Abstract
Background and Aims
Root hydrotropism is a response to water-potential gradients that makes roots bend towards areas of higher water potential. The gene MIZU-KUSSEI1 (MIZ1) that is essential for hydrotropism in Arabidopsis roots has previously been identified. However, the role of root hydrotropism in plant growth and survival under natural conditions has not yet been proven. This study assessed how hydrotropic response contributes to drought avoidance in nature.
Methods
An experimental system was established for the study of Arabidopsis hydrotropism in soil. Characteristics of hydrotropism were analysed by comparing the responses of the miz1 mutant, transgenic plants overexpressing MIZ1 (MIZ1OE) and wild-type plants.
Key Results
Wild-type plants developed root systems in regions with higher water potential, whereas the roots of miz1 mutant plants did not show a similar response. This pattern of root distribution induced by hydrotropism was more pronounced in MIZ1OE plants than in wild-type plants. In addition, shoot biomass and the number of plants that survived under drought conditions were much greater in MIZ1OE plants.
Conclusions
These results show that hydrotropism plays an important role in root system development in soil and contributes to drought avoidance, which results in a greater yield and plant survival under water-limited conditions. The results also show that MIZ1 overexpression can be used for improving plant productivity in arid areas.
Keywords: Arabidopsis thaliana, drought avoidance, hydrotropism, root system, MIZU-KUSSEI1 (MIZ1)
INTRODUCTION
The sessile nature of land plants does not allow them to move away from harsh conditions. To increase survival, plants have evolved numerous mechanisms for controlling directional growth in response to environmental cues. An example is tropism, which orients plant organs towards or away from an external stimulus. Tropism is thought to be a mechanism to adapt to unfavourable environmental conditions (Darwin and Darwin, 1880; Firn and Digby, 1980; Hart, 1990; Hangarter, 1997; Correll and Kiss, 2002; Kiss et al., 2003; Gilroy and Masson, 2008). A crucial environmental cue for plants is soil water status. Drought is one of the major factors limiting crop productivity (Boyer, 1982), and the growth and development of lateral roots are greatly influenced by environmental factors such as water deficiency (Deak and Malamy, 2005). Plants utilize hydrotropism to bend their roots towards moistened areas of soil in the presence of moisture gradients (Takahashi et al., 2009; Moriwaki et al., 2013). Because roots play an important role in water uptake, hydrotropism may help plants to obtain water efficiently under drought conditions. To date, physiological studies of hydrotropism reveal its interaction with gravitropism, and the involvement of abscisic acid (ABA) and auxin in hydrotropic responses (Takahashi and Suge, 1991; Takahashi, 1997; Takahashi et al., 2002, 2009; Kaneyasu et al., 2007; Moriwaki et al., 2013). An apparatus for sensing moisture gradients appears to reside in the root cap, and differential growth for the bending response takes place in the elongation zone, as observed in root gravitropism (Jaffe et al., 1985; Takahashi and Suge, 1991; Hirasawa et al., 1997; Miyazawa et al., 2008).
Hydrotropism studies with Arabidopsis thaliana have led to the isolation of ahydrotropic mutants such as no hydrotropic response 1 (nhr1), mizu-kussei1 (miz1) and miz2 (Eapen et al., 2003; Kobayashi et al., 2007; Miyazawa et al., 2009). Saucedo et al. (2012) reported an altered hydrotropic response 1 (ahr1) mutant with an enhanced hydrotropic response, in which the growth of primary roots was directed towards the source of higher water availability. Two genes involved in the hydrotropic response, MIZ1 and MIZ2, have been isolated (Kobayashi et al., 2007; Miyazawa et al., 2009). MIZ1 is composed of 297 amino acids and contains an uncharacterized domain of unknown function (DUF617), which is termed the MIZ domain (Kobayashi et al., 2007). The MIZ domain is conserved in land plants including mosses, but not in algae or animals (Kobayashi et al., 2007). The MIZ1 promoter is activated in root cap cells and the mature region of the roots, whereas MIZ1 protein localizes in the lateral root cap and the cortical cells of the root proper (Kobayashi et al., 2007; Moriwaki et al., 2012, 2013; Yamazaki et al., 2012). Light and ABA signalling participate in the hydrotropic response by up-regulating MIZ1 expression (Moriwaki et al., 2012, 2013). Transgenic plants overexpressing MIZ1 (MIZ1OE) exhibit significantly enhanced hydrotropic responses (Miyazawa et al., 2012).
The study of hydrotropism in Arabidopsis roots and the isolation of mutants defective in hydrotropism has enabled a molecular dissection of the hydrotropic response. However, these studies focused on primary roots, although the root system responsible for water acquisition consists primarily of lateral roots (Dittmer, 1937; Yamauchi et al., 1987). Iwata et al. (2012) established an experimental system to study hydrotropism of lateral roots in Arabidopsis seedlings, and showed that lateral roots display a hydrotropic response similar to that observed in primary roots. On the other hand, the responses to gravity differ between primary and lateral roots. Namely, primary roots display orthogravitropism, whereas lateral roots display diagravitropism, plagiogravitropism or orthogravitropism, depending upon the age of plants (Kiss et al., 2002; Baldwin et al., 2013). These results indicate that the mechanisms underlying root hydrotropism are probably common to the two types of roots, although different mechanisms are involved in their gravitropism.
A question arises as to whether hydrotropism of lateral roots plays a role in the development of the root system, because soil water status substantially influences root system development (Lynch, 1995). However, the potential role of hydrotropism in mediating adaptive plasticity of roots under natural conditions remains unknown. That is because most studies on hydrotropism have been done using its assay systems with agar media or aeroponic culture (Takahashi, 1997; Takahashi et al., 2009). Currently, contradictory results are reported on the role of hydrotropism in nature (Tsuda et al., 2003; Tsutsumi et al., 2003; Cole and Mahall, 2006). Tsuda et al. (2003) observed positive hydrotropism when a steep water-potential gradient was applied across roots of an agravitropic pea mutant grown in vermiculite. A model simulation based on growth experiments in soybean predicts that hydrotropism plays a major role in orienting plant roots in a suitable direction (Tsutsumi et al., 2003). By contrast, Cole and Mahall (2006) reported that a simulation experiment using coastal dune shrubs failed to find an ecological significance for hydrotropism.
This study aimed to clarify how the hydrotropic response contributes to the development of the root system in soil. We established moisture gradients in soil and analysed root system development of the miz1-1 mutant, MIZ1 overexpressor (MIZ1OE) and wild-type plants of Arabidopsis thaliana. Furthermore, we assessed the relationship between the hydrotropism-influenced root architecture and drought avoidance by examining the growth and survival rates of these plants.
