Abstract
Background
We conducted a systematic review of the literature to determine the efficacy and safety of denosumab in reducing skeletal-related events (SRE) in patients with bone metastases.
Methods
A literature search using MEDLINE, EMBASE, Web of Science and The Cochrane Collaboration Library identified relevant controlled clinical trials up-to-March 14, 2012. Two independent reviewers assessed studies for inclusion, according to predetermined criteria, and extracted relevant data. The primary outcomes of interest were SRE, time to first on-study SRE, and overall survival. Secondary outcomes included pain, quality of life, bone turnover markers (BTM), and adverse events.
Results
Six controlled trials including 6,142 patients were analyzed. Compared to zoledronic acid, denosumab had lower incidence of SRE with a risk ratio (RR) of 0.84 (95% confidence intervals (CI) 0.80-0.88), delayed the onset of first on-study SRE (RR 0.83; 95%CI 0.75-0.90) and time to worsening of pain (RR 0.84; 95%CI 0.77-0.91). No difference was observed in overall survival with pooled hazard ratio of 0.98 (95%CI 0.90-1.0). For total adverse events, denosumab was similar to zoledronic acid (RR 0.97; 95%CI 0.89-1.0). No significant differences were observed in the frequency of osteonecrosis of the jaw (RR 1.4; 95%CI 0.92-2.1). Patients on denosumab had a greater risk of developing hypocalcemia (RR 1.9; 95%CI 1.6-2.3).
Conclusions
Denosumab was more effective than zoledronic acid in reducing the incidence of SRE, and delayed the time to SRE. No differences were found between denosumab and zoledronic acid in reducing overall mortality, or in the frequency of overall adverse events.
Keywords: Denosumab (DB), Skeletal-Related Events (SRE), Bisphosphonates (BP), Zoledronic Acid (ZA), Bone Metastases
INTRODUCTION
Metastatic involvement of bone is a common complication of advanced cancer. Nearly 100% of patients with myeloma, 65 to 75% of patients with breast or prostate cancer, and 30 to 40% of those with lung cancer develop skeletal metastases.1 Half of these patients develop one or more complications collectively termed skeletal-related events (SRE) (i.e., bone pain, hypercalcemia, fracture, spinal cord compression, radiotherapy requirement for pain, and surgery for pathological fracture).2, 3 Since 2002, incidence of SRE has been used as the composite primary endpoint in the trials conducted to reduce skeletal complications among patients with bony metastases.4, 5 SRE cause significant morbidity reduced performance status, quality of life (QOL) and reduced survival.6, 7 They are estimated to cost 1.9 billion dollars every year in the United States, with the cost to treat a single SRE episode per patient varying from 6,973 to 11,979 USD.8-10
In addition to treating the primary cancer, bisphosphonates therapy has become an important strategy to reduce SRE among patients with myeloma, and bone metastases from breast, prostate and lung cancer.11-14 However, bisphosphonates reduce SRE by only 30-40% in patients with skeletal metastases, cause infusion-related reactions, osteonecrosis of the jaw (ONJ), and require intravenous administration and frequent renal monitoring.15-17
Receptor-activated nuclear factor kappa-B ligand (RANKL), one of the mediators of osteoclast differentiation, also attracts tumor cells into the bone, which in-turn interact with marrow stromal cells to produce more RANKL, creating a vicious cycle of osteoclast activation and bone destruction.18-21 Denosumab, a monoclonal antibody against RANKL, has shown efficacy in reducing osteolytic markers and SRE. It is administered as a subcutaneous injection and is not excreted through the kidney, a potential advantage compared to bisphosphonates for patients with chronic kidney disease.
Patients with metastatic breast, prostate and other cancers are living longer with the advent of newer and targeted therapies. Therefore, the role of supportive therapy to prevent and treat these bone complications is becoming more relevant. We conducted a systematic review and meta-analysis to evaluate the efficacy and safety of denosumab among patients with metastatic bone disease.
METHODS
Data sources and search strategy
We searched MEDLINE, EMBASE, the Cochrane Collaboration Library, and Web of Science with no language restrictions up to March 14, 2012. References of the included articles were also searched manually. The search strategy is provided in Appendix 1.
Study selection
Titles and abstracts of all retrieved citations were screened by two independent reviewers (GP and PP) to identify potentially relevant studies. Full texts were retrieved for relevant citations. Disagreements were resolved by consensus.
Inclusion criteria
Controlled clinical trials evaluating the efficacy of denosumab (at any dosage or frequency) for the treatment of cancer patients with skeletal metastases or myeloma were included, if they met the following criteria. 1) Participants of 18 years or older; and 2) Report of at least one of the following outcomes: a) Incidence of SRE, b) Time to first on-study SRE, c) Overall survival, d) Overall disease progression, e) Percent reduction in bone turnover markers (BTM), or f) Adverse events (AE).
We did not exclude studies based on trial duration or length of follow-up. Case reports, editorials, letters to the editors and studies with no comparison group were excluded. Abstracts of the conference proceedings were included if the journal article for the corresponding studies have not been published.
