Abstract
Background and Aims
Paleoclimatic data indicate that an abrupt climate change occurred at the Eocene–Oligocene (E–O) boundary affecting the distribution of tropical forests on Earth. The same period has seen the emergence of South-East (SE) Asia, caused by the collision of the Eurasian and Australian plates. How the combination of these climatic and geomorphological factors affected the spatio-temporal history of angiosperms is little known. This topic is investigated by using the worldwide sapindaceous clade as a case study.
Methods
Analyses of divergence time inference, diversification and biogeography (constrained by paleogeography) are applied to a combined plastid and nuclear DNA sequence data set. Biogeographical and diversification analyses are performed over a set of trees to take phylogenetic and dating uncertainty into account. Results are analysed in the context of past climatic fluctuations.
Key Results
An increase in the number of dispersal events at the E–O boundary is recorded, which intensified during the Miocene. This pattern is associated with a higher rate in the emergence of new genera. These results are discussed in light of the geomorphological importance of SE Asia, which acted as a tropical bridge allowing multiple contacts between areas and additional speciation across landmasses derived from Laurasia and Gondwana.
Conclusions
This study demonstrates the importance of the combined effect of geomorphological (the emergence of most islands in SE Asia approx. 30 million years ago) and climatic (the dramatic E–O climate change that shifted the tropical belt and reduced sea levels) factors in shaping species distribution within the sapindaceous clade.
Keywords: Biogeography, climate change, diversification, Eocene–Oligocene boundary; Sapindaceae; South-East Asia
INTRODUCTION
Although not considered as one of the ‘Big five’ mass extinctions (Jablonski, 2001), the abrupt cooling near the Eocene–Oligocene (E–O) boundary, approx. 33.7 million years ago (Ma), had great impacts on biodiversity (Katz et al., 2008; Zhonghui et al., 2009). During this period, Earth's climate shifted from a relatively ice-free world to one with glacial conditions in polar regions characterized by substantial ice sheets (Bowen, 2007). In a relatively short time span, high-latitude (45–70 ° in both hemispheres) temperatures decreased from approx. 20 °C to approx. 5 °C (Zhonghui et al., 2009). Explanations for this cooling include changes in ocean circulation due to the opening of Southern Ocean gateways, a decrease in atmospheric CO2 and a decrease in solar insulation (see Zhonghui et al., 2009, and references therein). This period also coincided with drought in southern regions (especially in Australia and Africa; Bowen, 2007) and subsequent reduction of the tropical belt (see Morley, 2003; Lohman et al., 2011). As a consequence, this abrupt cooling seemed to be related to a decrease in species diversity as shown, for instance, in the decline of the Neotropical floras (Jaramillo et al., 2006). In addition, Coetzee and Muller (1984) advocated that this event (whose effects lasted until the Miocene) disrupted previous phytogeographical connections between the southern hemisphere landmasses that were assembled during the Cretaceous (for more details on fossils from Antarctica, see also Cantrill and Pool, 2005).
During the same geological period, intensive volcanic activities were recorded in South-East (SE) Asia as a result of the collision of the Eurasian and Australian plates (Metcalfe, 1998; Hall, 2009). Although the western part of SE Asia (also known as Sundaland and referred to as proto-SE Asia by Buerki et al., 2011a) already formed a large emergent land area by the Late Cretaceous (including, for example, the older parts of Malaysia and Southwest Borneo), most of the islands at the margin of Sundaland and New Guinea, known as Wallacea (Hall, 2009), were created from this period onwards, with a peak of tectonic activity during the Miocene (Hall, 2009). Wallacea is at present a region of high endemism for plants and animals (for a review, see Richardson et al., 2012).
Currently, little is known about the consequences of the abrupt change in abiotic factors – the combination of climate change and intense tectonic/volcanic activities, especially in SE Asia – that occurred from the E–O boundary onwards on angiosperm biodiversity. Did sapindaceous lineages follow the same trend as marine biota (Rohde and Muller, 2005; Mayhew et al., 2012) and neotropical flora (Jaramillo et al., 2006) in declining in species diversity during this period? Alternatively, could the establishment of SE Asia have acted as a refugium for tropical angiosperms and triggered their diversification? In this study, we propose to investigate this topic by focusing on four closely related families – Xanthoceraceae, Aceraceae, Hippocastanaceae and Sapindaceae, hereafter referred to as the sapindaceous clade (Buerki et al., 2010). Recently, the authors have focused their effort in circumscribing generic entities within the worldwide sapindaceous clade and proposing a new familial classification based on molecular and morphological data (see, for example, Buerki et al., 2009, 2010). This clade was also used as a case study to assess the performance of various biogeographical methods and propose a worldwide stratified paleogeographical model (from Late Cretaceous to the present) to constrain biogeographical inference (Buerki et al., 2011a). Moreover, the four families within the sapindaceous clade are characterized by several features making them an ideal case study to investigate the effect of past climate and geomorphological change on the diversification and biogeography of flowering plants: (1) worldwide distribution (centres of diversity in South America and SE Asia); (2) available plastid and nuclear phylogeny at the generic level (Buerki et al., 2009); (3) occurrence of reliable fossils dating back to the Eocene (Buerki et al., 2011a); (4) fairly good taxonomic knowledge (see references in Buerki et al., 2009, 2010); and (5) a temporal framework compatible with the examination of processes that occurred at the E–O boundary (i.e. the clade originated during the Late Cretaceous; Buerki et al., 2011a).
Here, our aim is to (1) investigate the effect of the abrupt change in abiotic conditions at the E–O boundary on the spatio-temporal history of sapindaceous lineages and (2) examine how the evolutionary history of the clade was influenced by the geomorphological history of SE Asia. Although some work has been done on the sapindaceous clade, its spatio-temporal history was never properly investigated. To achieve this goal, we entirely re-analyse the plastid and nuclear data set of Buerki et al. (2011a) by performing a BEAST dating analysis (vs. a penalized-likelihood approach in the previous study) and running Lagrange (Ree et al., 2005; Ree and Smith, 2008) inferences on 100 randomly selected trees (vs. only one tree in the previous study). The biogeographical inferences are also constrained according to the same paleogeographical model as in Buerki et al. (2011a). This approach allows phylogenetic and dating uncertainty as well as paleogeography to be taken into account and therefore improves the estimation of the effect of abiotic factors on the evolution of the sapindaceous clade. We also propose an approach to extract major biogeographical trends in the sapindaceous clade by inspecting the genera stem ages in light of their current distribution. Finally, we estimate for the first time the diversification rate (i.e. speciation rate minus extinction rate) per genus within the sapindaceous clade using the estimator of Magallon and Sanderson (2001) [i.e. eqn (3)].
