Abstract
Backgrounds and Aims
The spatial separation of stigmas and anthers (herkogamy) in flowering plants functions to reduce self-pollination and avoid interference between pollen dispersal and receipt. Little is known about the evolutionary relationships among the three main forms of herkogamy – approach, reverse and reciprocal herkogamy (distyly) – or about transitions to and from a non-herkogamous condition. This problem was examined in Exochaenium (Gentianaceae), a genus of African herbs that exhibits considerable variation in floral morphology, including the three forms of herkogamy.
Methods
Using maximum parsimony and maximum likelihood methods, the evolutionary history of herkogamic and non-herkogamic conditions was reconstructed from a molecular phylogeny of 15 species of Exochaenium and four outgroup taxa, based on three chloroplast regions, the nuclear ribosomal internal transcribed spacer (ITS1 and 2) and the 5·8S gene. Ancestral character states were determined and the reconstructions were used to evaluate competing models for the origin of reciprocal herkogamy.
Key results
Reciprocal herkogamy originated once in Exochaenium from an ancestor with approach herkogamy. Reverse herkogamy and the non-herkogamic condition homostyly were derived from heterostyly. Distylous species possessed pendent, slightly zygomorphic flowers, and the single transition to reverse herkogamy was associated with the hawkmoth pollination syndrome. Reductions in flower size characterized three of four independent transitions from reciprocal herkogamy to homostyly.
Conclusions
The results support Lloyd and Webb's model in which distyly originated from an ancestor with approach herkogamy. They also demonstrate the lability of sex organ deployment and implicate pollinators, or their absence, as playing an important role in driving transitions among herkogamic and non-herkogamic conditions.
Keywords: Distyly, Exochaenium, floral evolution, Gentianaceae, herkogamy, heterostyly, phylogeny
INTRODUCTION
Animal-pollinated plants display diverse floral strategies that function to influence pollination and mating. One of the most widespread conditions is herkogamy, from the Greek herko (fence) and gamos (marriage), which involves variation in the spatial deployment of female and male sexual organs within and between flowers (Webb and Lloyd, 1986). Herkogamy is most commonly interpreted as a mechanism that restricts self-fertilization and the deleterious consequences of inbreeding depression on progeny fitness. Indeed, there is considerable evidence from self-compatible species that the distance separating stigmas and anthers within a flower influences mating patterns (e.g. Breese, 1959; Barrett and Shore, 1987; Takebayashi et al., 2006). However, many plant species with herkogamy are self-incompatible and are protected from the harmful effects of inbreeding. This has led to an alternative hypothesis for the function of herkogamy involving a reduction in sexual interference between pollen dispatch and pollen receipt (Webb and Lloyd, 1986; Barrett, 2002), a general problem of floral architecture in hermaphroditic flowers.
Although there are various forms of herkogamy (reviewed in Webb and Lloyd, 1986), three major types are commonly recognized – approach herkogamy, reverse herkogamy and reciprocal herkogamy. In populations with approach herkogamy, stigmas are positioned above anthers within a flower; whereas, in populations with reverse herkogamy the opposite pattern occurs. Both approach and reverse herkogamy are most commonly monomorphic conditions, although there can be significant variation among plants in the degree of stigma–anther separation, including continuous variation encompassing both forms of herkogamy (Forrest et al., 2011; Kulbaba and Worley, 2012). Among angiosperm families, approach herkogamy is considerably more frequent than reverse herkogamy (Webb and Lloyd, 1986).
The third major form of herkogamy is reciprocal herkogamy, more commonly referred to as distyly, in which populations are polymorphic for approach and reverse herkogamous morphs. Distyly is reported from approx. 28 angiosperm families (Darwin, 1877; Ganders, 1979; Barrett, 1992; Barrett and Shore, 2008; Weller, 2009; Cohen, 2010). Finally, the non-herkogamous condition homostyly commonly occurs as a derived condition in many heterostylous groups as a result of the evolutionary breakdown of distyly (Darwin, 1877; Ganders, 1979; Barrett, 1989; Naiki, 2012). In homostylous populations, flowers exhibit anthers and stigmas of the same height and, as a result, are often highly self-pollinating. Homostylous taxa usually have smaller flowers than their distylous ancestors.
Theoretical models of the evolution of reciprocal herkogamy emphasize different selective forces, involve different ancestral states and propose contrasting sequences in which the morphological and physiological components of the distylous syndrome are assembled (Charlesworth and Charlesworth, 1979a; Lloyd and Webb, 1992a, b; reviewed in Barrett and Shore, 2008). In the Charlesworth and Charlesworth (1979a) model, dimorphic incompatibility evolves prior to reciprocal herkogamy in a self-compatible ancestor in which stigmas and anthers are of the same height. In contrast, in the Lloyd and Webb model (1992b), reciprocal herkogamy evolves from an approach herkogamous ancestor, and the subsequent establishment of dimorphic incompatibility may or may not occur, depending on various selective forces. Both models include an intermediate stage involving stigma-height dimorphism. Determining the ancestral states and sequence of character evolution in heterostylous lineages is necessary to distinguish between these models of the evolution of distyly. The limited comparative evidence available tends to support the Lloyd and Webb model (Graham and Barrett, 2004; Pérez-Barrales et al., 2006; Ferrero et al., 2009), although these studies are currently restricted to a few taxa (Narcissus and Lithodora). Both of these genera have atypical forms of heterostyly, so it is unclear how representative these groups are with respect to transitions in other distylous taxa. Unfortunately, heterostylous clades often do not contain the three main herkogamic conditions (i.e. approach, reverse and reciprocal herkogamy), and this has limited opportunities to investigate the sequence of evolution among these conditions using comparative approaches (but see Cohen et al., 2012).