Data extraction
Primary outcomes were: 1)SRE, defined as pathological fracture (excluding major trauma), radiation therapy to bone, bone surgery or spinal cord compression. Hypercalcemia and pain were not included in this definition. We evaluated both incidence of SRE and time to first on-study SRE; 2) Overall survival (OS), defined as the time period from the point of entry into the study until death; and 3) Overall disease progression was analyzed, as reported by authors. Secondary outcomes included: 1) Pain evaluated as time to worsening, time to improvement, and time to improvement in physical activity, outcomes of pain were measured by any validated pain instrument or using visual analog scale;22, 23 2) Health-related quality of life (HRQL) was defined as the meaningful improvement in the composite scores of any instrument (MDASI or SDS) assessing physical, social, mental and functional wellbeing of an individual;24, 25 3) BTM such as urine N-telopeptide (uNTX) and serum bone-specific alkaline phosphatase (BSAP) are indicators for osteolysis and have shown linear correlation with SRE and death.22 Percentage reductions in the levels of BTM, proportion of patients who achieved reduction of uNTX >65% and time to achieve reduction in uNTX level >65% or <50 mmol/μmol creatinine were used as indicators to measure bone turnover outcome. uNTX level levels below 50 mmol/μmol creatinine are considered normal in young healthy individuals.26, 27 For patients with bone metastases, these levels are considered to represent a lower risk of developing SRE. The cut off level >65% was chosen based on the median percent reduction published on previous studies (59-65%);28, 29 and 4) AEs were defined as any unfavorable and unintended sign, symptom, abnormal laboratory finding, or disease associated with therapy. Grade 3 Common Terminology Criteria Adverse Events (CTCAE) requiring treatment discontinuation and serious AE (life threatening or requiring hospitalization) were considered when the information was available: a) Renal toxicity defined as an increase in blood urea or creatinine, acute or chronic renal failure, or decreased creatinine clearance, or proteinuria; b) Acute phase reactions defined as flu-like illness or any adverse events occurring within the first 3 days after the infusion; c) Hypocalcemia was defined as symptomatic or asymptomatic serum calcium below 8 mg/dl; d) ONJ is defined as appearance of necrotic bone in the oral cavity; and e) Incidence of new cancers and infections for both groups were analyzed as reported by authors.
Quality assessment
Each article that met eligibility criteria was independently assessed by two reviewers (PP and GP) for quality using the risk of bias tool. Attrition, confounding measurement, performance, selection and conflict of interest were graded as low risk, high risk and unable to determine.30
Data synthesis and analysis
All outcomes were pooled using STATA Software (version 11.2, StataCorp, College Station, TX).31 Dichotomous outcomes included rates or proportions from which pooled relative risk (RR) and 95% confidence intervals (CI) were estimated. Means and standard deviations (SD) were used to estimate mean differences and 95%CI. Medians were used instead of means when means were not reported. Standard deviation was estimated from the inter-quartile range when not available. If SD could not be derived through any method, missing data was imputed from other included studies. Pre calculated effect estimates (i.e. hazard ratio (HR)) and CI were pooled if median and SD were missing for time to event variables.24 Primary analyses were performed using a fixed effects model (Mantel-Haenszel method), and if there was study heterogeneity (I2 >40%), a random effects model was used. Number needed to treat (NNT) was also estimated.
RESULTS
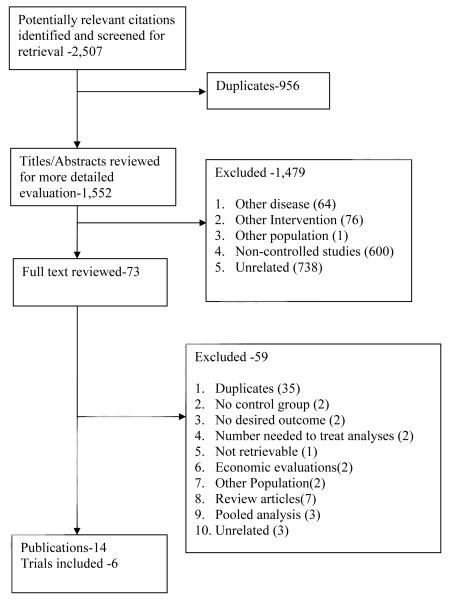
Our initial search identified 1,551 unique publications (Figure 1). Of these, only 14 met the inclusion criteria, providing data on 6 trials. Selection agreement between the two reviewers was 97.5% (κ= 0.7; Standard Error (SE) 0.02).
FIGURE 1.
Study characteristics
Six trials met our inclusion criteria; three were phase II28, 32, 33 and three phase III.34-36 The efficacy and safety of denosumab was compared with either intravenous zoledronic acid or pamidronate or ibandronate in these trials. The dosages and the frequency of the drugs administered, study population, length of follow-up and reported outcomes are shown in (Table 1).
Table 1.
Characteristics of the Included Studies.
| Study | Follow-up (Months) |
Population | Treated previously with IV Bisphosphonates |
Mean age (yrs) |
Intervention | Control | Outcomes |
|---|---|---|---|---|---|---|---|
| PHASE II TRIALS | |||||||
| Body 200632 | 2.8 | Myeloma Breast cancer |
No | 60.5 55.5 |
DB SQ 0.1,0.3,1.0,3.0 mg/kg (one dose) |
Pamidronate IV 90mg (one dose) |
BTM |
| Lipton 200728, 42 |
3 | Breast cancer | No | 58.7 | DB SQ 30,120,180 mg Q 4W 60, 180 mg Q 12W |
Bisphosphonates IV Q 4W |
Incidence of SRE BTM Safety |
| Fizazi 200933, 42, 60 |
3 | Prostate Breast Solid tumors (except lung) and uNTX > 50nM BCE/mM |
Yes | 60.5 | DB SQ 180 mg Q 4W or Q 12W |
Bisphosphonates IV Q 4W |
Incidence of SRE BTM Safety |
|
| |||||||
| PHASE III TRIALS | |||||||
|
| |||||||
| Stopeck 201034, 38, 41 |
34 | Breast cancer | No | 56.0 | DB SQ 120 mg Q 4W |
ZA IV 4 mg Q 4W |
Incidence of SRE. Time to first on-study SRE Time to first and subsequent on-study SRE Overall survival Overall disease progression Pain, HRQL BTM Safety |
| Fizazi 201135, 40 |
41 | Castrate resistant prostate cancer |
No | 71.0 | DB SQ 120 mg Q 4W |
ZA IV 4 mg Q 4W |
Incidence of SRE Time to first on-study SRE Time to first and subsequent on-study SRE Overall survival Overall disease progression Pain BTM Safety |
| Henry 201136, 39 |
34 | Solid tumors (except breast and prostate) Myeloma |
No | 60.5 | DB SQ 120 mg 4W |
ZA IV 4 mg Q 4W |
Incidence of SRE Time to first on-study SRE Time to first and subsequent on-study SRE Overall survival Overall disease progression Pain BTM Safety |
Q 4 W,12W, every 4 weeks and 12 weeks, DB, denosumab; ZA, zoledronic acid; BP, bisphosphonates; SQ, subcutaneous; IV, intravenous; uNTX, urine N-telopeptide; SRE, skeletal-related events.
