Abstract
Background
To quantify adherence to oral therapies in ethnically diverse and economically disadvantaged patients with rheumatoid arthritis (RA) using electronic medication monitoring, and to evaluate the clinical consequences of low adherence.
Methods
107 patients with RA enrolled in a 2-year prospective cohort study agreed to have their oral RA drug therapy intake electronically monitored, with the Medication Events Monitoring System (MEMS®). Adherence to disease-modifying antirheumatic drugs (DMARDs) and prednisone were determined as the percentage of days (or weeks for methotrexate) in which the patient took the correct dose as prescribed by the physician. Patient outcomes were assessed including the Modified Health Assessment Questionnaire (MHAQ), the Disease Activity Index 28 (DAS28), quality of life and radiological damage using Sharp-van der Heijde scores.
Results
Adherence to the treatment regimen as determined by percent of correct doses was 64% for DMARDs and 70% for prednisone. Patients who had better mental health were statistically more likely to be adherent. Only 23 (21%) of the patients had an average adherence to DMARDs ≥ 80%. These patients showed significantly better disease activity scores across 2 years of follow-up than those who were less adherent (DAS28 3.3±1.3 vs. 4.1±1.2, p<0.02). Radiological scores were also worse in non-adherent patients at baseline and 12 months.
Conclusions
Only one fifth of the RA patients had an overall adherence of at least 80%. Less than two thirds of the prescribed DMARD doses were correctly taken. Adherent patients had lower disease activity and radiological damage scores across the 2 years of follow-up.
Over the last decade, great advances have been made in the treatment of rheumatoid arthritis (RA) with the development of new biologic therapies (1). Despite these advances, oral disease modifying antirheumatic drugs (DMARDs), most commonly methotrexate, remain the cornerstone of therapy in RA, invariably used as initial therapy. Furthermore, it has been shown that most biologic therapies are more effective when administered in combination with methotrexate. To receive the full benefit of these treatments patients must adhere with the requirements of their prescribed drug regimens (2).
Adherence can be assessed using direct or indirect methods. Direct methods include performing biologic assays (e.g., serum drug concentration) and/or direct observation, but are difficult to implement because of their invasiveness, high cost and the variability in results owing to individual pharmacokinetics. (3) Patient observation only can be performed in infusion centers or through direct observation of the patient ingesting the medication (4). Indirect methods, including pharmacy data, patient diaries, pill counts, and patient interviews, are used more frequently than are direct methods (5-10). Self-report methods are relatively inexpensive and easy to use but can result in under or more commonly, overestimation of adherence (4, 11). Advances in the nanotechnology field enabled the development of a new indirect method based on electronic medication adherence monitoring. The cap of a medication bottle records the time and date of each opening through an integrated microcircuitry. This method has many advantages, including the noninvasive and automatic recording of data, decreased susceptibility to modifications, and it also provides a more complete and accurate measure of patient adherence (e.g., coverage time, distribution of doses, and gaps without medication) (12, 13).
To our knowledge, only two studies have utilized electronic monitoring to measure adherence in patients with RA (14, 15). In one study on 961 patients, the electronic monitoring data were combined with patient self-reporting, making it impossible to accurately extract the electronic monitoring results from the combined data. Furthermore, the follow-up of this study was only 14 days, limiting inferences about long-term adherence and its effects (15). The other study analyzed adherence in 81 RA patients and found a greater rate of adherence in patients taking methotrexate than in patients taking sulfasalazine (81% vs 55%, respectively). However, follow-up in this study was only 6 months and long-term consequences, or determinants of non-adherence were not assessed (14).
The purpose of our study was to quantify adherence to prescribed oral drug regimens in patients with RA over a 2-year period using an electronic medication monitoring system and to examine various determinants of adherence, as well as the clinical consequences of non-adherence.
PATIENTS AND METHODS
Patients
Patients in this study were participants of a larger prospective cohort of RA patients recruited from three publicly funded outpatient rheumatology clinics in Houston, TX, two of them within Harris County Hospital District, Ben Taub General Hospital and Lyndon B. Johnson General Hospital, and the other one, the Michael E. DeBakey Veterans Affairs Medical Center. The study was approved by the institutional review boards of participating institutions. All participants signed written informed consent.
Inclusion criteria were: age 18 to 80 years; fulfillment of American College of Rheumatology diagnostic criteria for RA (16); disease duration ≤ 15 years; current treatment with steroids and/or DMARDs (including methotrexate, leflunomide, sulfasalazine, hydroxychloroquine, and azathioprine), with or without concomitant administration of biologic agents; and English or Spanish language proficiency as determined by the interviewer. Consecutive patients in the clinic who met the inclusion criteria were invited to participate in the study and those who agreed chose whether they wanted to be monitored electronically. Only those patients who accepted electronic monitoring are included in this analysis.
