africa | * | ||||
| Leobordea (Synclistus) | Leobordea bolusii | Western and central South Africa | * | |||
| Leobordea polycephala | Western and central South Africa | * | ||||
| Lotononis s.s. (Oxydium) | Lotononis crumanina | Limestone or lime-rich soils in central southern Africa | * | |||
| Lotononis delicata | ND | * | ||||
| Lotononis falcata | Widespread in southern Africa | * | ||||
| Lotononis laxa | Widespread along the east coast of southern Africa | * | ||||
| Lotononis s.s. (Cleistogama) | Lotononis pungens | Central South Africa | * | |||
Accession numbers or cultivars, where relevant, are given in parenthes.
†Species distribution is according to van Wyk (1991).
ND, not determined.
General glasshouse procedures
Rhizobial strains were assessed for their capacity to nodulate and fix N2 with legume hosts using an axenic sand culture system (Howieson et al., 1995; Yates et al., 2004). Briefly, free-draining pots (1 kg) were lined with absorbent paper, filled with a 3:2 mix of yellow sand and washed river sand, moistened with deionized (DI) water and then sterilized by steam treatment or autoclaving. Each pot was flushed twice with hot, sterile DI water to remove inorganic nitrogen. Sterile polyvinyl chloride tubes (25 mm diameter) with lids were inserted into the sand mix for supply of water and nutrients. To maintain sterility, pots were covered with plastic film until inoculation and, post-inoculation, the soil surface was covered with sterile alkathene beads. Pots were watered as required with sterile DI water. Sterile nitrogen-free nutrient solution (20 mL) (Howieson et al., 1995) was supplied weekly to each pot. N+ controls received 5 mL of 0·1 m KNO3 per pot weekly. To evaluate Listia symbiotic specificity further, a closed vial system was used, with screw-topped polycarbonate vials (500 mL) that contained sterile mixed sand medium (400 g) and DI water (50 mL) (Yates et al., 2004). Each vial had a single dose of nutrient solution (20 mL) added at the time of planting. Seeds were surface sterilized and germinated as described by Yates et al. (2007), and aseptically sown into pots or vials when the radicles were 1–3 mm in length. Plants were inoculated with rhizobial cell suspensions (1 mL per plant, containing approx. 3·0 × 107–1·0 × 109 live cells mL−1) (Yates et al., 2007) and were grown in a naturally lit, controlled-temperature (maximum 24 °C) glasshouse.
Glasshouse experiment 1: nodulation studies
Before being included in a large-scale experiment to determine nodulation and N2 fixation with Lotononis s.l. hosts, the seven Leobordea and Lotononis s.s. isolates were assessed for their ability to nodulate a range of Leobordea and Lotononis s.s. species. Pots containing up to two seedlings of a given species were inoculated with a single rhizobial strain, and an uninoculated nitrogen-free control was included in the treatments. Because of the scarcity of host germplasm, only 1–4 plants of each species were included in the trial. Plants were assessed for nodulation, which was deemed to be effective if the plants were greener than the uninoculated control and the nodules were pink in colour.
Glasshouse experiment 2: nodulation and N2 fixation tests
Nodulation and N2-fixing capabilities of the nine Lotononis s.l. rhizobia on eight Listia, Leobordea or Lotononis s.s. species were determined using three replicate pots per treatment, with uninoculated nitrogen-free and supplied nitrogen (N+) treatments as controls. Each pot was sown with up to six germinated seeds and then thinned to four seedlings 3 weeks after planting (except for L. stipulosa, which produced insufficient germinated seeds). Because seed of L. bolusii was scarce, tip cuttings were taken from pot-grown plants maintained in the glasshouse. The cuttings (approx. 6 cm long) were stripped of their lower leaves, placed in DI water, then transferred to sodium hypochlorite (1 % w/v; 1 min) and rinsed twice in sterile DI water. The cut end was then dipped in striking powder [active ingredient 4-indol-3-yl butyric acid (0·3 % w/w)] and aseptically planted, with four cuttings per pot. Pots with cuttings were supplied as required with sterile DI water for the first 3 weeks, and then given a single dose (20 mL) of nutrient solution containing 0·25 g l−1 KNO3, to aid establishment. The rooted cuttings were subsequently supplied with sterile DI water and weekly doses of sterile nitrogen-free nutrient solution (20 mL). Pots containing germinated seeds were inoculated after the seedlings had emerged (7–10 d after sowing, depending on the growth rates of the different Lotononis s.l. species). Cuttings were inoculated 6 weeks after planting.
Glasshouse experiment 3: further evaluation of symbiotic specificity within Listia
Seedlings of L. angolensis, L. bainesii and L. heterophylla were grown in closed vials and inoculated separately with the rhizobia shown in Supplementary Data Table S1. Treatments were duplicated and an uninoculated control was used.
Glasshouse experiment 4: infection and nodule initiation in Listia angolensis and L. bainesii
Listia angolensis and L. bainesii were inoculated with their respective microsymbiont strains WSM3557T and WSM2598. Plants were grown in the glasshouse, either in pots (using the axenic sand culture system described above) or in gnotobiotic growth pouches (CYG Seed Germination Pouch, Mega International, West St. Paul, MN, USA) (Journet et al., 2001). Three-day-old seedlings (50–60) were incubated with 5 mL of late log phase ½ LA broth cultures (OD600nm = 0·5) for 1 h in sterile Petri dishes and then aseptically planted into growth pouches or pots.
Harvesting and light microscopy
Pot-grown plants were harvested 10 weeks post-inoculation and those in vials 6 weeks post-inoculation. The effectiveness of N2 fixation was determined by measuring the increase in above-ground plant biomass, which was excised, dried at 60 °C and then weighed. Nodules were assessed for colour, number and morphology. Selected nodules were prepared for nodule sectioning and light microscopy (Yates et al., 2007).
For infection and nodule initiation studies, both pot- and pouch-grown plants were harvested at regular intervals. Selected plants were cleared and stained with Brilliant Green to highlight root and nodule initials (O'Hara et al., 1988). Sections of fresh plants that included the hypocotyl and the upper portion of the main root were excised and prepared for sectioning and light microscopy (Yates et al., 2007).
Amplification and sequencing of 16S rRNA and nodA genes
The primers used for DNA amplification and sequencing are described in Table 3. PCR amplification of the nearly full-length 16S rRNA gene was performed using the universal eubacterial primers FGPS6 and FGPS1509 (Normand et al., 1992). DNA template was obtained from whole cells, using fresh plate culture resuspended in 0·89 % (w/v) NaCl to an OD600nm of 2·0. The reaction mixture contained 1 µL of bacterial cells, 5 µL of 5× PCR Polymerisation Buffer (Fisher Biotech), 1·5 mm MgCl2, 1·0 µm of each of the two primers FGPS6 and FGPS1509 and 1 U of Taq DNA polymerase (Invitrogen) in a final volume of 25 µL. Thermal cycling conditions were 5 min at 94 °C, then 35 cycles of 30 s at 94 °C, annealing for 30 s at 55 °C and extension for 1 min at 70 °C, with a final 7 min extension at 72 °C. Amplicons were purified and sequenced as described by Yates et al. (2007), using FGPS6 and FGPS1509 and the internal primers described by Yanagi and Yamasato (1993).
Table 3.
Primers used in this study
| Primer | Sequence | Reference |
|---|---|---|
| 16S rRNA | ||
| FGPS6 | 5'-GGAGAGTTAGATCTTGGCTCAG-3' | Normand et al. (1992) |
| FGPS1509 | 5'-AAGGAGGGGATCCAGCCGCA-3' | Normand et al. (1992) |
| 420F | 5'-GATGAAGGCCTTAGGGTTGT-3' | Yanagi and Yamasato (1993) |
| 800F | 5'-GTAGTCCACGCCGTAAACGA-3' | Yanagi and Yamasato (1993) |
| 1100F | 5'-AAGTCCCGCAACGAGCGCAA-3' | Yanagi and Yamasato (1993) |
| 520R | 5'-GCGGCTGCTGGCACGAAGTT-3' | Yanagi and Yamasato (1993) |
| 920R | 5'- CCCCGTCAATTCCTTTGAGT-3' | Yanagi and Yamasato (1993) |
| 1190R | 5'-GACGTCATCCCCACCTTCCT-3' | Yanagi and Yamasato (1993) |
| 16S-1924r | 5′-GGCACGAAGTTAGCCGGGGC-3′ | Sy et al. (2001) |
| 16S-1080r | 5′-GGGACTTAACCCAACATCT-3′ | Sy et al. (2001) |
| nodA | ||
| M4-46 nodD 89-109f* | 5'-ATTGCGGGCTGGCTTAGGTTG-3' | This study |
| M4-46 nodB 29-48r2* | 5'-CGCGCCAATGACACAGAACG-3' | This study |
| nodA-1 | 5'-TGCRGTGGAARNTRNNCTGGGAAA-3' | Haukka et al. (1998) |
| nodA-2 | 5'-GGNCCGTCRTCRAAWGTCARGTA-3' | Haukka et al. (1998) |
R, A, G; W, A, T; N, all.
*The position of the primer in the corresponding sequence of the Methylobacterium sp. 4-46 target gene.
