Abstract
Purpose
Bisphosphonates are known to prevent skeletal-related events (SREs) in advanced breast cancer, prostate cancer and multiple myeloma. This systematic review assessed the efficacy of bisphosphonates in preventing SREs, controlling pain, and overall survival in patients with bone metastases from lung cancer.
Methods
We searched MEDLINE, EMBASE, Web of Science, and the Cochrane Library databases through November 10, 2011, for controlled trials that included lung cancer patients with bone metastases treated with bisphosphonates. Two reviewers independently extracted data on pain control, survival, SREs and evaluated the quality of each study. Meta-analyses were performed when there were two or more trials with similar outcomes.
Results
Twelve trials, met our inclusion criteria, and included 1,767 patients. Studies were placebo-controlled or compared bisphosphonates with other modalities (chemotherapy, radiation therapy, or radioisotope therapy), or used different bisphosphonates as active controls. Randomized controlled trials did not report adequate descriptions of randomization procedures, allocation concealment, and blinding, resulting in low quality scores. Patients treated with zoledronic acid + chemotherapy had fewer SREs than those receiving chemotherapy alone (relative risk (RR) 0.81, 95% confidence interval (CI) 0.67-0.97). Pain control improved when a bisphosphonate was added to another treatment modality (chemotherapy or radiation; RR 1.18, 95%CI 1.0-1.4). Bisphosphonate therapy improved survival compared to controls, but the difference failed to reach statistical significance (mean of 72 days, 95%CI −8.9-152.9).
Conclusions
Treatment with bisphosphonates reduced SREs, improved pain control and showed a trend to increased survival. Bisphosphonates should be used in the treatment of patients with lung cancer and bone metastases.
Keywords: Bisphosphonates, Lung cancer/neoplasm, Bone metastases, Randomized controlled trials, Meta-analysis
Despite years of research, tobacco prevention programs, and various new treatment modalities, lung cancer remains the leading cause of cancer-related deaths, with a median overall survival time of only 5.8 months after diagnosis and 1- and 2-year survival rates of 22% and 7%, respectively [1]. Thirty percent of patients with lung cancer can develop osteoblastic metastases and up to 40 percent of patients can develop osteolytic or mixed bone metastases [2]; 55% of these will experience one or more skeletal-related events (SRE) over a median follow-up period of 6 months [3];. Those, who already have experienced an SRE, are at higher risk of developing subsequent events [3, 4]. SREs include pathologic fractures, spinal cord compression, bone radiation or bone surgery, and hypercalcemia of malignancy [3-8], and they are associated with significant reductions in physical, functional, and emotional well-being, quality of life and performance status [3, 9]. Furthermore, pathological fractures or other SREs can render patients ineligible for anti-neoplastic treatments, resulting in further tumor progression and a decrease in overall survival [10, 11].
Since the 1990s, bisphosphonates have become a mainstay of the management of bone metastases from various cancers [12-14]. Bisphosphonates are synthetic analogues of pyrophosphate that bind to hydroxyapatite and are then internalized by osteoclasts, inducing apoptosis of the osteoclasts [15]. There are three Cochrane systematic reviews examining the efficacy of bisphosphonates in metastatic bone disease in breast and prostate cancer, and in multiple myeloma [8, 16, 17], but little is known about the effects of bisphosphonates on bone metastases from other solid tumors such as lung cancer, bladder cancer, gastrointestinal malignancies, renal cell carcinoma, melanoma and metastatic cancer with unknown primary [3, 9]. There is also a gross under usage of the bisphosphonates reported in non-small cell lung cancer (NSCLC) patients with metastatic bone disease [18]. The primary objective of this study was to systematically review the efficacy of various bisphosphonates in reducing SREs and bone pain, improving survival in patients with bone metastases from lung cancer. Additionally, we also summarized the evidence on other secondary outcomes (e.g., biomarkers, disease progression, and quality of life) and head-to-head comparisons of the different bisphosphonates used in lung cancer.
METHODS
Study Design
We followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement to guide us in our review and reporting of findings [19].