Patient reported outcomes and assessment
Patients were followed for 2 years and were assessed at baseline and at 3, 6, 12, 18, and 24 months. We collected baseline sociodemographic characteristics, including ethnicity, age, sex, marital status, education level, number of household members, income, employment, and health insurance status. At each visit patients completed the Modified Health Assessment Questionnaire (MHAQ; a higher score representing greater disability) (17), self-reported visual analogue scale (VAS) of general health, comorbidities, pain and disease activity. Additional self-report questionnaires were completed at baseline, 12 and 24 months. Health related quality of life (HRQoL) was assessed using the Medical Outcome Study (MOS) 12-item short form health survey (SF12) (18). Social support was ascertained with the MOS social support survey (1-5 scale in which 1 represented the worst social support ) (19). Depression was measured with the Center for Epidemiologic Studies Depression Scale 10-item survey (CESD10; 0-30 scale in which 30 represented the most depressed) (20). We also recorded number, type and quantity of drugs used for diseases other than RA.
Many patients spoke only Spanish. Questionnaires were translated into Spanish using forward and backward translation by independent translators followed by bilingual group evaluation and consensus on item translation.
Clinical assessment
At each patient visit, the physician evaluated the patient’s general health, disease activity and level of pain using a visual analogue scale (VAS), and 28-joint counts (tenderness and swelling). Laboratory testing was performed at baseline, 12 and 24 months including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). An index disease activity score (DAS28) was calculated for baseline and each annual visit (21).
Radiographs of both hands were requested at baseline and at years 1 and 2, and were scored using the van der Heijde modified Sharp method (22). Only patients who had a baseline and at least one follow-up radiograph were analyzed. Radiographic progression was determined as the difference between baseline and follow-up scores.
Assessment of adherence
Medication adherence was defined as “the extent to which a patient acts in accordance with the prescribed interval, dose, and dosing regimen” (23). Only adherence for oral DMARDs and steroids was recorded. No effort was made to monitor adherence to nonsteroidal anti-inflammatory drugs (NSAIDs) or drug therapies for comorbid conditions. Adherence was assessed using the medication event monitoring system (MEMS®; AARDEX. Ltd. Zug, Switzerland). The MEMS® cap, designed to compile dosing histories of oral medications, has a microcircuitry that records the time and date each time the bottle is opened. The system assumes that every opening of the bottle signifies the prescribed dose is taken. Additional software (QuickRead and PowerView, AARDEX. Ltd.) and a MEMS® reader was used to capture data and perform analysis. MEMS® caps are designed for oral drugs, therefore, adherence to biologic therapies, which are only administered as injections or infusions, was not assessed, although we recorded whether patients were receiving these agents.
Measures of adherence were:
Doses taken as prescribed: Percentage of days (or weeks for methotrexate) in which the patient took the correct dose as prescribed, calculated as total number of days or weeks with the correct number of doses, divided by total number of monitored days multiplied by 100.
Underdosing: Percentage of days or weeks in which the patient took fewer doses than prescribed, calculated as total number of days or weeks with fewer recorded doses divided by total number of monitored days multiplied by 100.
Overdosing: Percentage of days or weeks in which the patient took more doses than prescribed, calculated as total number of days or weeks with more recorded doses divided by total number of monitored days multiplied by 100.
The MEMS cap software was set in order to allow a margin of error of 25% in time between doses. For example, if the drug had to be taken every 12 hours, the dose was considered “taken as prescribed” when the interval between doses ranged from 9 to 15 hours (+/- 25% or 3 hours). Occasional openings of the container unrelated to drug taking (e.g., opening for refills) were excluded from the analysis. These openings were assessed by patient interview and direct comparison with the pharmacy refill register. During the initial visit, patients were informed about the features of the MEMS®, so they were aware of the monitoring capability of the bottle.
Adherence was also measured using the Compliance Questionnaire Rheumatology (CQR). Comparison of baseline CQR scores between patients who accepted electronic monitoring and those who declined was carried out, looking for bias in group selection according to baseline adherence (e.g., whether patients who accepted electronic monitoring were more adherent than those who did not) (11).
Statistical analysis
Adherence measures were analyzed as a continuous variable and then dichotomized in two categories according to an 80% adherence cut-off, which has been traditionally used in chronic disease studies (11). Categorical variables were compared using the chi-square test or the Fisher exact test. For bivariate continuous variables we used the Student t test, Pearson correlation coefficients, and one-way analysis of variance (ANOVA). Tukey’s standardized difference, Bonferroni and Scheffe multiple comparison tests were used for post hoc analysis. A stepwise multiple regression analysis using the percent of doses taken as prescribed as the dependent variable was performed to identify predictors of adherence (variable significance entry criterion of p<0.15 and removal criterion of p>0.05). Only patients who completed 2 years of follow-up were included in the outcome analysis examining the association of adherence with clinical measures. We performed a multiple regression analysis using DAS28 at 24-months as dependent variable and prescribed doses taken of DMARDs as the independent variable adjusting for age, gender, disease duration, prednisone cumulative dose, baseline DAS28, number of DMARDs and whether the patient had received a biologic agent. Missing DAS28 values at 24-months (n=21, 19.6%) were imputed using last observation carried forward. A two-sided p value of 0.05 was considered statistically significant.Data analyses were performed using SAS version 9.1.3.