MATERIALS AND METHODS
Data set and divergence time estimation
The data set used to estimate lineage divergence times and ancestral ranges in the sapindaceous clade is based on Buerki et al. (2011a) and contains seven plastid regions and the nuclear ITS (internal transcribed spacer) region. This data set includes >60 % of the generic diversity of the group (147 samples) and one outgroup taxon, Harrisonia abyssinica (Simaroubaceae; for more details, see Buerki et al., 2009). We have included only one outgroup taxon based on previous phylogenetic inferences that strongly supported the monophyly of the sapindaceous clade (e.g. Gadek et al., 1996; APG III, 2009; Buerki et al., 2011b).
A partitioned Bayesian inference approach implemented in the package BEAST v.1.5.4 (Drummond and Rambaut, 2007) was used to infer a temporal framework for the evolution of the sapindaceous clade. Two partitions (plastid and nuclear) were defined following Buerki et al. (2011a) with an uncorrelated relaxed molecular clock assuming a log normal distribution of rates and a Yule speciation model. Two runs of 20 × 106 generations were performed, sampling one tree every 1000th generation. Average branch lengths and 95 % confidence intervals on nodes were calculated using TreeAnnotator v.1.5.4 (Drummond and Rambaut, 2007) after burn-in and reported on a majority rule consensus tree. Six calibration points based on fossil records (see Buerki et al., 2011a) were used to constrain the BEAST analysis (see below). With the exception of the calibration point associated with the root, all points were modelled as follows: log normal distribution, mean = 0, s.d. = 1, offset = fossil age (see below). In the case of the root calibration point, (a) a normal distribution was applied with the mean fixed at 125 million years and s.d. = 1. The calibration points are depicted in Supplementary Data Fig. S1 and the offset values fixed as follows: (b) the stem group of Acer, Aesculus and Dipteronia was constrained with an offset of 55·8 Ma; (c) the stem group of Dodonaea and Diplopeltis was constrained with an offset of 37·2 Ma; (d) the stem group of Koelreuteria was constrained with an offset of 37·2 Ma; (e) the stem group of Pometia was constrained with an offset of 5·33 Ma; and (f) the stem group of Cardiospermum, Paullinia and Serjania was constrained with an offset of 37·2 Ma; see Buerki et al. (2011a) for more details on fossil records.
Diversification analyses
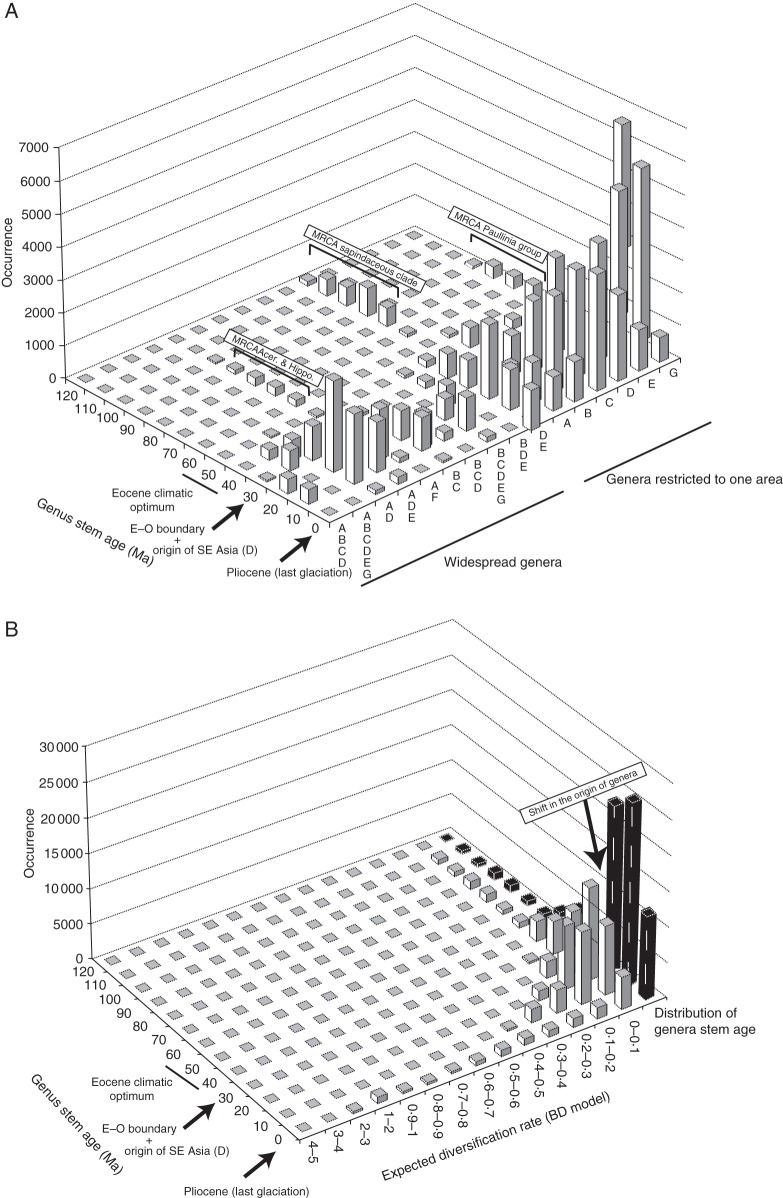
Major trends in diversification and biogeographical patterns were first investigated by extracting the stem age of every genus from a set of 900 randomly selected trees from the BEAST analysis. A 3-D barplot showing the stem age of the genera as a function of their distribution and occurrence was built based on these data. This graph allowed the extraction of major biogeographical trends in the sapindaceous clade using a simple visual representation of the raw data.