Exochaenium (Gentianaceae), a small genus of 22 species endemic to Africa, provides a valuable opportunity to investigate the evolutionary relationships among the three main herkogamous conditions as each is represented within the genus. Moreover, species of Exochaenium exhibit considerable floral diversity (Fig. 1), including variation in the length and form of the corolla tube (infundibuliform or cylindrical), corolla colour (white or bright yellow to salmon) and flower presentation (inclined/pendulous or erect). This diversity provides an opportunity to assess to what extent variation in floral traits may be associated with evolutionary transitions among herkogamic conditions, an approach that has proven to be valuable in other groups (e.g. Graham and Barrett, 2004).
Fig. 1.
Selected species of Exochaenium investigated in this study illustrating variation in flower morphology. (A) Exochaenium grande with large inclined distylous flowers. (B, C) Exochaenium teucszii with erect flowers and long, narrow corolla tubes associated with hawkmoth pollination. (D) Infundibuliform corolla tube of distylous E. exiguum. (E) Yellow, erect flower of homostylous E. clavatum. (F, G) Tiny, erect flowers of homostylous E. baumianum. Scale bars = 1 cm.
Here, we use a molecular phylogeny of Exochaenium to reconstruct the evolutionary history of herkogamous and non-herkogamous conditions. Our study addresses the following specific questions. (1) Has reciprocal herkogamy evolved more than once in Exochaenium and what are the ancestral states and intermediate stages that are involved? (2) What are the evolutionary relationships between approach, reverse and reciprocal herkogamy, and also between herkogamous and non-herkogamous conditions? (3) Are there associations between these conditions and particular floral traits? We discuss the implications of our findings for models of the evolution of distyly and also evaluate the functional basis for several of the floral associations revealed by our study.
MATERIALS AND METHODS
Study group
Exochaenium is endemic to sub-Saharan Africa, with species occurring in most tropical and sub-tropical regions of the continent, particularly on the Katanga plateau (Angola, Democratic Republic of Congo and Zambia), with many extending to the Sudano-Zambesian and Guineo-Congolian regions (Kissling, 2012). All species are erect, insect-pollinated annuals with populations occurring in a wide variety of habitats, including tropical forest (E. oliganthum), grasslands and savannas (most of the species), marshes or water-logged areas (e.g. E. teucszii and E. clavatum) or sandy riverbanks (e.g. E. debile). Annual population sizes fluctuate considerably owing to seasonal variation in moisture availability. The flowers of Exochaenium species are white, rarely yellow or salmon, and protandrous, with a tendency for zygomorphy in species with pendent flowers. Heterostyly has long been recognized in the genus (Welwitsch, 1869; Gilg, 1895; Schinz, 1906; Hill, 1908; Vogel, 1955; Raynal, 1967; Ornduff, 1974; Nemomissa, 2002; Wolfe et al., 2009), but there appears to be considerable variation in expression both within and among species. For example, E. oliganthum produces both chasmogamous and underground cleistogamous flowers (Raynal, 1967). The chasmogamous flowers are reported to be distylous, whereas the cleistogamous flowers are much reduced in size.
Sampling, data collection and sequencing
We sampled leaf material from 15 out of 22 species of Exochaenium (68 % of the genus) from natural populations during 2004–2010, attempting to maximize the ecological and morphological variation within the genus. We followed sampling methods and material preservation previously described in Kissling et al. (2009a). The seven species that were not collected are all rare, often only known from the type locality, and, despite having made the effort to visit these localities, we were unable to find them. Additionally, representative species from four related genera (Exacum, Lagenias, Sebaea and Tachiadenus) were used as outgroups in our phylogenetic reconstructions.
Our data matrix consisted of three chloroplast regions (trnL intron, trnL-F spacer and the atpB-rbcL spacer) as well as the nuclear ribosomal internal transcribed spacer (ITS1 and 2) and the 5·8S gene. When available, we used previously published DNA sequence data of the Exaceae (Kissling et al., 2009a, b). To these pre-existing data, we added 41 new sequences (GenBank accession numbers KC763515–KC763547; Supplementary Data Table S1) following methods previously described for Exaceae (see Yuan et al., 2003; Kissling et al., 2009a). To detect errors and correct uncertainties in the computer-generated sequence, we compared aligned tracefiles in CHROMASPRO version 1·33 (Technelysium Pty Ltd, Queensland, Australia). Alignment was performed using CLUSTAL W (Thompson et al., 1994) as implemented in GENEIOUS version pro 5·6·6 (Biomatters Ltd, New Zealand) with subsequent manual improvement. The data matrix containing the aligned sequences is available on TreeBase (study ID: 13986).