MATERIALS AND METHODS
Plant materials
Arabidopsis thaliana ecotype ‘Columbia’ was used in this study. The ahydrotropic mutant miz1-1 and a transgenic plant overexpressing MIZ1 (MIZ1OE) (OE7) were described previously (Kobayashi et al., 2007; Moriwaki et al., 2011; Miyazawa et al., 2012).
Plant growth conditions
Seeds were surface sterilized with a solution of 5 % (v/v) sodium hypochlorite, washed with distilled water, and germinated on half-strength Murashige and Skoog medium (Sigma-Aldrich, St Louis, MO, USA) with 0·8 % (w/v) agar (Wako Pure Chemical Industries, Osaka, Japan). Then, 14-d-old plants were transplanted in the soil (Fig. 1). In brief, seedlings were placed in transparent polystyrene rectangular dishes (14 × 10 × 1·5 cm) and covered with commercially available soil (Metro-Mix 360; 0·07 g N, 0·07 g P, 0·1 g K kg−1; Hyponex, Osaka, Japan) that was moistened at 65 % (v/v) water content. A block of water-absorbable plastic foam, PVA sponge (type D; 1 × 1 × 11 cm) (AION, Osaka, Japan) containing 10 mL distilled water was placed on the two longest sides of the soil, parallel to the root system. To maintain the soil at higher water content, the dish was entirely covered with a lid. This set-up was the control chamber (Fig. 1B). To establish experimental moisture gradients, the dish had the block of water-absorbable plastic foam only on one side of the soil, and the side containing the plastic foam was half-covered with a lid. This set-up was the hydrostimulated chamber (Fig. 1C). To maintain the moisture gradient in the hydrostimulated chamber for a relatively long period of time, 10 mL water was supplied to the plastic foam through an injection port every 5 d during the experiment. All chambers were placed at an angle of 5 ° from the vertical to promote root growth along the bottom surface of the chamber. Plants were grown for 30 d at 23 °C under continuous fluorescent light (50 µmol m−2 s−1) with a relative humidity of 60 %.
Fig. 1.
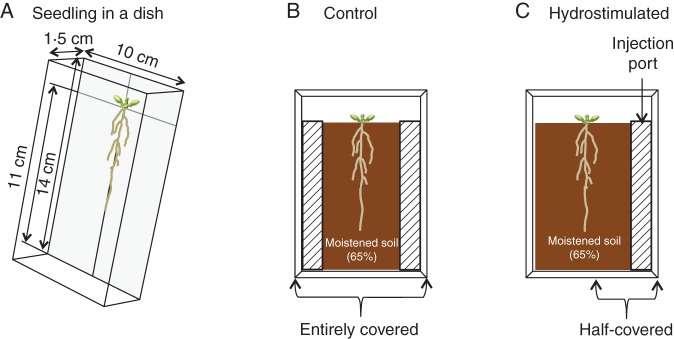
Experimental system for the study of hydrotropism-influenced root system development in soil. (A) Fourteen-day-old plants were placed in the bottom of a transparent rectangular dish (14 × 10 × 1·5 cm). (B, C) The seedling roots were covered with soil (1·5 cm depth and 11 cm length). To maintain uniformly higher water content (Control), a block of water-absorbent plastic foam (1 × 1 × 11 cm; indicated by hatched bars) containing 10 mL distilled water was placed on each side of the soil, and the dish was entirely covered with a lid (B). To establish moisture gradients (Hydrostimulated), the water-absorbent plastic foam containing 10 mL distilled water was placed only on one side of the soil, and the half of the container with the plastic foam was covered with a lid (C). To maintain the moisture gradient in the hydrostimulated chamber for a longer time, 10 mL water was supplied to the plastic foam through the injection port every 5 d during the experiment. The chambers were held at an angle of 5 ° from the vertical to promote root growth along the transparent bottom side of the dish. Plants were grown under these conditions for 30 d.
Measurement of water distribution in soil
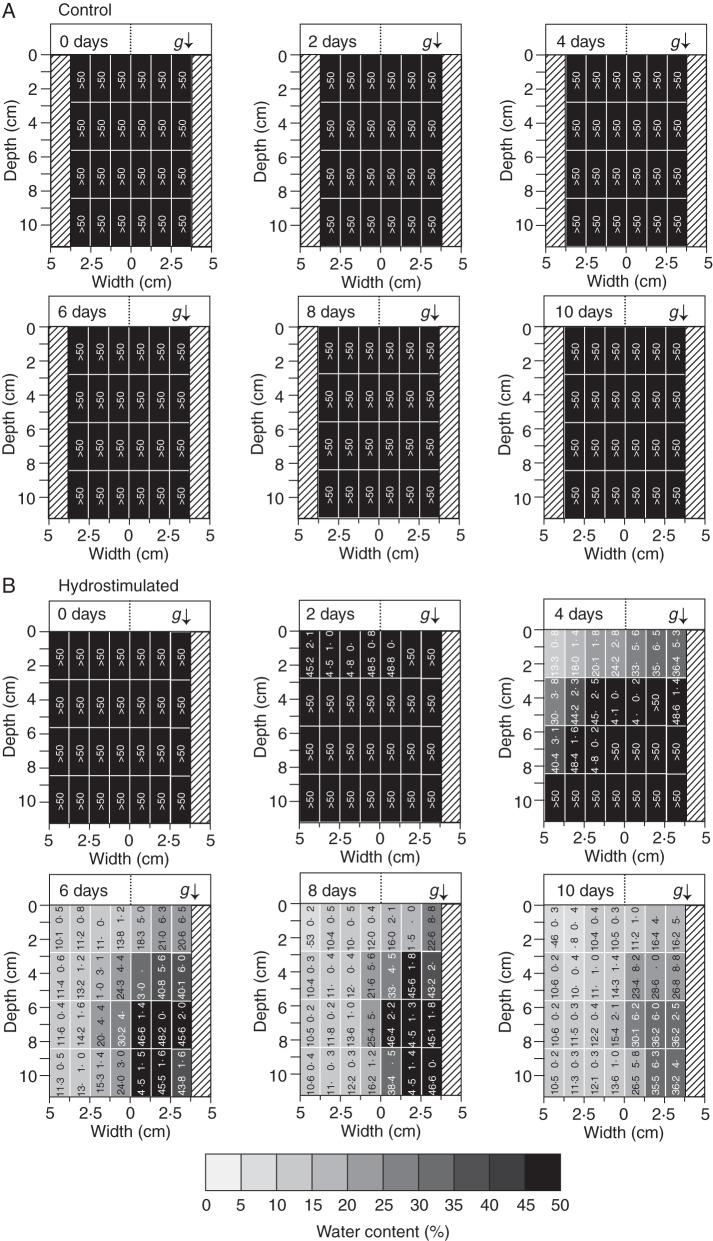
The entire soil media in the control and hydrostimulated chambers were divided into 24 and 28 zones, respectively (superficially, 1·25-cm width × 2·75-cm height for each zone). Then, the soil water content of the zone was measured with a moisture meter (M-291; AS ONE, Tokyo, Japan) based on the change in permittivity at time zero; measurements were taken at 0, 2, 4, 6, 8, and 10 d after transplanting seedlings. A measurable range by this moisture meter is <50 % (v/v) according to the manufacturer. Data are shown as means ± s.e. (n = 4).