To avoid any bias in the diversification analyses due to uneven taxon sampling between lineages (here genera), we pruned the 900 BEAST trees at the generic level (one species per genus) and assigned species richness to each genus based on data presented in Buerki et al. (2009; see table 1 in this publication). Over the set of 900 BEAST trees, the expected diversification rate for each genus was inferred using the estimator proposed by Magallon and Sanderson (2001), i.e. expected diversification rate = log(species richness of a given genus)/stem age. A 3-D barplot was also constructed to display the age of the genera as a function of their expected diversification rates, distribution and occurrence. Finally, a set of two graphs showing the relationship between the generic species richness of a lineage and (1) its mean expected diversification rate or (2) its average age (in Ma) were built.
Biogeographical inferences
Seven geographical areas were used (as in Buerki et al., 2011a; Supplementary Data Fig. S2): (A) Eurasia, from Western Europe to Indochina; (B) Africa; (C) Madagascar, including Comoros and Mascarene islands; (D) SE Asia, including India, the Malaysian Peninsula, Philippines, Sumatra, Borneo and the Inner Banda Arc, as well as the Pacific Islands (e.g. Hawaii); (E) Australia, including New Guinea, New Caledonia and New Zealand; (F) North America; and (G) South America, including Central America and the West Indies. The numbers of genera per area have been estimated based on recent literature (e.g. Buerki et al., 2009). These values provide a first insight into the current spatial generic richness and will serve as a basis for the biogeographical discussion. In this study, we have included India in area D due to the low taxonomic richness of the sapindaceous clade in this area (Buerki et al., 2009). In addition, no genera are endemic to India and most of the species are shared with SE Asia (Buerki et al., 2009). For these reasons and to avoid an unnecessary over-parameterization of the model, we have not considered India as an independent biogeographical area, but we would like to redirect the reader to another more complete paleogeographical model including India as an area (Buerki et al., 2013). The paleogeographical model used in this study was sub-divided into four time slices as follows: (1) Early to Late Cretaceous (120–80 Ma); (2) Late Cretaceous to Early Paleocene (80–61·7 Ma); (3) Middle Paleocene to Late Eocene (61·7–33·9 Ma); and (4) Early Oligocene to the present (33·9–0 Ma) (for more details, see Buerki et al., 2011a, 2013).
The dispersal–extinction–cladogenesis (DEC) likelihood model implemented in Lagrange v. 2.0.1 (Ree et al., 2005; Ree and Smith, 2008) was used to investigate the biogeographical history of the sapindaceous clade following Buerki et al. (2011a) and constraining the reconstruction with a paleogeographical model. To also take phylogenetic and dating uncertainty into account while inferring the biogeographical scenario, the analysis was run on 100 randomly selected BEAST trees (using a collection of R scripts developed in Espindola et al., 2012). The biogeographical scenario was subsequently summarized on the dated majority rule consensus tree of BEAST using pie charts. Finally, the effect of past climate change on range changes was investigated by plotting the number of dispersals through time together with the variation of isotopic O18 composition in the last 60 million years (Zachos et al., 2001).
RESULTS
Phylogenetic inference and divergence time estimation
The BEAST majority rule consensus tree with 95 % confidence intervals on nodes is shown in Supplementary Data Fig. S1. All the informal groups within Sapindaceae were retrieved monophyletic (for more details, see Buerki et al., 2009; Supplementary Data Fig. S1). As shown previously, familial relationships between Xanthoceraceae, Aceraceae + Hippocastanaceae and Sapindaceae are not well resolved (for a full discussion, see Buerki et al., 2010). The tempo of divergence between lineages is highly congruent with the penalized-likelihood estimation provided in Buerki et al. (2011a). The origin of the sapindaceous clade is estimated to have occurred during the Cretaceous, with a differentiation of the four families later during this period (Supplementary Data Fig. S1). Most of the genera within Sapindaceae, for the most part belonging to the Cupania and Paullinia groups, originated from the Eocene onwards, with a peak in the Miocene (Fig. 1, Supplementary Data Fig. S1).
Fig. 1.
(A) 3-D barplot showing the stem age of the genera of the sapindaceous clade (x-axis) as a function of their distribution (y-axis) and occurrence (z-axis). Abbreviations: A, Eurasia from Western Europe to Indochina; B, Africa; C, Madagascar, including the Comoro Islands and the Mascarene Islands; D, South-East Asia including India, the Malaysian Peninsula, Philippines, Sumatra, Borneo, and the Inner Banda Arc, as well as the Pacific Islands (e.g. Hawaii); E, Australia including New Guinea, New Caledonia and New Zealand; F, North America; and G, South America including Central America and the West Indies. (B) 3-D barplot displaying the age of the genera of the sapindaceous clade (x-axis) as a function of their expected birth–death diversification rates (y-axis) and occurrence (z-axis). The distribution of generic stem ages through time (in black) is also presented on the graph. See Fig. 3 for a map of area distribution. BD, birth–death; Ma, million years ago.
Diversification rate analyses
The 3-D barplot displaying the stem age of genera according to their distribution (Fig. 1A) and together with the dated phylogenetic inference (Supplementary Data Fig. S1) supported (1) an origin of the sapindaceous clade in Eurasia during the Cretaceous [i.e. most recent common ancestor (MRCA) of Xanthoceras plus the rest of the sapindaceous clade]; (2) the occurrence of genera of Aceraceae + Hippocastanaceae in North America during the Cretaceous; (3) the dispersal of the MRCA of the Paullinia group to South America during the Eocene; and (4) the origin of almost all the genera from the E–O boundary onwards. This analysis also supported Eurasia as a secondary centre of diversification during the last 30 Ma. Although most of the genera are restricted to one area, the barplot indicated an origin of the widespread genera between 20 and 10 Ma and showed that they are generally occurring in SE Asia and shared with either Eurasia or Australia and to some extent Africa. Taxonomically, these genera mainly belonged to the Litchi, Schleichera and Cupania groups (Supplementary Data Fig. S1, and see below for more details). The barplot showing the stem age of the genera through time (in black in Fig. 1B) supported a rise in the number of genera from the Paleocene onwards, with a substantial increase in their occurrence after the E–O boundary. When diversification analyses were applied, most of the expected diversification rates per genus were retrieved with low values (<0.2) and they were mainly distributed after the E–O boundary (Fig. 1B). This pattern suggested a constant rate of speciation through time, with some young genera (during the Miocene onwards) having increased rates of diversification (Fig. 1B). In fact, if the diversification rate is constant, we expect a plot of stem age vs log(species richness) to be a straight line, with the slope revealing the diversification rate. However, our data clearly deviate from a straight line, with young genera having substantially more species than expected (Fig. 2). As a consequence, applying a standard diversification model (either with a birth–death or a Yule speciation process) with diversification rate changes affecting all lineages in the tree equally (e.g. Morlon et al., 2011; Stadler, 2011; Etienne et al., 2012) is not suitable here, as such models predict that older clades will have more species. In contrast, a scenario with clade-specific diversification rate changes where young genera have accelerated diversification rates is compatible with the data.