Risk of bias
All studies were randomized controlled trials and had a low risk for bias for the various items assessed. However, three did not adequately report allocation concealment28, 33, 34 and two had open label study design for the drug administered.28, 33 All were funded by industry (Table 2).
Table 2.
Risk of Bias Assessment.
| Authors | Randomized | Analyzed | Withdrawn | ITT | Power calculation |
Selection bias |
Performance bias |
Measurement bias |
Funding Bias |
|
|---|---|---|---|---|---|---|---|---|---|---|
| E | S | |||||||||
|
Body
2006 32 |
54 | 54 | 54 | 5 | Yes | No | Unclear | Low risk | Low risk | Amgen |
|
Lipton
2007 28 |
255 | 254 | 254 | 1 | No | Yes | High risk | High risk | Unclear | Amgen |
|
Fizazi
2009 33 |
111 | 105 | 108 | NR | No | Yes | High risk | Low risk | Unclear | Amgen |
|
Stopeck
2010 34 |
2,046 | 2,046 | 2,046 | 5 | Yes | Yes | Unclear | Low risk. | Low risk | Amgen & Daichi |
|
Fizazi
2011 35 |
1,904 | 1,901 | 1,898 | 3 | No | Yes | Low risk | Low risk | Low risk | Amgen |
|
Henry
2011 36 |
1,779 | 1,776 | 1,756 | 3 | Yes | Yes | Low risk | Low risk | Low risk | Amgen |
E, efficacy; S, safety; NR, not reported; Unclear, if allocation concealment or random sequence generation or outcome assessment is not reported; High risk, if study has no blinding.
Participants
The six trials included 6,142 participants, of whom 3,191 received denosumab and 2,951 received intravenous bisphosphonates (either zoledronic acid or pamidronate or ibandronate). Weighted mean ages of the patients in the denosumab and bisphosphonate groups were 62.2 and 62.3 years, respectively. Prognostic factors including European cooperative oncology group performance status (ECOG), median times from the initial diagnosis of bone metastases to study allocation, proportion of patients with prior SRE, therapy and tumor histology were comparable in both groups (Table 3).
Table 3.
Characteristics of Study Participants
| Demographics | Denosumab (N=3,191) |
Bisphosphonates (N=2,951) |
|---|---|---|
| Mean age (years) | 62.2 | 62.3 |
| ECOG 0-1 | 2,837 | 2,617 |
| ECOG 2 | 303 | 312 |
| Prior SRE | 1,168 | 1,109 |
| Presence of visceral metastases | 1,187 | 1,154 |
ECOG, European Cooperative Oncology Group SRE, skeletal-related event
Outcomes
Incidence of SRE
This outcome was reported in five trials, all comparing denosumab to bisphosphonates (Table 4). Three of these measured SRE by a central radiological committee who were blinded to the intervention.34-36 For the remaining two trials, it was unclear if the SRE assessment was blinded.28, 33 There were 1,389 SRE (44%) in the denosumab-treated group and 1,628 SRE (55%) in the bisphosphonate-treated group with an absolute risk reduction of 11% (95%CI 8.6%-13.5%). The overall pooled RR for denosumab versus bisphosphonates was 0.84 (95%CI 0.80-0.88). Pathological fracture was the most common SRE for both denosumab and bisphosphonates (48.1-50.2%) followed by radiation (40.5-43.1%), surgery (2.7-2.8%) and spinal cord compression (6.0%). Patients on denosumab had lower likelihood of receiving radiation to the bone for pain relief (RR 0.81; 95% CI 0.72-0.92).37
Table 4.