Observation of root system development
Images of root architecture were obtained by scanning the roots observed on the bottom surface of the transparent plastic container with an image scanner (CanoScan 8800F; Canon, Tokyo, Japan). Scans were performed at time zero and after exposing plants to moisture gradients for 2, 4, 6, 8, 10 and 30 d. The scanned images of roots were traced by visual discrimination of roots from the soil background using PowerPoint 2008 (Fig. 2). Then, the root system areas were measured using ImageJ (ver. 1·42) software.
Fig. 2.
Diagram of the experimental set-up for viewing the root system architecture. Root architecture was scanned two-dimensionally (A), and the image was subjected to computer-assisted analysis (B). PowerPoint2012 software enabled discrimination of the root system from the background (C).
Analysis of biomass production
The above-ground shoots were harvested 30 d after transplanting the seedlings into the soil for both control and hydrostimulated chambers. Shoots were dried at 28 °C for 2 weeks in a constant-temperature oven (DK63; Yamato, Tokyo, Japan). Then, dried shoots were weighed using an analytical balance (AB265-S/FACT; Mettler Toledo, Switzerland) for the measurement of shoot biomass. The experiment was repeated three times.
Assessment of plant survival rate under water-stressed conditions
To assess survival under drought conditions, 14-d-old plants were transplanted into soil in transparent polystyrene rectangular dishes. For these experiments, plant roots were covered with soil moistened to 65 % (v/v) water content, and moisture gradients were developed by covering half of the chamber with a lid. The formation of water distribution was determined by the moisture meter. The control chamber was fully covered with a lid to maintain a uniform and higher water content. The control and experimental chambers were kept at 23 °C under continuous light for 12 d without any additionally supplied water. Then, plants were re-watered until soil water content reached >50 %, and the survival rate was calculated 2 d after re-watering. The experiment was repeated three times.
RESULTS AND DISCUSSION
We established an experimental system to induce and measure the hydrotropic response in soil to investigate its role in root system development (Figs 1 and 2). Figure 3 shows the soil water distribution for the control and hydrostimulated chambers. In the control, the soil kept a constantly high water content in all zones for 10 d (Fig. 3A). Water can move to the lower zone of the inclined chamber due to gravity, but >50 % water content was maintained even in the uppermost zone of the soil. Accordingly, the results show that there were no detectable gradients in water content in the control chamber. By contrast, in the hydrostimulated chamber, the uncovered side lost water content much sooner than the covered side, which resulted in the formation of water-content gradients (Fig. 3B). Water gradient formation was observed 4 d after the start of the treatment. The water gradient was apparent in the upper zones (soil water contents were approx. 20 %), and probably formed in co-operation with gravity-forced water movement in the inclined chambers. On day 6, the soil water-content gradients were clear both longitudinally and laterally. The uncovered side was relatively dry (approx. 12 %), whereas the covered side ranged from 15 to 45 % water content towards the plastic foam, values that were maintained until the end of experiment. Thus, the results show that these experimental techniques successfully induced gradients in soil water contents. Therefore, we grew Arabidopsis plants under these conditions to examine the role of hydrotropic responses in the formation of root architecture, biomass production and survival under drought conditions.
Fig. 3.
Soil water distribution in control and hydrostimulated chambers. (A) Water distribution in the absence of hydrostimulation (Control) and (B) in the presence of hydrostimulation (Hydrostimulated) are shown. The soil media in control and hydrostimulated chambers were divided into 24 and 28 zones, respectively, and the water content in each zone was measured at time zero and 2, 4, 6, 8, and 10 d after hydrotropic stimulation. The bottom bar shows water contents as grey scales, which correspond to those of the water distribution map shown above. The arrow (g) indicates the direction of the gravitational force. Data show the means ± s.e. (n = 4).
Contribution of hydrotropic response to root system development
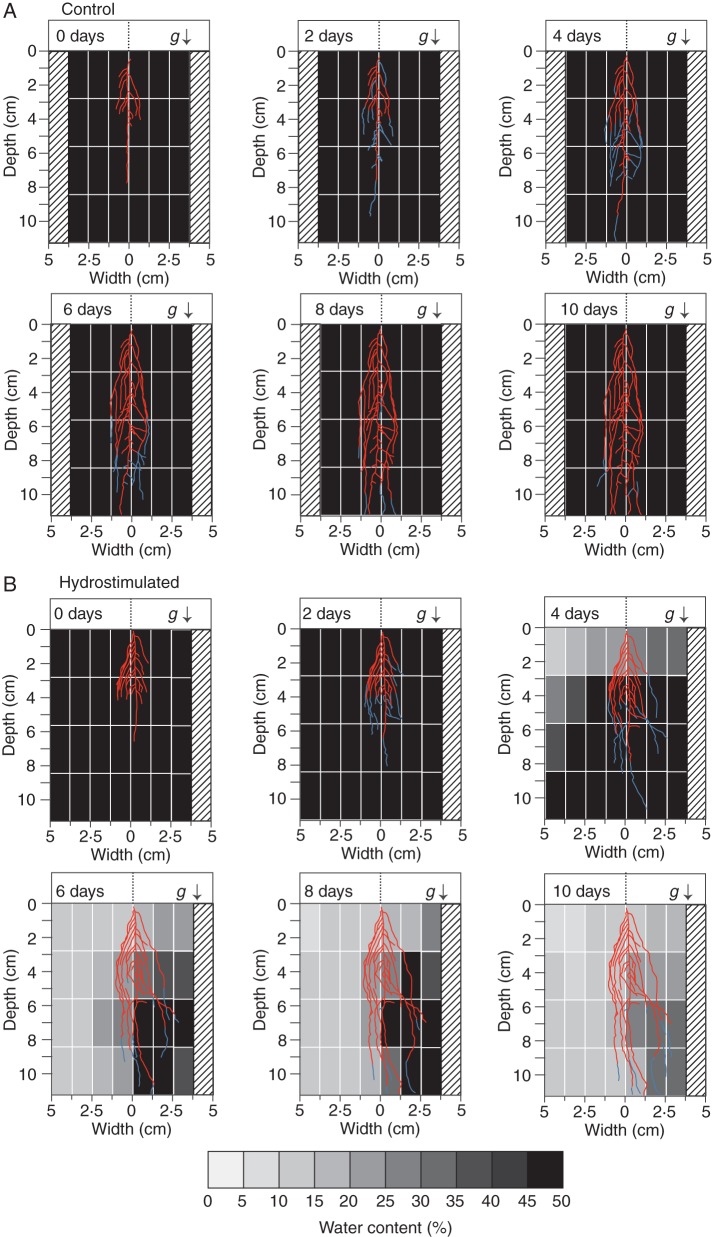
We obtained images of root architecture at 0, 2, 4, 6, 8 and 10 d after the start of hydrostimulation, and merged the images with the data for soil water distribution in the chambers (Fig. 4). When wild-type plants were incubated under water-saturated conditions in the control chambers, they developed lateral roots almost symmetrically, within a 1·25-cm-wide zone from the primary roots (Fig. 4A). By contrast, in the presence of water-content gradients, lateral roots developed to approx. 8·0 cm deep in the moist side and did not exceed the 1·25-cm wide growth on the dry side (Fig. 4B). This asymmetrical distribution of lateral roots was attributed to the hydrotropic response to the presence of water-content gradients in the soil. Previous work demonstrated that Arabidopsis lateral roots exhibit hydrotropism in response to moisture gradients, similar to the hydrotropic response of primary roots (Iwata et al., 2012). Thus, it is probable that differences in the root system architecture between control and hydrostimulated chambers are caused by the absence or presence of hydrotropic responses, respectively. That is, the hydrotropism could result in an increased development of the root system towards the moist side of the chamber. This result supports previous work that shows that the site of water supply affects the morphology of root system development and architecture (Tsutsumi et al., 2003). The asymmetric root architecture was observed on day 4 when soil water contents were apparently still uniform. This suggests that plants can perceive water-content gradients that induce a hydrotropic response by day 4, but these gradients are below the detection limit of the moisture meter used in this study.