Phylogenetic analysis
We employed Bayesian inference using MRBAYES version 3·1·2 (Huelsenbeck et al., 2001; Ronquist and Huelsenbeck, 2003) with data partitioned by ‘gene’. The most appropriate model of sequence evolution for each partition was determined using MRMODELTEST version 2·2 (Posada and Crandall, 1998; Nylander, 2004; Posada and Buckley, 2004). Default priors were used for the base frequency parameters. Two independent analyses, each with four Markov chains, three heated and one cold, and starting from a random tree, were run simultaneously for 10 million generations, with trees sampled every 1000 generations. We used the online program AWTY (Wilgenbusch et al., 2004) to check for stationarity. Trees generated before the four Markov chains reach stationarity (the ‘burn-in’) were discarded. The remaining trees were used to construct a 50 % majority rule consensus tree. The congruence between the chloroplast and nuclear data was assessed by visually comparing the topology of both trees.
Character coding
The characters were coded (Table 1) based on field observations and surveys of herbarium specimens and the literature. Sampling effort per population is provided in Supplementary Data Table S2. The variation in herkogamy condition was coded as: (a) approach herkogamy; (b) reverse herkogamy; (c) reciprocal herkogamy; and (d) anthers and stigma at the same level (homostyly). These codings were straightforward for most species, except for the following, which were coded as polymorphic: (a) E. grande – populations of this species included those with reciprocal herkogamy but also populations with stigma-height dimorphism (anthers at the same level but stigmas dimorphic in height) and populations monomorphic for plants with stigmas and anthers at a similar height; (b) E. oliganthum – this species has been described as possessing reciprocal herkogamy but also with several populations with short styles only (reverse herkogamy; Raynal, 1967); (c) E. perparvum has been described as ‘heterostylous’ (Nemomissa, 2002), but additional populations we observed were monomorphic for plants exhibiting stigmas and anthers at the same level; and (d) E. platypterum is usually non-herkogamous, but we observed a few populations with approach herkogamy.
Table 1.
Herkogamic and non-herkogamic condition (HC), flower presentation (FP), flower colour (FC), flower size (FS), corolla shape (CS) and corolla tube depth (CL) for all taxa of Exochaenium examined in this study
| Taxon | HC | FP | FC | FS | CS | CL |
|---|---|---|---|---|---|---|
| Exochaenium baumianum | D | 1 | 0 | 0 | 1 | 0 |
| Exochaenium clavatum | D | 1 | 1 | 1 | 0 | 0 |
| Exochaenium debile | C | 0 | 0 | 1 | 0 | 0 |
| Exochaenium exiguum | C | 0 | 0 | 1 | 0 | 0 |
| Exochaenium fernandesianum | C | 0 | 0 | 1 | 0 | 0 |
| Exochaenium gracile | C | 0 | 0 | 1 | 0 | 0 |
| Exochaenium grande a | C | 0 | 0 | 2 | 0 | 0 |
| Exochaenium grande b | D | 0 and 1 | 1 | 1 and 2 | 0 | 0 |
| Exochaenium grande c | C | 0 | 1 | 2 | 0 | 0 |
| Exochaenium lineariforme | C | 0 | 1 | 1 | 0 | 0 |
| Exochaenium macropterum | C | 0 | 0 | 1 | 0 | 0 |
| Exochaenium oliganthum | B and C | 1 | 0 | 0 | 1 | 0 |
| Exochaenium perparvum | C and D | 1 | 0 | 0 | 0 | 0 |
| Exochaenium platypterum | A and D | 0 | 0 | 0 | 1 | 0 |
| Exochaenium primulaeflorum | C | 0 | 1 | 1 | 0 | 0 |
| Exochaenium sp. nov. | D | 1 | 1 | 1 | 0 | 0 |
| Exochaenium teucszii | B | 1 | 0 | 2 | 1 | 1 |
Herkogamic and non-herkogamic condition: (A) approach herkogamy, (B) reverse herkogamy, (C) reciprocal herkogamy and (D) stigmas and anthers at the same level (homostyly). Flower presentation: (0) inclined/pendulous, (1) erect. Flower colour: (0) white, (1) pigmented (yellow or salmon). Flower size: (0) small (1–4 mm), (1) medium (4–15 mm) and (2) large (>15 mm). Corolla tube shape: (0) infundibuliform, (1) cylindrical. Corolla tube depth: (0) short (<20 mm), (1) long (>25 mm).
Exochaenium grande a, b and c refer to three different populations, as listed in Supplementary Data Table S1.
We coded variation in corolla shape as being either infundibuliform = funnel shaped (0) or cylindrical and narrow (1), whereas flower colour variation was coded as white (0) or (1) pigmented (yellow to salmon) and flower presentation as either inclined/pendulous (0) or erect (1). The depth of the corolla tube was coded as (0) short (<20 mm) and (1) long (>25 mm). Finally, the width of the flower was coded as (0) small (1–4 mm), (1) medium (4–15 mm) and (2) large (>15 mm). The size cut-offs we chose for these floral traits were based on natural gaps in the range of variation (see Kissling, 2012) and probably reflect functionally important divisions in plant–pollinator interactions.