PCR amplification of the nodA gene of Lotononis s.l. rhizobia was performed using the primers M4-46 nodD 89-109f and M4-46 nodB 29-48r2 (this study) and the nodA primers developed by Haukka et al. (1998). M4-46 nodD 89-109f and M4-46 nodB 29-48r2 were designed based on conserved regions of the nodD and nodB genes in the sequenced genome of the L. bainesii microsymbiont Methylobacterium sp. 4-46. The nodA PCR reaction components were the same as those used for PCR amplification of the 16S rRNA gene, but used nodA primers and 1·0 mm MgCl2. Thermal cycling conditions using the M4-46 nodA primers were 4 min at 94 °C, then 35 cycles of 30 s at 94 °C, annealing for 30 s at 52 °C and extension for 30 s at 70 °C, with a final 5 min extension at 72 °C. The optimized cycling conditions for the nodA-1 and nodA-2 primers were modified slightly from the original conditions cited by Haukka et al. (1998) and consisted of 4 min at 94 °C, then 35 cycles of 45 s at 94 °C, annealing for 45 s at 55 °C and extension for 2 min at 68 °C, with a final 5 min extension at 70 °C. Amplicons were purified and sequenced as for the 16S rRNA gene.
Phylogenetic analyses
Sequences were manually edited using Genetool Lite (version 1·0; Double Twist Inc., Oakland, CA, USA). Searches for sequences with high sequence similarity were conducted using BLASTN (Altschul et al., 1990) against sequences deposited in the National Centre for Biotechnology Information GenBank database. Phylogenetic trees and molecular analyses were determined using MEGA version 5·0 (Tamura et al., 2011). 16S rRNA sequences were trimmed to the same length and aligned. The nodA sequence alignments were based on full-length, or nearly full-length gene sequences.
Phylogenetic trees were generated by Neighbor–Joining (NJ; Saitou and Nei, 1987) maximum likelihood (ML) and parsimony methods, and bootstrapped with 1000 replicates.
RESULTS
Identification of rhizobia associated with Lotononis s.l.
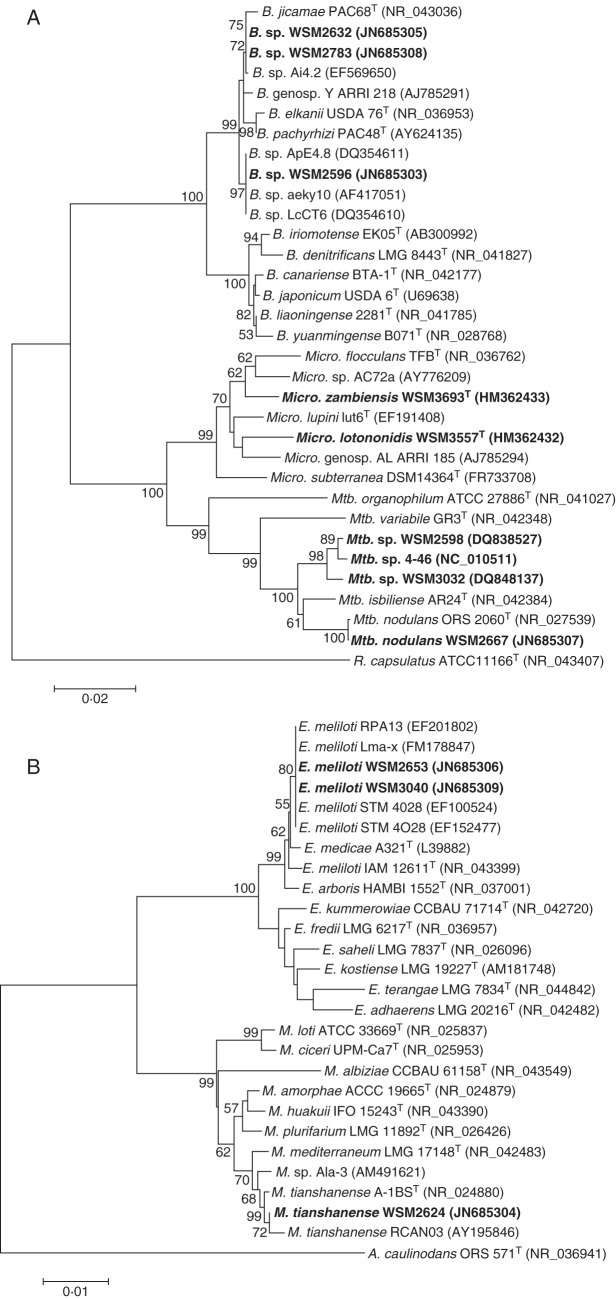
The 16S rRNA gene sequences of WSM2598 and WSM3557T have been described previously (Yates et al., 2007; Ardley et al., 2012). Nearly full-length portions of the 16S rRNA gene were amplified and sequenced for the remaining seven Lotononis s.l. strains. The resulting NJ phylogenetic trees were based on a 1388 bp alignment of the nine Lotononis s.l. rhizobia, closely related strains and reference strains (Fig. 1A, B). Phylogenetic trees reconstructed using maximum-parsimony (MP) or minimal-evolution (ME) methods gave similar topologies.
Fig. 1.
NJ phylogenetic tree, bootstrapped with 1000 replicates, showing the relationships of (A) Bradyrhizobium, Methylobacterium and Microvirga and (B) Ensifer and Mesorhizobium strains isolated from Lotononis s.l. species (in bold type), with related strains and type strains of Bradyrhizobium, Ensifer, Mesorhizobium, Methylobacterium and Microvirga, based on a 1388 bp alignment of the 16S rRNA genes. GenBank accession numbers are in parentheses. Bootstrap values are indicated on branches only when higher than 50 %. Scale bars: (A) = 2 % sequence divergence (two substitutions per 100 nucleotides); (B) = 1 % sequence divergence (one substitution per 100 nucleotides). A., Azorhizobium; B., Bradyrhizobium; E., Ensifer; M., Mesorhizobium; Mtb., Methylobacterium; Micro., Microvirga; R., Rhodobacter; T = type strain.
The rhizobia associated with Lotononis s.l. are remarkably diverse. WSM2598 is a pigmented Methylobacterium sp., most closely related to other pigmented Listia methylobacteria and to Methylobacterium nodulans (Yates et al., 2007). Analysis of the nearly full-length (1416 bp) 16S rRNA gene sequence of WSM2667 also identified this strain as a species of Methylobacterium, with 99·9 % sequence identity with the type strain of M. nodulans ORS 2060T, isolated from Crotalaria podocarpa in Senegal (Sy et al., 2001). WSM3557T is the type strain of Microvirga lotononidis (Methylobacteriaceae) (Ardley et al., 2012). In addition to grouping with the rhizobial species Microvirga lupini (strain Lut6T) and Microvirga zambiensis (strain WSM3693T) and described non-symbiotic species of Microvirga, WSM3557T was also closely related to two authenticated rhizobial strains that grouped within the Microvirga clade (Fig. 1A). These were AC72a, isolated from nodules of Phaseolus vulgaris growing in Ethiopia (Wolde-Meskel et al., 2005), and ARRI 185 (genospecies AL), from northern Australian Indigofera linifolia (Lafay and Burdon, 2007).
Strains WSM2596, WSM2632 and WSM2783 were identified as bradyrhizobia that grouped, with high bootstrap support, within a cluster containing Bradyrhizobium elkanii, B. jicamae and B. pachyrhizae (Fig. 1A), rather than with the cluster of B. japonicum, B. canariense, B. liaoningense and B. yuanmingense strains [Group II and Group I, respectively, according to the phylogeny of Menna and Hungria (2011)]. Within Group II, strain WSM2596 was placed in a lineage that included strains ApE4·8, LcCT6 and aeky10 and was separate from the B. elkanii, B. jicamae and B. pachyrhizae type strains. Strains WSM2632 and WSM2783 had 100 % sequence identity and grouped with strains Ai4·2, ARRI 218 and PAC68T.
Strains WSM2653 and WSM3040 had identical 16S rRNA gene sequences and were closely related to Ensifer (syn. Sinorhizobium) meliloti strains, with 99·7 % sequence identity to the type strain (Fig. 1B). The sequences contained the specific primer sequence that differentiates E. meliloti from E. medicae (Garau et al., 2005). WSM2624 was most closely related to strains of Mesorhizobium tianshanense (Fig. 1B). A 1452 bp fragment of the 16S rRNA gene had sequence identity of 99·8 % with the M. tianshanense type strain A-1BST (Chen et al., 1995; Jarvis et al., 1997).
Amplification and sequencing of nodA
PCR amplification and sequencing using the primers M4-46 nodD 89-109f and M4-46 nodB 29-48r2 yielded sequences of 937 and 965 bp for WSM2598 and WSM2667, respectively. These contained a portion of nodD, the NodD nod box-binding region, the complete nodA gene and a portion of nodB. Both strains had 645 bp nodA genes, which is the same length as the published nodA sequences of the rhizobial methylobacteria strains Methylobacterium sp. 4-46, which nodulates Listia bainesii, and M. nodulans ORS 2060T. The sequence identity of the nodA gene of WSM2598 was <80 % of the WSM2667 nodA gene. The WSM2598 nodA gene had the highest sequence identity to strain 4-46 (99·4 %), while the WSM2667 nodA gene was most closely related to that of M. nodulans ORS 2060T (99·5 %). The proteins deduced from the Methylobacterium nodA gene sequences contained 214 amino acids.