RESULTS
We screened over 1700 patients; of these, 201 had RA, fulfilled the inclusion criteria and agreed to participate (refusal rate was 32%); 107 of the 201 patients were on DMARDs and agreed to electronic monitoring of their oral therapies using MEMS® caps over the 2 years of the study. When compared to patients who did not want to be electronically monitored, participants in the MEMS® group were younger, and more likely to be female and/or of Hispanic ethnicity. There were no statistically significant differences between patients who accepted electronic monitoring and those who did not with regard to baseline disease activity, functional status, radiological damage, quality of life measures, depression, comorbidities, and self-reported adherence (CQR). Most patients (92%) were patients at a Harris County District Hospital. Patients were primarily non-white (65% Hispanic; 19% African-American; 16% White), female (87%), had a mean disease duration of 8±6 years, moderate disease activity (DAS28=4.7±1.6), a low educational status (45% had not completed high school), and 67% had an income < $20,000/year.
Methotrexate was the most prescribed DMARD (81%), followed by leflunomide (43%), hydroxychloroquine (36%), sulfasalazine (15%), and azathioprine (2%). Seventy-six patients (68%) also received prednisone, and 63 patients (57%) were treated with biologic agents. Fifty patients (45%) were taking one DMARD, 40 patients (36%) two, 17 patients (15%) three DMARDs and 4 patients (3%) received four DMARDs. During the two years of follow-up, 93 (28%) of 336 RA-related drugs (excluding NSAIDs and/or analgesics) were discontinued (22% DMARDs, 22% prednisone, and 36% biologic agents), most commonly because of adverse events or lack of efficacy.
Adherence assessment
A total 205 (75%) of 273 prescribed drugs (DMARDs and/or prednisone) were monitored in the MEMS® group. Table 1 shows the adherence measures across two years of follow-up. Mean adherence ranged from 58% to 71% for each DMARD and was 70% for prednisone. Patients took less than the prescribed dose 22% to 41% of the time. Overdosing occurred less frequently, varying from 1% for sulfasalazine to 14% for methotrexate. Only 23 (21%) of 107 patients took DMARDs as prescribed at least 80% of the time, and 23 (41%) of 56 patients took prednisone as prescribed at least 80% of the time.
Table 1.
Mean adherence during the 2-year follow-up for individual DMARDS and prednisone
| Methotrexate | Leflunomide | Hydroxy- chloroquine |
Sulfasalazine | All DMARDs | Prednisone | |
|---|---|---|---|---|---|---|
|
Number of patients who received
the prescribed drug |
76 | 35 | 29 | 8 | 107 | 56 |
| Prescribed days, mean (SD) | 650 (185) | 503 (255) | 637 (205) | 577 (242) | 591 (198) | 603 (230) |
|
Days under electronic monitoring mean (SD) |
451 (254) | 379 (269) | 494(245) | 303 (334) | 421 (247) | 437 (263) |
|
% Doses taken as prescribed mean* (SD) |
63(20) | 71(18) | 63 (23) | 58 (29) | 64 (19) | 70 (20) |
|
% Underdosing mean* (SD) |
22(18) | 24(16) | 34 (23) | 41 (30) | 26 (19) | 27 (20) |
|
% Overdosing mean* (SD) |
14(10) | 4 (8) | 2 (2) | 1 (2) | 9 (8) | 3 (2) |
Only one patient using azathioprine was monitored (not showed in the table). All DMARDs: Patients receiving at least one DMARD.
mean of the % doses taken by each patient for each drug.
Baseline predictors of nonadherence
Bivariate analysis with regard to baseline sociodemographic variables showed that adherence to DMARDs was associated with being married or with significant other, and with living with someone (Table 2). Table 3 shows the bivariate associations between baseline clinical variables and adherence. Adherence was associated with lower depression, better mental health, lower disease activity (low DAS28 score), and better function (lower MHAQ scores). Therapy with biologic agents was not associated with adherence.
Table 2.