Ancestral state reconstruction and correlated evolutionary changes
Ancestral state reconstructions depend on outgroup selection. However, in the tribe Exaceae, all genera exhibit approach herkogamy, except Exochaenium and Sebaea.
We reconstructed character state evolution using MESQUITE 2·75 (Maddison and Maddison, 2011), the Bayesian phylograms using both maximum parsimony and maximum likelihood approaches (Pagel, 1999; Maddison and Maddison, 2000), and assuming that all character state transitions occur at the same rate and are unordered. We manually resolved a basal polytomy formed by outgroups, according to the relationships obtained in Kissling et al. (2009a). We further tested for correlated evolution among floral traits using Pagel's (1994) likelihood approach for discrete characters as implemented in MESQUITE 2·75. Traits originally coded as multistate were recoded as binary, so that each state was transformed into a new trait coded as presence/absence, because MESQUITE does not allow evolutionary correlations to be performed between multistate characters. One hundred searches were carried out, with the P-values being estimated from 1000 repeated simulations.
RESULTS
Phylogenetic relationships
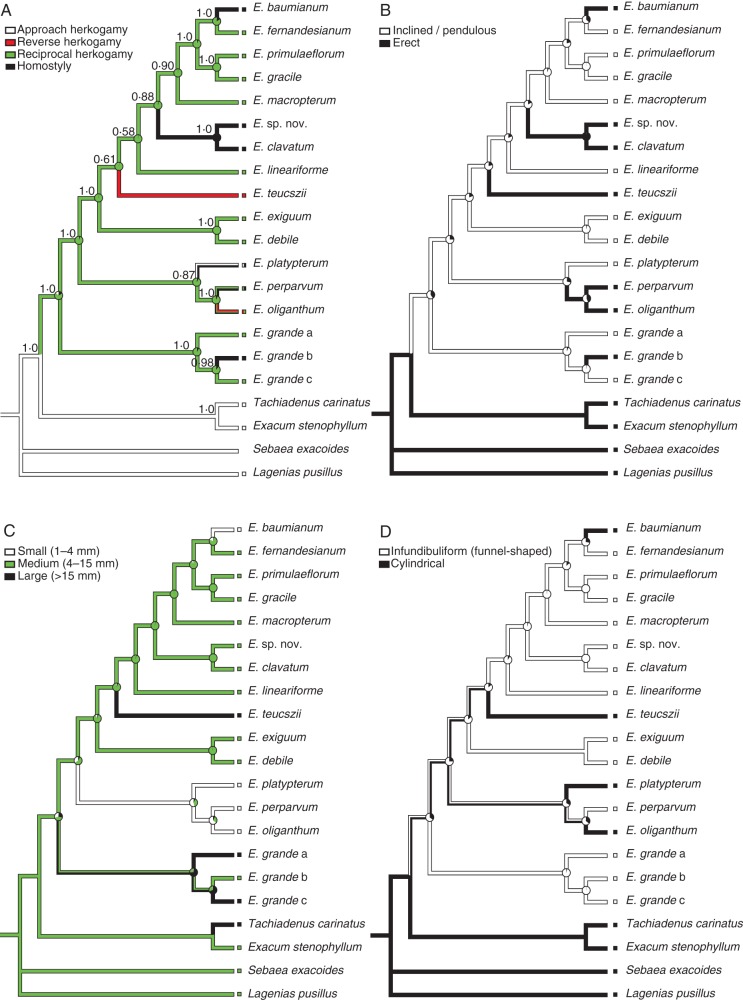
The phylogeny of Exochaenium was statistically well supported (Fig. 2), and the posterior probabilities (hereafter pp) of each clade are indicated at nodes in the tree (arithmetic mean of the loglikelihood of trees sampled after the burn-in = –7211·41; harmonic mean = 7211·001). Exochaenium grande is sister to the rest of the genus (pp = 1·0), which is further divided into two main clades. The first (pp = 0·88) is composed of E. oliganthum, E. perparvum and E. platypterum; the second (pp = 1·0) constitutes the majority of the genus. Exochaenium exiguum is closely related to E. debile (pp = 0·97) and sister to the remaining species.
Fig. 2.
Parsimony and likelihood reconstructions of ancestral states of herkogamic conditions, using the Bayesian consensus tree based on the chloroplast trnL intron, trnL-F spacer, atpB-rbcL spacer, the nuclear internal transcribed spacer regions and the 5·8S gene. Bayesian posterior probabilities are indicated above branches in (A), while proportional likelihoods of ancestral states at nodes are represented by pie charts. Reconstructions of (A) herkogamic and non-herkogamic character states, (B) flower presentation, (C) flower size, and (D) corolla shape. Exochaenium grande a, b and c refer to three different populations, as listed in Supplementary Data Table S1.
Reconstructions of floral traits
Ancestral character state reconstruction indicated that reciprocal herkogamy (distyly) arose once in Exochaenium from an ancestor with approach herkogamy (Fig. 2). The transition to distyly is correlated with inclined (P < 0·05), infundibuliform (P < 0·05) and, to a lesser extent, white (0·05 < P < 0·1) flowers. Distyly was subsequently lost on five occasions, once resulting in reverse herkogamy, and four resulting in the non-herkogamic condition homostyly (style and anthers at the same level). Three of the transitions to homostyly were accompanied by reductions in flower size (P < 0·05). There were no significant associations between morphological characters and transitions between herkogamous conditions, with the exception of the breakdown of distyly to reverse herkogamy. In this case, the loss of stylar dimorphism to monomorphism was associated with the acquisition of erect flowers, long cylindrical floral tubes (P < 0·01) and night flowering, thus suggesting a transition to hawkmoth pollination.