Fig. 4.
Effects of moisture gradients on root system development of wild-type plants grown in soil. (A) Root systems in the absence of hydrostimulation (Control) and (B) in the presence of hydrostimulation (Hydrostimulated) are shown. The images of root architecture were obtained at time zero and 2, 4, 6, 8, and 10 d after hydrotropic stimulation. These images were merged with images of the water distribution shown in Fig. 2. Blue lines indicate the root regions that elongated during the preceding 2 d of growth (when the previous image was captured). The bottom bar shows water contents as grey scales, which correspond to those of the water distribution map shown above. The root system of a representative seedling is shown for each time point of imaging. Numbers of seedlings used and experiments repeated correspond to those of Table 1. The arrow (g) indicates the direction of the gravitational force.
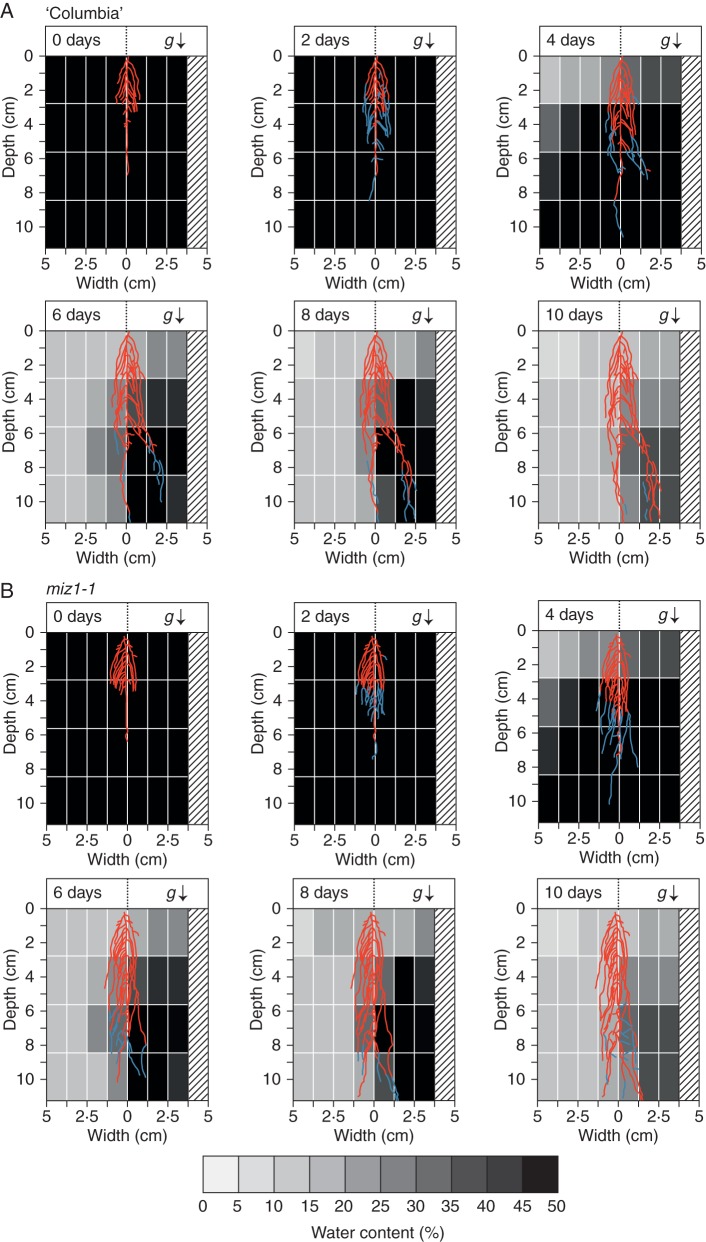
We previously showed that MIZ1 is essential for hydrotropism in both primary and lateral roots of Arabidopsis (Iwata et al., 2012). To verify that hydrotropism induced the observed root system architecture, we observed root system development of the ahydrotropic mutant miz1-1 grown in control and hydrostimulated chambers (Fig. 5). In comparison with the root architecture of wild-type plants in hydrostimulated chambers, that of the miz1-1 mutant did not show such asymmetrical development. In the soil water-content gradients of the hydrostimulated chambers, the miz1-1 mutant developed a more symmetrical root system extending <1·25 cm in width from the primary root (Fig. 5). These results suggest that the hydrotropic response mediated by MIZ1 functions to modify the root system architecture.
Fig. 5.
Effects of the miz1-1 mutation on root system development of plants grown in the hydrostimulation chambers. The root architecture images of wild-type (A) and miz1-1 (B) plants were obtained at time zero and 2, 4, 6, 8 and 10 d after hydrotropic stimulation, and these images were merged with images of the water distribution shown in Fig. 2. Blue lines indicate the root regions that elongated during the preceding 2 d of growth (when the previous image was captured). The bottom bar shows water contents as grey scales, which correspond to those of the water distribution map shown above. The root system of a representative seedling is shown for each time point of imaging. Numbers of seedlings used and experiments repeated correspond to those of Table 1. The arrow (g) indicates the direction of the gravitational force.