Association between categorical baseline variables and adherence during the 2-year follow-up
| All patients (n=lll) |
DMARDs: % doses taken as prescribed (107 patients) |
Prednisone: % doses taken as prescribed (56 patients) |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline variables | n (%) | n | Mean % (SD) | P* | n | Mean % (SD) | p* | |
| Education | < High school | 50 (45%) | 47 | 67 (19) | 0.20 | 29 | 69 (22) | >0.20 |
| ≥High school | 61 (55%) | 60 | 62 (20) | 27 | 71 (17) | |||
|
| ||||||||
|
Marital
status |
Widowed/separated | 42 (38%) | 41 | 56 (19) | <0.01¥ | 15 | 62 (19) | >0.20 |
| Single never married | 13 (12%) | 13 | 72 (16) | 10 | 74 (18) | |||
| Married or significant other |
56 (50%) | 53 | 69 (18) | 31 | 72(20) | |||
|
| ||||||||
| Ethnicity | Hispanic | 71 (64%) | 67 | 66 (17) | >0.20 | 42 | 70 (21) | >0.20 |
| White | 18 (16%) | 18 | 64 (20) | 5 | 75 (13) | |||
| African American | 21 (19%) | 21 | 60 (24) | 8 | 69 (20) | |||
| Other | 1 (1%) | 1 | 52 (NA) | 1 | 65 (NA) | |||
|
| ||||||||
| Insurance * | Uninsured | 23 (21%) | 22 | 65 (23) | >0.20 | 14 | 72 (19) | >0.20 |
| Private insurance | 3 (3%) | 3 | 66 (13) | 1 | 85 (NA) | |||
| Medicaid/Medicare | 81 (76%) | 78 | 64 (19) | 40 | 69 (20) | |||
|
| ||||||||
|
Employment
status £ |
Employed | 19 (18%) | 19 | 64 (15) | >0.20 | 9 | 57 (21) | 0.10 |
| Homemaker | 38 (35%) | 35 | 68 (17) | 26 | 70 (22) | |||
| Unemployed | 8 (7%) | 8 | 63 (14) | 6 | 78 (7) | |||
| Disabled/retired | 40 (37%) | 39 | 61 (23) | 13 | 77 (12) | |||
| Other | 3 (3%) | 3 | 77 (22) | 1 | 46 (NA) | |||
|
| ||||||||
| Income § | < $20,000/year | 74 (67%) | 70 | 62 (19) | >0.20 | 36 | 72 (17) | 0.11 |
| > $20,000/year | 20 (18%) | 20 | 69 (20) | 8 | 60 (27) | |||
|
| ||||||||
|
Household
members |
Living alone | 17 (15%) | 17 | 56 (21) | 0.05 | 7 | 70 (15) | >0.20 |
| Not living alone | 94 (85%) | 90 | 66 (19) | 49 | 70 (20) | |||
|
| ||||||||
| Language | English or English/Spanish |
66 (59%) | 65 | 64 (21) | >0.20 | 26 | 71 (18) | >0.20 |
| Spanish only | 45 (41%) | 42 | 65 (16) | 30 | 69 (21) | |||
|
| ||||||||
| Biologic agent used | No | 64 (58%) | 63 | 63 (21) | >0.20 | 35 | 73 (20) | 0.19 |
| Yes | 47 (42%) | 44 | 66 (17) | 21 | 65 (19) | |||
DMARD = disease-modifying anti-rheumatic drug; SD = standard deviation.
ANOVA and Student t test were used.
Widowed or separated patients had significantly less adherence than those married or living with significant other or single (never married).
Data missing for 4 patients.
Data missing for 3 patients.
Data missing for 17 patients.
Table 3.
Correlations between continuous baseline variables and adherence throughout the 2-year follow-up
| DMARD % prescribed doses accurately taken (n=107) |
Prednisone % prescribed doses accurately taken (n=56) |
|||||
|---|---|---|---|---|---|---|
| Baseline variables | n | r* | P | n | r* | p |
| Age | 107 | −0.07 | >0.20 | 56 | 0.04 | >0.20 |
| Disease duration | 107 | 0.08 | >0.20 | 56 | −0.06 | >0.20 |
| MHAQ | 107 | −0.20 | 0.04 | 56 | −0.03 | >0.20 |
| DAS28 | 90 | −0.27 | 0.01 | 49 | 0.10 | >0.20 |
| Sharp/van Der Heijde score | 79 | −0.06 | >0.20 | 45 | −0.04 | >0.20 |
| CESD10 | 107 | −0.19 | 0.05 | 56 | −0.19 | 0.17 |
| MOS | 107 | 0.17 | 0.08 | 56 | 0.24 | 0.07 |
| SF-12-PCS | 105 | 0.07 | >0.20 | 55 | −0.30 | <0.03 |
| SF-12-MCS | 105 | 0.34 | 0.0004 | 55 | 0.24 | 0.08 |
| Comorbidities | 107 | −0.06 | >0.20 | 56 | −0.02 | >0.20 |
| Number of RA-related drugs 1 | 107 | 0.05 | >0.20 | 56 | 0.09 | >0.20 |
| Pills per day of RA-related drugs 1 | 107 | 0.08 | >0.20 | 56 | 0.32 | 0.02 |
| Number of non-RA related drugs | 107 | −0.17 | 0.07 | 56 | 0.05 | >0.20 |
| Pills per day of non-RA-related drugs | 107 | −0.15 | 0.12 | 56 | 0.14 | >0.20 |
Pearson correlation coefficients.