Amplification and sequencing of M. lotononidis WSM3557T nodA has been described previously and gave 100 % sequence identity with a 562 bp portion of nodA of M. zambiensis WSM3693T, which is also a microsymbiont of L. angolensis (Ardley et al., 2012). A partial sequence of the nodA and nodB genes was obtained for WSM2632, WSM2653, WSM2783 and WSM3040 using the primers nodA-1 and nodA-2. No amplification products could be obtained for WSM2596 or WSM2624. The 638 bp sequences of the bradyrhizobial strains WSM2632 and WSM2783 were identical. The 631 bp sequences obtained from the E. meliloti strains WSM2653 and WSM3040 also had 100 % sequence identity. Alignment of a 565 bp nodA fragment showed that the Bradyrhizobium, Ensifer, M. nodulans, pigmented Methylobacterium and Microvirga strains isolated from Lotononis s.l. hosts all possessed distinct nodA sequences, with ≤84·9 % sequence identity between each of these groups. Microvirga lotononidis WSM3557T and the Bradyrhizobium sp. strains WSM2632 and WSM2783 were characterized by the deletion of a nucleotide triplet at positions 460–462 of the bradyrhizobia nodA sequence. This deletion was also a feature of the nodA sequences of the Azorhizobium, Ensifer, Mesorhizobium, Microvirga lupini and Rhizobium reference strains used in the alignment.
The high-quality draft genome sequences of WSM3557T and WSM2783 have recently become available on the Joint Genome Institute (JGI) website (http://img.jgi.doe.gov/cgi-bin/geba/main.cgi), allowing complete nodA sequences to be obtained for these strains. Both had nodA genes of 630 bp, with deduced proteins of 210 amino acids. This length is characteristic of the nodA genes found in the majority of Bradyrhizobium strains and of Burkholderia sp. strain STM678, rather than the shorter length nodA genes of Azorhizobium, Ensifer, Mesorhizobium and Rhizobium, and is due to the presence of codons for additional amino acids at the N-terminal part of the deduced NodA protein (Moulin et al., 2004)
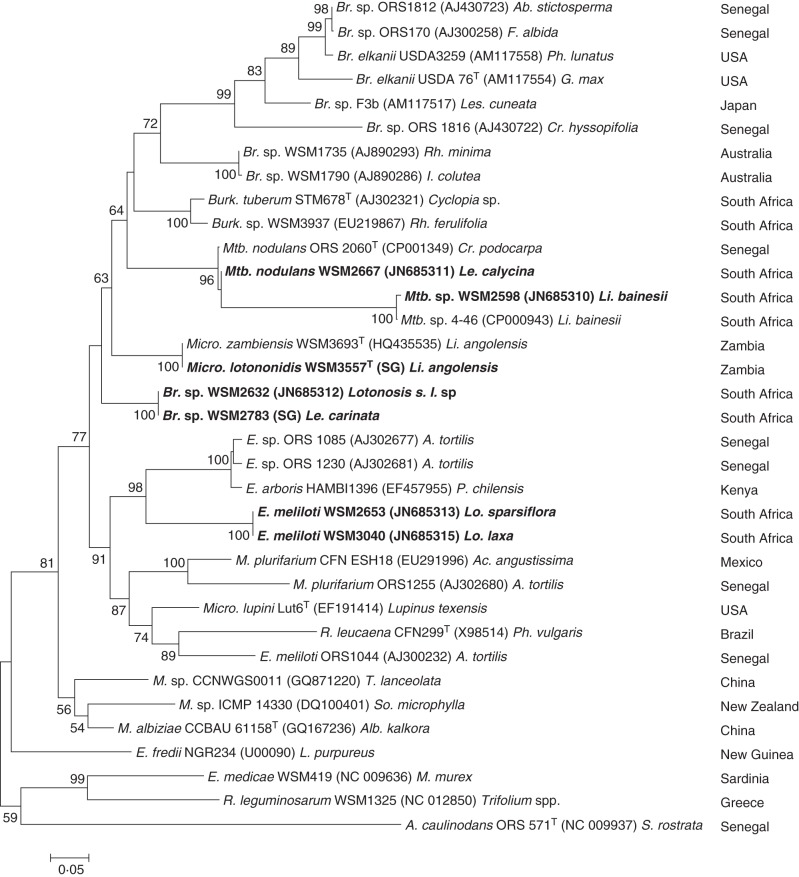
Phylogenetic analyses of the complete or nearly complete nodA sequences of the Lotononis s.l. strains were performed by distance, parsimony and ML methods. Rhizobial strains with high BLASTN nodA sequence similarity and several reference strains were included in the analysis. The resulting trees all had similar topology. The ML phylogenetic tree is shown in Fig. 2. The Lotononis s.l. strains formed five clearly defined clusters that were well supported by high bootstrap values. However, the low values obtained for higher nodes indicated that the position of the clusters within the tree was not well supported and the nodA phylogenetic relationships therefore could not be determined with confidence.
Fig. 2.
ML nodA phylogenetic tree, bootstrapped with 1000 replicates, showing the relationships of the Lotononis s.l.-associated rhizobia (in bold type) and other rhizobia. GenBank accession numbers are in parentheses. Complete nodA gene sequences were used for all strains except WSM2632, WSM2653 and WSM3040. For WSM2783 and WSM3557, complete nodA gene sequences obtained from high-quality draft genome sequences (available on the JGI website, http://img.jgi.doe.gov/cgi-bin/w/main.cgi) were used instead of the nodA sequences deposited in GenBank (accession nos JN685314 and HQ435534, respectively). The original host and the geographical location of the strain are also shown. Scale bar = 5 % sequence divergence (five substitutions per 100 nucleotides). A., Azorhizobium; Br., Bradyrhizobium; Burk., Burkholderia; E., Ensifer; M., Mesorhizobium; Mtb., Methylobacterium; Micro., Microvirga; R., Rhizobium. A., Acacia; Ab., Abrus; Ac., Acaciella; Alb., Albizia; Cr., Crotalaria; F., Faidherbia; G., Glycine; I., Indigofera; L., Lablab; Le., Leobordea; Les., Lespedeza; Li., Listia; Lo., Lotononis; Lu., Lupinus; M., Medicago; P., Prosopis; Ph., Phaseolus; Rh., Rhynchosia; S., Sesbania; So., Sophora; T., Thermopsis; Tr., Trifolium; T = type strain.
Analysis of nodA of the E. meliloti strains WSM2653 and WSM3040 revealed that they formed a sister group to a cluster of African Ensifer strains that nodulate Acacia [syn. Senegalia (Seigler et al., 2006)] and Prosopis (Nick et al., 1999; Ba et al., 2002), with sequence identities ranging from 76 to 78 %. The Lotononis s.l. bradyrhizobia WSM2632 and WSM2783 were in a lineage that was well separated from Bradyrhizobium strains isolated from Australian native legumes (WSM1735 and WSM1790) and from the clade of remaining bradyrhizobia, which included a number of strains isolated from native African legumes. The Microvirga strains WSM3557T and WSM3693T formed a sister group to the Lotononis s.l. bradyrhizobia. The methylobacterial WSM2598 and WSM2667 were included in a clade that contained the other rhizobial Methylobacterium strains ORS 2060T and 4-46, but there was a large divergence between the M. nodulans strains (ORS 2060T and WSM2667) and the strains isolated from Listia bainesii (4-46 and WSM2598).
Glasshouse experiment 1: nodulation studies
Rhizobial strains isolated from Leobordea and Lotononis s.s. hosts were assessed for their ability to nodulate a range of Leobordea and Lotononis s.s. species. All strains were able to nodulate at least one species of either Leobordea or Lotononis s.s., and several were effective for N2 fixation on some species (Supplementary Data Table S2).
Glasshouse experiment 2: nodulation and N2 fixation tests
The symbiotic relationships between Lotononis s.l. species and their associated rhizobia were assessed by measurement of nodulation and N2 fixation of nine strains on eight taxonomically diverse Lotononis s.l. species (Table 4). Quantification of nodulation and N2 fixation of rhizobial strains on the individual Lotononis s.l. species is given in Supplementary Data Fig. S1a–h. All strains were able to nodulate several Lotononis s.l. species. Moreover, all except WSM2624 and WSM2667 were at least partially effective for N2 fixation with one or more of these hosts, i.e. plants were green and larger than the uninoculated control, although shoot biomass was noticeably less than that of N+ control plants. WSM2667, however, is able to form partially effective nodules on Leobordea mollis (Supplementary Data Table S2).
Table 4.