Fig. 2.
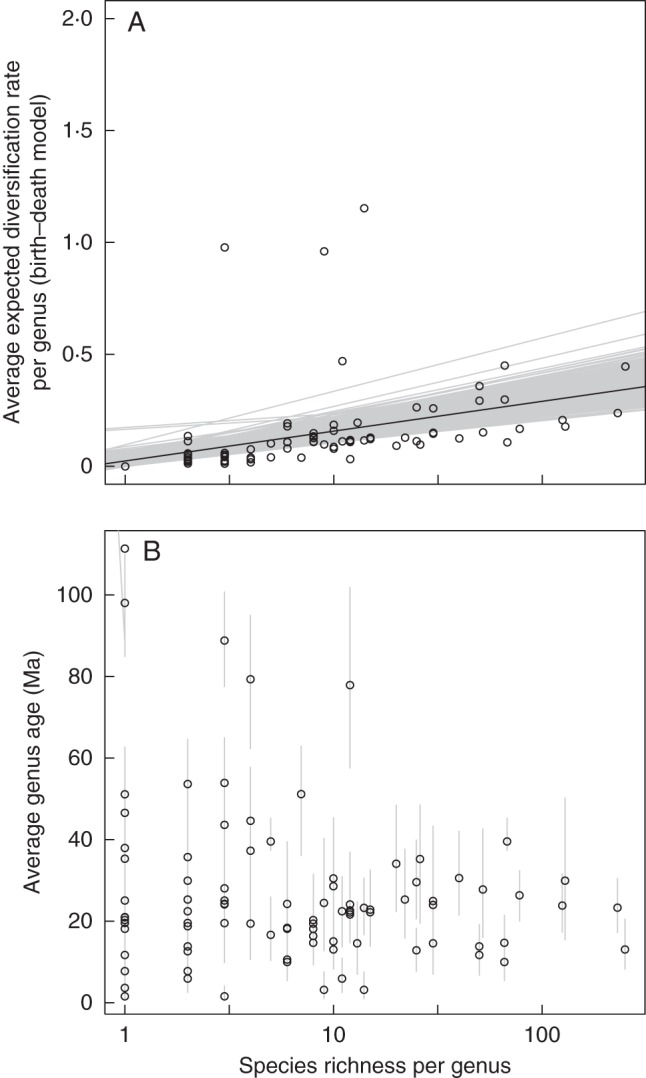
(A) Average expected diversification rate (based on a birth–death model) per genus of the sapindaceous clade displayed according to the generic species richness based on the 900 BEAST trees. Linear correlations (in grey) are also represented. (B) Average genus stem age (in Ma) as a function of species richness per genus. Error bars indicate 95 % confidence intervals on the node. For both panels the species richness per genus was log-transformed.
Biogeographical inferences
Most genera in the sapindaceous clade are restricted in distribution; of the 142 currently described genera, 96 are restricted to one area as defined here, 33 are distributed in two areas, seven in three areas, two in four areas and three in five areas. The current generic richness for each area is represented in Supplementary Data Fig. S2. The highest generic richness within the sapindaceous clade is found in SE Asia (45 genera, but only seven endemic genera), whereas the lowest occurred in North America (four genera; Supplementary Data Fig. S2). South America had the highest percentage of endemism (32 genera), with 34.4 % of monotypic genera (Supplementary Data Fig. S2).
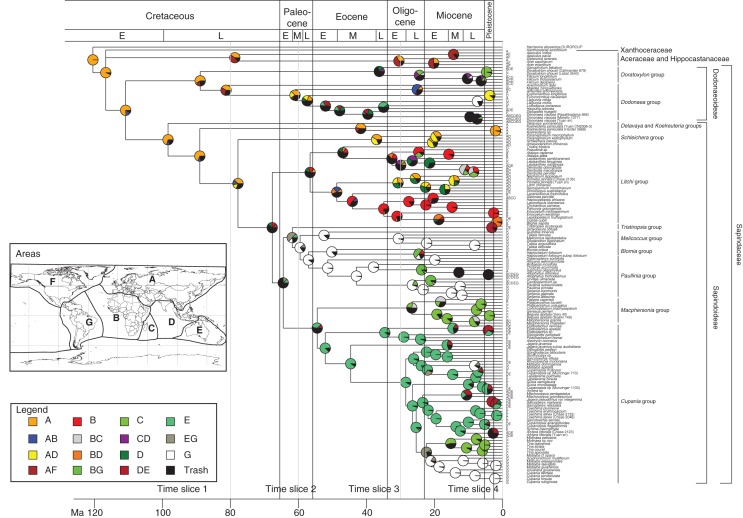
Results from the biogeographical analysis (encompassing the 100 Lagrange analyses) is presented on the BEAST majority rule consensus tree (Fig. 3). Dating uncertainty did not influence ancestral area reconstructions. The highest number of dispersals took place during time slice 4 (with 53 dispersals out of 69), whereas only one dispersal event was inferred during time slice 1. The 44 extinction events inferred by Lagrange are distributed as follows: zero in time slice 1, five in time slice 2, 11 in time slice 3 and 28 in time slice 4. Africa and SE Asia have the highest number of inferred extinctions, with 12 and ten extinctions, respectively, whereas only one extinction event was estimated in North America.
Fig. 3.
Biogeographical reconstruction of the sapindaceous clade based on 100 Lagrange analyses constrained with the paleogeographical model published in Buerki et al. (2011a) and plotted on the BEAST majority rule consensus tree. Pie charts on nodes represent probabilities for assigned ancestral area(s). The classification follows Buerki et al. (2009, 2010). See the Materials and Methods section for more details on the paleogeographical model and Fig. 1 for area definitions. Ma, million years ago.
