Abstract
We evaluated the capability of soluble cardiac biomarkers to predict tolerability and outcomes of IMiD-containing treatments among 106 patients treated on clinical trials. Baseline elevations in troponin T (TnT) and N-terminal brain naturietic protein (NT-proBNP) predicted for an inability to tolerate IMiD-based regimens. The best predictors for early attrition during cycle 1 were TnT ≥ 0.07 μg/L and NT-proBNP ≥ 11,939 ng/L. NT-proBNP-response under-performed TnT-response as a predictor for overall survival (OS), but both predicted for early protocol attrition. Despite hematologic response, IMiD-treated patients were at higher risk for NT-proBNP rises and early drug discontinuation than a control population but not for early death. These observations prompt two questions: (1) does IMiD-based therapy lead to increased fluid retention and/or cardiac toxicity and (2) is an NT-proBNP-driven cardiac response system valid in IMiD-treated amyloidosis patients? Recognition of potential drug-induced cardiac toxicity is important so that increased cardiac surveillance and drug dose-adjustment or discontinuation may be implemented.
Introduction
As progress is made in treating multiple myeloma, slower progress has been made in treating patients with immunoglobulin light chain amyloidosis (AL). Advancements in the therapy of AL are hindered by its low prevalence, the heterogeneity of the disorder, and the narrow therapeutic window experienced by these patients. Advent of the novel therapies is a potential boon to these patients [1,2], but concerns exist about how best to use these drugs and about whether they may be toxic in a subset of patients with AL [3,4].
Baseline levels of cardiac biomarkers predict for overall survival (OS) of AL [5,6], and there is an initiative to improve on cardiac response criteria in AL patients implementing these soluble biomarkers. Using the combination of melphalan and dexamethasone, the Pavia group demonstrated that decrements in serum NT-proBNP coincide with decreases in serum immunoglobulin free light chain (FLC) and improved OS [7]. This observation has been substantiated in part by others [8-10]. Based on anecdotal observation, IMiDs may contribute to cardiac decompensation in a minority of patients [1,3,4,11-13]. Therefore, we formally evaluated the importance of baseline and serial cardiac biomarkers among patients treated with IMiDs on clinical trials.
Methods
From October 2004 to November 2009, 78 patients with AL were treated at Mayo Clinic Rochester on one of three IRB-approved IMiD containing treatment trials (seven patients enrolled on two trials consecutively): 38, lenalidomide and dexamethasone (NCT00166413-Rd); 26, cyclophosphamide and Rd (NCT00564889-CRd); and 21, pomalidomide and dexamethasone (NCT00558896-Pd) [1,4,11,13]. A secondary dataset of 21 melphalan–dexamethasone (M-Dex)-treated patients participating in an ongoing randomized control comparing M-Dex to high-dose melphalan with stem cell transplantation (NCT00477971) was included as a comparator arm (alkylator-based vs. IMiD-based therapy). According to protocol, serial cardiac biomarkers were performed every 3 months, see Supporting Information Table 1.
Our analyses focused on two questions. The first one was whether there were baseline thresholds at which attrition rates were high among patients treated with IMiD-based therapy. The second one was whether a relationship between FLC response and cardiac biomarker improvements existed [7]. Any increase or decrease in troponin T (TnT) was defined as measurable change from baseline. Changes in NT-proBNP were defined as 30% from baseline (but at least 200 ng/L). FLC changes were according to published criteria, that is, a 50% reduction in the involved FLC, as long as the baseline FLC was ≥7.5 mg/dL [14]. Cardiac biomarker stage is as previously defined using the thresholds TnT ≥ 0.035 μg/L and NT-proBNP ≥ 332 ng/L resulting in Stages I, II, and III if neither, either, or both are above threshold [6]. All statistical analyses were performed using JMP software (SAS, Carey, NC).
Results and Discussion
The baseline characteristics of the 106 participants on one of three IMiD-based and an M-Dex clinical trial are given in Table 1. Time from diagnosis to enrollment was significantly different among the studies (p <0.0001). Baseline TnT, NT-proBNP, creatinine, serum FLC, and cardiac stage were similar among the four trials. There was a trend toward more cardiac biomarker Stage III patients in the IMiD trials than the M-Dex trial (p = 0.06).
TABLE I.