Efficacy Outcomes
| Outcome | Tumor Type | Denosumab (n/N) |
Bisphosphonates (n/N) |
RR | 95%CI | I2 |
|---|---|---|---|---|---|---|
| Incidence of SRE | ||||||
| Denosumab vs. Pamidronate | ||||||
| Body 200632 | Breast Myeloma |
1/44 | 0/10 | 0.73 | 0.03, 16.8 | |
| Denosumab vs. Zoledronic Acid/Pamidronate/Ibandronate e | ||||||
| Lipton 200728 | Breast | 25/212 | 7/43 | 0.72 | 0.33, 1.5 | |
| Fizazi 200933 | Prostate Breast Solid tumorsb |
6/73 | 6/37 | 0.51 | 0.18, 1.4 | |
| Denosumab vs. Zoledronic Acid | ||||||
| Stopeck 201034 | Breast | 471/1,026 | 595/1,020 | 0.79 | 0.72, 0.86 | |
| Fizazi 201135 | Prostate | 494/950 | 584/951 | 0.85 | 0.78. 0.92 | |
| Henry 201136 | All tumors,c Myeloma |
392/886 | 436/890 | 0.90 | 0.82, 1.0 | |
| Pooled | 0.84 | 0.78,0.91 | 53% | |||
| OVERALL POOLED ESTIMATE | 0.84 | 0.80, 0.88 | 7% | |||
|
| ||||||
| Time to first on-study SRE |
Median
time (months) |
Median time
(months) |
HR | 95%CI | I2 | |
| Denosumab vs. Zoledronic Acid | ||||||
| Stopeck 201034 | Breast | Not reached | 26.4 | 0.82 | 0.71, 0.95 | |
| Fizazi 201135 | Prostate | 20.7 | 17.1 | 0.82 | 0.71, 0.95 | |
| Henry 201136 | All tumors,c Myeloma |
20.6 | 16.3 | 0.84 | 0.71, 0.98 | |
| Pooled | 0.83 | 0.75, 0.90 | 0% | |||
|
| ||||||
| Overall survival | ||||||
| Denosumab vs. Zoledronic Acid | ||||||
| Stopeck 201034 | Breast | Not reached | Not reached | 0.95 | 0.81, 1.1 | |
| Fizazi 201135 | Prostate | 19.4 | 19.8 | 1.0 | 0.91, 1.1 | |
| Henry 201136 | All tumors,c Myeloma |
13 | 13 | 0.95 | 0.83, 1.0 | |
| Pooled | 0.98 | 0.90, 1.0 | 0% | |||
|
| ||||||
| Time to worsening of paind,e | HR | 95%CI | I2 | |||
| Denosumab vs. Zoledronic Acid | ||||||
| Stopeck 201038 | Breast(1,042) | 9.7 | 5.7 | .78 | 0.67, 0.92 | |
| Brown 201140 | Prostate (1,901) | 5.8 | 4.8 | .89 | 0.77, 1.0 | |
| von Moos 201039 | All tumors,c Myeloma (1,776) |
5.5 | 4.7 | .85 | 0.73, 0.98 | |
| Pooled | 0.84 | 0.77, 0.91 | 0% | |||
Insufficient information to analyze the data for each bisphosphonates;
Except lung;
Except breast and prostate cancer;
Time to worsening from no/mild to moderate/severe pain;
Outcome measured at 73 weeks for Stopeck et al., and von Moos et al. Brown et al reported at 45 weeks. SRE, skeletal-related event.
Time to on-study SRE
Time to on-study SRE was reported in three phase III trials.34-36 Denosumab resulted in a greater delay to on-study SRE compared with zoledronic acid, with a pooled HR of 0.83 (95%CI 0.75-0.90). Henry et al.,36 reported HR of 0.84 for non-small cell lung cancer (NSCLC) (95%CI 0.64-1.1), 1.0 for myeloma (95%CI 0.68-1.6) and 0.79 for other solid tumors (95%CI 0.62-0.99).
Overall survival
Only three randomized studies reported survival, with no differences between denosumab and zoledronic acid.34-36 However, Henry et al.,36 examined overall survival stratifying patients according to tumor type and found that HR were 0.79 for NSCLC (95%CI 0.65-0.95), 2.3 for myeloma (95%CI 1.1-4.5) and 1.1 for other solid tumors (95%CI 0.90-1.3).
Overall disease progression
This outcome was reported as the time to worsening of the disease, in three phase III RCTs comparing denosumab and zoledronic acid, no difference was noted between the denosumab and zoledronic acid groups with an HR of 1.0 (95%CI 0.95-1.0)34-36 In one study disease progression was defined as progression of either localized or regional cancer, visceral metastases, worsening of prostate specific antigen concentration and bone turnover markers, progression of the disease due to skeletal related events was not included in this definition.35 Two studies did not report the definition of this outcome.34, 36
Pain
Three phase III trials reported time to worsening of pain and time to improvement.34-36, 38-40 All three studies used the Brief Pain Inventory (BPI), a 7-item self-reported tool ranging from 0 (no interference) to 10 (complete interference) to evaluate pain. Change on the BPI inventory was considered to indicate pain worsening (>4 points increase) or improvement (> 2 point decrease). There was a greater delay in worsening of pain level among the patients receiving denosumab (5.5-9.7 months) compared with patients on zoledronic acid (4.7-5.7 months), with a pooled HR of 0.84 (95%CI 0.77-0.91).38-40 However, there was no difference in the median times for the improvement of pain level between the two study groups.38, 40
HRQL
Only one phase III trial reported HRQL for patients with metastatic breast cancer.34 Patients completed FACT-G questionnaires at baseline, on day 8 and before each monthly visit to assess HRQL. Higher scores are associated with better HRQL (range 0-108). A 5-point increase from the baseline is considered as meaningful improvement. At 25 weeks 37.1% in denosumab and 31.4% of zoledronic acid groups had noted 5 point improvement on the FACT-G scores (P < 0.02). Mean scores improved from baseline through week 73 in both groups with an average of 3.2% more patients experiencing improvement in the denosumab treated group (range 1-7% from week 5-73). 41
BTM
Six trials reported BTM at 13 weeks. Denosumab was superior to bisphosphonates in reducing BTM in both bisphosphonate-naïve and patients previously treated with bisphosphonates (Table-5).28, 32-36, 42 Median time to achieve uNTX levels below 50 mmol/μmol creatinine was reported in metastatic breast cancer patients by Body et al.42 In patients previously treated with bisphosphonates, the median time was shorter for denosumab compared to bisphosphonates (9 vs. 65 days). However, in bisphosphonate-naïve patients the median time to achieve reduction was similar in both groups (9 and 8 days, respectively). Median time from baseline for >65% reduction in uNTX/Cr was 13 days (95%CI 10-29 days) for denosumab compared to 29 days (95% CI 9-86 days) for bisphosphonates.28
Table 5.