DISCUSSION
We investigated variation and evolution of herkogamy in the poorly known African genus Exochaenium of the Gentianaceae. Our phylogenetic reconstructions of herkogamic and non-herkogamic conditions indicate that reciprocal herkogamy arose once from an approach herkogamous ancestor, and that the distylous polymorphism has broken down on multiple occasions, resulting in either homostyly or reverse herkogamy (Fig. 2). Both of these transitions were associated with changes to floral traits other than the sex organs, suggesting modifications in the reproductive biology of populations. We now discuss the implications of these findings for the evolution and breakdown of heterostyly and consider the role that pollinators may have played in the evolutionary transitions we have documented.
Evolution of heterostyly
Although the breakdown of heterostyly to various derived conditions has been well documented since Darwin's (1877) seminal work, our knowledge of the evolutionary build-up of the polymorphism is still poorly understood. This gap in our understanding is largely because the origin of heterostyly is a relatively rare event, whereas its breakdown has occurred on numerous occasions in most heterostylous groups, including on multiple occasions within species (reviewed in Ganders, 1979; Barrett, 1989; Weller, 1992; Barrett et al., 2009; Naiki, 2012). Given the complexity of the heterostylous syndrome, it is unsurprising that our reconstructions revealed a single origin within Exochaenium, although phylogenetic evidence from other lineages of Gentianaceae in which the polymorphism occurs suggests that heterostyly may have had multiple origins within the family (J. Kissling, unpubl. res.). As yet it is unclear what intrinsic and extrinsic conditions may have favoured the evolution of distyly in Exochaenium. However, general features of the flower, such as the relatively long ‘depth-probed’ corolla tube, inserted stamens and the location of the stigma above the anthers, were probably shared by the common ancestor of the tribe (Kissling et al., 2009a, and unpubl. res.) and these traits may have favoured the selection of distyly, as postulated by Lloyd and Webb (1992a, b). The transition to distyly was accompanied by changes in a suite of floral characters including flower shape, colour and presentation, which seem likely to have also been driven by pollinator-mediated selection.
Consistent with Lloyd and Webb's (1992a, b) pollen transfer model of the evolution of distyly, our reconstructions indicated that reciprocal herkogamy arose from approach herkogamy, the ancestral condition of the Exaceae, but a form of herkogamy generally absent from the species of Exochaenium sampled in this study. In contrast to Lloyd and Webb (1992a), the Charlesworth and Charlesworth (1979a) model of the evolution of distyly postulates a homostylous ancestor. However, this ancestral condition seems rather unlikely because it commonly promotes high rates of self-fertilization and this would prevent strong inbreeding depression, a necessary condition in their model for the evolution of diallelic incompatibility (Ganders, 1979). Our character reconstructions did not consider whether stigma-height dimorphism was an intermediate stage in the transition from approach to reciprocal herkogamy, as postulated in both models and supported by comparative evidence (Graham and Barrett, 2004; Ferrero et al., 2009). Significantly, our observations of the basal species E. grande indicate that some populations possess stigma-height dimorphism, as well as distyly (see Wolfe et al., 2009), so it seems quite likely that in this group stylar polymorphism may indeed be an intermediate condition on the pathway to distyly.
Evolutionary breakdown of distyly to homostyly
Our historical reconstructions of the evolution of herkogamic and non-herkogamic conditions in Exochaenium indicated five losses of floral dimorphism (Fig. 2). Four of the five transitions involved the evolution of populations with anthers and stigmas at roughly the same height within a flower (homostyly). In three of these cases, the transition to stylar monomorphism is not complete as species still exhibit populations with stylar dimorphism. For example, E. grande is a widespread species growing throughout Africa from Mali to Ethiopia to South Africa. Most populations are distylous or have stigma-height dimorphism, but some are monomorphic with stamens and anthers at more or less the same level [formerly described by Hill (1908) as E. macranthum]. The complete loss of heterostyly apparently has only occurred in the common ancestor of E. clavatum and Exochaenium sp. nov. The occurrence of both heterostylous and homostylous populations is commonplace in heterostylous species (e.g. Baker, 1966; Charlesworth and Charlesworth, 1979b; Barrett et al., 1989), and such intraspecific variation can provide important insights into the ecology and genetics of the breakdown process.
The remaining three transitions from distyly to homostyly in Exochaenium were associated with changes to flower presentation. The distylous taxa exhibit pendulous flowers whereas the non-herkogamic species possess erect flowers. Pendulous flowers may be associated with more effective pollinator handling by long-tongued pollinators, and this association also occurs in heterostylous Narcissus species (Pérez-Barrales et al., 2004). However, the functional basis of repeated switches from pendulous to erect flowers in homostylous taxa is unclear.