Data Sources and Search Strategy
Comprehensive electronic searches in MEDLINE, EMBASE, Web of Science, and Cochrane Library databases were performed until November 10, 2011, with no language restrictions. The detailed search strategy is presented in Appendix 1. Relevant published abstracts and articles were selected. Additionally, hand searching was done to identify relevant studies in the retrieved articles. In addition, a review of the clinicaltrials.gov website was performed to identify other ongoing or completed trials eligible for review. Authors of abstracts were contacted to obtain additional data but none responded to our request.
Study Selection
Studies were screened and independently selected by 3 reviewers (NS, MLO and GP) in pairs and disagreements were solved by consensus. Eligible studies were controlled clinical trials including lung cancer patients with bone metastases as confirmed by the authors treated with a bisphosphonate in at least one of the intervention groups, either alone or combined with other treatments such as chemotherapy, radiation therapy, or radioisotope therapy, and a comparison (control) group. Because in the real world, patients will receive multi-modality treatments concomitantly, supportive treatments and co-interventions prior to or after the bisphosphonate treatments were allowed. Any type of bisphosphonate was considered eligible, without restrictions on dose, route, frequency, or duration of treatment. The control group could have received placebo or an active control (a different bisphosphonate, chemotherapy, radiation therapy, radioisotope therapy, or any combination of these modalities). We excluded studies that did not include patients with bone metastases from lung cancer or those with the main objective not involving bisphosphonates. Additionally, we excluded observational studies, basic science studies, mixed population trials with non-retrievable data for lung cancer patients.
Data Extraction
Data were extracted independently by 3 reviewers (NAS, MLO and GP) including: 1) General study information; 2) Characteristics of participants; 3) Characteristics of intervention; 4) Characteristics of control; and 5) Outcome variables: SRE included fracture, radiation or surgery to bone, cord compression and/or hypercalcemia of malignancy. Any methods for pain measurement were allowed (visual analogue scale, numerical rating scale, verbal rating scale, 6-point McGill-Melzack pain questionnaire). We used the category “pain controlled” for categories defining effective control, significant improvement, complete or partial remission; and “pain not controlled” for categories with no improvement, exacerbation or no effect. Overall survival was measured in days since patients were allocated to study group. Secondary outcomes included biomarkers (i.e., serum N-telopeptide (NTX) and serum C-telopeptide (CTX) of collagen type I, urine NTX and bone alkaline phosphatase), time to first SRE, bone lesion progression, overall disease progression, performance status, quality of life, and toxicity reports. Appendix 2 shows the different definitions of SRE and other outcome measures used by each included study. The quality of each trial was evaluated independently by 3 reviewers (NS, MLO and GP) using the Cochrane Back Review Group questionnaire to assess risk of bias (0 referred to lowest quality and 11 to highest quality) [20, 21]. We evaluated each trial using 1=“yes” and 0=“no or don’t know” for selection, performance, attrition, detection, and reporting biases. A trial with a cumulative score of 0 to 6 was considered “low-quality” with a higher risk of bias, and a trial with a cumulative score of 7 to 11 was considered “high-quality” with a lower risk of bias.
Data Analysis and Synthesis
For this review, we have use only published data (full text or abstracts). We used STATA (version 10; College Station, Texas, USA) to perform the analysis [22]. Data was pooled in a meta-analysis when there were more than one trial reporting on the same outcome. A qualitative synthesis was provided for those outcomes reported only by one trial. The I-squared (I2) statistic was used to assess heterogeneity, an I2>40% was considered to indicate heterogeneous results. In the absence of heterogeneity, fixed effects models were used to pool results. When heterogeneity was present, random effects models were used [23]. The Mantel-Haenszel method was used to pool the relative risk (RR) for dichotomous outcomes, and the inverse variance method was used to pool the mean differences (MD) for continuous variables. We set the significance level at α=0.05 for pooled data. Comprehensive Meta-Analysis (version 2; Biostat, Englewood, NJ, USA ) was used to compute effect sizes, standard errors, and variances for the survival outcome when data were limited [24].