Patient Characteristics and Responsesa
| All IMiD | Rd | CRd | Pd | M-Dex | Pa,b | |
|---|---|---|---|---|---|---|
| N with baseline measurement | 85 | 38 | 26 | 21 | 21 | NA |
| Diagnosis to enrollment (months) | 8.9 (0.1–234) | 9.4 (0.4–234) | 1.8 (0.1–129) | 45.8 (4.2–106) | 1.5 (0.2–6.7) | <0.0001 |
| BL TnT (μg/L) | 0.02 (0.01–0.55) | 0.03 (0.01–0.55) | 0.015 (0.01–0.22) | 0.01 (0.01–0.12) | 0.01 (0.01–0.12) | NS |
| ≥ 0.07 μg/L (%) | 29.4 | 26.3 | 42.3 | 19 | 14.3 | NS |
| BL NT-proBNP (ng/L) | 1683 (69–42,844) | 2494 (88.3–42,844) | 1320 (69–15,675) | 1449 (88–36,498) | 1597 (90–24,947) | NS |
| ≥12,000 ng/L (%) | 9.4 | 10.5 | 7.7 | 9.5 | 14.3 | NS |
| Baseline Cr (mg/dL) | 1.2 (0.5–2.9) | 1.3 (0.7–2.9) | 1.2 (0.5–2.8) | 1.0 (0.7–2.4) | 1.1 (0.5–2.4) | NS |
| Informative BL FLC [N] (mg/dL) | [78] 25(7.5–278) | [35] 27 (8.29–278) | [23] 25 (10–182) | [19] 20 (7.5–80) | [19] 31.4 (8.9–326) | NS |
| Cardiac stages I, II, III [N], n (%) | 15/36/34 (18/42/40) | 6/16/16 (16/42/42) | 46/8/12 (23/31/46) | 43/12/6 (14/57/29) | 4/13/4 (19/62/19) | NS |
| Months on study | 7.9 (0.2–61+) | 15.3(0.2–61+) | 10.8 (0.9–24+) | NR (0.2–13+) | NR (2.8–15) | 0.002 |
| Off drug within 1 month, n (%) | 10 (11.8) | 9 (23.7) | 3 (11.5) | 2 (10) | 0 | NS |
| Off drug within 3 months, n (%) | 23 (27.1%) | 15 (39.5%) | 6 (23.1%) | 2 (9.5%) | 0 | 0.007 |
| Assessablec by FLC, n | 52 | 21 | 17 | 14 | 17 | NA |
| FLC down, n (%) | 29 (56) | NA | NA | NA | 12 (71) | NS |
| FLC up, n (%) | 2 (3.8) | NA | NA | NA | 1 (5.9) | NS |
| NT-proBNP down, n(%)d | 3 (7.5) | NA | NA | NA | 5 (35.7) | 0.021 |
| NT-proBNP up, n (%) | 30 (58) | NA | NA | NA | 5 (29.4) | 0.038 |
| TnT down, n (%)e | 6 (27.3) | NA | NA | NA | 2 (33.3) | NS |
| TnT up, n (%) | 22 (42) | NA | NA | NA | 7 (41.1) | NS |
IMiD, immune modulatory drugs; Rd, lenalidomide and dexamethasone; CRd, cyclophosphamide, lenalidomide, and dexamethasone; Pd, pomalidomide and dexamethasone; BL, baseline: NA, not assessed; NR, not reached; NS, not significant.
The IMiD trials allowed previously treated or untreated patients except for the Pd trial which excluded previously untreated patients. The M-Dex trial included only previously untreated patients who were potentially eligible for autologous stem cell transplantation.
Comparisons between IMiD-treated and M-Dex-treated patients.
Patients were considered assessable for serial measurements if they had an informative baseline involved FLC that was at least 7.5 mg/dL and had at least one serial measurement after 3 months on study drugs.
As 15 patients had minimally elevated NT-proBNP, they could not be eligible for an NT-proBNP response; therefore, the respective denominators for the IMiDs and the M-Dex are 40 and 14, respectively.
Since 33 patients had troponin T ≤ 0.01, they could not be eligible for a troponin T response; therefore, the respective denominators for the IMiDs and the M-Dex are 22 and 6, respectively.