Percent Reduction in BTM at 13 weeks. Denosumab vs. Bisphosphonates.
| BTM | Denosumab (N) |
Bisphosphonates (N) |
Pooled Mean Difference |
95%CI | I2 | P-value |
|---|---|---|---|---|---|---|
| Denosumab vs. Zoledronic Acid/Pamidronate/Ibandronate a | ||||||
|
uNTX
a
28,
32-36 |
2980 | 2719 | −14.9 | −19.2,−10.7 | 78% | <0.0001 |
| BSAP 34-36 | 2771 | 2609 | −6.5 | −8.9,−4.2 | 13% | <0.0001 |
| Denosumab vs. Zoledronic Acid | ||||||
| uNTX | 2650 | 2629 | −12.5 | −14.8,−10.3 | 53% | 0.001 |
| BSAP | 2554 | 2552 | −7.6 | −9.9,−5.2 | 0% | <0.0001 |
Insufficient information to analyze data separately for each bisphosphonates.
Body et al.,32 reported percent reductions in uNTX for different doses of denosumab (0.1,0.3,1.0,3.0 mg/kg). uNTX, urine N-telopeptide; BSAP, serum bone-specific alkaline phosphatase.
Adverse events
Patients on denosumab had lower incidence of renal toxicity (RR 0.76; 95%CI 0.59-0.98) and acute phase reactions than those on bisphosphonates (RR 0.42; 95%CI 0.37-0.49).34-36, 43 No difference was observed between the two groups in the occurrence of CTCAE grade 3 adverse events, ONJ, new cancers and the incidence of infections (Table 6).
Table 6.
Adverse Events
| Outcome | Denosumab n/N |
Bisphosphonates n/N |
Pooled relative risk (RR) |
95%CI | P value | I2 |
|---|---|---|---|---|---|---|
| CTCAE grade 3 AE28, 32-36, 42 |
2,041/3,170 | 2,003/2,926 | 0.97 | 0.89,1.0 | 0.51 | 74% |
| AE-associated hospitalization28, 32-36, 42 |
1,575/3,176 | 1,646/2,930 | 0.95 | 0.91,1.0 | 0.04 | 0% |
| AE leading to Rx discontinuation28, 32-36, 42 |
336/3,176 | 402/2,942 | 0.82 | 0.72, 0.94 | 0.005 | 0% |
| Acute phase reactions28, 32-36, 42 |
264/3,170 | 586/2,939 | 0.42 | 0.37, 0.49 | <0.00001 | 37.9 % |
| Renal toxicity33-36 | 262/2,841 | 335/2,836 | 0.76 | 0.59, 0.98 | 0.03 | 61% |
| Hypocalcemia28, 32-36, 42 | 295/3,170 | 143/2,926 | 1.9 | 1.6, 2.3 | <0.00001 | 0% |
| New cancers34-36 | 28/2,841 | 18/2,836 | 1.6 | 0.86, 2.8 | 0.14 | 0% |
| Infections28, 33-36 | 1,474/3,125 | 1,646/2,930 | 1.0 | 0.93, 1.1 | 0.76 | 48% |
| ONJ32, 34-36 | 52/2,885 | 37/2,846 | 1.4 | 0.92, 2.1 | 0.11 | 0% |
AE, adverse events; Rx, treatment; ONJ, osteonecrosis of jaw.
Hypocalcemia
Denosumab treated patients had increased likelihood of developing hypocalcemia including CTCAE grade 3 and 4 (RR 1.9; 95%CI 1.6-2.3). Majority of patients in both groups developed transient hypocalcemia in the first weeks or within 6 months after treatment initiation and large number of patients remained asymptomatic. ONJ: all cases were ascertained based on serial oral examinations twice yearly by an independent expert panel. Although denosumab had similar risk to bisphosphonates in the occurrence of ONJ, an increased trend in the denosumab group was noted (1.8 vs. 1.3%, respectively). Patients with ONJ in both groups had several risk factors: (i) they were receiving either chemotherapy (54-67%) or anti-angiogenic therapy (9.6-21.6%); (ii) prior oral bisphosphonate therapy (7.6%); or (iii) had history of tooth extraction, bad oral hygiene and use of dental appliance (72.9-85.5%). Resolution of ONJ was observed in 27% of denosumab and 8% of zoledronic acid treated patients, however, the difference was not statistically significant (p=0.48).34-36 New cancers: incidence of new primary cancers was low in both groups (<1%). Bladder, lung, colorectal and skin cancers were reported as new malignancies by Fizazi et al.35
When denosumab was compared to zoledronic acid only, results were similar, except for the overall grade 3 adverse events. Patients in the denosumab group experienced lesser grade-3 adverse events than patients receiving zoledronic acid (HR 0.87; 95%CI 0.77-0.97).34-36
DISCUSSION
In this systematic review, subcutaneous admini stration of denosumab significantly reduced the incidence, as well as delayed the onset of SRE in patients with skeletal metastases when compared to intravenous bisphosphonates. Our results show that compared with bisphosphonates 9 (95%CI 7-11) additional people need to be treated to prevent one SRE, suggesting that this difference is clinically significant, given the morbidity and costs associated with SRE. Although the benefit of SRE reduction was consistently noted in all the trials included in our analysis, this effect among myeloma patients could not be assessed separately, due to lack of subgroup data from the published trials. Patients on denosumab also noted a significant delay in worsening of pain and a trend towards improvement in quality of life. Additionally, denosumab had a greater reduction in BTM (uNTX), which have been shown to be surrogate biomarkers for predicting SRE.44, 45 The effect of greater reduction in BTM (uNTX) by denosumab was maintained in both bisphosphonatenaïve and patients previously treated with bisphosphonates, suggesting that direct inhibition of the RANK ligand might be more effective in suppressing osteoclast activity. High levels of bone specific alkaline phosphatase correlate with bone formation, and elevated uNTX levels correlate with osteolysis and extent of bone disease in cancer patients. Failure to achieve reduction or normalization of BTM (uNTX) is associated with adverse clinical outcomes like increased pain, increased number of SRE and increased death.22