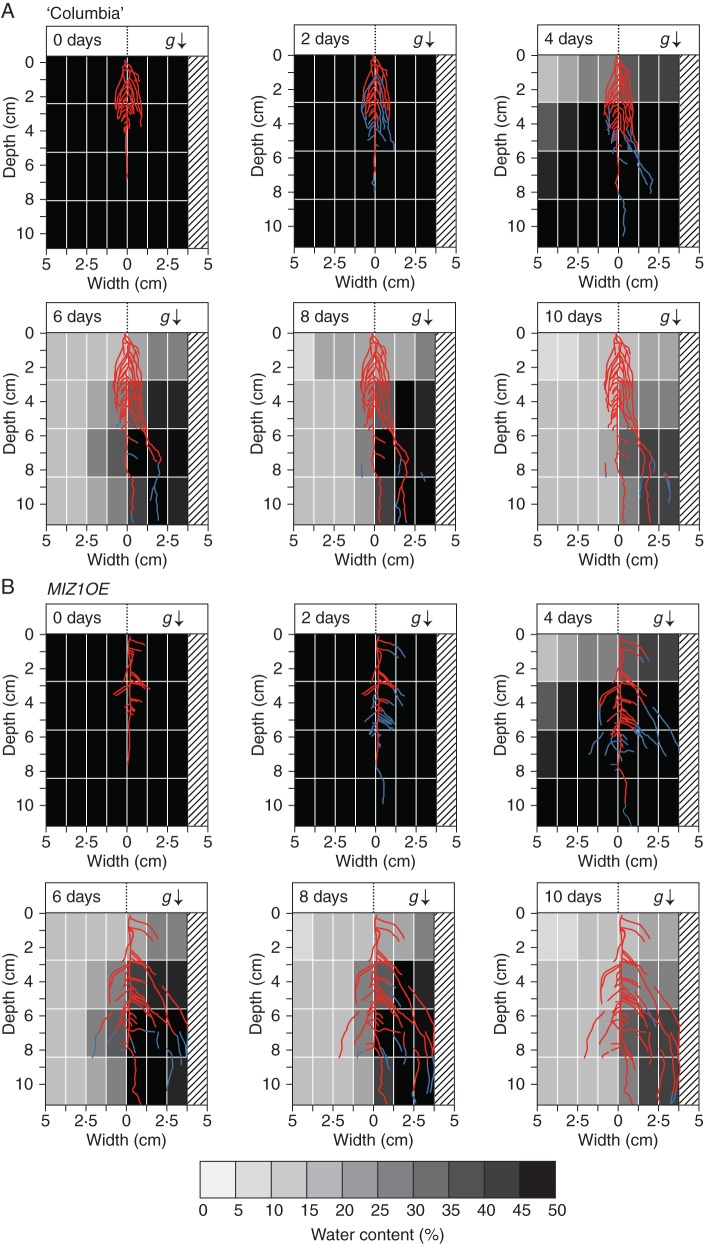
To elucidate further the effect of hydrotropism on root system architecture, transgenic plants overexpressing MIZ1 (MIZ1OE) were examined. Overexpression of MIZ1 substantially enhanced hydrotropism in Arabidopsis primary roots (Miyazawa et al., 2012). MIZ1 overexpression also resulted in an inhibition of lateral roots (Moriwaki et al., 2011). The inhibition of lateral root formation was observed at an early age of seedling growth in soil, but it became indistinguishable by 4–6 d after transplanting seedlings into soil. In the hydrostimulated chambers, a number of the wild-type roots grew towards the moist side with a width range greater than 1·25 cm, which became obvious on days 2–6 (Fig. 6A). This asymmetrical root growth was increased in MIZ1OE plants, and the lateral roots that were hydrotropically bending reached the plastic foam used as the water source (Fig. 6B). In MIZ1OE plants, several lateral roots grew far from the 1·25-cm distance even on the dry side of the chamber. This implies that the hydrotropic response enhanced by MIZ1 overexpression causes an increase in the size of the whole root system. However, the extent of root growth was similar between wild-type and MIZ1OE plants when they were grown in control chambers (data not shown). These results indicate that root hydrotropism occurs in the soil and plays an important role in the formation of root architecture.
Fig. 6.
Effects of MIZ1 overexpression on root system development of plants grown in the hydrostimulation chambers. The root system images of (A) wild-type and (B) MIZ1OE plants were obtained at time zero and 2, 4, 6, 8 and 10 d after hydrotropic stimulation, and these images were merged with images of the water distribution shown in Fig. 2. Blue lines indicate the root regions that elongated during the preceding 2 d of growth (when the previous image was captured). The bottom bar shows water contents as grey scales, which correspond to those of the water distribution map shown above. The root system of a representative seedling is shown for each time point of imaging. Numbers of seedlings used and experiments repeated correspond to those of Table 1. The arrow (g) indicates the direction of gravitational force.
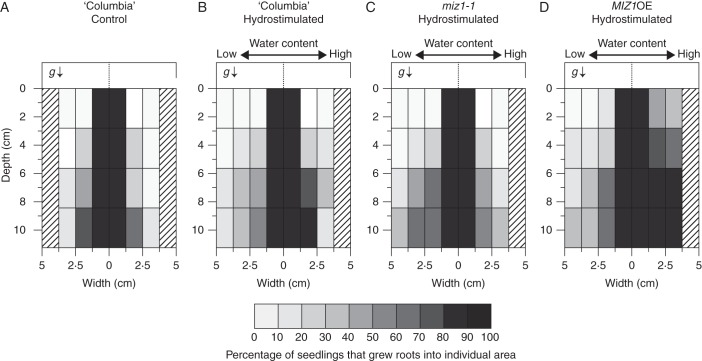
We examined root system architecture at 30 d after the start of hydrostimulation, and the percentage of plants that grew roots into individual zones in the rectangular dish was calculated (n =12–25). In the control chamber, more than 80 % of wild-type plants developed symmetrical lateral roots within the 1·25-cm-wide zone from the primary root (Fig. 7A). More lateral roots grew into the moist zones in hydrostimulated chambers; approx. 80 % of wild-type plants developed roots that extended beyond the 1·25-cm-wide zone in the moist side of the chamber (Fig. 7B). By contrast, even in the presence of soil water-content gradients, miz1-1 mutant roots developed symmetrical lateral roots within a 1·25-cm-wide zone, similar to that of wild-type plants in control chambers (Fig. 7C). Contrary to the ahydrotropic mutant, MIZ1OE transgenic plants showed a much greater distribution of lateral roots in the moist side of the chamber; more than 80 % of MIZ1OE plants developed roots that extended beyond the 2·5-cm zone (Fig. 7D).
Fig. 7.
The root system development of wild-type (‘Columbia’), miz1-1 and MIZ1OE plants after hydrotropic stimulation for 30 d. The root system images of (A, B) wild-type (‘Columbia’), (C) miz1-1 and (D) MIZ1OE plants were obtained after growing in the absence (A) or presence of hydrostimulation (B–D) for 30 d. The percentage of plants that grew roots into each zone of a rectangular dish was calculated, and is represented by grey scales according to the classification indicated in the bottom bar. Numbers of seedlings used and experiments repeated correspond to those of Table 1. The arrow (g) indicates the direction of gravitational force.