In three of the four transitions, the flowers of homostylous species were smaller than in their distylous ancestors. The association between homostyly and small flowers is widely reported in the literature on heterostyly and is usually the result of a shift in mating system from outcrossing to predominant selfing (e.g. Baker, 1966; Barrett et al., 1989; Schoen et al., 1997). However, reduced flower size is not always associated with the acquisition of homostyly, and in some cases derived homostylous populations have flowers of equivalent size to heterostylous relatives and possess mixed mating systems (e.g. Barrett and Shore, 1987). This may be especially likely if homostyly is of recent origin and changes in sex allocation are only weakly developed (Vallejo-Marín and Barrett, 2009). Studies of the mating system of homostylous populations of Exochaenium species would be useful to confirm that the loss of herkogamy has indeed resulted in high selfing rates.
Although the selective mechanisms responsible for the evolutionary breakdown of distyly to homostyly in Exochaenium are not known, it seems probable that this transition is associated with uncertain pollinator service. The annual life history of Exochaenium species, combined with the fluctuations in population size that are typical of annual species, may have led to selection for reproductive assurance if pollinators became unreliable owing to changes in demographic conditions. The vast majority of heterostylous species are perennial, and it may be significant that the few that are annual are most often self-compatible and appear to be particularly prone to the breakdown of heterostyly and the origin of selfing (e.g. Barrett et al., 1989; Schoen et al., 1997). Self-compatibility has been reported from Exacum (Riesman et al., 2006) and Sebaea (Hill, 1913), but, with the exception of E. grande, which possesses a dimorphic incompatibility system (Wolfe et al., 2009), nothing is known about the compatibility systems of the remaining distylous Exochaenium species.
Transition to reverse herkogamy
Reverse herkogamy is a much less frequent condition than approach herkogamy among angiosperm families, and is most commonly associated with long narrow floral tubes and lepidopteran pollination, particularly by hawkmoths (Grant and Grant, 1965; Webb and Lloyd, 1986; Endress, 1994; Kulbaba and Worley, 2012). Our reconstructions indicated a single transition from distyly to reverse herkogamy involving E. teucszii. This species has very long (>20 mm), narrow, cylindrical floral tubes and erect white flowers that are characteristic of the hawkmoth pollination syndrome (Faegri and van der Pijl, 1971). We observed hawkmoths visiting flowers at twilight, so it seems probable that this species is primarily hawkmoth pollinated. Long funnel-shaped floral tubes characterize distylous species of Exochaenium, and it is possible that Lepidoptera are also pollinators of some species. Such ‘depth-probed’ pollination by long-tongued pollinators is important for mediating disassortative pollen transfer in heterostylous species (Lloyd and Webb, 1992b). If this is true, the transition to reverse herkogamy in Exochaenium may have been a relatively simple switch involving the loss of the long-styled morph from populations and adjustments to floral morphology, including a narrowing and lengthening of the floral tube and changes to the orientation of flowers. However, if the ancestral distylous species possessed dimorphic incompatibility, loss of intramorph incompatibility in the S-morph would also be required. Experimental manipulation of flowers from an upright to a pendent position in Aquilegia pubescens resulted in a reduction in hawkmoth visitation by an order of magnitude (Fulton and Hodges, 1999), supporting the interpretation that in E. teuczii the change from pendent to erect flowers is associated with the transition to hawkmoth pollination. Elsewhere in Gentianaceae other transitions to reverse herkogamy are evident in the tribe Saccifolieae, which contains distylous species, and also in Sebaea and Tachiadenus (J. Kissling, unpubl. res.), and it would be worthwhile investigating if such transitions are also associated with erect flowers and hawkmoth pollination.
In conclusion, our reconstructions of the evolutionary history of herkogamous and non-herkogamous conditions in Exochaenium support the hypothesis that modifications in the floral traits of populations are associated with the pollination biology of populations, including shifts in the types of pollinators visiting flowers, or insufficient visitation to maintain fertility. Nevertheless, more sampling is clearly desirable to determine the extent of intraspecific variation in floral traits, including the types of herkogamy. Several species that we investigated exhibit variation in stigma–anther position, and future studies could profitably examine the pollination and mating biology of populations in these species.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Dirk Bellstedt for providing samples, the following herbaria for allowing access or loans: BM, BOL, BR, C, DSM, G, GRA, K, MO, MRSC, MPR, NBG, NEU, PRE, S, WAG and Z, and the Mount Makulu Central Research Station for allowing the collection of plants in Zambia. We acknowledge grants to J.K. from the Swiss National Science Foundation (no. PA00P3_129140) and the Velux Stiftung (Project no. 679), and a Discovery Grant to S.C.H.B. from the Natural Sciences and Engineering Research Council of Canada.