Although SRE such as bone fractures and spinal cord compression increase the risk of death among cancer patients,7, 46 the benefit of reduction in SRE achieved by denosumab showed improved survival only for NSCLC patients, but did not have an impact on overall survival in other groups, in fact myeloma patients had decreased survival (HR 2.2; 95% CI 1.13-4.50).36 The differences in overall survival among NSCLC and myeloma could be related to prognostic factors that might have been different between these groups at ba seline, and cannot be ascertained by our analysis. Of interest, zoledronic acid appears to have a direct inhibitory action on myeloma cells in mice.47-49 On the other hand although blocking the RANK ligand has been demonstrated to prevent the development of progesterone induced mammary epithelial tumors,50 the role of anti RANKL i.e. osteoprotegerin (OPG) a decoy receptor for RANK molecule in the prevention of bone cancers remains controversial, as it is associated with both pro and anti-tumor effects in the in vitro models.51
Studies in mice show that RANKL inhibition effectively reduced skeletal tumor burden by promoting tumor cell apoptosis and decreasing the tumor proliferation rate.52, 53 Likewise, mice without RANKL showed weak cell-mediated immunity and were noted to have high rates of infections and development of new cancers.54, 55 In our review we did not observe any difference in overall disease progression between the two study groups (RR 1.0; 95%CI 0.95-1.0), no increased rates for infections or the occurrence of new cancers in patients treated with denosumab was noted, but long-term post marketing surveillance is necessary to establish the risk for these low frequency events.56
Patients receiving denosumab had a significantly lower incidence of acute phase reactions and renal toxicity, and a small reduction in hospitalizations from adverse events. No differences were observed in the occurrence of ONJ between patients receiving denosumab or bisphosphonates. More patients in the denosumab group developed hypocalcemia a worrisome event which can cause cardiac and neurological complications, although no such complications were documented in these trials.57 Denosumab was associated with a decrease in SRE and BTM, and more frequent hypocalcemia, possibly reflecting superior inhibition of osteoclasts compared with zoledronic acid because of a different mechanism of action.18, 58, 59
To our knowledge, no systematic review has analyzed the safety and efficacy of denosumab in patients with skeletal metastases. Although our analysis included only six trials, the sample size was large enough and the data was obtained from fairly good quality RCTs to provide meaningful conclusions. However, as with any other literature based meta-analyses our study is limited by information available in the included trials, as the, pain and HRQOL were not reported in all the trials, evidence comparing the effectiveness of the two study drugs is limited for these outcome measures. Similarly, the evidence comparing the efficacy of denosumab and zoledronic acid on the incidence of SRE among the subgroups like lung cancers, colon cancer, and multiple myeloma could not be provided because of insufficient information..
In summary, denosumab was superior to zoledronic acid in reducing and delaying the onset of SRE, and decreasing BTM compared with bisphosphonates. However, a survival benefit was only observed for patients with NSCLC in one trial, and increased mortality was reported for patients with myeloma. Furthermore, superior efficacy of denosumab for SRE reduction may not be generalized for the patients previously treated with bisphosphonates, as this group constituted only small proportion and had very few events reported (6 events in each group). Reduced renal toxicity was observed for denosumab, suggesting that it is a more appropriate therapy than bisphosphonates for patients with chronic kidney disease. Considering the superior efficacy and safety in several outcome measures, denosumab will be one of the promising treatment options for patients with skeletal metastases. However, physicians should consider its cost effectiveness and also excise caution using in myeloma patients until further research.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Shana Palla for checking the accuracy of statistical methodology and Michael Worley for editing the manuscript.
Dr. Suarez-Almazor has a K24 career award from the National Institute for Arthritis, Musculoskeletal and Skin Disorders (NIAMS: grant # AR053593).
Funding source: Supported in part by a Cancer Center Support grant (CA016672) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests: All authors report no conflicts of interests.
AUTHOR CONTRIBUTIONS M. E. Suarez-Almazor had full access to all of the data in the study and takes responsibility for the integrity and the accuracy of the data analysis.
Study concept and design: M. E. Suarez-Almazor, M. A. Lopez-Olivo, P. Peddi.
Acquisition of data: P. Peddi, G. F Pratt, M. A. Lopez-Olivo, M. E. Suarez-Almazor.
Analysis and interpretation of data: M. A. Lopez-Olivo, P. Peddi, M. E. Suarez-Almazor.
Drafting of the manuscript: M. A. Lopez-Olivo, P. Peddi, M. E. Suarez-Almazor.
Critical revision of the manuscript for important intellectual content: M.A. Lopez-Olivo, P. Peddi, M. E. Suarez-Almazor.
Statistical analysis: M. A. Lopez-Olivo, P. Peddi, M. E. Suarez-Almazor.
Obtained funding: M. E. Suarez-Almazor.
Administrative, technical, or material support: M. E. Suarez-Almazor.
Study supervision: M. E. Suarez-Almazor.