We determined the total root area for each individual seedling (Table 1). In hydrostimulated chambers, wild-type and MIZ1OE transgenic plants showed greater root growth in the moist side of the chamber (13·2 and 21·1 cm2, respectively) than in the dry side of the chamber (8·72 and 10·8 cm2, respectively). By contrast, miz1-1 plants did not show such asymmetry in root growth between moist and dry sides of the chamber (approx. 10 cm2 for each) (Table 1). MIZ1OE plants developed a greater root system towards the moist side of the chamber than wild-type plants (P < 0·001). By contrast, root system development was similar among wild-type, miz1-1 mutant and MIZ1OE transgenic plants in control chambers (Table 1). These results suggest that the strength of influence on hydrotropism-mediated development of root architecture occurs in the order miz1 mutant < wild type < MIZ1OE. This implies that plant root hydrotropism could function in normal soil environments and play an important role in the development of root system architecture.
Table 1.
Root areas of wild-type (‘Columbia’), miz1-1 mutant and MIZ1OE transgenic plants grown in control and hydrostimulated chambers for 30 d
| Control (cm2) |
Hydrostimulated (cm2) |
|||||
|---|---|---|---|---|---|---|
| Left side | Right side | n | Dry side | Moist side | n | |
| Columbia | 10·5 ± 0·9 | 9·7 ± 0·7 | 12 | 8·7 ± 0·5 | 13·2 ± 0·9*** | 20 |
| miz1-1 | 11·4 ± 1·1 | 9·8 ± 0·6 | 14 | 11·1 ± 0·7 | 10·1 ± 0·8 | 20 |
| MIZ1OE | 12·7 ± 1·4 | 11·8 ± 1·4 | 14 | 10·1 ± 1·3 | 21·2 ± 1·9**** | 20 |
Data are the mean root area per plant ± s.e. No statistically significant differences between the left and right sides of the control chambers were observed for all plants tested. In hydrostimulated chambers, ‘Columbia’ and MIZ1OE plants grew more roots in the moist side of the chamber, but miz1-1 did not show such asymmetrical root growth. The experiments were repeated three times. Statistical analyses were performed using Student's t-test. Asterisks represent highly significant values (***P < 0·001, ****P < 0·0001).
Effects of root hydrotropism on biomass production and survival under water-limited conditions
Plant productivity depends on environmental variables, especially during water-stress conditions (Chalmers et al., 1981; Lynch, 1995). Root hydrotropism is considered to play a role in drought avoidance, but the relationship between the hydrotropic response and plant production under water-limited conditions remains unknown. Therefore, we examined whether the hydrotropism-influenced architecture of the root system ultimately affects biomass production.
Figure 8 shows the shoot dry weight at the end of the 30-d experiment. In control chambers, no differences in shoot biomass were observed among wild-type, miz1-1 mutant and MIZ1OE transgenic plants. In hydrostimulated chambers, shoot dry weights decreased compared with those grown in control chambers. However, the decrease in shoot dry weight was significantly less for MIZ1OE plants than for miz1 plants. Shoot biomass weight for the miz1-1 mutant did not differ statistically from that of wild-type plants (Fig. 8). These results suggest that root hydrotropism does influence the growth of plant biomass under conditions of soil water-content gradients. Water deficit is a common environmental stress experienced by plants, and it strongly impairs their production (Boyer, 1982; Passioura, 1996). Root architecture is considered to be linked to the acquisition of water (Lynch, 1995). Our results show that hydrotropism is a factor that is involved in root system development, which results in better yield under water-limited conditions.
Fig. 8.
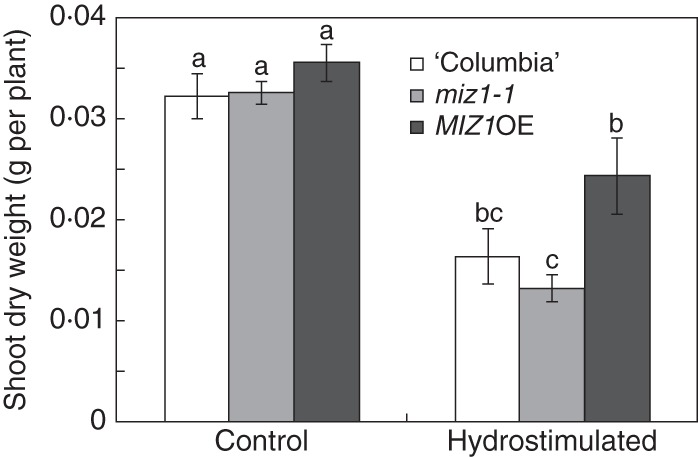
Effect of hydrotropic response on biomass production of wild-type (‘Columbia’), miz1-1 and MIZ1OE plants. Seedlings were grown in the absence (Control) and presence (Hydrostimulated) of hydrotropic stimulation for 30 d, and the dry weights of the shoots were measured. Data are means ± s.e. (n = 11–20). Different letters above the bars indicate statistically significant differences at P < 0·05 by Tukey's HSD.
We next examined the survival rate of wild-type, miz1-1 mutant and MIZ1OE plants when they were grown in control and hydrostimulated chambers. For these experiments, 12-d-old plants were transplanted to control (covered) and hydrostimulated (partially uncovered) chambers (Fig. 1), and then the hydrostimulated chambers were gradually subjected to drought stress by withholding water for 12 d. Wet conditions were maintained in control chambers, whereas drought stress gradually became severe in the hydrostimulated chamber (Supplementary data Fig. S1). In the hydrostimulated chamber, plants were exposed to both soil water-content gradients and water stress. After 12 d of drought stress, plants were re-watered until soil water content reached >50 % and allowed to grow for 2 d before the survival rates were determined.
Under the control condition, no water-stressed plants were observed, and 100 % of wild-type, miz1-1 and MIZ1OE plants survived (Fig. 9). When plants were grown in the hydrostimulated chambers and subjected to drought stress for 12 d, the survival rate of miz1-1 mutant plants significantly decreased, compared with that of wild-type plants (Fig. 9). By contrast, MIZ1OE plants showed an enhanced tolerance to drought stress and had a significantly greater survival rate than wild-type plants (Fig. 9).
Fig. 9.