RESULTS
Eligible Trials and Study Characteristics
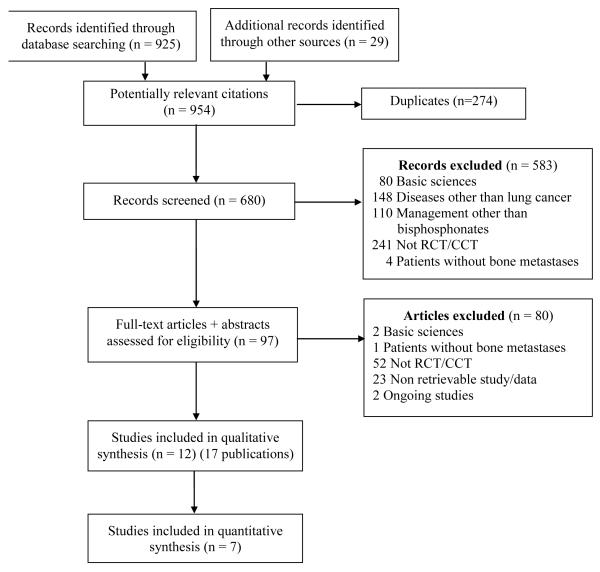
Of the 925 records identified from the electronic database searches, 680 abstracts were selected for further review. Twelve studies (17 publications) met the inclusion criteria, including a total of 1,767 participants (Figure 1) [6, 7, 18, 25-39]. Of these, only seven studies reported sufficient data for the meta-analysis. Four studies were published in Chinese and were translated by 3 people (see acknowledgements). Table 1 shows participant and design characteristics of the studies included and Table 1 summarizes the comparison groups used in each study.
Figure 1.
Study selection flowchart following PRISMA recommendations[19].
Table 1.
Study and Patient Characteristics
| Study | Country | Center | Follow-up | Groups (n) | Mean age,years |
Male, % |
NSCLC Stage |
Bias Scalea |
|---|---|---|---|---|---|---|---|---|
| Zhang [29] | China | Single | 21 days | Pamidronate (13) Pamidronate + radiation (12) Pamidronate + Chemotherapy (15) Radiation (40) |
55.5 | 58.7 | NRf | 2 |
| Su [30] | China | Single | 3 months | Clodronate (28) Radioisotope (89SrCl2) (19) Clodronate + Radioisotope (20) |
58.9 | 65.7 | NRf | 2 |
| Li [28] | China | Single | NR | Radiation (20) Radioisotope (16) Chemotherapy (19) Clodronate (15) Radiation + chemotherapy (22) Radiation + Radioisotope (25) Clodronate + chemotherapy (24) |
58.5 | 69.5 | NR | 1 |
| Zheng [32] | China | Single | NR | Pamidronate + chemotherapy (18) Pamidronate + radiation (16) Pamidronate + radioisotope (12) Chemotherapy (15) Radiation (10) |
48.5 | 64.8 | NRf | 2 |
| Guo [26] | China | Single | 42 days | Ibandronate (44) Clodronate (42) |
51.4 | 53 | NR | 2 |
| Kritikosg,i [34] | Greece | Single | 24 months | Zolendronic acid (NR) Placebo (NR) |
NR | NR | NRf | - |
| Rosen [7][6][27] | USA, UK, Canada |
Multi | 9 months | Zoledronicacid (259) Placebo (123) |
63c | 67 | NR | 4 |
| Zarogoulidis [31, 35] | Greece | Single | 12 months | Zoledronic acid + Chemotherapy (87) Chemotherapy (57) |
62c | NR | IV | 2 |
| Francini [25] | US, Canada |
Multi | 3 months | Zoledronic acid (28) Ibandronate (27) |
64 | 63.2 | IIIB or IV | 2 |
| Pandyah [33] | Italy | Single | 12 months | Zolendronic acid + Chemotherpay (100) Chemotherapy (52) |
70c | 75 | IV | 3 |
| Hirai [36] | Japan | Multi | 12 months | Zolentronic acid + Chemotherapy (50) Chemotherapy (50) |
NR | NR | NR | - |
| Scagliottib [37-39] | Europe | Multi | 24 months | Zolendronic acid (226) No treatmentb (211) |
59.6 | 70.3 | IIIA or IIIB | 3 |
NR, not reported; NSCLC, non-small cell lung cancer;
Cochrane Back Review group risk of bias (range 0 to 11, higher values indicate lower bias[20];
All patients had completed primary treatment (surgery, chemotherapy or radiotherapy).