REFERENCES
- 1.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–94. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, et al. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol. 1998;16:593–602. doi: 10.1200/JCO.1998.16.2.593. [DOI] [PubMed] [Google Scholar]
- 3.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–68. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 4.Johnson JR, Williams G, Pazdur R. End points and United States Food and Drug Administration approval of oncology drugs. J Clin Oncol. 2003;21:1404–11. doi: 10.1200/JCO.2003.08.072. [DOI] [PubMed] [Google Scholar]
- 5.Major PP, Cook R. Efficacy of bisphosphonates in the management of skeletal complications of bone metastases and selection of clinical endpoints. Am J Clin Oncol. 2002;25:S10–8. doi: 10.1097/00000421-200212001-00003. [DOI] [PubMed] [Google Scholar]
- 6.Oefelein MG, Ricchiuti V, Conrad W, Resnick MI. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol. 2002;168:1005–7. doi: 10.1016/S0022-5347(05)64561-2. [DOI] [PubMed] [Google Scholar]
- 7.Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860–7. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 8.Xie J, Namjoshi M, Wu EQ, Parikh K, Diener M, Yu AP, et al. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. J Manag Care Pharm. 2011;17:621–43. doi: 10.18553/jmcp.2011.17.8.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groot MT, Boeken Kruger CG, Pelger RC, Uyl-de Groot CA. Costs of prostate cancer, metastatic to the bone, in the Netherlands. Eur Urol. 2003;43:226–32. doi: 10.1016/s0302-2838(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 10.Delea T, Langer C, McKiernan J, Liss M, Edelsberg J, Brandman J, et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology. 2004;67:390–6. doi: 10.1159/000082923. [DOI] [PubMed] [Google Scholar]
- 11.Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042–57. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Berenson JR, Hillner BE, Kyle RA, Anderson K, Lipton A, Yee GC, et al. American Society of Clinical Oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2002;20:3719–36. doi: 10.1200/JCO.2002.06.037. [DOI] [PubMed] [Google Scholar]
- 13.Basch EM, Somerfield MR, Beer TM, Carducci MA, Higano CS, Hussain MH, et al. American Society of Clinical Oncology endorsement of the Cancer Care Ontario Practice Guideline on nonhormonal therapy for men with metastatic hormone-refractory (castration-resistant) prostate cancer. J Clin Oncol. 2007;25:5313–8. doi: 10.1200/JCO.2007.13.4536. [DOI] [PubMed] [Google Scholar]
- 14.Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial--the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21:3150–7. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim A, Scher N, Williams G, Sridhara R, Li N, Chen G, et al. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin Cancer Res. 2003;9:2394–9. [PubMed] [Google Scholar]
- 16.Mauri D, Valachis A, Polyzos IP, Polyzos NP, Kamposioras K, Pesce LL. Osteonecrosis of the jaw and use of bisphosphonates in adjuvant breast cancer treatment: a meta-analysis. Breast Cancer Res Treat. 2009;116:433–9. doi: 10.1007/s10549-009-0432-z. [DOI] [PubMed] [Google Scholar]
- 17.Diel IJ, Bergner R, Grotz KA. Adverse effects of bisphosphonates: current issues. J Support Oncol. 2007;5:475–82. [PubMed] [Google Scholar]
- 18.Silva I, Branco JC. Rank/Rankl/opg: literature review. Acta Reumatol Port. 2011;36:209–18. [PubMed] [Google Scholar]
- 19.Fili S, Karalaki M, Schaller B. Mechanism of bone metastasis: the role of osteoprotegerin and of the host-tissue microenvironment-related survival factors. Cancer Lett. 2009;283:10–9. doi: 10.1016/j.canlet.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–6. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 21.Mancino AT, Klimberg VS, Yamamoto M, Manolagas SC, Abe E. Breast cancer increases osteoclastogenesis by secreting M-CSF and upregulating RANKL in stromal cells. J Surg Res. 2001;100:18–24. doi: 10.1006/jsre.2001.6204. [DOI] [PubMed] [Google Scholar]
- 22.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–21. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Herr K. Pain assessment strategies in older patients. J Pain. 2011;12:S3–S13. doi: 10.1016/j.jpain.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JAC. Meta-Analysis in Stata: An Updated Collection from the Stata Journal. Stata Press. 2009:3–54. 61, 101–2, 20, 44–5. [Google Scholar]
- 25.Kirkova J, Davis MP, Walsh D, Tiernan E, O’Leary N, LeGrand SB, et al. Cancer symptom assessment instruments: a systematic review. J Clin Oncol. 2006;24:1459–73. doi: 10.1200/JCO.2005.02.8332. [DOI] [PubMed] [Google Scholar]
- 26.Coleman RE, Major P, Lipton A, Brown JE, Lee KA, Smith M, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23:4925–35. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 27.Coleman R, Costa L, Saad F, Cook R, Hadji P, Terpos E, et al. Consensus on the utility of bone markers in the malignant bone disease setting. Crit Rev Oncol Hematol. 2011;80:411–32. doi: 10.1016/j.critrevonc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25:4431–7. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 29.Berenson JR, Vescio R, Henick K, Nishikubo C, Rettig M, Swift RA, et al. A Phase I, open label, dose ranging trial of intravenous bolus zoledronic acid, a novel bisphosphonate, in cancer patients with metastatic bone disease. Cancer. 2001;91:144–54. doi: 10.1002/1097-0142(20010101)91:1<144::aid-cncr19>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 30.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. [Google Scholar]
- 31.StataCorp . Stata Statistical Software: Release 11. StataCorp LP; College Station, TX: 2009. [Google Scholar]
- 32.Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M, et al. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12:1221–8. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- 33.Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–71. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 34.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin KdB, Lichinitser RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–9. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 35.Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–22. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–32. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 37.Amgen [Accessed on: May 04, 2012];Highlights of prescribing information. XGEVA. 2010 Available from: http://wwwext.amgen.com/patients/products_xgeva.html.