Median time on treatment for all participants was 12.2 months. The 9% who discontinued therapy within 1 month of registration had significantly higher TnT and NT-proBNP values than those remaining on study (p < 0.002). The best cut-points to predict for withdrawal during the first cycle were ≥0.07 μg/L for TnT and ≥11,939 ng/L for NT-proBNP. Patients receiving IMiD-based therapy were more likely to end protocol therapy prematurely but were no more likely to die prematurely (Fig. 1A–C). These observations held true on multivariate analysis, regardless of baseline cardiac biomarker stage, individual cardiac biomarkers, serum creatinine, and time between diagnosis and enrollment. In addition, variables predicting for OS on univariate analysis including all 106 patients were in decreasing significance: baseline TnT, FLC reduction, stage, baseline NT-proBNP, and serum creatinine. IMiD-based therapy versus M-Dex was not significant. On multivariate analysis, only cardiac stage (HR 14.1, 95%CI 4.55, 50.8, p < 0.0001), FLC reduction (HR 0.23, 95%CI 0.95, 0.48, p<0.0001), and baseline NT-proBNP (HR 4.97, 95%CI 1.82, 12.3, p = 0.003) predicted for OS.
Figure 1.
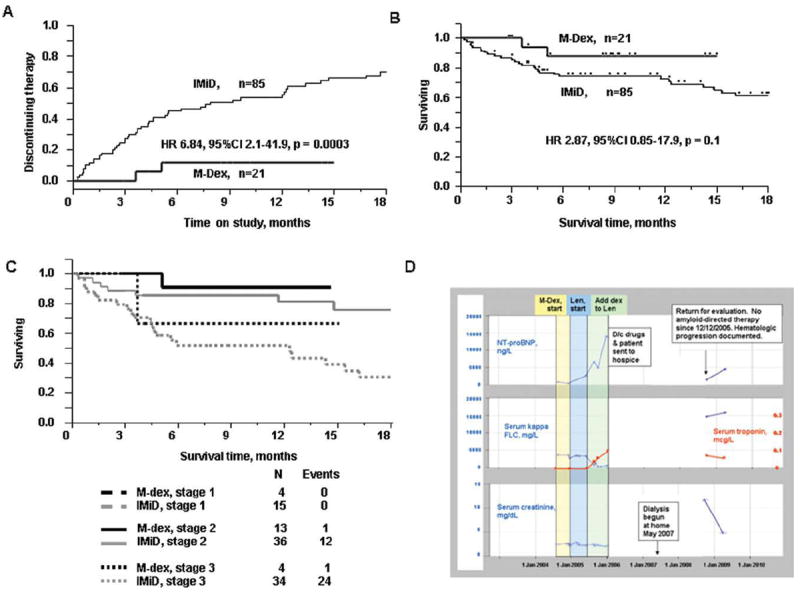
Impact of treatment on clinical trial attrition and OS IMiD, immune modulatory drug containing regimen; M-Dex, melphalan dexamethasone treatment. (A) Time to study regimen discontinuation, (B) OS, (C) OS, correcting for baseline cardiac stage. Cardiac biomarker stage is as previously defined, using the thresholds TnT ≥ 0.035 μg/L and NT-proBNP ≥ 332 ng/L resulting in Stages I, II, and III if neither, either, or both are above threshold [6], and (D) Example of putative IMiD-based therapy induced biochemical changes. Mr. J. was diagnosed with renal AL in December 2003. He received three cycles of melphalan plus dexamethasone with modest impact on his M-spike and no impact on his kappa FLC. He then enrolled on the lenalidomide with on-demand dexamethasone study. As there was no hematologic response after three cycles of lenalidomide alone, dexamethasone was added according to protocol. Despite a kappa FLC response (decreased from 325 to 47.2 mg/dL), he had toxicity that included rash, myelosuppression, diarrhea, and ultimately progressive dyspnea and progressive nephrotic syndrome, which led to discontinuation of drugs and referral to hospice in December 2005. His functional status improved while in hospice, but his renal function declined such that in Mayo of 2007 dialysis was instituted. He remained off all therapies directed at his plasma cell and presented back to our clinic in September 2008 for further active management.