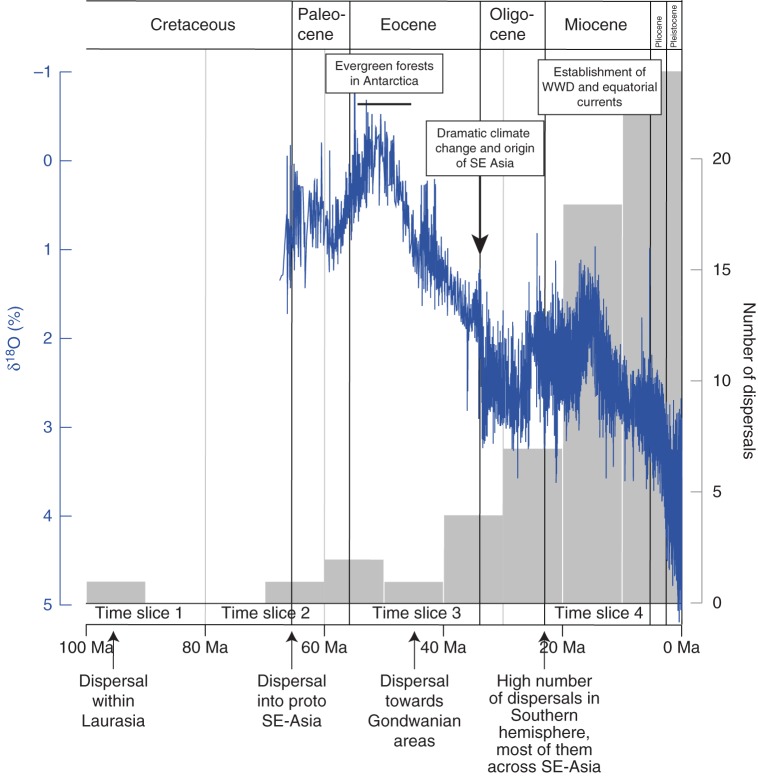
Figure 4 depicts the biogeographical scenario for the four time slices plotted on paleogeographical maps, and dispersals are provided in Fig. 5. The biogeographical reconstruction supported an origin of the sapindaceous clade in Eurasia in the Early Cretaceous (Figs 1A and 4). With the exception of one dispersal event that was inferred before 80 Ma (corresponding to the dispersal of the MRCA of Aceraceae + Hippocastanaceae in North America), the sapindaceous lineages remained in Laurasia until the Late Paleocene (Figs 1A and 4A). After migrating to proto-SE Asia during time slice 2, a first colonization event of the southern hemisphere from this area (that only included the Malay Peninsula and part of Borneo at that time) took place during time slice 3 (Fig. 4). This region acted as an important catalyser during the evolutionary history of the sapindaceous clade, by connecting northern and southern hemispheres, especially during time slice 4 (Fig. 4C, D). This pattern was also suggested by only taking into account the stem age of the genera and their current distribution (Fig. 1A). During time slice 3 a single long dispersal from Australia to South America (via Antarctica) gave rise to the radiation of the speciose Paullinia group (Fig. 4C). During time slices 3 and 4, the spread of sapindaceous lineages has been favoured by the Gondwanan break-up: for instance, the collision of the African and Eurasian plates and the northern drift of India that occurred during time slice 3, as well as the emergence of SE Asian islands, mainly resulting from the collision of the Australian and Eurasian plates during time slice 4 (Fig. 4). In the latter case, SE Asia acted as a bridge between Australia and Eurasia (Fig. 4). In addition, the establishment of the West Wind Drift (WWD) and equatorial currents might also have mediated long-distance dispersal (LDD) events during time slice 4 (Fig. 4D). When the number of dispersals through time is compared with the variation of isotopic O18 composition from the last 60 million years, a trend is observed shortly after the abrupt climate change at the E–O boundary, followed by a progressive increase of dispersals, especially during the Miocene onwards (Fig. 5).
Fig. 4.
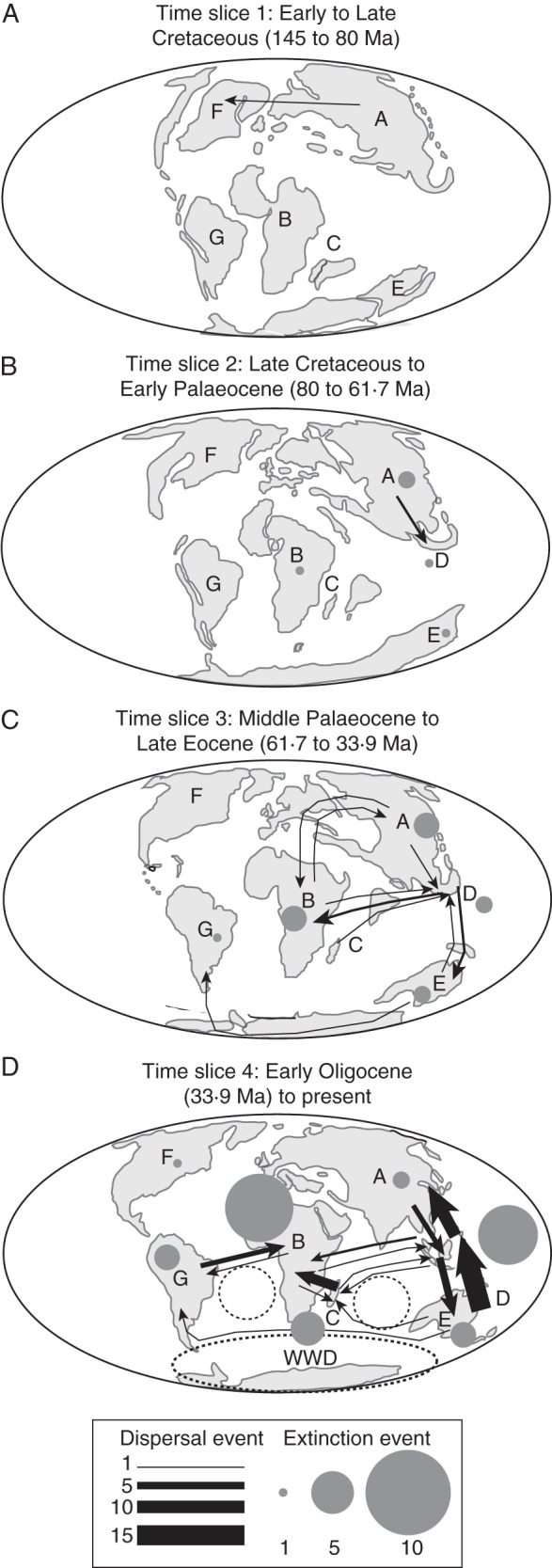
Biogeographical scenario of the sapindaceous clade inferred from the 100 Lagrange analyses (see Supplementary Fig. S2). Dispersals and extinctions are represented in proportion to their occurrence by arrows and circles, respectively. Paleogeographical maps were adapted from Buerki et al. (2011a). See Figs 1 and 3 for abbreviations.