Summary of nodulation and N2 fixation of Lotononis s.l. hosts by rhizobia isolated from Lotononis s.l. species
| Inoculant strain |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bradyrhizobium sp. | Ensifer meliloti | Mesorhizobium tianshanense |
Methylobacterium |
Microvirga lotononidis | ||||||
|
M. sp. |
M. nodulans |
|||||||||
| Host Lotononis s.l. section | Host genus and species | WSM 2596 | WSM 2632 | WSM 2783 | WSM2653 | WSM 3040 | WSM 2624 | WSM 2598 | WSM 2667 | WSM 3557T |
| Listia | Listia angolensis | N– | N– | N– | N– | N– | N– | N + F– | N ± F– | N + F + |
| Listia bainesii | N– | N– | N– | N– | N– | N– | N + F + | N– | N– | |
| Digitata | Leobordea longiflora | N ± F– | N ± F– | N + F– | N ± F– | N– | N ± F– | N + F– | N + F– | N + F– |
| Leobordea | Leobordea platycarpa | N– | N– | N– | N + F ± | N + F– | N– | N– | N– | N ± F ± |
| Leobordea stipulosa | N + F– | N + F– | N + F– | N– | N– | N + F– | N + F– | N + F– | N + F– | |
| Synclistus | Leobordea bolusii | N ± F ± | N + F + | N + F + | N– | N ± F– | N ± F– | N + F– | N ± F– | N ± F ± |
| Oxydium | Lotononis crumanina | N ± F ± | N ± F– | N ± F– | N + F + | N + F + | N ± F– | N– | N– | N + F ± |
| Lotononis falcata | N + F– | N ± F– | N ± F– | N– | N ± F– | N– | N– | N– | N + F– | |
N–, no nodulation; N ± F–, some plants nodulated, but no N2 fixation; N + F–, all plants nodulated, but no N2 fixation; N ± F ± ,. some plants nodulated and some nodulated plants were partially effective for N2 fixation; N + F ± , all plants nodulated and some nodulated plants were effective or partially effective for N2 fixation; N + F + , all plants nodulated and all were effective for N2 fixation.
A notable feature of these symbiotic relationships was the extreme specificity exhibited by L. bainesii (Table 4; Supplementary Data Fig. S1b), which was nodulated only by the pigmented Methylobacterium strain WSM2598. The symbiosis was highly effective, producing plant biomass equivalent to that obtained for the N+ control. Listia angolensis was less specific, as host plants formed ineffective nodules with the methylobacterial strains WSM2598 and WSM2667. Effective nodulation was observed only with M. lotononidis WSM3557T (Table 4; Supplementary Data Fig. S1a).
In contrast, many of the Leobordea and Lotononis s.s. species could be considered promiscuous, as they were nodulated by a range of Lotononis s.l. rhizobia, with no obvious taxonomically based patterns of symbiotic specificity. Leobordea platycarpa, for example, was comparatively specific, being nodulated only by WSM3557T and the Ensifer strains WSM2653 and WSM3040, yet L. stipulosa, in the same taxonomic section (Leobordea) as L. platycarpa, was nodulated by all inoculants except the two Ensifer strains. Nodulation of the Leobordea and Lotononis s.s. species was seldom effective. Moreover, the effectiveness was in most cases only partial, as plants were green but biomass was much smaller than for the N+ control. In several instances, the response to the inoculant strain was variable, as within a treatment some individual plants were effectively nodulated, while in others nodulation was only partially effective or ineffective.
Glasshouse experiment 3: further evaluation of symbiotic specificity within Listia
Inoculation of L. angolensis, L. bainesii and L. heterophylla with phylogenetically diverse rhizobia (Supplementary Data Table S1) confirmed the symbiotic specificity of these species. Listia bainesii and L. heterophylla were nodulated only by WSM2598, and nodulation was always effective. Listia angolensis was effectively nodulated only by WSM3557T, but consistently, or occasionally, formed ineffective nodules with WSM2598 and M. nodulans ORS 2060T, respectively.
Glasshouse experiment 4: infection and nodule initiation in Listia angolensis and L. bainesii
Nodule initials were clearly visible 16–17 days after inoculation (dai) on L. angolensis and L. bainesii plants grown in both pots and growth pouches, most frequently on the border of the hypocotyl just above the root hair zone (Fig. 3), but also further down the taproot. Nodule initials arose in the outer cortex. Lateral root initials could also be seen along the hypocotyl and taproot, but could be distinguished from nodule initials by their connection to the plant stele (Fig. 3). Curled or deformed root hairs were not observed.
Fig. 3.
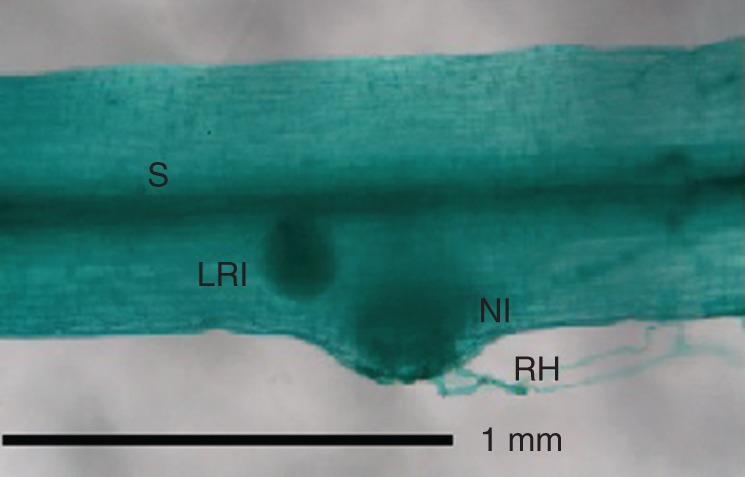
Light micrograph of a cleared and stained Listia bainesii plant, 16 d after inoculation, showing lateral root and nodule initials. Abbreviations: H, hypocotyl; LRI, lateral root initial; NI, nodule initial; RH, root hair; S, stele.
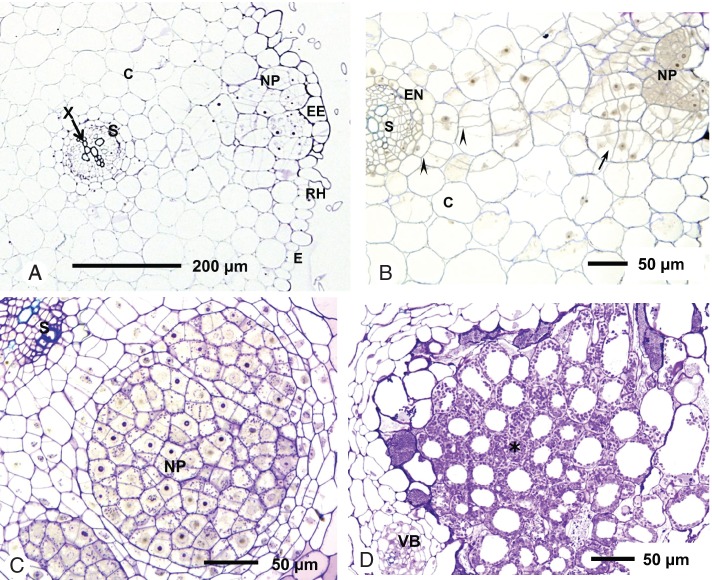
Transverse sections of the hypocotyl of L. angolensis seedlings showed root hairs, although these were not curled or deformed and infection threads were not observed (Fig. 4A). The nodule primordium developed in the outer cortical layer. At 6 dai, there was a proliferation of cells in the outer cortex directly under an enlarged epidermal cell (Fig. 4A, B). Both anticlinal and periclinal cell division was observed, and cells in the infection zone had prominent nuclei and dense cytoplasm. There was no simultaneous division of pericycle cells; rather, cell division appeared to spread from the outer to the inner cortex. A similar pattern of nodule development occurred in L. bainesii. At 10 dai, the central tissue of the nodule primordium was infected with bacteria (Fig. 4C). Infection pockets, caused by the collapse and death of cortical cells, were not seen in either L. angolensis or L. bainesii.
Fig. 4.
Nodule development in Listia species. (A–C) Light micrographs of Listia angolensis and Listia bainesii hypocotyl sections (transverse to the primary root axis) showing development of nodule primordia after inoculation with WSM3557T and WSM2598, respectively. (A) Whole section of L. angolensis, 6 days after inoculation (dai). (B) Section of the same nodule at higher magnification. Cells in the nodule primordium have divided repeatedly and show cytoplasmic staining and nuclei (arrow). Cell division can also be seen in the inner cortex (arrowheads). (C) Section of L. bainesii at 10 dai; cells in the nodule primordium are infected with bacteria. Another locus of infection is visible in the bottom left-hand corner. (D) Light micrograph of a section of a 10-week-old N2-fixing nodule of L. bainesii. The central tissue (*) contains infected cells only. Abbreviations: C, cortex; E, epidermis; EE, enlarged epidermal cell; EN, endodermis; NP, nodule primordium; RH, root hair; S, stele; VB, vascular bundle; X, xylem.
Nodule morphology and structure
Nodule morphology clearly differentiated Listia species from the other Lotononis s.l. taxa. Both L. angolensis and L. bainesii formed lupinoid nodules, primarily on the border of the hypocotyl, just above the root hair zone and on the taproot, whereas nodules of Leobordea and Lotononis s.s. species were indeterminate and distributed throughout the root system. Sections of nodules taken from 10-week-old inoculated Listia, Leobordea and Lotononis s.s. host species were examined by light microscopy to compare their internal structure. All effective nodules contained uniformly infected central tissue with no uninfected interstitial cells, whether from the lupinoid nodules of L. angolensis and L. bainesii (Fig. 4D) or from the indeterminate nodules of Leobordea and Lotononis s.s. species. Infected cells of L. bainesii nodules were highly vacuolated.