The next analyses focused on serial FLC and cardiac biomarker assessments, see Supporting Information Table 2. These 69 patients were different from the 37 who were excluded for lack of serial measurements in that they had lower baseline troponins (0.01 vs. 0.03 μg/L, p = 0.006), NT-proBNP (1285 vs. 3507 ng/L, p = 0.01), serum creatinine (1.0 vs. 1.3 mg/dL, p = 0.02), and FLC (17.6 vs. 28.5 mg/dL, p = 0.008). Although in IMiD and M-Dex cohorts (Table 1), there were comparable numbers of FLC progressors (3.8 vs. 5.9%) and responders (56 vs. 71%), more IMiD-treated patients had a rise in their NT-proBNP (58 vs. 29.4%), and the IMiD-treated patients were less likely to have a decrease in their NT-proBNP (7.9 vs. 35.7%), Fig. 1D. The number of patients with documented changes in TnT did not differ between treatment groups.
We found no association between FLC response and NT-proBNP response as previously reported with alkylator and corti-costeroid therapy [7] or as we have observed after autologous stem cell transplantation [10]. Others report that with chemotherapy, NT-proBNP can rise at least transiently but asymptomatically [9,15]. This is in sharp contrast to our findings. Among our population, the median time on clinical trial for those patients with and without an NT-proBNP increase was 12.2 versus 33.8 months, p = 0.001, respectively, and for TnT increase was 12.1 and 23.5 months, p = 0.002, both of which translated into inferior OS on univariate analysis. Unexpectedly, TnT increase outperformed NT-proBNP increase as a predictor for OS, but on multivariate only baseline cardiac biomarker stage (HR5.8, 95%CI 2.2–17.2, p = 0.003) and FLC response (HR 0.17, 95%CI 0.05–0.52, p = 0.0015) were prognostic, whereas TnT increase was of borderline significance (3.09, 95%CI 0.91–14.1, p = 0.07).
Not only there are major differences between our study and that of others [7,9,15] in terms of results but also methodology is different. Our patients had participated on one of four IRB-approved prospective therapeutic trials allowing for a uniform follow-up schedule and information about patient censoring. Although our analysis of serial measurements was not prospectively designed, we can account for the 37 excluded patients, that is, principally attrition before completion of three cycles of therapy due to early death or toxicity. In contrast, when the UK group analyzed NT-proBNP levels at 0, 6, and 12 months after the commencement of either cyclophosphamide–thalidomide–dexamethasone or M-Dex as a part of clinical practice [9], they found: NT-proBNP rose from baseline in 71% at 6 months, but dropped by 12 months in 92%; comparable proportions of patients with NT-proBNP increase among the M-Dex-treated patients (6/9) and cyclophosphamide–thalidomide–dexamethasone-treated patients (30/42); and no difference in OS for those who did and did not have an NTpro-BNP increase at 6 months. Our experience would question whether all patients were accounted for in these analyses.
In conclusion, we found that high baseline cardiac biomarkers predicted for a high rate of IMiD discontinuation and that the majority of patients receiving IMiDs have an increase in their cardiac biomarkers discordant from their FLC response—a decoupling that was not evident in patients treated with M-Dex and that did not translate into OS on multivariate analysis. Therefore, it is unclear whether more frequent NT-proBNP-increases observed with IMiDs are related to potential drug-induced cardiotoxicity or IMiD-induced fluid retention. Finally, our data suggest that the proposed system of NT-proBNP response as a measure of cardiac response may not be valid for patients treated with IMiD-based therapy and that a troponin-based cardiac response system may be more informative. Larger prospective studies will be required to clarify these points. It is essential, however, that physicians be aware of this phenomenon so that increased cardiac surveillance and drug dose-adjustment or discontinuation can be implemented if the clinical picture is consistent with cardiac decompensation.
Supplementary Material
Acknowledgments
Contract grant sponsor: National Cancer Institute and the Robert A. Kyle Hematologic Malignancies Fund; Contract grant numbers: CA 125614, CA111345, CA062242, CA107476, CA150831, CA93842, CA83724, CA100080, and CA100707.
Footnotes
Author Contributions
AD designed the study, collected and analyzed the data and wrote the paper. SKK and MAG contributed data from their respective trials, helped analyze the data, and contributed to the review of the manuscript. DD and SVR contributed to the design of the study and the review of the manuscript. MQL, SRH, FB, SZ, NL, KDS, JAL, SJR, and RAK contributed patients and to the review of the manuscript.