Fig. 5.
Occurrence of dispersal events through time for the sapindaceous clade inferred by the Lagrange analyses (grey bars). Climatic oscillations (blue lines), estimated from the variation of oxygen-18 isotope concentration through time, is also displayed (Zachos et al., 2001). Ma, million years ago.
DISCUSSION
Laurasian origin of the sapindaceous clade with subsequent dispersals into the southern hemisphere mediated by proto-SE Asia and the Gondwanan break-up
Our results suggest an origin of the sapindaceous clade in Eurasia sometime during the Late Cretaceous, with subsequent dispersals into the southern hemisphere during the Late Paleocene mediated by the Gondwanan break-up and the emergence of proto-SE Asia (Figs 1A, 3 and 4). Currently, >80 % of the generic diversity of this clade (corresponding to Sapindaceae) is restricted to tropical and sub-tropical ecosystems of the southern hemisphere. Three main routes of dispersals were used by sapindaceous lineages to colonize the southern hemisphere (Fig. 4C): (1) a first route of dispersal connected Eurasia with Africa and resulted from the collision of the African and Eurasian plates; (2) a second route was established between proto-SE Asia, Africa and Madagascar, and resulted from the break-up of India and Madagascar and the northern raft of India; (3) a third route connected proto-SE Asia and Australia and was facilitated by the existence of a myriad of archipelagos (see, for example, Hall, 2009; Fig. 4C). From this period to the end of the Eocene, the climate was warm (Bowen, 2007; Zhonghui et al., 2009) and might have favoured the proliferation of sapindaceous lineages in the southern hemisphere. Unlike Cucurbitaceae that extended to South America from Asia (through Africa; Schaefer et al., 2009), South American lineages of the sapindaceous clade (in this case, the MRCA of the Paullinia group) used the third route of dispersal and further dispersed through Antarctica (Fig. 4C). This dispersal was estimated to have occurred during the Middle Eocene (approx. 44 Ma). The warm climate during this period (with ice probably only occurring in the Antarctic highlands and within and around the Arctic Ocean in the north; e.g. Bowen, 2007; Zhonghui et al., 2009; Figs 4 and 5) combined with specific tectonic configuration mediated this LDD (Fig. 4C). Once established in South America, the Paullinia group diversified shortly after the E–O boundary and currently contributes to approx. 30 % of the entire sapindaceous richness (approx. 600 species; Buerki et al., 2009). This unique diversification within the sapindaceous clade is associated with two morphological synapomorphies: the development of zygomorphic flowers (vs. actinomorphic flowers in most lineages) and a liana habit (vs. a shrub to tree habit in the other lineages). As demonstrated by two studies, these synapomorphies are usually associated with (1) an increase in successful animal pollination (Sargent, 2004) and (2) higher species diversities in liana clades compared with their sister tree clades (Gianoli, 2004). These morphological features could therefore explain the impressive success of the Paullinia group. Finally, although most of the species within this group are restricted to South America, taxa assigned to Allophylus subsequently colonized the paleotropics, most probably during the Miocene onwards mediated by the WWD and sub-equatorial currents (e.g. Takayama et al., 2008).
Interestingly, 75 % of the sampled genera occurring in Africa are found in the Litchi group (see also Blomia and Macphersonia groups for the remaining genera; Supplementary Data Fig. S2) and exhibit close relationships with taxa widely distributed over SE Asia and tropical Eurasia (e.g. Dimocarpus and Litchi) and to some extent Madagascar (e.g. Deinbollia; Fig. 3). These taxa originated sometime between the Late Paleocene and Early Eocene, and seem to have colonized Africa either using route 1 in the case of the endemic African genera (e.g. Chytranthus and Pancovia) or using route 2 in the case of taxa mainly shared with SE Asia and to some extent Madagascar (e.g. Lepisanthes and Deinbollia) (Figs 3 and 4C). However, further investigations on the Litchi group (based on an expanded sampling in SE Asia and Africa) have to be conducted to confirm these preliminary results.
Effect of the E–O boundary climate change and the emergence of islands in SE Asia on the fate of sapindaceous lineages
The spatio-temporal history of sapindaceous lineages appears to have been strongly linked with paleoclimatic changes and tectonic movements that allowed recurrent events of dispersal and isolation among neighbouring land masses. From the E–O boundary to the Miocene, the biogeographical scenario strongly indicates that SE Asia has acted as a bridge allowing sapindaceous lineages (more specifically taxa of Sapindaceae) to escape the effect of the abrupt climate change and remain within the tropical belt (Fig. 4). Although a west to east route of dispersal was identified in this region in other plant groups [e.g. Alocasia (Araceae), Nauheimer et al. (2012); Aglaia (Meliaceae), Muellner et al. (2008); Begonia (Begoniaceae), Thomas et al., (2012); Margaritopsis (Rubiaceae), Barrabé et al. (2012)], our study shows that a majority of the sapindaceous lineages initiated an important northward migration (14 dispersals from Australia to SE Asia and seven dispersals from SE Asia to Eurasia; Fig. 4D) in response to the northwards shift of tropical zones at the E–O boundary and the formation of Wallacea (Morley, 2003; Hall, 2009). A similar pattern was observed in several angiosperm families such as Monimiaceae (Renner et al., 2010), Myrtaceae (Sytsma et al., 2004) and Proteaceae (Barker et al., 2007). Despite the occurrence of extinctions during this geological period (especially in Africa and SE Asia; Fig. 4D), the abrupt climate change coupled with the emergence of most SE Asian islands seems to have opened up new routes of dispersals and appears to be associated with the origin of several genera (especially in subfamily Sapindoideae; Figs 1A and 4). The genera found in SE Asia (and in the neighbouring areas) mainly belong to the Litchi, Schleichera and Cupania groups (Fig. 3). Genera of the former two groups originated in Eurasia and subsequently dispersed to SE Asia, whereas the genera of the Cupania group used a northern route of dispersal from Australia to SE Asia (Fig. 4). Interestingly, all these genera reached SE Asia at the same time during the Early Oligocene onwards (Figs 1A, 4D and 5). In Borneo, these genera are found in sympatry, but recent fieldwork conducted by the first author suggested that these genera do not produce fruits during the same period: genera of the Litchi and Schleichera groups (characterized by an indehiscent fruit with a fleshy arillode, e.g. Litchi) are fruiting in August/September, whereas the other genera (the Cupania group is mostly characterized by a dehiscent fruit with a dry arillode, e.g. Elattostachys; for more details see Buerki et al., 2011a) are producing fruits much earlier. This trend could be reminiscent of the different spatial origins of these genera and has to be further investigated (Fig. 3). Although taxa of the sapindaceous clade are mainly restricted to humid tropical ecosystems (Sapindaceae), several genera of subfamily Dodonaeoideae are currently mostly found in dry Australian ecosystems (Dodonaea group; Buerki et al., 2009; Fig. 3). Our analysis suggests that the origin of some of these genera (e.g. Diplopeltis) is concomitant with the creation of new open habitats in Australia (associated with adaptation to drought; Bowen, 2007).