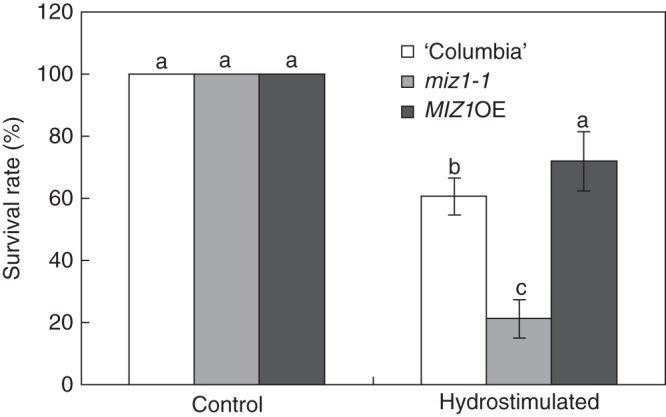
Survival rates of wild-type (‘Columbia’), miz1-1 and MIZ1OE plants after treatment with 12-d drought stress. Fourteen-day-old plants were transplanted into moistened soil, and chambers were either entirely covered (Control) or were half-covered (Hydrostimulated) as shown in Fig. 1. Then, plants were grown without further water supply for 12 d. The plants were re-watered until soil water content reached >50 %, and the survival rate was counted 2 d after re-watering. Data are means ± s.e. (n = 21–29). Different letters above the bars indicate statistically significant differences at P < 0·05 by Tukey's HSD.
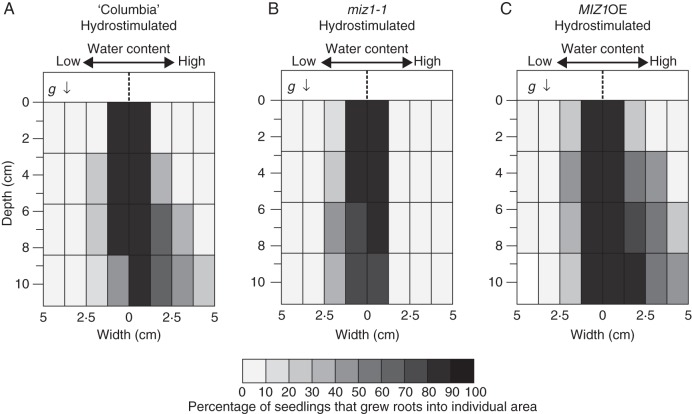
In these experiments, we also assessed the development of the root system as shown in Fig. 10. More than 80 % of wild-type plants did not develop lateral roots that extended beyond the 1·25-cm-wide zone in the dry side, whereas more than 60 % of the plants developed lateral roots that extended beyond the 1·25-cm-wide zone in the moist side (Fig. 10A). The root system distribution of miz1-1 mutant plants was symmetrical, with more than 60 % of plants developing lateral roots within the 1·25-cm-wide zone (Fig. 10B). By contrast, more than 80 % of MIZ1OE plants developed root systems that extended beyond the 1·25-cm-wide zone, and more than 50 % of MIZ1OE plants developed root systems that extended beyond the 2·5-cm-wide zone (Fig. 10C). These results indicate that the hydrotropic response occurred during drought stress treatment, and suggest that the drought tolerance shown in Fig. 9 correlates with the hydrotropism-influenced root system architecture shown in Fig. 10.
Fig. 10.
Effects of hydrotropic response and drought stress on the development of root system of wild-type (‘Columbia’), miz1-1 and MIZ1OE plants. Fourteen-day-old plants were transplanted into moistened soil, and the chambers were half-covered (Hydrostimulated) as shown in Fig. 1. They were kept without further water supply for 10 d. The percentage of plants that grew roots into each zone of soil medium was calculated and represented by grey scales according to the classification indicated in the bottom bar. The arrow (g) indicates the direction of gravitational force.
Furthermore, the results suggest strongly that the hydrotropic response contributes to drought avoidance by promoting root growth towards the moistened region. Saucedo et al. (2012) discuss the potential of a crop fitness increase by altering pathways that regulate the response of roots to water-potential gradients. However, Cole and Mahall (2006) could find no evidence for the ecological significance of root hydrotropism in seedlings of two species of dune shrubs. In their report, it is not clear whether the roots of the observed dune shrubs are able to display hydrotropic responses by overcoming gravitropic responses. The ability of roots to respond to moisture gradients should be experimentally assessed in dune shrubs to examine the ecological significance of hydrotropism in these plant species.
Conclusions
This study has demonstrated that root hydrotropism plays a role in root system development in soil. In addition, the hydrotropism-influenced root system architecture appears to affect plant growth and survival under water-limited conditions. Root hydrotropism could function during plant responses to water-limited conditions by modifying root architecture for increased acquisition of water, which ultimately determines plant productivity. Our study suggests that constitutive expression of MIZ1 could potentially increase crop productivity in arid areas. This technology could be applied to plant breeding programmes to improve drought tolerance.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank our laboratory members, Drs Tomokazu Yamazaki, Teppei Moriwaki and Akie Kobayashi, for their helpful discussions and suggestions. This work was supported by the Japan Society of the Promotion of Science (Grants-in-Aid for Scientific Research B, No. 20370017, to H.T.), the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grants-in-Aid for Scientific Research on Priority Areas, No. 19039005, and Grants-in-Aid for Scientific Research on Innovative Areas, No. 22120004, to H.T.), and the Cabinet Office, Government of Japan, through its Funding Program for Next Generation World-Leading Researchers (GS0002 to Y.M.). This work was carried out as part of the Global COE Program J03 (Ecosystem Management Adapting to Global Change).