LITERATURE CITED
- Baker HG. The evolution, functioning and breakdown of heteromorphic incompatibility systems. I. The Plumbaginaceae. Evolution. 1966;20:349–368. doi: 10.1111/j.1558-5646.1966.tb03371.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH. The evolutionary breakdown of heterostyly. In: Linhart Y, Bock J, editors. The evolutionary ecology of plants. Boulder, CO: Westview Press; 1989. pp. 151–169. [Google Scholar]
- Barrett SCH. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992. [Google Scholar]
- Barrett SCH. Sexual interference of the floral kind. Heredity. 2002;88:154–159. doi: 10.1038/sj.hdy.6800020. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Shore JS. Variation and evolution of breeding systems in the Turnera ulmifolia L. complex (Turneraceae) Evolution. 1987;41:340–354. doi: 10.1111/j.1558-5646.1987.tb05802.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Shore JS. New insights on heterostyly: comparative biology, ecology and genetics. In: Franklin-Tong V, editor. Self-incompatibility in flowering plants: evolution, diversity and mechanisms. Berlin: Springer-Verlag; 2008. pp. 3–32. [Google Scholar]
- Barrett SCH, Morgan MT, Husband BC. Dissolution of a complex genetic polymorphism: the evolution of self-fertilization in tristylous Eichhornia paniculata (Pontederiaceae) Evolution. 1989;43:1398–1416. doi: 10.1111/j.1558-5646.1989.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Ness RW, Vallejo-Marín M. Evolutionary pathways to self-fertilization in a tristylous species. New Phytologist. 2009;183:546–556. doi: 10.1111/j.1469-8137.2009.02937.x. [DOI] [PubMed] [Google Scholar]
- Breese EL. Selection for differing degrees of out-breeding in Nicotiana rustica. Annals of Botany. 1959;23:331–344. [Google Scholar]
- Charlesworth D, Charlesworth B. A model for the evolution of distyly. American Naturalist. 1979a;114:467–498. [Google Scholar]
- Charlesworth D, Charlesworth B. The maintenance and breakdown of distyly. American Naturalist. 1979b;114:499–513. [Google Scholar]
- Cohen JI. ‘A case to which no parallel exists’: the influence of Darwin's Different Forms of Flowers. American Journal of Botany. 2010;97:701–716. doi: 10.3732/ajb.0900395. [DOI] [PubMed] [Google Scholar]
- Cohen JI, Litt A, Davis JI. Comparative floral development in Lithospermum (Boraginaceae) and implications for the evolution and development of heterostyly. American Journal of Botany. 2012;99:797–805. doi: 10.3732/ajb.1100329. [DOI] [PubMed] [Google Scholar]
- Darwin CR. The different forms of flowers on plants of the same species. London: John Murray; 1877. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Faegri K, van de Pijl L. The principles of pollination ecology. Oxford: Pergamon; 1971. [Google Scholar]
- Ferrero V, Arroyo J, Vargas P., Thompson JD, Navarro L. Evolutionary transitions of style polymorphisms in Lithodora (Boraginaceae) Perspectives in Plant Ecology, Evolution and Systematics. 2009;11:111–125. [Google Scholar]
- Forrest JRK, Ogilvie JE, Gorischek AM, Thomson JD. Seasonal change in a pollinator community and the maintenance of style length variation in Mertensia fusiformis (Boraginaceae) Annals of Botany. 2011;108:1–12. doi: 10.1093/aob/mcr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton M, Hodges SA. Floral isolation between Aquilegia formosa and Aquilegia pubescens. Proceedings of the Royal Society B: Biological Sciences. 1999;266:2247–2252. [Google Scholar]
- Ganders FR. The biology of heterostyly. New Zealand Journal of Botany. 1979;17:607–635. [Google Scholar]
- Gilg E. Gentianaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. Vol. 4. Leipzig: Verlag con Wilhem Engelmann; 1895. pp. 50–180. [Google Scholar]
- Graham SW, Barrett SCH. Phylogenetic reconstruction of the evolution of stylar polymorphisms in Narcissus (Amaryllidaceae) American Journal of Botany. 2004;91:1007–1021. doi: 10.3732/ajb.91.7.1007. [DOI] [PubMed] [Google Scholar]
- Grant V, Grant KA. Flower pollination in the phlox family. New York: Columbia University Press; 1965. [Google Scholar]
- Hill AW. Notes on Sebaea and Exochaenium. Bulletin of Miscellaneous Information (Royal Gardens, Kew) 1908;1908:317–341. [Google Scholar]
- Hill AW. The floral morphology of the genus Sebaea. Annals of Botany. 1913;27:479–490. [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Kissling J. Taxonomy of Exochaenium and Lagenias: two resurrected genera of tribe Exaceae (Gentianaceae) Systematic Botany. 2012;37:238–253. [Google Scholar]
- Kissling J, Yuan Y-M, Küpfer P, Mansion G. The polyphyletic genus Sebaea (Gentianaceae): a step forward in understanding the morphological and karyological evolution of the Exaceae. Molecular Phylogenetics and Evolution. 2009a;53:734–748. doi: 10.1016/j.ympev.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Kissling J, Endress PK, Bernasconi G. Ancestral and monophyletic presence of diplostigmaty in Sebaea (Gentianaceae) and its potential role as a morphological mixed mating strategy. New Phytologist. 2009b;184:303–310. doi: 10.1111/j.1469-8137.2009.03000.x. [DOI] [PubMed] [Google Scholar]