DISCUSSION
Nodule morphology and structure as taxonomic markers
Within Lotononis s.l., the distinct differences in nodule morphology suggest that the lupinoid nodule is synapomorphic for the genus Listia. Nodule sections of all the Lotononis s.l. host species had a central mass of infected tissue with no uninfected interstitial cells, as found in nodules of other genistoid legumes (Sprent, 2009).
Infection and nodule initiation in Listia angolensis and L. bainesii
Infection and nodule organogenesis in L. angolensis and L. bainesii appear to be similar to the process observed in the genistoid legumes Lupinus albus and Lupinus angustifolius, where rhizobia penetrate at the junction between epidermal cells and subsequently invade a cortical cell immediately beneath the epidermis (Tang et al., 1992; González-Sama et al., 2004). Unlike the nodules of the dalbergioid legumes Aeschynomene and Stylosanthes or (under waterlogged conditions) the aquatic robinioid legume Sesbania rostrata (Chandler et al., 1982; Alazard and Duhoux, 1990; Lavin et al., 2001; D'Haeze et al., 2003), nodulation in L. angolensis and L. bainesii was not associated with lateral roots, nor did intercellular infection involve the collapse and death of cortical root cells. Neither root hair curling nor infection threads were seen during L. angolensis and L. bainesii nodulation, similar to the pattern observed in rhizobial infection of Lupinus albus (González-Sama et al., 2004) and Crotalaria podocarpa (Renier et al., 2011). The salient features of infection and nodule organogenesis in Listia species – epidermal infection, a lack of infection threads and a central mass of uniformly infected tissue – appear to be characteristic of the basal genistoid and dalbergioid clades (Sprent and James, 2007) and support the proposed taxonomic value and evolutionary significance of infection pathways and nodule structure in legumes (Sprent, 2007).
Rhizobia associated with Lotononis s.l. species are taxonomically diverse
This is the first report and description of N2-fixing microsymbionts of Leobordea and Lotononis s.s. species. Moreover, it demonstrates that Lotononis s.l. species are nodulated by and can form effective N2-fixing symbioses with a remarkable diversity of rhizobia. Some variability in the microsymbiont genotype would be expected, given that South Africa is the centre of diversity for Lotononis s.l. and centres of rhizobial diversity are thought to coincide with those of their legume hosts (Lie et al., 1987; Andronov et al., 2003). Other studies have also reported genotypic diversity in the natural populations of rhizobia that nodulate indigenous legumes (Sylla et al., 2002; Rasolomampianina et al., 2005; Rincón et al., 2008; Lorite et al., 2010; Zhao et al., 2010). What is unusual about the Lotononis s.l. symbiotic associations, however, is first, the wide taxonomic diversity of the rhizobia (belonging to five different genera of Alphaproteobacteria) and, secondly, the very different levels of symbiotic specificity seen within these associations.
Lotononis s.l. appears to have two specificity groups: Listia species are specifically nodulated by unusual Methylobacterium and Microvirga rhizobia, while species of Leobordea and Lotononis s.s. are generally promiscuous and nodulated by species of the classic legume symbionts Bradyrhizobium, Ensifer and Mesorhizobium, in addition to Methylobacterium and Microvirga strains. The small number of isolates from Leobordea and Lotononis s.s. hosts prevents a full examination of the possible biogeographical and symbiotic patterns of these rhizobia. However, the Bradyrhizobium strains isolated from Lotononis s.l. hosts are not closely related to bradyrhizobia from other African legumes (Fig. 1A) (Sylla et al., 2002; Steenkamp et al., 2008; Boulila et al., 2009; Garau et al., 2009), but instead group with isolates from host plants that are phylogenetically and geographically distant from Lotononis s.l. species (Qian et al., 2003; Parker and Kennedy, 2006; Parker, 2008; Ramírez-Bahena et al., 2009). Similarly, M. tianshanense, the species with the highest 16S rRNA sequence identity to WSM2624, was originally described from strains that nodulate diverse Chinese legume hosts (Chen et al., 1995) and was considered to be endemic to the Xinjiang region (Han et al., 2010). It has not previously been reported as associated with African legumes. In contrast, effective strains of E. meliloti have been common isolates of diverse African host species (Ba et al., 2002; Ben Romdhane et al., 2007; Mnasri et al., 2007; León-Barrios et al., 2009; Mnasri et al., 2009; Zurdo-Piñeiro et al., 2009; Fterich et al., 2011; Ourarhi et al., 2011).
Unlike Bradyrhizobium, Ensifer and Mesorhizobium strains, which have been isolated from a wide diversity of legume hosts, rhizobial methylobacteria appear to be associated exclusively with African crotalarioid legumes. Only two lineages have so far been discovered: the pigmented strains from Listia spp. (Jaftha et al., 2002; Yates et al., 2007) and the non-pigmented M. nodulans, which specifically nodulates species of Senegalese Crotalaria (Sy et al., 2001). The isolation of WSM2667 from Leobordea calycina and the demonstration of its ability to nodulate other Lotononis s.l. species is the first report of the extension of the M. nodulans host range to species outside the genus Crotalaria. The Microvirga strain WSM3557T appears to have a broad host range within Lotononis s.l., nodulating all hosts except L. bainesii and fixing N2 with three of these species in addition to its original host.
Rhizobia associated with Lotononis s.l. species have diverse nodA sequences
Several previous studies of legume–rhizobia symbiotic relationships have found a correlation between nod gene phylogeny and host range (Dobert et al., 1994; Haukka et al., 1998; Laguerre et al., 2001; Suominen et al., 2001;Ba et al., 2002; Lu et al., 2009). In other studies, however, this association is not as strong, and nod gene phylogeny is more closely aligned with the rhizobial chromosomal background than with the host (Han et al., 2010; Lorite et al., 2010; Zhao et al., 2010). In the Lotononis s.l. rhizobia, there are five different nodA lineages, each associated with a specific rhizobial chromosomal background and interspersed with nodA from rhizobia isolated from taxonomically diverse hosts (Fig. 2). This indicates, first, that the symbiotic genes of Lotononis s.l. rhizobia were derived from different sources or have evolved divergently and, secondly, that the promiscuous species of Leobordea and Lotononis s.s. do not have stringent requirements for a particular rhizobial nodA genotype. In contrast, the long branch length of the pigmented Methylobacterium nodA gene (Fig 2) suggests an ancient origin of this highly specific symbiosis and possible co-evolution of the two partners.
The 100 % nodA sequence similarity found within strains of similar chromosomal background (Bradyrhizobium strains WSM2632 and WSM2783; Ensifer strains WSM2653 and WSM3040; and Microvirga strains WSM3557T and WSM3693T) suggests horizontal gene transfer of symbiotic loci between closely related rhizobia. The nodA genes of WSM2783 and WSM3557T display features characteristic of both bradyrhizobial nodA [the signature length of the N-terminal segment (Moulin et al., 2004)] and of fast-growing rhizobia (nucleotide triplet deletion).
A model for the development of symbiotic specificity?
Leobordea and Lotononis s.s. species appear to be able to interact (often ineffectively) with rhizobia of diverse chromosomal and symbiotic lineages. (The variable response of individual plants to inoculation may be due to the genetic variability of the wild seed stocks, rather than cultivars, that were used in the experiments.) In contrast, specificity appears to have developed within the genus Listia, whose species associate exclusively with strains of Methylobacterium or Microvirga. The present study has confirmed the extreme symbiotic specificity of L. bainesii, which probably extends to the other host species in the Listia–Methylobacterium cross-inoculation group, and supports Sprent's (2008) association of specificity with effectiveness
The Lotononis s.l. isolates in this study were collected from a diversity of bioregions (Table 1). The genotypic diversity of these rhizobia may be due to topo-edaphic heterogeneity, seen for example in South Africa's Cape Floristic Region, and proposed as a driver of the diversification and speciation of the flora of this region (Cowling et al., 2009). Studies on legumes nodulated by taxonomically diverse rhizobia have found a correlation between eco-regions and/or edaphic factors and the microsymbiont genotype (Garau et al., 2005; Bala and Giller, 2006; Diouf et al., 2007; Han et al., 2009; Lu et al., 2009). For Listia, the adaptation of this genus to seasonally waterlogged habitats (van Wyk, 1991) may have resulted in the selection of microsymbionts that are also adapted to these environments. Members of the genus Methylobacterium are commonly found in water (Green, 1992; Hiraishi et al., 1995; Gallego et al., 2005, 2006) as well as soil and air environments. Microvirga species have similarly been isolated from aquatic or potentially waterlogged environments; additionally, the tolerance of described Microvirga species to relatively high temperatures correlates well with the isolation of non-symbiotic species from thermal waters or sub-tropical regions (Kanso and Patel, 2003; Takeda et al., 2004; Zhang et al., 2009; Weon et al., 2010) and rhizobial Microvirga strains from tropically or sub-tropically distributed hosts (Wolde-Meskel et al., 2005; Lafay and Burdon, 2007; Ardley et al., 2012).