Median age, years;
Standard Deviation;
Included both small cell lung cancer and non-small cell lung cancer;
Data published in abstract form only were included;
Did not include patients with bone metastases;
assuming total number of patients equally distributed between groups (26 + 26 =52).
Quality Appraisal
Quality scores of the studies ranged from 1 to 4. None of the studies described the method of randomization (e.g., random number table) or allocation concealment (e.g., sealed envelopes). Only three trials described baseline similarity among the groups [25, 27, 36]. Blinding was often not feasible, especially when non-drug treatment modalities (e.g., radiation therapy) were included. One study mentioned adequate double-blinding but did not specify whether the patient or the outcome assessor or care provider were blinded [27]. Because all treatments were multi-modal, none of the trials could avoid co-interventions. Although none of the trials mentioned patient adherence to treatment, 2 trials described patient drop-out owing to side effects or other adverse events such as renal impairment, gastrointestinal problems, or osteonecrosis of the jaw [25, 31]. Overall, seven trials included all randomized or assigned patients in the final analysis, thus providing intention-to-treat analysis [27-32, 37-39]. Two trials were only available in abstract format; therefore no quality assessment was performed [34, 36].
Efficacy outcomes
Table 2 describes efficacy outcomes: SRE incidence, pain control and overall survival, time to first SRE and disease progression, in individual and pooled data from included trials.
Table 2.
Major efficacy outcomes
| Intervention | Control | Effect size (95% CI) | I2 | p-value | |||
|---|---|---|---|---|---|---|---|
| Events/ n | % | Events/ n | % | ||||
| SRE INCIDENCE |
RR for reduced
|
SREs | |||||
| Zoledronic acid vs. placebo | |||||||
| Kritikosc [34] | 10/26 | 38 | 13/26 | 50 | 0.77 (0.41-1.4) | ||
| Zoledronic acid + chemotherapy vs. chemotherapy | |||||||
| Zarogoulidis[31, 35] | 4/87 | 5 | 3/57 | 5 | 0.87 (0.20-3.8) | ||
| Rosen [7] [6] [27] | 124/259 | 48 | 74/123 | 60 | 0.80 (0.66-0.96) | ||
| Hirai [36] | 15/50 | 30 | 20/50 | 39 | 0.75 (0.44-1.3) | ||
| Scagliottid [37-39] | 5/226 | 2 | 3/211 | 1 | 0.74 (0.39-1.4) | ||
| Pooled M-H | 148/622 | 24 | 100/441 | 23 | 0.81(0.67-0.97) | 0 | 0.02 |
| Zoledronic acid vs. ibandronate | |||||||
| Francini [25] | 5/26 | 19 | 7/27 | 30 | 0.74 (0.27-2.0) | ||
|
| |||||||
| PAIN CONTROL | RR for better pain control | ||||||
| Clodronate vs. radioisotope | |||||||
| Su [30] | 23/28 | 82 | 16/19 | 84 | 0.98 (0.75-1.3) | ||
| Li [28] | 8/15 | 53 | 9/16 | 56 | 0.95 (0.50-1.8) | ||
| Pooled M-H | 31/43 | 72 | 25/35 | 71 | 0.97 (0.74-1.3) | 0 | 0.80 |
| Bisphosphonate alone (clodronate or pamidronate) vs. other modalities a | |||||||
| Su [30] | 23/28 | 82 | 16/19 | 84 | 0.98 (0.75-1.3) | ||
| Li [28] | 8/15 | 53 | 64/102 | 63 | 0.85 (0.52-1.4) | ||
| Zhang [29] | 11/13 | 85 | 33/40 | 83 | 1.0 (0.78-1.4) | ||
| Pooled M-H | 42/56 | 75 | 113/161 | 70 | 0.95 (0.78-1.2) | 0 | 0.62 |
| Bisphosphonate combined (clodronate or pamidronate) vs. other modalities b | |||||||
| Su [30] | 18/20 | 90 | 16/19 | 84 | 1.1 (0.84-1.4) | ||
| Li [28] | 18/24 | 75 | 64/102 | 63 | 1.2 (0.91-1.6) | ||
| Zhang [29] | 24/27 | 89 | 33/40 | 83 | 1.1 (0.89-1.3) | ||
| Zheng [32] | 41/46 | 89 | 16/25 | 64 | 1.4 (1.0-1.9) | ||