LITERATURE CITED
- Baldwin KL, Strohm AK, Masson PH. Gravity sensing and signal transduction in vascular plant primary roots. American Journal of Botany. 2013;100:126–142. doi: 10.3732/ajb.1200318. [DOI] [PubMed] [Google Scholar]
- Boyer JS. Plant productivity and environment. Science. 1982;218:443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- Chalmers DJ, Mitchell PD, Vanheek L. Control of peach tree growth and productivity by regulated water supply, tree density and summer pruning. Journal of the American Society for Horticultural Science. 1981;106:307–312. [Google Scholar]
- Cole ES, Mahall BE. A test for hydrotropic behavior by roots of two coastal dune shrubs. New Phytologist. 2006;172:358–368. doi: 10.1111/j.1469-8137.2006.01822.x. [DOI] [PubMed] [Google Scholar]
- Correll M, Kiss JZ. Interactions between gravitropism and phototropism in plants. Journal of Plant Growth Regulation. 2002;21:89–101. doi: 10.1007/s003440010056. [DOI] [PubMed] [Google Scholar]
- Darwin C, Darwin F. The power of movement in plants. London: John Murray; 1880. [Google Scholar]
- Deak KI, Malamy J. Osmotic regulation of root system architecture. The Plant Journal. 2005;43:17–28. doi: 10.1111/j.1365-313X.2005.02425.x. [DOI] [PubMed] [Google Scholar]
- Dittmer HJ. A quantitative study of the roots and root hairs of a winter rye plant (Secale cereale) American Journal of Botany. 1937;24:417–420. [Google Scholar]
- Eapen D, Barroso ML, Campos ME, et al. A no hydrotropic response root mutant that responds positively to gravitropism in Arabidopsis. Plant Physiology. 2003;131:536–546. doi: 10.1104/pp.011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firn RD, Digby J. The establishment of tropic curvatures in plants. Annual Review of Plant Physiology. 1980;31:131–148. [Google Scholar]
- Gilroy S, Masson PH. Plant tropisms. Oxford: Blackwell Publishing; 2008. [Google Scholar]
- Hangarter RP. Gravity, light and plant form. Plant, Cell and Environment. 1997;20:796–800. doi: 10.1046/j.1365-3040.1997.d01-124.x. [DOI] [PubMed] [Google Scholar]
- Hart JW. Plant tropism and growth movement. London: Unwin Hyman; 1990. [Google Scholar]
- Hirasawa T, Takahashi H, Suge H, Ishihara K. Water potential, turgor and cell wall properties in elongating tissues of the hydrotropically bending roots of pea, Pisum sativum L. Plant, Cell and Environment. 1997;20:381–386. [Google Scholar]
- Iwata S, Miyazawa Y, Takahashi H. MIZU-KUSSEI1 plays an essential role in the hydrotropism of lateral roots in Arabidopsis thaliana. Environmental and Experimental Botany. 2012;75:167–172. [Google Scholar]
- Jaffe MJ, Takahashi H, Biro RL. A pea mutant for the study of hydrotropism in roots. Science. 1985;230:445–447. doi: 10.1126/science.230.4724.445. [DOI] [PubMed] [Google Scholar]
- Kaneyasu T, Kobayashi A, Nakayama M, Fujii N, Takahashi H, Miyazawa Y. Auxin response, but not its polar transport, plays a role in hydrotropism of Arabidopsis roots. Journal of Experimental Botany. 2007;58:1143–1150. doi: 10.1093/jxb/erl274. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Miller KM, Ogden LA, Roth KK. Phototropism and gravitropism in lateral roots of Arabidopsis. Plant and Cell Physiology. 2002;43:35–43. doi: 10.1093/pcp/pcf017. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Mullen JL, Correll MJ, Hangarter RP. Phytochrome A and B mediate red-light-induced positive phototropism in roots. Plant Physiology. 2003;131:1411–1417. doi: 10.1104/pp.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Takahashi A, Kakimoto Y, et al. A gene essential for hydrotropism in roots. Proceedings of the National Academy of Sciences, USA. 2007;104:4724–4729. doi: 10.1073/pnas.0609929104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. Root architecture and plant productivity. Plant Physiology. 1995;109:7–13. doi: 10.1104/pp.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa Y, Sakashita T, Funayama T, et al. Effects of locally targeted heavy-ion and laser microbeam on root hydrotropism in Arabidopsis thaliana. Journal of Radiation Research. 2008;49:373–379. doi: 10.1269/jrr.07131. [DOI] [PubMed] [Google Scholar]
- Miyazawa Y, Takahashi A, Kobayashi A, Kaneyasu T, Fujii N, Takahashi H. The GNOM-mediated vesicular trafficking plays an essential role in hydrotropism of Arabidopsis roots. Plant Physiology. 2009;149:835–840. doi: 10.1104/pp.108.131003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa Y, Moriwaki T, Uchida M, Kobayashi A, Fujii N, Takahashi H. Overexpression of MIZU-KUSSEI1 enhances root hydrotropic response by retaining cell viability under hydrostimulated condition in Arabidopsis thaliana. Plant and Cell Physiology. 2012;53:1926–1933. doi: 10.1093/pcp/pcs129. [DOI] [PubMed] [Google Scholar]
- Moriwaki T, Miyazawa Y, Kobayashi A, et al. Hormonal regulation of lateral roots development in Arabidopsis modulated by MIZ1 and requirement of GNOM activity for MIZ1 function. Plant Physiology. 2011;157:1209–1220. doi: 10.1104/pp.111.186270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki T, Miyazawa Y, Fujii N, Takahashi H. Light and abscisic acid signaling are integrated by MIZ1 gene expression and regulate hydrotropic response in roots of Arabidopsis thaliana. Plant, Cell and Environment. 2012;35:1359–1368. doi: 10.1111/j.1365-3040.2012.02493.x. [DOI] [PubMed] [Google Scholar]
- Moriwaki T, Miyazawa Y, Kobayashi A, Takahashi H. Molecular mechanisms of hydrotropism in seedling roots of Arabidopsis thaliana (Brassicaceae) American Journal of Botany. 2013;100:25–34. doi: 10.3732/ajb.1200419. [DOI] [PubMed] [Google Scholar]
- Passioura JB. Drought and drought tolerance. Plant Growth Regulation. 1996;20:79–83. [Google Scholar]
- Saucedo M, Ponce G, Campos ME, et al. An altered hydrotropic response (ahr1) mutant of Arabidopsis recovers root hydrotropism with cytokinin. Journal of Experimental Botany. 2012;63:3587–3601. doi: 10.1093/jxb/ers025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H. Hydrotropism: the current state of our knowledge. Journal of Plant Research. 1997;1110:163–169. doi: 10.1007/BF02509304. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Suge H. Root hydrotropism of an agravitropic pea mutant, ageotropum. Physiologia Plantarum. 1991;82:24–31. [Google Scholar]
- Takahashi N, Goto N, Okada K, Takahashi H. Hydrotropism in abscisic acid, wavy, and gravitropic mutants of Arabidopsis thaliana. Planta. 2002;216:203–211. doi: 10.1007/s00425-002-0840-3. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Miyazawa Y, Fujii N. Hormonal interactions during root tropic growth: hydrotropism versus gravitropism. Plant Molecular Biology. 2009;69:489–502. doi: 10.1007/s11103-008-9438-x. [DOI] [PubMed] [Google Scholar]
- Tsuda S, Miyamoto N, Takahashi H, Ishihara K, Hirasawa T. Roots of Pisum sativum L. exhibit hydrotropism in response to a water potential gradient in vermiculite. Annals of Botany. 2003;92:767–770. doi: 10.1093/aob/mcg200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi D, Kosugi K, Mizuyama T. Effect of hydrotropism on root system development in soybean (Glycine max): growth experiments and a model simulation. Journal of Plant Growth Regulation. 2003;21:441–458. [Google Scholar]
- Yamauchi A, Kono Y, Tatsumi J. Quantitative analysis on root system structures of upland rice and maize. Japanese Journal of Crop Science. 1987;56:608–617. [Google Scholar]
- Yamazaki T, Miyazawa Y, Kobayashi A, Moriwaki T, Fujii N, Takahashi H. MIZ1, an essential protein for root hydrotropism, is associated with the cytoplasmic face of the endoplasmic reticulum membrane in Arabidopsis root cells. FEBS Letters. 2012;586:398–402. doi: 10.1016/j.febslet.2012.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.