The particular habitat favoured by Listia species may promote the development of symbiotic specificity. In mutualisms, factors that are suggested to align the interests of the symbiont and the host are: vertical transmission of symbionts, genotypic uniformity of symbionts within individual hosts, spatial structure of populations leading to repeated interactions between would-be mutualists, and restricted options outside the relationship for both partners. Conversely, horizontal transmission, multiple symbiont genotypes and varied options decrease symbiotic stability (Herre et al., 1999). The legume–rhizobia symbiosis falls into the latter category (Kiers et al., 2008). The specialized Listia habitat may both reduce the diversity of rhizobial partners available for selection by the host plant and restrict the opportunity for horizontal gene transfer of symbiotic loci (Papke and Ward, 2004), thus contributing to symbiotic isolation. Thrall et al. (2000) have noted that Acacia species with more limited distributions or tighter ecological requirements have a greater degree of specificity than widespread species. In such cases, the selection pressures may favour the co-evolution of the host and microsymbiont towards a more effective and specific symbiosis.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
J.A. is the recipient of a Murdoch University Research Scholarship. We thank Regina Carr and Gordon Thomson (School of Biological Sciences and Biotechnology, Murdoch University) for skilled technical assistance. We gratefully acknowledge Professor Janet Sprent for useful discussions.
LITERATURE CITED
- Alazard D, Duhoux E. Development of stem nodules in a tropical forage legume, Aeschynomene afraspera. Journal of Experimental Botany. 1990;41:1199–1206. [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Andronov EE, Terefework Z, Roumiantseva ML, et al. Symbiotic and genetic diversity of Rhizobium galegae isolates collected from the Galega orientalis gene center in the Caucasus. Applied and Environmental Microbiology. 2003;69:1067–1074. doi: 10.1128/AEM.69.2.1067-1074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardley JK. Symbiotic specificity and nodulation in the southern African legume clade Lotononis s.l. and description of novel rhizobial species within the Alphaproteobacterial genus Microvirga. Murdoch, WA, Australia: Murdoch University; 2012. PhD thesis. [Google Scholar]
- Ardley J, O'Hara G, Reeve W, Yates R, Dilworth M, Tiwari R, Howieson J. Root nodule bacteria isolated from South African Lotononis bainesii, L. listii and L. solitudinis are species of Methylobacterium that are unable to utilize methanol. Archives of Microbiology. 2009;191:311–318. doi: 10.1007/s00203-009-0456-0. [DOI] [PubMed] [Google Scholar]
- Ardley JK, Parker MA, De Meyer SE, et al. Microvirga lupini sp. nov., Microvirga lotononidis sp. nov. and Microvirga zambiensis sp. nov. are alphaproteobacterial root-nodule bacteria that specifically nodulate and fix nitrogen with geographically and taxonomically separate legume hosts. International Journal of Systematic and Evolutionary Microbiology. 2012;62:2579–2588. doi: 10.1099/ijs.0.035097-0. [DOI] [PubMed] [Google Scholar]
- Ba S, Willems A, De Lajudie P, et al. Symbiotic and taxonomic diversity of rhizobia isolated from Acacia tortilis subsp raddiana in Africa. Systematic and Applied Microbiology. 2002;25:130–145. doi: 10.1078/0723-2020-00091. [DOI] [PubMed] [Google Scholar]
- Bala A, Giller KE. Relationships between rhizobial diversity and host legume nodulation and nitrogen fixation in tropical ecosystems. Nutrient Cycling in Agroecosystems. 2006;76:319–330. [Google Scholar]
- Ben Romdhane S, Tajini F, Trabelsi M, Aouani ME, Mhamdi R. Competition for nodule formation between introduced strains of Mesorhizobium ciceri and the native populations of rhizobia nodulating chickpea (Cicer arietinum) in Tunisia. World Journal of Microbiology and Biotechnology. 2007;23:1195–1201. [Google Scholar]
- Beringer JE. R factor transfer in Rhizobium leguminosarum. Journal of General Microbiology. 1974;84:188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Boatwright JS, le Roux MM, Wink M, Morozova T, van Wyk BE. Phylogenetic relationships of tribe Crotalarieae (Fabaceae) inferred from DNA sequences and morphology. Systematic Botany. 2008;33:752–761. [Google Scholar]
- Boatwright JS, Tilney PM, Van Wyk BE. The generic concept of Lebeckia (Crotalarieae, Fabaceae): reinstatement of the genus Calobota and the new genus Wiborgiella. South African Journal of Botany. 2009;75:546–556. [Google Scholar]
- Boatwright JS, Wink M, van Wyk B-E. The generic concept of Lotononis (Crotalarieae, Fabaceae): reinstatement of the genera Euchlora, Leobordea and Listia and the new genus Ezoloba. Taxon. 2011;60:161–177. [Google Scholar]
- Boulila F, Depret G, Boulila A, Belhadi D, Benallaoua S, Laguerre G. Retama species growing in different ecological–climatic areas of northeastern Algeria have a narrow range of rhizobia that form a novel phylogenetic clade within the Bradyrhizobium genus. Systematic and Applied Microbiology. 2009;32:245–255. doi: 10.1016/j.syapm.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Chandler MR, Date RA, Roughley RJ. Infection and root-nodule development in Stylosanthes species by Rhizobium. Journal of Experimental Botany. 1982;33:47–57. [Google Scholar]
- Chen WX, Wang E, Wang SY, Li YB, Chen XQ, Li YB. Characteristics of Rhizobium tianshanense sp. nov., a moderately and slowly growing root-nodule bacterium isolated from an arid saline environment in Xinjiang, People's Republic of China. International Journal of Systematic Bacteriology. 1995;45:153–159. doi: 10.1099/00207713-45-1-153. [DOI] [PubMed] [Google Scholar]
- D'Haeze W, De Rycke R, Mathis R, et al. Reactive oxygen species and ethylene play a positive role in lateral root base nodulation of a semiaquatic legume. Proceedings of the National Academy of Sciences, USA. 2003;100:11789–11794. doi: 10.1073/pnas.1333899100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diouf D, Samba-Mbaye R, Lesueur D, et al. Genetic diversity of Acacia seyal Del. rhizobial populations indigenous to Senegalese soils in relation to salinity and pH of the sampling sites. Microbial Ecology. 2007;54:553–566. doi: 10.1007/s00248-007-9243-0. [DOI] [PubMed] [Google Scholar]
- Dobert RC, Breil BT, Triplett EW. DNA sequence of the common nodulation genes of Bradyrhizobium elkanii and their phylogenetic relationship to those of other nodulating bacteria. Molecular Plant-Microbe Interactions. 1994;7:564–572. doi: 10.1094/mpmi-7-0564. [DOI] [PubMed] [Google Scholar]
- Eagles DA, Date RA. The CB Rhizobium/Bradyrhizobium strain collection. Genetic Resources Communication No. 30. St Lucia, Queensland, Australia: CSIRO Tropical Agriculture; 1999. [Google Scholar]
- Elliott GN, Chen WM, Bontemps C, et al. Nodulation of Cyclopia spp. (Leguminosae, Papilionoideae) by Burkholderia tuberum. Annals of Botany. 2007;100:1403–1411. doi: 10.1093/aob/mcm227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fterich A, Mahdhi M, Caviedes M, et al. Characterization of root-nodulating bacteria associated to Prosopis farcta growing in the arid regions of Tunisia. Archives of Microbiology. 2011;193:385–397. doi: 10.1007/s00203-011-0683-z. [DOI] [PubMed] [Google Scholar]
- Gallego V, García MT, Ventosa A. Methylobacterium isbiliense sp nov., isolated from the drinking water system of Sevilla, Spain. International Journal of Systematic and Evolutionary Microbiology. 2005;55:2333–2337. doi: 10.1099/ijs.0.63773-0. [DOI] [PubMed] [Google Scholar]
- Gallego V, García MT, Ventosa A. Methylobacterium adhaesivum sp. nov., a methylotrophic bacterium isolated from drinking water. International Journal of Systematic and Evolutionary Microbiology. 2006;56:339–342. doi: 10.1099/ijs.0.63966-0. [DOI] [PubMed] [Google Scholar]
- Garau G, Reeve WG, Bräu L, et al. The symbiotic requirements of different Medicago spp. suggest the evolution of Sinorhizobium meliloti and S. medicae with hosts differentially adapted to soil pH. Plant and Soil. 2005;276:263–277. [Google Scholar]
- Garau G, Yates RJ, Deiana P, Howieson JG. Novel strains of nodulating Burkholderia have a role in nitrogen fixation with papilionoid herbaceous legumes adapted to acid, infertile soils. Soil Biology and Biochemistry. 2009;41:125–134. [Google Scholar]