| Pooled M-H | 101/117 | 86 | 129/186 | 69 | 1.2 (1.0-1.4) | 0 | 0.01 |
| Bisphosphonate (combined) vs. bisphosphonate (alone) | |||||||
| Su [30] | 18 /20 | 90 | 23/28 | 82 | 1.1 (0.87-1.4) | ||
|
|
|||||||
| Li [28] | 18/24 | 75 | 8/15 | 53 | 1.4 (0.83-2.4) | ||
|
|
|||||||
| Zhang [29] | 24/27 | 89 | 11/13 | 85 | 1.1 (0.80-1.4) | ||
|
|
|||||||
| Pooled M-H | 60/71 | 85 | 48/56 | 86 | 1.2 (0.96-1.4) | 0 | 0.14 |
| Zoledronic acid vs. ibandronate | |||||||
| Francini [25] | 11/18 | 61 | 9/16 | 56 | 1.1 (0.62-1.9) | ||
| Ibandronate vs. clodronate | |||||||
| Guo [26] | 36/46 | 78 | 31/42 | 74 | 1.1(0.88-1.4) | ||
| SURVIVAL | Mean difference (days) | ||||||
|
Zoledronic acid + chemotherapy vs.
chemotherapy |
n | days e | n | days e | |||
| Zarogoulidis [31, 35] | 87 | 578 | 57 | 384 | 194.0 (80.9-307.2) | ||
| Rosen [7][6][27] | 259 | 187 | 123 | 157 | 30.0 (−65.6-125.6) | ||
| Pandyaf [33] | 100 | 266 | 52 | 206 | 60.0 (−108.2-228.2) | ||
| Hirai [36] | 50 | 312 | 50 | 291 | 21.0 (−61.7-103.7) | ||
| Pooled I-V | 496 | 282 | 72.0 (−8.9-152.9) | 55 | 0.08 | ||
| TIME TO DISEASE PROGRESSION | Mean difference (days) | ||||||
|
Zoledronic acid + chemotherapy vs.
chemotherapy |
n | days e | n | days e | |||
| Zarogoulidis [31, 35] | 87 | 265 | 144 | 150 | 115.0 (47.9-182.1) | ||
| Pandya [33] | 100 | 132 | 150 | 131 | 0.00 (−0.04-0.04) | ||
| Hirai [36] | 50 | 81 | 100 | 78 | 3.0 (−39.4-45.4) | ||
| Scagliottid [37-39] | 226 | 270 | 437 | 339 | −69.0 (−109.8- −28.2) | ||
| Pooled I-V | 463 | 831 | 5.2 (−41.5-51.9) | 87 | 0.83 | ||
n= sample size; SRE, Skeletal Related Events; M-H, Mantel-Haenzel; I-V, Inverse Variance;
Other modalities include: chemotherapy, radiation and /or radioisotopes;
Combined: bisphosphonates with chemotherapy, radiation and/or radioisotopes;
Assuming total number of patients equally distributed between groups;
All patients had completed primary treatment (surgery, chemotherapy or radiotherapy);
Median survival time in days; fDid not include patients with bone metastases.
SRE incidence
Six studies including 1,170 participants reported SRE. Pooled estimates showed a statistically significant 19% reduction in the risk of developing new SREs within the first 2 years of treatment with zoledronic acid (RR 0.81, 95%CI 0.67-0.97) [6, 7, 27, 31, 35, 36]. Zoledronic acid did not demonstrate a statistically significant difference in the risk of developing SREs compared to ibandronate.
Pain Control
Six studies including 500 participants evaluated pain control (patient-reported pain). All studies used 3 levels to categorize pain control (see Appendix 2). Pooled estimates were not statistically significant. However, from individual studies, when bisphosphonates were added to chemotherapy or radiation therapy, patients in the combined-modality group had significantly better pain control than patients in the group receiving chemotherapy or radiation therapy alone [28-30, 32]. Also, more rapid reduction in pain scores was found in patients receiving zoledronic acid (41.6% vs. 29.3%, p=0.05) compared to ibandronate, which disappeared at three months (66.2% vs. 61.8%, p=0.31) [25]. In a recent small head-to-head comparison, treatment with ibandronate did not lead to significantly better pain control than clodronate (RR 1.1, 95%CI 0.88-1.4), but the authors mentioned that the monthly intravenous injection schedule of ibandronate was more convenient for patients than the daily intravenous injection of clodronate [26].