Includes disease-modifying antirheumatic drugs, prednisone, non-steroidal anti-inflammatory drugs and/or analgesics.
DMARD = disease-modifying antirheumatic drug; MHAQ = Modified Health Assessment Questionnaire; DAS28 = disease activity score 28-joint index; CESD10 = Center for Epidemiologic Studies Depression score; MOS = Medical Outcome Study; SF-12-PCS = Physical health score on the SF12; SF-12-MCS = mental health score on the SF12; NSAIDs=non-steroidal anti-inflammatory drugs.
With stepwise multivariate regression analysis using the percentage of correct DMARD doses taken as the dependent variable, adherence was significantly associated with being married/having significant other not being widowed or separated (b=-10.22, p<0.01), having lower disease activity (b=-2.41, p<0.05) and better mental health (b=0.40, p<0.05).
Relationship between adherence and outcomes
One hundred and two patients (95%) of the 107 enrolled patients completed the 2 years of follow-up. There were no statistically significant differences between patients who completed the 2-year follow-up and patients who did not with regard to age, sex, disease duration, ethnicity, baseline disease activity, comorbidities, baseline MHAQ and baseline self-reported adherence.
When comparing with baseline, at 24 months patients presented progression of radiographic damage (Sharp-van der Heijde score difference in means= 7.8 (11.6); p=0.0001) and improvement in disease activity score (DAS28 difference in means= -0.7 (1.7); p = 0.0002). Adherence to DMARDs had a statistically significant association with the DAS28 activity score (adherent patients had lower disease activity; r=-0.26, p=0.01). After adjusting for age, gender, disease duration, cumulative dose of prednisone, number of DMARDs and biologic agents used, adherence to DMARDs remained independently associated with DAS28 disease activity score at 2-years (Table 4).
Table 4.
Multiple regression model with DAS28 at 2 years as the dependent variable
| Dependent variable: DAS28 at 2-years | ||||
|---|---|---|---|---|
|
| ||||
| b | 95% CI | Beta | p | |
| DMARDs: % prescribed dose taken | −0.02 | −0.03, −0.001 | −0.2 | 0.03* |
| Age | 0.02 | −0.01, 0.05 | 0.15 | 0.13 |
| Female | 1.45 | 0.75, 2.15 | 0.37 | <0.01* |
| Disease duration (years) | −0.05 | −0.1, −0.006 | −0.21 | 0.03* |
| Cumulative dose of prednisone (grams) | 0.04 | −0.07, 0.15 | 0.07 | 0.5 |
| Received biologics agents | 0.53 | −0.04, 1.09 | 0.18 | 0.07 |
| Number of DMARDs used | 0.02 | −0.32, 0.36 | 0.01 | 0.90 |
| Baseline DAS28 | 0.31 | 0.14, 0.49 | 0.33 | <0.01* |
| Intercept | −0.2 | 0.87 | ||
|
| ||||
| R2=0.44 | ||||
CI= Confidence Interval; DMARD = disease-modifying antirheumatic drug; DAS28 = disease activity score 28-joint index
statistically significant.
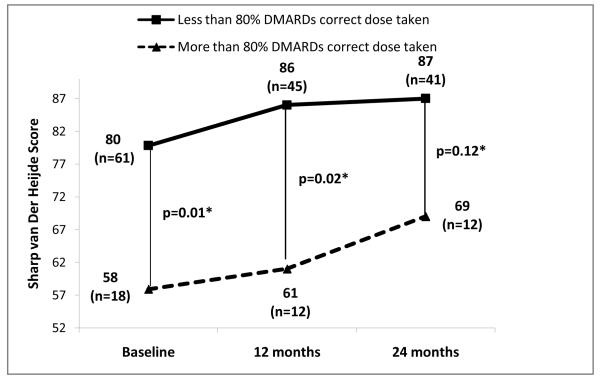
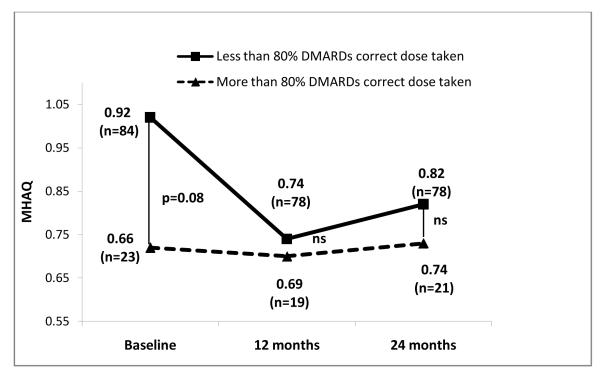
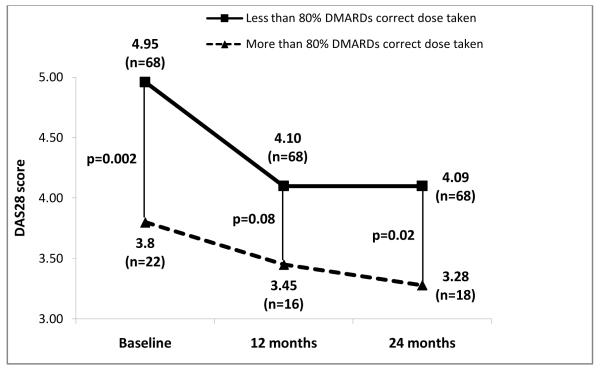
Figures 1, 2 and 3 show radiographic scores, MHAQ and DAS28 at baseline, 12 and 24 months in patients who were at least 80% adherent and those who were not. Patients with better adherence showed less disease activity throughout the study (Figure 3). Radiographic progression was also decreased in adherent compared to nonadherent patients, but the difference lost statistical significance at 24 months (Figure 1).