Additional supporting information may be found in the online version of this article.
Conflict of interest: AD has received research dollars and speaking honoraria from Celgene and the Binding Site, SKK, MQL, SRH, FB, SZ, NL, KDS, DD, JAL, SVR, and SJR have nothing to disclose. RAK participates on disease monitoring committees for Celgene and has received speaking honoraria from the Binding Site. MAG serves on advisory committees for Amgen and Easai advisory and has received honoraria from Celgene and Millenium.
References
- 1.Dispenzieri A, Lacy MQ, Zeldenrust SR, et al. The activity of lenalidomide with or without dexamethasone in patients with primary systemic amyloidosis. Blood. 2007;109:465–470. doi: 10.1182/blood-2006-07-032987. [DOI] [PubMed] [Google Scholar]
- 2.Sanchorawala V, Wright DG, Rosenzweig M, et al. Lenalidomide and dexamethasone in the treatment of AL amyloidosis: Results of a phase 2 trial. Blood. 2007;109:492–496. doi: 10.1182/blood-2006-07-030544. [DOI] [PubMed] [Google Scholar]
- 3.Dispenzieri A, Lacy M, Zeldenrust S, et al. Cardiac biomarkers predict for ability to tolerate and complete therapy with lenalidomide {+/−} dexamathosone in AL amyloidosis. ASH Annu Meet Abstr. 2006;108:130. [Google Scholar]
- 4.Dispenzieri A, Lacy M, Zeldenrust SR, et al. Long term follow-up of patients with immunoglobulin light chain amyloidosis treated with lenalidomide and dexamethasone. Blood (ASH Annu Meet Abstr) 2008;112:1737. [Google Scholar]
- 5.Dispenzieri A, Kyle RA, Gertz MA, et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet. 2003;361:1787–1789. doi: 10.1016/S0140-6736(03)13396-X. [DOI] [PubMed] [Google Scholar]
- 6.Dispenzieri A, Gertz MA, Kyle RA, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: A staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22:3751–3757. doi: 10.1200/JCO.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 7.Palladini G, Lavatelli F, Russo P, et al. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood. 2006;107:3854–3858. doi: 10.1182/blood-2005-11-4385. [DOI] [PubMed] [Google Scholar]
- 8.Wechalekar A, Merlini G, Gillmore JD, et al. Role of NT-ProBNP to assess the adequacy of treatment response in AL amyloidosis. Blood (ASH Annu Meet Abstr) 2008;112:1689. [Google Scholar]
- 9.Gibbs SDJ, De Cruz M, Sattianayagam PT, et al. Transient post chemotherapy rise in NT Pro-BNP in AL Amyloidosis : Implications for organ response assessment. Blood (ASH Annu Meet Abstr) 2009;114:1791. [Google Scholar]
- 10.Madan S, Kumar S, Dispenzieri A, et al. Outcomes with high dose therapy and peripheral blood stem cell transplantation for light chain (AL) amyloidosis with cardiac involvement. ASH Annu Meet Abstr. 2009;114:534. [Google Scholar]
- 11.Dispenzieri A, Gertz MA, Hayman SR, et al. A Pilot study of pomalidomide and dexamethasone in previously treated light chain amyloidosis patients. Blood (ASH Annu Meet Abstr) 2009;114:3854. [Google Scholar]
- 12.Dispenzieri A, Lacy MQ, Rajkumar SV, et al. Poor tolerance to high doses of thalidomide in patients with primary systemic amyloidosis. Amyloid. 2003;10:257–261. doi: 10.3109/13506120309041743. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Hayman SR, Buadi F, et al. A Phase II Trial of Lenalidomide, Cyclophosphamide and dexamethasone (RCD) in patients with light chain amyloidosis. Blood (ASH Annu Meet Abstr) 2009;114:3853. [Google Scholar]
- 14.Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79:319–328. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- 15.Palladini G, Russo P, Bragotti LZ, et al. A Phase II Trial of Cyclophosphamide, Lenalidomide and Dexamethasone (CLD) in Previously Treated Patients with AL Amyloidosis. ASH Annu Meet Abstr. 2009;114:2863. doi: 10.3324/haematol.2012.073593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