Overall Survival
Four studies including 778 participants compared survival following treatment with zoledronic acid plus chemotherapy versus chemotherapy alone [6, 7, 27, 31, 33, 36]. An estimated difference of 72.0 days in median survival was observed favoring the zoledronic acid group compared to controls, but this did not reach statistical significance (p=0.08).
Time to first SRE
Zoledronic acid significantly delayed the time to first SRE compared to placebo (MD 163 days; 95%CI 45.2-278.8) [34]. Similarly, patients on zoledronic acid + chemotherapy showed a non-statistically significant longer time to first SRE compared to chemotherapy alone (MD 36 days; 95%CI −312.6-384.6) [36]. Patients in the zoledronic acid group developed their first SRE later than patients in the ibandronate group (median time to develop first SRE=10.2 months for zoledronic acid vs. 9.4 months for ibandronate, p=0.03) [25].
Progression of bone lesions
Two studies reported progression of bone lesions, where ‘Improvement’ was measured as complete remission or partial remission compared to ‘no improvement’ as no change or progressive disease by imaging modalities [26, 30]. The proportion of patients with no disease progression with or without zolendronic acid was similar (41% vs 39%, p=0.80) [33]. Patients treated with a combination of clodronate and radioisotope therapy had less progression 3 months after treatment began compared to patients who received radioisotope therapy alone (p< 0.05) [30]. After 2 cycles of ibandronate or clodronate, bone disease progression was comparable between the two treatment groups [26].
Overall disease progression
When compared to chemotherapy alone, zoledronic acid + chemotherapy did not result in significantly different time to disease progression (MD 5, 95%CI −41.5-51.9, p=0.8) [31, 33, 36-39]. Tumor response to zoledronic acid and to ibandronate was comparable using Response Evaluation Criteria In Solid Tumors criteria at 3-month follow-up [25].
Performance status and quality of life
Two studies reported improvement in quality of life and functional status (data not shown). Treatment with clodronate combined with radioisotope therapy was more effective than radioisotope therapy alone at improving quality of life at 1 month after treatment was started (75% vs. 47.3%, p<0.05)[30]. Ibandronate and clodronate were similarly effective at improving daily living [26].
Biomarkers
Three trials examined biomarkers including serum NTX and CTX of collagen type I, urine NTX and bone alkaline phosphatase [25, 27, 31]. In a post-hoc analysis zoledronic acid and placebo groups were divided into high (≥64 nmol/mmol creatinine), and normal/low-NTX (<64 nmol/mmol creatinine) subgroups. Within both the zoledronic acid and placebo groups, high NTX levels, compared to normal/low levels, were associated with increased adverse events, although not consistently statistically significant including: SREs (zoledronic acid: RR = 1.3, p=0.3; placebo: RR = 1.5, p=0.2), bone disease progression (zoledronic acid: RR = 1.4. p=0.2; placebo: RR = 2.2, p=0.04), experiencing all-time SREs (zoledronic acid: RR = 1.8, p=0.01; placebo: RR = 1.6, p=0.07), and death (zoledronic acid: RR = 1.3, p=0.1; placebo: RR = 2.4, p=0.001) [27]. When analyzing only patients with high baseline NTX levels, the zoledronic acid group had a significantly reduced risk of death (35%, p=0.02) compared to the placebo group [27]. Patients treated with zoledronic acid had significantly lower median serum CTX levels at 1 month compared to those in the ibandronate group (reduction in levels = 54.8% vs. 38.2%, p=0.03); however, this difference disappeared at 3 months (reduction in levels = 72.6% vs. 66.4%, p=0.22) [25]. Reductions in median bone alkaline phosphatase levels were not significantly different at 1 and 3 months [25]. Urinary NTX levels were reduced in the zoledronic acid treated group by more than half in the first 3 months compared to placebo [31]. Moreover, those who had lower urinary NTX levels at baseline showed maximum reduction of levels lasting up to 9 months when compared to those with higher levels.