Figure 1.
Comparison of Sharp van der Heijde radiological score progression between patients with greater than and less than 80% adherence to DMARDs.
Figure 2.
Comparison of modified Health Assessment Questionnaire (MHAQ) scores between patients with greater than and less than 80% adherence to DMARDs.
Figure 3.
Comparison of disease activity scores (DAS28) between patients with greater than and less than 80% adherence to DMARDs.
DISCUSSION
To our knowledge, this is the first study to measure adherence in patients with RA across 2 years of follow-up, and to identify the correlates and consequences of poor adherence. Our population had low socioeconomic status, were ethnically diverse and most of them had less than optimal access to medical care. Using an electronic medication monitoring system, which is considered by many the gold standard for the measurement of compliance (13, 14, 24), we found that overall mean adherence to DMARDs and prednisone was low (64% and 70%, respectively). These percentages were lower than those reported by de Klerk in Europe (11), who found that 55% and 81% of patients took sulfasalazine and methotrexate correctly. However, that study’s follow-up was only 6 months and showed an adherence decline of 35% for methotrexate and 7% for sulfasalazine from baseline. In a similar disadvantaged clinical setting, we previously reported a low adherence rate to oral medications in patients with systemic lupus erythematosus, ranging from 61 to 74% (25). We observed no differences between DMARD and prednisone adherence, despite the faster effects of steroids, which one could hypothesize could lead to better adherence. However, our study was not designed or powered to compare adherence patterns between these drugs.
Having better mental health and not being widowed or separated were associated with higher adherence. Other studies have shown that sociodemographic and clinical characteristics including shorter disease duration, less adverse events, and older age were also related with better adherence (26, 27). However, it should be noted that these studies used patient self-report for measuring adherence, making it difficult to compare with our study.
Patients with better adherence showed less disease activity throughout the study. This association was also significant at baseline, suggesting that patients with less DAS28 were already adherent to prescribed drugs before the study began (mean disease duration at baseline was 7 years). This observation can be explained in part by the “adherer effect” theory. This theory argues that people who are compliant to a physician’s prescriptions have better outcomes, regardless of the underlying treatment (including a placebo) (28, 29). The “adherer effect” theory is based on the finding that behaviors of adherent people are different than the behaviors of people who are non-adherent. Adherent people have healthier lifestyles, do not engage in risky behaviors, and are also more adherent to non-pharmacological prescriptions (e.g., diet, exercise), and therefore have better global health outcomes (not only outcomes related to the disease under investigation). Overall, changes in DAS were lower than what is reported in clinical trials of patients initiating DMARDS, also suggesting that low adherence in the ‘real world’ could partially explain the smaller changes in disease activity observed in this community setting.
Our study was unable to measure adherence to biologic agents because these are administered subcutaneously or as infusions, and we only captured adherence to oral therapies. Nevertheless, when we compared patients who had received biologics and those had not, no differences were observed with respect to adherence. While biologic therapies have changed the treatment of RA, methotrexate remains the initial drug of choice and most trials suggest that they are most effective when received in combination with methotrexate and other DMARDs. Furthermore, economically disadvantaged and underinsured patients might not be able to receive biologic therapies because of their higher costs. Therefore, adequate adherence to methotrexate therapy remains a major therapeutic goal in RA.
All methods proposed to measure adherence can be susceptible to patient manipulation or bias (4, 6-11, 24, 30). MEMS® are considered by many as one of the best methods. It is not invasive, can provide the exact time of drug intake, and is insensitive to “white-coat compliance”. It is assumed that that every time the bottle was opened, the the prescribed dose was taken. While this cannot be assured, it would require willingness to deceit, and strong discipline on the part of the patient to open the bottle at the prescribed times without taking the pill, during a long period of follow-up, such as the 2 years in our study (14). Our study has limitations. Not all patients we approached agreed to have their medication intake electronically assessed. We included patients with less than 15 years of disease, and not those with early active arthritis exclusively. It can be argued that the most relevant clinical effects of non-adherence would occur early on but our study cannot ascertain this. It is also difficult to disentangle causal relationships with a prevalent cohort study, and it could be argued that poor disease control could lead to low adherence. We believe nevertheless that the most reasonable explanation, or at least the one with the largest effect, is that better adherence improved outcomes. Patients were seen by their physicians throughout the study, at least every 6 months, and those who did not respond to therapy would have been given another treatment, which in many cases would have worked as can be seen in trials of non-responders where about half of participants respond to a different therapy. If disease activity had been the major determinant of adherence (and not the other way around) adherence would have improved, as disease activity improved over 2 years, which was not the case. Finally, our patient population had low socio-economic status, was ethnically diverse, and most patients were underinsured. Thus, the results cannot be generalizable to the population at large.