- González-Sama A, Lucas MM, de Felipe MR, Pueyo JJ. An unusual infection mechanism and nodule morphogenesis in white lupin (Lupinus albus) New Phytologist. 2004;163:371–380. doi: 10.1111/j.1469-8137.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- Green PN. The genus Methylobacterium. In: Balows A, Trüper HG, Dworkin M, Harder W, Schliefer K-H, editors. The prokaryotes: a handbook on the biology of bacteria: ecophysiology, isolation, identification, applications. New York: Springer-Verlag; 1992. pp. 2342–2349. [Google Scholar]
- Gyaneshwar P, Hirsch AM, Moulin L, et al. Legume-nodulating betaproteobacteria: diversity, host range, and future prospects. Molecular Plant-Microbe Interactions. 2011;24:1276–1288. doi: 10.1094/MPMI-06-11-0172. [DOI] [PubMed] [Google Scholar]
- Han LL, Wang ET, Han TX, Liu J, Sui XH, Chen WF, Chen WX. Unique community structure and biogeography of soybean rhizobia in the saline–alkaline soils of Xinjiang, China. Plant and Soil. 2009;324:291–305. [Google Scholar]
- Han TX, Tian CF, Wang ET, Chen WX. Associations among rhizobial chromosomal background, nod genes, and host plants based on the analysis of symbiosis of indigenous rhizobia and wild legumes native to Xinjiang. Microbial Ecology. 2010;59:311–323. doi: 10.1007/s00248-009-9577-x. [DOI] [PubMed] [Google Scholar]
- Haukka K, Lindström K, Young JPW. Three phylogenetic groups of nodA and nifH genes in Sinorhizobium and Mesorhizobium isolates from leguminous trees growing in Africa and Latin America. Applied and Environmental Microbiology. 1998;64:419–426. doi: 10.1128/aem.64.2.419-426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herre EA, Knowlton N, Mueller UG, Rehner SA. The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends in Ecology and Evolution. 1999;14:49–53. doi: 10.1016/s0169-5347(98)01529-8. [DOI] [PubMed] [Google Scholar]
- Hiraishi A, Furuhata K, Matsumoto A, Koike KA, Fukuyama M, Tabuchi K. Phenotypic and genetic diversity of chlorine-resistant Methylobacterium strains isolated from various environments. Applied and Environmental Microbiology. 1995;61:2099–2107. doi: 10.1128/aem.61.6.2099-2107.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howieson JG, Loi A, Carr SJ. Biserrula pelecinus L. – a legume pasture species with potential for acid, duplex soils which is nodulated by unique root-nodule bacteria. Australian Journal of Agricultural Research. 1995;46:997–1009. [Google Scholar]
- Howieson JG, Yates RJ, Foster KJ, Real D, Besier RB. Prospects for the future use of legumes. In: Dilworth MJ, James EK, Sprent JI, Newton WE, editors. Nitrogen-fixing leguminous symbioses. Dordrecht, The Netherlands: Springer; 2008. pp. 363–393. [Google Scholar]
- Howieson JG, De Meyer SE, Vivas-Marfisi A, Ratnayake S, Ardley JK, Yates RJ. Novel Burkholderia bacteria isolated from Lebeckia ambigua – a perennial suffrutescent legume of the fynbos. Soil Biology and Biochemistry. 2013;60:55–64. [Google Scholar]
- Jaftha JB, Strijdom BW, Steyn PL. Characterization of pigmented methylotrophic bacteria which nodulate Lotononis bainesii. Systematic and Applied Microbiology. 2002;25:440–449. doi: 10.1078/0723-2020-00124. [DOI] [PubMed] [Google Scholar]
- Jarvis BDW, Van Berkum P, Chen WX, et al. Transfer of Rhizobium loti, Rhizobium huakuii, Rhizobium ciceri, Rhizobium mediterraneum, and Rhizobium tianshanense to Mesorhizobium gen. nov. International Journal of Systematic and Evolutionary Microbiology. 1997;47:895–898. [Google Scholar]
- Journet E-P, Barker D, Harrison HJ, Kondorosi E. M. truncatula as biological material. EMBO practical course on the new plant model system Medicago truncatula. 2001 http://www.isv.cnrs-gif.fr/embo01/manuels/pdf/module1.pdf . [Google Scholar]
- Kanso S, Patel BKC. Microvirga subterranea gen. nov., sp. nov., a moderate thermophile from a deep subsurface Australian thermal aquifer. International Journal of Systematic and Evolutionary Microbiology. 2003;53:401–406. doi: 10.1099/ijs.0.02348-0. [DOI] [PubMed] [Google Scholar]
- Kiers ET, West SA, Denison RF. Maintaining cooperation in the legume–rhizobia symbiosis: identifying selection pressures and mechanisms. In: Dilworth MJ, Newton WE, James EK, Sprent JI, editors. Nitrogen-fixing leguminous symbioses. Dordrecht, The Netherlands: Springer; 2008. pp. 59–76. [Google Scholar]
- Lafay B, Burdon JJ. Molecular diversity of legume root-nodule bacteria in Kakadu National Park, Northern Territory, Australia. PLoS ONE. 2007;2:pe277. doi: 10.1371/journal.pone.0000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguerre G, Nour SM, Macheret V, Sanjuan J, Drouin P, Amarger N. Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiology. 2001;147:981–993. doi: 10.1099/00221287-147-4-981. [DOI] [PubMed] [Google Scholar]
- Lavin M, Pennington RT, Klitgaard BB, Sprent JI, de Lima HC, Gasson PE. The dalbergioid legumes (Fabaceae): delimitation of a pantropical monophyletic clade. American Journal of Botany. 2001;88:503–533. [PubMed] [Google Scholar]
- León-Barrios M, Lorite MJ, Donate-Correa J, Sanjuán J. Ensifer meliloti bv. lancerottense establishes nitrogen-fixing symbiosis with Lotus endemic to the Canary Islands and shows distinctive symbiotic genotypes and host range. Systematic and Applied Microbiology. 2009;32:413–420. doi: 10.1016/j.syapm.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Lie TA, Göktan D, Engin M, Pijnenborg J, Anlarsal E. Co-evolution of the legume–Rhizobium association. Plant and Soil. 1987;100:171–181. [Google Scholar]
- Lindström K, Murwira M, Willems A, Altier N. The biodiversity of beneficial microbe–host mutualism: the case of rhizobia. Research in Microbiology. 2010;161:453–463. doi: 10.1016/j.resmic.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Lorite MJ, Donate-Correa J, del Arco-Aguilar M, Galdona RP, Sanjuán J, León-Barrios M. Lotus endemic to the Canary Islands are nodulated by diverse and novel rhizobial species and symbiotypes. Systematic and Applied Microbiology. 2010;33:282–290. doi: 10.1016/j.syapm.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Lu YL, Chen WF, Wang ET, Guan SH, Yan XR, Chen WX. Genetic diversity and biogeography of rhizobia associated with Caragana species in three ecological regions of China. Systematic and Applied Microbiology. 2009;32:351–361. doi: 10.1016/j.syapm.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Menna P, Hungria M. Phylogeny of nodulation and nitrogen-fixation genes in Bradyrhizobium: supporting evidence for the theory of monophyletic origin, and spread and maintenance by both horizontal and vertical transfer. International Journal of Systematic and Evolutionary Microbiology. 2011;61:3052–3067. doi: 10.1099/ijs.0.028803-0. [DOI] [PubMed] [Google Scholar]
- Mnasri B, Mrabet M, Laguerre G, Aouani ME, Mhamdi R. Salt-tolerant rhizobia isolated from a Tunisian oasis that are highly effective for symbiotic N2-fixation with Phaseolus vulgaris constitute a novel biovar (bv. mediterranense) of Sinorhizobium meliloti. Archives of Microbiology. 2007;187:79–85. doi: 10.1007/s00203-006-0173-x. [DOI] [PubMed] [Google Scholar]
- Mnasri B, Badri Y, Saidi S, de Lajudie P, Mhamdi R. Symbiotic diversity of Ensifer meliloti strains recovered from various legume species in Tunisia. Systematic and Applied Microbiology. 2009;32:583–592. doi: 10.1016/j.syapm.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Moulin L, Béna G, Boivin-Masson C, Stepkowski T. Phylogenetic analyses of symbiotic nodulation genes support vertical and lateral gene co-transfer within the Bradyrhizobium genus. Molecular Phylogenetics and Evolution. 2004;30:720–732. doi: 10.1016/S1055-7903(03)00255-0. [DOI] [PubMed] [Google Scholar]
- Mucina L, Rutherford MC. The vegetation of South Africa, Lesotho and Swaziland. Pretoria: South African National Biodiversity Institute; 2006. [Google Scholar]
- Normand P, Cournoyer B, Simonet P, Nazaret S. Analysis of a ribosomal RNA operon in the actinomycete Frankia. Gene. 1992;111:119–124. doi: 10.1016/0378-1119(92)90612-s. [DOI] [PubMed] [Google Scholar]
- Norris DO. A red strain of Rhizobium from Lotononis bainesii Baker. Australian Journal of Agricultural Research. 1958;9:629–632. [Google Scholar]
- O'Hara GW, Dilworth MJ, Boonkerd N, Parkpian P. Iron-deficiency specifically limits nodule development in peanut inoculated with Bradyrhizobium sp. New Phytologist. 1988;108:51–57. doi: 10.1111/j.1469-8137.1988.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Ourarhi M, Abdelmoumen H, Guerrouj K, et al. Colutea arborescens is nodulated by diverse rhizobia in Eastern Morocco. Archives of Microbiology. 2011;193:115–124. doi: 10.1007/s00203-010-0650-0. [DOI] [PubMed] [Google Scholar]