Toxicity
Table 3 shows the frequency of adverse events in the included trials, most commonly transient flu-like and gastrointestinal symptoms. Furthermore, patients assigned to the group with zoledronic acid were 17.8 times more likely to develop flu-like syndrome compared to the group without it. Renal impairment was reported in up to 15% of the patients treated with zoledronic acid [25, 31]. There were also reports of reversible bone marrow suppression with the combination of clodronate, pamidronate and zoledronic acid with other therapeutic modalities [26, 30, 33]. Incidence of osteonecrosis of the jaw was observed in 4 of 87 (5%) patients who receiving zoledronic acid [31].
Table 3.
Major toxicity outcomes
| Intervention | Control | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Adverse event (study) | Comparison group | Events/n | % | Events /n | % | RRa (95% CI) | ARa |
| Flu-like illness | |||||||
| Zheng [32] | Pamidronate (combined)a vs. chemoradiation | 4/46 | 9 | 3/25 | 13 | 0.7 (0.18-3.0) | 4 |
| Guo[26] | Ibandronate vs. clodronate | 3/44 | 7 | 5/42 | 12 | 0.6 (0.15-2.3) | 5.1 |
| Shucai [29] | Pamidronate (single or combined)a vs. radiation | 1/40 | 3 | 2/40 | 5 | 0.5 (0.05-5.3) | 2.5 |
| Zarogoulidis [31] | Zoledronic acid + chemotherapy vs. chemotherapy | 13/87 | 17 | 0b/57 | 0 | 17.8 (1.1-293.6) | 17 |
| Francini [25] | Zoledronic acid vs. ibandronate | 6/26 | 23 | 2/27 | 7 | 3.1 (0.69-14.1) | 15.6 |
| Scagliotti [37-39] | Zoledronic acid vs. no treatment | 12/224 | 5 | 3/213 | 1 | 3.8 (1.1-2.1) | 4 |
| Gastrointestinal illness | |||||||
| Su [30] | Clodronate vs. radioisotope | 6/48 | 13 | 2/19 | 11 | 1.2 (0.26-5.4) | 2 |
| Li [28] | Clodronate (single or combined) vs. other modalities | 38/43 | 88 | 44/63 | 70 | 1.3 (1.0-1.5) | 19 |
| Shucai [29] | Pamidronate (single or combined)a vs. radiation | 5/40 | 13 | 9/40 | 23 | 0.56 (0.20-1.5) | 10 |
| Zarogoulidis [31] | Zoledronic acid + chemotherapy vs. chemotherapy | 15/87 | 17 | 0b/57 | 0 | 20.4 (1.3-334.9) | 17 |
| Francini [25] | Zoledronic acid vs. ibandronate | 0b/26 | 0 | 1/27 | 4 | 0.35 (0.01-8.1) | 3.8 |
| Scagliotti [37-39] | Zoledronic Acid vs. no treatment | 100/224 | 9 | 65/213 | 4 | 1.5 (0.08-0.23) | 15.3 |
| Kidney impairment c | |||||||
| Zarogoulidis [31] | Zoledronic acid + chemotherapy vs. chemotherapy | 4/87 | 5 | 0b/57 | 0 | 5.9 (0.33-108.1) | 5 |
| Francini [25] | Zoledronic acid vs. ibandronate | 4/26 | 15 | 1/27 | 4 | 4.2 (0.50-34.8) | 11.6 |
| Scagliotti [37-39] | Zoledronic Acid vs. no treatment | 1/224 | 5 | 1/213 | 1 | 0.95 (0.06-15.1) | 0.02 |
| Decrease in blood counts/bone marrow suppression | |||||||
| Su [30] | Clodronate vs. radioisotope | 4/48 | 8 | 4/19 | 21 | 0.4 (0.11-1.4) | 12.7 |
| Guo [26] | Ibandronate vs. clodronate | 1/44 | 2 | 1/42 | 2 | 0.95 (0.06-14.8) | 0.1 |
| Pandyad [33] | Zoledronic acid + chemotherapy vs. chemotherapy | 29/98 | 30 | 14/52 | 27 | 1.1 (0.64-1.9) | 2.7 |
| Osteonecrosis of jaw | |||||||
| Zarogoulidis [31] | Zoledronic acid + chemotherapy vs. chemotherapy | 4/87 | 5 | 0b/57 | 0 | 5.9 (0.33-108.2) | 5 |
| Francini [25] | Zoledronic acid vs. ibandronate | 1/26 | 4 | 0b/27 | 0 | 3.1 (0.13-73.1) | 3.8 |
| Scagliotti [37-39] | Zoledronic Acid vs. no treatment | 1/224 | 1 | 1/213 | 1 | 0.95 (0.06-15.1) | 0.02 |
AR, Absolute Risk; CI, confidence interval; RR, relative risk;
Single: no additional treatment modalities used, combined: additional modalities (chemotherapy, radiation therapy, or radioisotope therapy) used;
Numbers not reported in study, ‘0’ events were assumed;
Authors reported: increase in creatinine ≤1 mg/dl (Zarogoulidis et al. [62]), decrease renal function (Francini et al. [23]), and renal failure (Scagliotti et al. [53-55]);
Did not include patients with bone metastases.