In summary, we found that adherence to oral DMARDs and steroid therapy in patients with RA was low, ranging from 58% to 71%, with only one-fifth of patients showing greater than 80% adherence. Non-adherent patients had higher disease activity scores across the 2 years of follow-up and increased radiographic damage, with a trend towards lower function as well. Our findings should alert physicians about the need to integrate adherence assessments into daily practice, to explore potential barriers to non-adherence and engage in discussions with patients to highlight the importance of taking medications as prescribed to reach therapeutic target goals.
ACKNOWLEDGEMENTS
This study was funded by NIAMS R01AR047858. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAMS or NIH. Dr. Suarez-Almazor is the recipient of a K24 award from NIAMS (K24AR053593).
Footnotes
Conflict of Interest Disclosures: None
Footnote for all: DMARDs= Disease modifying antirrheumatic drugs; n= number of patients; ns = not statistically significant.
Contributor Information
Christian A. Waimann, The University of Texas, M.D. Anderson Cancer Center, Houston, TX, USA
Maria F. Marengo, The University of Texas, M.D. Anderson Cancer Center, Houston, TX, USA
Sofia de Achaval, The University of Texas, M.D. Anderson Cancer Center, Houston, TX, USA
Vanessa L. Cox, The University of Texas, M.D. Anderson Cancer Center, Houston, TX, USA
Araceli Garcia-Gonzalez, The University of Texas, M.D. Anderson Cancer Center, Houston, TX, USA
John D. Reveille, The University of Texas Health Sciences Center, Houston, TX, USA
Marsha N. Richardson, The University of Texas, M.D. Anderson Cancer Center, Houston, TX, USA.
Maria E. Suarez Almazor, The University of Texas, M.D. Anderson Cancer Center, Houston, TX, USA
References
- 1.Klarenbeek NB, Allaart CF, Kerstens PJ, Huizinga TW, Dijkmans BA. The BeSt story: on strategy trials in rheumatoid arthritis. Current Opinion in Rheumatology. 2009;21(3):291–8. doi: 10.1097/BOR.0b013e32832a2f1c. [DOI] [PubMed] [Google Scholar]
- 2.Shi L, Hudges M, Yurgin N, Boye KS. Impact of dose frequency on compliance and health outcomes: A literature review (1966-2006) Expert Review of Pharmacoeconomics and Outcomes Research. 2007;7(2):187–202. doi: 10.1586/14737167.7.2.187. [DOI] [PubMed] [Google Scholar]
- 3.Costedoat-Chalumeau N, Amoura Z, Hulot JS, Aymard G, Leroux G, Marra D, et al. Very low blood hydroxychloroquine concentration as an objective marker of poor adherence to treatment of systemic lupus erythematosus. Annals of the Rheumatic Diseases. 2007;66(6):821–4. doi: 10.1136/ard.2006.067835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26(5):331–42. doi: 10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- 5.Borah BJ, Huang X, Zarotsky V, Globe D. Trends in RA patients’ adherence to subcutaneous anti-TNF therapies and costs. Current Medical Research and Opinion. 2009;25(6):1365–77. doi: 10.1185/03007990902896386. [DOI] [PubMed] [Google Scholar]
- 6.Curkendall S, Patel V, Gleeson M, Campbell RS, Zagari M, Dubois R. Compliance with biologic therapies for rheumatoid arthritis: do patient out-of-pocket payments matter? Arthritis & Rheumatism. 2008;59(10):1519–26. doi: 10.1002/art.24114. [DOI] [PubMed] [Google Scholar]
- 7.de Klerk E, van der Heijde D, van der Tempel H, van der Linden S. Development of a questionnaire to investigate patient compliance with antirheumatic drug therapy. Journal of Rheumatology. 1999;26(12):2635–41. [PubMed] [Google Scholar]
- 8.Grijalva CG, Chung CP, Arbogast PG, Stein CM, Mitchel EF, Jr., Griffin MR, et al. Assessment of adherence to and persistence on disease-modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis. Medical Care. 2007;45(10 Supl 2):S66–76. doi: 10.1097/MLR.0b013e318041384c. [DOI] [PubMed] [Google Scholar]
- 9.Harley CR, Frytak JR, Tandon N. Treatment compliance and dosage administration among rheumatoid arthritis patients receiving infliximab, etanercept, or methotrexate. American Journal of Managed Care. 2003;9(SUPPL. 6) [PubMed] [Google Scholar]
- 10.Ward MM, Lotstein DS, Bush TM, Lambert RE, van Vollenhoven R, Neuwelt CM. Psychosocial correlates of morbidity in women with systemic lupus erythematosus. Journal of Rheumatology. 1999;26(10):2153–8. [PubMed] [Google Scholar]