- Papke RT, Ward DM. The importance of physical isolation to microbial diversification. FEMS Microbiology Ecology. 2004;48:293–303. doi: 10.1016/j.femsec.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Parker MA. Symbiotic relationships of legumes and nodule bacteria on Barro Colorado Island, Panama: a review. Microbial Ecology. 2008;55:662–672. doi: 10.1007/s00248-007-9309-z. [DOI] [PubMed] [Google Scholar]
- Parker MA, Kennedy DA. Diversity and relationships of bradyrhizobia from legumes native to eastern North America. Canadian Journal of Microbiology. 2006;52:1148–1157. doi: 10.1139/w06-076. [DOI] [PubMed] [Google Scholar]
- Phalane F, Steenkamp ET, Law IJ, Botha WJ. The diversity of root nodule bacteria associated with Lebeckia species in South Africa. In: Dakora FD, Chimphango SBM, Valentine AJ, Elmerich C, Newton WE, editors. Biological nitrogen fixation: towaards poverty alleviation through sustainable agriculture. Dordrecht, The Netherlands: Springer; 2008. pp. 119–120. [Google Scholar]
- Pueppke SG, Broughton WJ. Rhizobium sp. strain NGR234 and R. fredii USDA257 share exceptionally broad, nested host ranges. Molecular Plant-Microbe Interactions. 1999;12:293–318. doi: 10.1094/MPMI.1999.12.4.293. [DOI] [PubMed] [Google Scholar]
- Qian JH, Kwon SW, Parker MA. rRNA and nifD phylogeny of Bradyrhizobium from sites across the Pacific Basin. FEMS Microbiology Letters. 2003;219:159–165. doi: 10.1016/S0378-1097(03)00043-0. [DOI] [PubMed] [Google Scholar]
- Ramírez-Bahena MH, Peix A, Rivas R, et al. Bradyrhizobium pachyrhizi sp. nov. and Bradyrhizobium jicamae sp. nov., isolated from effective nodules of Pachyrhizus erosus. International Journal of Systematic and Evolutionary Microbiology. 2009;59:1929–1934. doi: 10.1099/ijs.0.006320-0. [DOI] [PubMed] [Google Scholar]
- Rasolomampianina R, Bailly X, Fetiarison R, et al. Nitrogen-fixing nodules from rose wood legume trees (Dalbergia spp.) endemic to Madagascar host seven different genera belonging to α- and β-Proteobacteria. Molecular Ecology. 2005;14:4135–4146. doi: 10.1111/j.1365-294X.2005.02730.x. [DOI] [PubMed] [Google Scholar]
- Renier A, Jourand P, Rapior S, et al. Symbiotic properties of Methylobacterium nodulans ORS 2060(T): a classic process for an atypical symbiont. Soil Biology and Biochemistry. 2008;40:1404–1412. [Google Scholar]
- Renier A, De Faria SM, Jourand P, et al. Nodulation of Crotalaria podocarpa DC. by Methylobacterium nodulans displays very unusual features. Journal of Experimental Botany, 2011;62:3693–3697. doi: 10.1093/jxb/err083. [DOI] [PubMed] [Google Scholar]
- Rincón A, Arenal F, González I, Manrique E, Lucas MM, Pueyo JJ. Diversity of rhizobial bacteria isolated from nodules of the gypsophyte Ononis tridentata L. growing in Spanish soils. Microbial Ecology. 2008;56:223–233. doi: 10.1007/s00248-007-9339-6. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor–joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Seigler DS, Ebinger JE, Miller JT. The genus Senegalia (Fabaceae: Mimosoideae) from the New World. Phytologia. 2006;88:38–93. [Google Scholar]
- Sprent JI. Evolving ideas of legume evolution and diversity: a taxonomic perspective on the occurrence of nodulation. New Phytologist. 2007;174:11–25. doi: 10.1111/j.1469-8137.2007.02015.x. [DOI] [PubMed] [Google Scholar]
- Sprent JI. Evolution and diversity of legume symbiosis. In: Dilworth MJ, James EK, Sprent JI, Newton WE, editors. Nitrogen-fixing leguminous symbioses. Dordrecht, The Netherlands: Springer; 2008. pp. 1–21. [Google Scholar]
- Sprent JI. Legume nodulation: a global perspective. Oxford: Wiley-Blackwell; 2009. [Google Scholar]
- Sprent JI, James EK. Legume evolution: where do nodules and mycorrhizas fit in? Plant Physiology. 2007;144:575–581. doi: 10.1104/pp.107.096156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp ET, Stepkowski T, Przymusiak A, Botha WJ, Law IJ. Cowpea and peanut in southern Africa are nodulated by diverse Bradyrhizobium strains harboring nodulation genes that belong to the large pantropical clade common in Africa. Molecular Phylogenetics and Evolution. 2008;48:1131–1144. doi: 10.1016/j.ympev.2008.04.032. [DOI] [PubMed] [Google Scholar]
- Suominen L, Roos C, Lortet G, Paulin L, Lindström K. Identification and structure of the Rhizobium galegae common nodulation genes: evidence for horizontal gene transfer. Molecular Biology and Evolution. 2001;18:907–916. doi: 10.1093/oxfordjournals.molbev.a003891. [DOI] [PubMed] [Google Scholar]
- Sy A, Giraud E, Jourand P, et al. Methylotrophic Methylobacterium bacteria nodulate and fix nitrogen in symbiosis with legumes. Journal of Bacteriology. 2001;183:214–220. doi: 10.1128/JB.183.1.214-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylla SN, Samba RT, Neyra M, et al. Phenotypic and genotypic diversity of rhizobia nodulating Pterocarpus erinaceus and P. lucens in Senegal. Systematic and Applied Microbiology. 2002;25:572–583. doi: 10.1078/07232020260517715. [DOI] [PubMed] [Google Scholar]
- Takeda M, Suzuki I, Koizumi JI. Balneomonas flocculans gen. nov., sp nov., a new cellulose-producing member of the α-2 subclass of Proteobacteria. Systematic and Applied Microbiology. 2004;27:139–145. doi: 10.1078/072320204322881745. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Robson AD, Dilworth MJ, Kuo J. Microscopic evidence on how iron deficiency limits nodule initiation in Lupinus angustifolius L. New Phytologist. 1992;121:457–467. doi: 10.1111/j.1469-8137.1992.tb02946.x. [DOI] [PubMed] [Google Scholar]
- Thrall PH, Burdon JJ, Woods MJ. Variation in the effectiveness of symbiotic associations between native rhizobia and temperate Australian legumes: interactions within and between genera. Journal of Applied Ecology. 2000;37:52–65. [Google Scholar]
- Weon H-Y, Kwon S-W, Son J-A, et al. Description of Microvirga aerophila sp. nov. and Microvirga aerilata sp. nov., isolated from air, reclassification of Balneimonas flocculans Takeda et al. 2004 as Microvirga flocculans comb. nov. and emended description of the genus Microvirga. International Journal of Systematic and Evolutionary Microbiology. 2010;60:2596–2600. doi: 10.1099/ijs.0.018770-0. [DOI] [PubMed] [Google Scholar]
- Wolde-Meskel E, Terefework Z, Frostegård A, Lindström K. Genetic diversity and phylogeny of rhizobia isolated from agroforestry legume species in southern Ethiopia. International Journal of Systematic and Evolutionary Microbiology. 2005;55:1439–1452. doi: 10.1099/ijs.0.63534-0. [DOI] [PubMed] [Google Scholar]
- van Wyk B-E. A synopsis of the genus Lotononis (Fabaceae: Crotalarieae) Cape Town, South Africa: Rustica Press; 1991. [Google Scholar]
- Yanagi M, Yamasato K. Phylogenetic analysis of the family Rhizobiaceae and related bacteria by sequencing of 16S rRNA gene using PCR and DNA sequencer. FEMS Microbiology Letters. 1993;107:115–120. doi: 10.1111/j.1574-6968.1993.tb06014.x. [DOI] [PubMed] [Google Scholar]
- Yates RJ, Howieson JG, Nandasena KG, O'Hara GW. Root-nodule bacteria from indigenous legumes in the north-west of Western Australia and their interaction with exotic legumes. Soil Biology and Biochemistry. 2004;36:1319–1329. [Google Scholar]
- Yates RJ, Howieson JG, Reeve WG, et al. Lotononis angolensis forms nitrogen fixing, lupinoid nodules with phylogenetically unique, fast-growing, pink-pigmented bacteria, which do not nodulate L. bainesii or L. listii. Soil Biology and Biochemistry. 2007;39:1680–1688. [Google Scholar]
- Zhang J, Song F, Xin YH, Zhang J, Fang C. Microvirga guangxiensis sp. nov., a novel Alphaproteobacterium from soil, and emended description of the genus Microvirga. International Journal of Systematic and Evolutionary Microbiology. 2009;59:1997–2001. doi: 10.1099/ijs.0.007997-0. [DOI] [PubMed] [Google Scholar]
- Zhao LF, Deng ZS, Yang WQ, Cao Y, Wang ET, Wei GH. Diverse rhizobia associated with Sophora alopecuroides grown in different regions of Loess Plateau in China. Systematic and Applied Microbiology. 2010;33:468–477. doi: 10.1016/j.syapm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Zurdo-Piñeiro JL, García-Fraile P, Rivas R, et al. Rhizobia from Lanzarote, the Canary Islands, that nodulate Phaseolus vulgaris have characteristics in common with Sinorhizobium meliloti isolates from mainland Spain. Applied and Environmental Microbiology. 2009;75:2354–2359. doi: 10.1128/AEM.02811-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.