- 38.Stopeck A, Fallowfield L, Patrick D, Cleeland CS, De Boer RH, Steger GG, et al. Effects of denosumab versus zoledronic acid (ZA) on pain in patients (pts) with metastatic breast cancer: Results from a phase III clinical trial. Journal of Clinical Oncology. 2010;28(15 Suppl):1024. [Google Scholar]
- 39.von Moos R, Patrick D, Fallowfield L, Cleeland CS, Henry DH, Qian Y, et al. Effects of denosumab versus zoledronic acid (ZA) on pain in patients (pts) with advanced cancer (excluding breast and prostate) or multiple myeloma (MM): Results from a randomized phase III clinical trial. Journal of Clinical Oncology. 2010;(7 Suppl) abstract 9043. [Google Scholar]
- 40.Brown JE, Cleeland CS, Fallowfield LJ, Patrick DL, Fizazi K, Smith MR, et al. Pain outcomes in patients with bone metastases from castrate-resistant prostate cancer: Results from a phase 3 trial of denosumab vs. zoledronic acid. European Urology. 2011;10(2 Suppl):336. [Google Scholar]
- 41.Fallowfield L, Patrick D, Body J, Lipton A, Tonkin KS, Qian Y, et al. Effects of denosumab versus zoledronic acid (ZA) on health-related quality of life (HRQL) in metastatic breast cancer: Results from a randomized phase III trial. Journal of Clinical Oncology. 2010;28(15 Suppl):1025. [Google Scholar]
- 42.Body JJ, Lipton A, Gralow J, Steger GG, Gao G, Yeh H, et al. Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. J Bone Miner Res. 2010;25:440–6. doi: 10.1359/jbmr.090810. [DOI] [PubMed] [Google Scholar]
- 43.Campbell-Baird C, Lipton A, Sarkeshik M, Ma H, Jun S. Incidence of acute phase adverse events following denosumab or intravenous bisphosphonates: Results from a randomized, controlled phase II study in patients with breast cancer and bone metastases. Community Oncology. 2010;7:85–9. [Google Scholar]
- 44.Clemons M, Cole DE, Gainford MC. Can bone markers guide more effective treatment of bone metastases from breast cancer? Breast Cancer Res Treat. 2006;97:81–90. doi: 10.1007/s10549-005-9094-7. [DOI] [PubMed] [Google Scholar]
- 45.Lein M, Miller K, Wirth M, Weissbach L, May C, Schmidt K, et al. Bone turnover markers as predictive tools for skeletal complications in men with metastatic prostate cancer treated with zoledronic acid. Prostate. 2009;69:624–32. doi: 10.1002/pros.20917. [DOI] [PubMed] [Google Scholar]
- 46.Yong M, Jensen AO, Jacobsen JB, Norgaard M, Fryzek JP, Sorensen HT. Survival in breast cancer patients with bone metastases and skeletal-related events: a population-based cohort study in Denmark (1999-2007) Breast Cancer Res Treat. 2011;129:495–503. doi: 10.1007/s10549-011-1475-5. [DOI] [PubMed] [Google Scholar]
- 47.Sorscher SM, Lockhart AC. Ras inhibition and the survival benefit favoring zoledronic acid compared with denosumab in patients with multiple myeloma. J Clin Oncol. 2011;29:2735–6. doi: 10.1200/JCO.2011.35.8333. author reply 6-8. [DOI] [PubMed] [Google Scholar]
- 48.Croucher PI, De Hendrik R, Perry MJ, Hijzen A, Shipman CM, Lippitt J, et al. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J Bone Miner Res. 2003;18:482–92. doi: 10.1359/jbmr.2003.18.3.482. [DOI] [PubMed] [Google Scholar]
- 49.Guenther A, Gordon S, Tiemann M, Burger R, Bakker F, Green JR, et al. The bisphosphonate zoledronic acid has antimyeloma activity in vivo by inhibition of protein prenylation. Int J Cancer. 2010;126:239–46. doi: 10.1002/ijc.24758. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103–7. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 51.Lamoureux F, Moriceau G, Picarda G, Rousseau J, Trichet V, Redini F. Regulation of osteoprotegerin pro- or anti-tumoral activity by bone tumor microenvironment. Biochim Biophys Acta. 2010;1805:17–24. doi: 10.1016/j.bbcan.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Dougall WC. Molecular Pathways: Osteoclast-Dependent and Osteoclast-Independent Roles of the RANKL/RANK/OPG Pathway in Tumorigenesis and Metastasis. Clin Cancer Res. 2012;18:326–35. doi: 10.1158/1078-0432.CCR-10-2507. [DOI] [PubMed] [Google Scholar]
- 53.Miller RE, Roudier M, Jones J, Armstrong A, Canon J, Dougall WC. RANK ligand inhibition plus docetaxel improves survival and reduces tumor burden in a murine model of prostate cancer bone metastasis. Mol Cancer Ther. 2008;7:2160–9. doi: 10.1158/1535-7163.MCT-08-0046. [DOI] [PubMed] [Google Scholar]
- 54.Terpos E, Dimopoulos MA. Interaction between the skeletal and immune systems in cancer: mechanisms and clinical implications. Cancer Immunol Immunother. 2011;60:305–17. doi: 10.1007/s00262-011-0974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrari-Lacraz S, Ferrari S. Do RANKL inhibitors (denosumab) affect inflammation and immunity? Osteoporos Int. 2011;22:435–46. doi: 10.1007/s00198-010-1326-y. [DOI] [PubMed] [Google Scholar]
- 56.Almenoff JS, Pattishall EN, Gibbs TG, DuMouchel W, Evans SJ, Yuen N. Novel statistical tools for monitoring the safety of marketed drugs. Clin Pharmacol Ther. 2007;82:157–66. doi: 10.1038/sj.clpt.6100258. [DOI] [PubMed] [Google Scholar]
- 57.Abramson EC, Gajardo H, Kukreja SC. Hypocalcemia in cancer. Bone Miner. 1990;10:161–9. doi: 10.1016/0169-6009(90)90259-i. [DOI] [PubMed] [Google Scholar]
- 58.Dominguez LJ, Di Bella G, Belvedere M, Barbagallo M. Physiology of the aging bone and mechanisms of action of bisphosphonates. Biogerontology. 2011;12:397–408. doi: 10.1007/s10522-011-9344-5. [DOI] [PubMed] [Google Scholar]
- 59.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–9. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 60.Fizazi K, Bosserman L, Gao G, Skacel T, Markus R. Denosumab treatment of prostate cancer with bone metastases and increased urine N-telopeptide levels after therapy with intravenous bisphosphonates: results of a randomized phase II trial. J Urol. 2009;182:509–15. doi: 10.1016/j.juro.2009.04.023. discussion 15-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.