Conclusions and perspectives
The increase in dispersal events and number of genera that originated in the sapindaceous clade from the E–O boundary onwards might be explained by two main factors: (1) the stable tropical climate of SE Asia (Sohdi et al., 2004; Lohman et al., 2011) and (2) the emergence of new islands suitable to offer new niches for the establishment of sapindaceous lineages (especially in the Wallacea region; Hall, 2009; Richardson et al., 2012). This pattern is in line with angiosperm fossil data indicating an increase in taxa diversity in this area during this period (Morley, 2003). Unfortunately, there is currently very little reliable fossil evidence of Sapindaceae in this region and further investigations are required. It is therefore highly likely that SE Asia might have acted as a refugium during the E–O boundary for taxa restricted to tropical ecosystems (see, for example, Bush and Flenley, 2007; Fig. 4D). In addition to its role as a refugium, the region might have provided new niches for angiosperm lineages to diversify, as suggested by the increase of new genera at this period.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We wish to thank Philippe Küpfer and Isabel Sanmartin for their enthusiasm, comments and help that contributed to the elaboration of this study. We also would like to thank Martin Callmander (Missouri Botanical Garden, USA) for his valuable support with taxonomic queries. Finally, we are grateful to two anonymous reviewers who provided valuable comments to improve our manuscript. Financial support to S.B. was provided by a Marie Curie Intra-European Fellowship (CRADLE; no 253866). N.A. was funded by the Swiss National Science Foundation (fellowship PZ00P3_126624).
LITERATURE CITED
- APG III. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009;161:105–121. [Google Scholar]
- Barker NP, Weston PH, Rutschmann F, Sauquet H. Molecular dating of the ‘Gondwanan’ plant family Proteaceae is only partially congruent with the timing of the break-up of Gondwana. Journal of Biogeography. 2007;34:2012–2027. [Google Scholar]
- Barrabé L, Buerki S, Mouly A, Davis AP, Munzinger J, Maggia L. Delimitation of the genus Margaritopsis (Rubiaceae) in the Asian, Australasian and Pacific region, based on molecular phylogenetic inference and morphology. Taxon. 2012;61:1251–1258. [Google Scholar]
- Bowen GJ. Palaeoclimate – when the world turned cold. Nature. 2007;445:607–608. doi: 10.1038/445607a. [DOI] [PubMed] [Google Scholar]
- Buerki S, Forest F, Acevedo-Rodríguez P, et al. Plastid and nuclear DNA markers reveal intricate relationships at subfamilial and tribal levels in the soapberry family (Sapindaceae) Molecular Phylogenetics and Evolution. 2009;51:238–258. doi: 10.1016/j.ympev.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Buerki S, Lowry PP, II, Alvarez N, Razafimandimbison SG, Küpfer P, Callmander MW. Phylogeny and circumscription of Sapindaceae revisited: molecular sequence data, morphology and biogeography support recognition of a new family, Xanthoceraceae. Plant Ecology and Evolution. 2010;143:148–161. [Google Scholar]
- Buerki S, Forest F, Alvarez N, Nylander JAA, Arrigo N, Sanmartín I. An evaluation of new parsimony-based versus parametric inference methods in biogeography: a case study using the globally distributed plant family Sapindaceae. Journal of Biogeography. 2011a;38:531–550. [Google Scholar]
- Buerki S, Forest F, Salamin N, Alvarez N. Comparative performance of supertree algorithms in large data sets using the soapberry family (Sapindaceae) as a case study. Systematic Biology. 2011b;60:32–44. doi: 10.1093/sysbio/syq057. [DOI] [PubMed] [Google Scholar]
- Buerki S, Devey DS, Callmander MW, Phillipson PB, Forest F. Spatio-temporal history of the endemic genera of Madagascar. Botanical Journal of the Linnean Society. 2013;171:304–329. [Google Scholar]
- Bush MB, Flenley JR. Tropical rainforest responses to climatic change. Berlin: Springer; 2007. [Google Scholar]
- Cantrill DJ, Poole I. Taxonomic turnover and abundance in Cretaceous to Tertiary wood floras of Antarctica: implications for changes in forest ecology. Palaeogeography, Palaeoclimatology, Palaeoecology. 2005;215:205–219. [Google Scholar]
- Coetzee JA, Muller J. The phytogeographic significance of some extinct Gondwana pollen types from the Tertiary of the southwestern Cape (South Africa) Annals of the Missouri Botanical Garden. 1984;71:1088–1099. [Google Scholar]
- Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espindola A, Buerki S, Alvarez N. Ecological and historical drivers of diversification in the fly genus Chiastocheta Pokorny. Molecular Phylogenetics and Evolution. 2012;63:466–474. doi: 10.1016/j.ympev.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Etienne RS, Haegeman B, Stadler T, et al. Diversity-dependence brings molecular phylogenies closer to agreement with the fossil record. Proceedings of the Royal Society B: Biological Sciences. 2012;279:1300–1309. doi: 10.1098/rspb.2011.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek PA, Fernando ES, Quinn CJ, et al. Sapindales: molecular delimitation and infraordinal groups. American Journal of Botany. 1996;83:802–811. [Google Scholar]
- Gianoli E. Evolution of a climbing habit promotes diversification in flowering plants. Proceedings of the Royal Society B: Biological Sciences. 2004;271:2011–2015. doi: 10.1098/rspb.2004.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. Southeast Asia's changing palaeogeography. Blumea. 2009;54:148–161. [Google Scholar]