- Kulbaba MW, Worley AC. Selection on floral design in Polemonium brandegeei (Polemoniaceae): female and male fitness under hawkmoth pollination. Evolution. 2012;66:1344–1359. doi: 10.1111/j.1558-5646.2011.01536.x. [DOI] [PubMed] [Google Scholar]
- Lloyd DG, Webb CJ. The evolution of heterostyly. In: Barrett SCH, editor. Evolution and function of heterostyly. Berlin: Springer; 1992a. pp. 151–178. [Google Scholar]
- Lloyd DG, Webb CJ. Evolution and function of heterostyly. Berlin: Springer; 1992b. The selection of heterostyly. Barrett SCH; pp. 179–208. [Google Scholar]
- Maddison DR, Maddison WP. MacClade 4: analysis of phylogeny and character evolution. Sunderland, MA: Sinauer Associates; 2000. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. 2011 Version 2·75; http://mesquiteproject.org . [Google Scholar]
- Naiki A. Heterostyly and the possibility of its breakdown by polyploidization. Plant Species Biology. 2012;27:3–29. [Google Scholar]
- Nemomissa S. Gentianaceae. In: Beentje HJ, Smith SAL, editors. Flora of tropical East Africa. Rotterdam: AA Balkema; 2002. pp. 1–68. [Google Scholar]
- Nylander JAA. MrModeltest v2. 2004 Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Sweden. http://www.abc.se/~nylander/ [Google Scholar]
- Ornduff R. Heterostyly in South African flowering plants: a conspectus. Journal of South African Botany. 1974;40:169–187. [Google Scholar]
- Pagel M. The maximum likelihood approach to reconstructing ancestral states of discrete characters on phylogenies. Systematic Biology. 1999;48:612–622. [Google Scholar]
- Pagel M. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proceedings of the Royal Society B: Biological Sciences. 1994;255:37–45. [Google Scholar]
- Pérez-Barrales R, Vargas P, Arroyo J. Convergent evolution of flower polymorphism in Narcissus (Amaryllidaceae) New Phytologist. 2004;161:235–252. [Google Scholar]
- Pérez-Barrales R, Vargas P, Arroyo J. New evidence for the Darwinian hypothesis of heterostyly: breeding systems and pollinators in Narcissus sect. Apodanthi. New Phytologist. 2006;171:553–567. doi: 10.1111/j.1469-8137.2006.01819.x. [DOI] [PubMed] [Google Scholar]
- Posada D, Buckley TR. Model selection and model averaging in phylogenetics: advantages of the Akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology. 2004;53:793–808. doi: 10.1080/10635150490522304. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Raynal A. Sur un Sebaea africain saprophyte (Gentianaceae) Adansonia sér. 2. 1967;7:207–219. [Google Scholar]
- Riseman A, Sumanasinghe VA, Craig R. Cytology, crossability, and pollen fertility of Sri Lankan Exacum (Gentianaceae) and their hybrids. International Journal of Plant Sciences. 2006;167:191–199. [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Schinz H. Beiträge zur Kentniss der Afrikanischen Flora. Gentianaceae. Bulletin de l'Herbier Boissier sér. 2. 1906;6:714–746. [Google Scholar]
- Schoen DJ, Johnson MO, L'Heureux A, Marsolais JV. Evolutionary history of the mating system in Amsinckia (Boraginaeae) Evolution. 1997;51:1090–1099. doi: 10.1111/j.1558-5646.1997.tb03956.x. [DOI] [PubMed] [Google Scholar]
- Takebayashi N, Wolf DE, Delph LF. Effect of variation in herkogamy on outcrossing within a population of Gilia achilleifolia. Heredity. 2006;96:159–165. doi: 10.1038/sj.hdy.6800780. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo-Marín M, Barrett SCH. Modification of flower architecture during early stages in the evolution of self-fertilization. Annals of Botany. 2009;103:951–962. doi: 10.1093/aob/mcp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. Über den Blutendimorphismus einiger südafrikanischer Pflanzen. Österreichische Botanische Zeitschrift. 1955;102:486–500. [Google Scholar]
- Webb CJ, Lloyd DG. The avoidance of interference between the presentation of pollen and stigmas in angiosperms II. Herkogamy. New Zealand Journal of Botany. 1986;24:163–178. [Google Scholar]
- Weller SG. Evolutionary modifications of tristylous breeding systems. In: Barrett SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992. pp. 247–272. [Google Scholar]
- Weller SG. The different forms of flowers – what have we learned since Darwin? Botanical Journal of the Linnean Society. 2009;160:249–261. [Google Scholar]
- Welwitsch F. Sertum angolense. Transactions of the Linnean Society of London. 1869;27:47–49. [Google Scholar]
- Wilgenbusch JC, Warren DL, Swofford DL. AWTY: a system for graphical exploration of MCMC convergence in Bayesian phylogenetic inference. 2004 doi: 10.1093/bioinformatics/btm388. http://ceb.csit.fsu.edu/awty . [DOI] [PubMed] [Google Scholar]
- Wolfe LM, Massinga PH, Johnson SD. A quantitative evaluation of the distylous syndrome in Sebaea grandis (Gentianaceae) South African Journal of Botany. 2009;75:785–790. [Google Scholar]
- Yuan YM, Wohlhauser S, Moller M, et al. Monophyly and relationships of the tribe Exaceae (Gentianaceae) inferred from nuclear ribosomal and chloroplast DNA sequences. Molecular Phylogenetics and Evolution. 2003;28:500–517. doi: 10.1016/s1055-7903(03)00068-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.