- 11.de Klerk E, van der Heijde D, Landewe R, van der Tempel H, van der Linden S, de Klerk E, et al. The compliance-questionnaire-rheumatology compared with electronic medication event monitoring: a validation study. Journal of Rheumatology. 2003;30(11):2469–75. [PubMed] [Google Scholar]
- 12.Feinstein AR. On white-coat effects and the electronic monitoring of compliance. Arch Intern Med. 1990;150(7):1377–8. [PubMed] [Google Scholar]
- 13.Urquhart J. The electronic medication event monitor. Lessons for pharmacotherapy. Clin Pharmacokinet. 1997;32(5):345–56. doi: 10.2165/00003088-199732050-00001. [DOI] [PubMed] [Google Scholar]
- 14.de Klerk E, van der Heijde D, Landewe R, van der Tempel H, Urquhart J, van der Linden S, et al. Patient compliance in rheumatoid arthritis, polymyalgia rheumatica, and gout.[erratum appears in J Rheumatol. 2003 Feb;30(2):423] Journal of Rheumatology. 2003;30(1):44–54. [PubMed] [Google Scholar]
- 15.Dunbar-Jacob J, Holmes JL, Sereika S, Kwoh CK, Burke LE, Starz TW, et al. Factors associated with attrition of African Americans during the recruitment phase of a clinical trial examining adherence among individuals with rheumatoid arthritis. Arthritis & Rheumatism. 2004;51(3):422–8. doi: 10.1002/art.20411. [DOI] [PubMed] [Google Scholar]
- 16.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 17.Pincus T, Summey JA, Soraci SA, Jr., Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26(11):1346–53. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 19.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–14. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 20.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 21.Fuchs HA, Brooks RH, Callahan LF, Pincus T. A simplified twenty-eight-joint quantitative articular index in rheumatoid arthritis. Arthritis & Rheumatism. 1989;32(5):531–7. doi: 10.1002/anr.1780320504. [DOI] [PubMed] [Google Scholar]
- 22.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. Journal of Rheumatology. 1999;26(3):743–5. [PubMed] [Google Scholar]
- 23.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, et al. Medication compliance and persistence: terminology and definitions. Value in Health. 2008;11(1):44–7. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 24.de Achaval S, Suarez-Almazor ME. Treatment adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and systemic lupus erythematosus. Int J Clin Rheumtol. 2010;5(3):313–26. doi: 10.2217/ijr.10.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marengo M, Waimann C, Achaval S, Zhang H, Garcia-Gonzalez A, Richardson M, et al. Measuring therapeutic adherence in systemic lupus erythematosus with electronic monitoring. Lupus. 2012;21(11):1158–65. doi: 10.1177/0961203312447868. [DOI] [PubMed] [Google Scholar]
- 26.Tuncay R, Eksioglu E, Cakir B, Gurcay E, Cakci A, Tuncay R, et al. Factors affecting drug treatment compliance in patients with rheumatoid arthritis. Rheumatology International. 2007;27(8):743–6. doi: 10.1007/s00296-006-0299-9. [DOI] [PubMed] [Google Scholar]
- 27.van den Bemt BJ, van den Hoogen FH, Benraad B, Hekster YA, van Riel PL, van Lankveld W. Adherence Rates and Associations with Nonadherence in Patients with Rheumatoid Arthritis Using Disease Modifying Antirheumatic Drugs. Journal of Rheumatology. 2009 doi: 10.3899/jrheum.081204. [DOI] [PubMed] [Google Scholar]
- 28.Dormuth CR, Patrick AR, Shrank WH, Wright JM, Glynn RJ, Sutherland J, et al. Statin adherence and risk of accidents: a cautionary tale. Circulation. 2009;119(15):2051–7. doi: 10.1161/CIRCULATIONAHA.108.824151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Coronary Drug Project Research Group Influence of adherence to treatment and response of cholesterol on mortality in the coronary drug project. N Engl J Med. 1980;303(18):1038–41. doi: 10.1056/NEJM198010303031804. [DOI] [PubMed] [Google Scholar]
- 30.Borah BJ, Huang X, Zarotsky V, Globe D, Borah BJ, Huang X, et al. Trends in RA patients’ adherence to subcutaneous anti-TNF therapies and costs. Current Medical Research & Opinion. 2009;25(6):1365–77. doi: 10.1185/03007990902896386. [DOI] [PubMed] [Google Scholar]