- Jablonski D. Lessons from the past: evolutionary impacts of mass extinctions. Proceedings of the National Academy of Sciences, USA. 2001;98:5393–5398. doi: 10.1073/pnas.101092598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo C, Rueda MJ, Mora G. Cenozoic plant diversity in the neotropics. Science. 2006;311:1893–1896. doi: 10.1126/science.1121380. [DOI] [PubMed] [Google Scholar]
- Katz ME, Miller KG, Wright JD, et al. Stepwise transition from the Eocene greenhouse to the Oligocene icehouse. Natural Geosciences. 2008;1:329–334. [Google Scholar]
- Lohman DJ, De Bruyn M, Page T, et al. Biogeography of the Indo-Australian archipelago. Annual Review of Ecology, Evolution and Systematics. 2011;42:205–226. [Google Scholar]
- Magallon S, Sanderson MJ. Absolute diversification rates in angiosperm clades. Evolution. 2001;55:1762–1780. doi: 10.1111/j.0014-3820.2001.tb00826.x. [DOI] [PubMed] [Google Scholar]
- Mayhew PJ, Bell MA, Benton TG, McGowan AJ. Biodiversity tracks temperature over time. Proceedings of the National Academy of Sciences, USA. 2012;109:15141–15145. doi: 10.1073/pnas.1200844109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe I. Paleozoic and Mesozoic geological evolution of the SE Asia region: multidisciplinary constraints and implications for biogeography. In: Halle R, Holloway JD, editors. Biogeography and geological evolution of SE Asia. Leiden: Backhuys Publisher; 1998. pp. 25–41. [Google Scholar]
- Morley RJ. Interplate dispersal paths for megathermal angiosperms. Perspectives in Plant Ecology, Evolution and Systematics. 2003;6:5–20. [Google Scholar]
- Morlon H, Parsons TL, Plotkin J. Reconciling molecular phylogenies with the fossil record. Proceedings of the National Academy of Sciences, USA. 2011;108:16327–16332. doi: 10.1073/pnas.1102543108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellner AN, Pannell CM, Coleman A, Chase MW. The origin and evolution of Indomalesian, Australasian and Pacific island biotas: insights from Aglaieae (Meliaceae, Sapindales) Journal of Biogeography. 2008;35:1769–1789. [Google Scholar]
- Nauheimer L, Boyce PC, Renner SS. Giant taro and its relatives: a phylogeny of the large genus Alocasia (Araceae) sheds light on Miocene floristic exchange in the Malesian region. Molecular Phylogenetics and Evolution. 2012;63:43–51. doi: 10.1016/j.ympev.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Ree RH, Smith SA. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology. 2008;57:4–14. doi: 10.1080/10635150701883881. [DOI] [PubMed] [Google Scholar]
- Ree RH, Moore BR, Webb CO, Donoghue MJ. A likelihood framework for inferring the evolution of geographic range on phylogenetic trees. Evolution. 2005;59:2299–2311. [PubMed] [Google Scholar]
- Renner SS, Strijk JS, Strasberg D, Thebaud C. Biogeography of the Monimiaceae (Laurales): a role for East Gondwana and long-distance dispersal, but not West Gondwana. Journal of Biogeography. 2010;37:1227–1238. [Google Scholar]
- Richardson JE, Costion CM, Muellner AM. The Malesian floristic interchange: plant migration patterns across Wallace's line. In: Gower DJ, Richardson JE, Rosen BR, Rüber L, Williams ST, editors. Biotic evolution and environmental change in Southeast Asia. Cambridge: Cambridge University Press; 2012. pp. 138–163. [Google Scholar]
- Rohde RA, Muller RA. Cycles in fossil diversity. Nature. 2005;434:208–210. doi: 10.1038/nature03339. [DOI] [PubMed] [Google Scholar]
- Sargent RD. Floral symmetry affects speciation rates in angiosperms. Proceedings of the Royal Society B: Biological Sciences. 2004;271:603–608. doi: 10.1098/rspb.2003.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer H, Heibl C, Renner SS. Gourds afloat: a dated phylogeny reveals an Asian origin of the gourd family (Cucurbitaceae) and numerous oversea dispersal events. Proceedings of the Royal Society B: Biological Sciences. 2009;276:843–851. doi: 10.1098/rspb.2008.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi NJ, Koh LP, Brook BW, Ng PKL. South East Asian biodiversity: an impending disaster. Trends in Ecology and Evolution. 2004;19:654–660. doi: 10.1016/j.tree.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Stadler T. Mammalian phylogeny reveals recent diversification rate shifts. Proceedings of the National Academy of Sciences, USA. 2011;108:6187–6192. doi: 10.1073/pnas.1016876108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytsma KJ, Litt A, Zjhra ML, Pires JC, Nepokroeff M, Conti E, Walker J, Wilson PG. Clades, clocks, and continents: historical and biogeographical analysis of Myrtaceae, Vochysiaceae, and relatives in the Southern Hemisphere. International Journal of Plant Sciences. 2004;165:S85–S105. [Google Scholar]
- Takayama K, Tateishi Y, Murata J, Kajita T. Gene flow and population subdivision in a pantropical plant with sea-drifted seeds Hibiscus tiliaceus and its allied species: evidence from microsatellite analyses. Molecular Ecology. 2008;17:2730–2742. doi: 10.1111/j.1365-294X.2008.03799.x. [DOI] [PubMed] [Google Scholar]
- Thomas DC, Hughes M, Phutthai T, et al. West to east dispersal and subsequent rapid diversification of the mega-diverse genus Begonia (Begoniaceae) in the Malesian archipelago. Journal of Biogeography. 2012;39:98–113. [Google Scholar]
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- Zhonghui L, Pagani M, Zinniker D, et al. Global cooling during the Eocene–Oligocene climate transition. Science. 2009;323:1187–1190. doi: 10.1126/science.1166368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.