DISCUSSION
Bisphosphonates are commonly used in patients with breast or prostate cancer with bone metastases or multiple myeloma, and have been shown to be effective in reducing bone pain and the occurrence of SREs, either when used alone or concomitantly with radiation therapy [8, 16, 17, 40-43]. However, bisphosphonates are seldom used in patients with lung cancer and metastatic bone disease. Our systematic review and meta-analysis revealed that bisphosphonates can reduce the risk of developing SREs, and help control bone pain in these patients. We found that patients treated with zoledronic acid, compared to placebo, were 19% less likely to develop SREs. Our findings are consistent with the findings of studies assessing the efficacy of zoledronic acid in treating bone metastases from other types of solid tumors [6, 7, 44, 45]. While subgroup analysis was not possible in our review because data was not available, a prior exploratory analysis showed that duration of bone metastasis (≥2 months vs. <1 month), predominant lesion type (osteolytic vs. osteoblastic), a lymphocyte level >14% were associated with increased risk of developing an SRE in patients with NSCLC [46]. In multivariate models, NTX ≥64 nmol/mmol was associated with a >3-fold increased risk of developing a pathologic fracture [46]. Conceivably, patients with these risk factors could benefit the most from bisphosphonate therapy.
Pain control is a primary objective of the use of bisphosphonates in patients with bone metastases [8, 43, 47]. Pamidronate combined with radiation have been reported to provide better pain relief than radiation alone in patients with breast and lung cancers metastatic to bone [47]. A double-blinded, placebo-controlled randomized trial showed that, compared to placebo, 1,600 mg/day of oral clodronate reduced pain scores and analgesic requirements in patients with bone metastases from tumors that were poorly responsive to chemotherapy [9]. When a bisphosphonate alone was compared to other modalities, treatments such as chemotherapy, radiation therapy, or radioisotope therapy provided better pain control. A meta-analysis of 30 randomized controlled trials in 3,682 subjects evaluating the role of bisphosphonates for the relief of bone pain secondary to metastases from various cancers found that 11 patients would need to be treated for 4 weeks or 7 patients for 12 weeks in order to observe the best pain response in 1 patient [43]. Our meta-analysis in patients with lung cancer revealed that when a bisphosphonate was added to standard treatments as mentioned earlier, the combination treatment resulted in significantly better pain control (18% reduction) than the standard treatments without the bisphosphonate. However, use of bisphosphonate alone was not better than the use of other modalities alone. This suggests that bisphosphonates are most effective for pain control when combined with other therapies.
Four trials in our review reported survival, with a pooled overall survival benefit of more than 2 months when comparing the patients treated with zoledronic acid plus chemotherapy to chemotherapy alone, albeit the samples were small with a total of 778 participants, and the difference did not reach statistical significance. Due to the shorter life expectancy among patients with metastatic lung cancer compared to the life expectancy of patients with some other metastatic cancers, survival measurement may be challenging. Larger, well-performed randomized trials or well controlled cohort studies may be required to strengthen the evidence on overall survival. Coleman et al., reported the results of 3 randomized controlled trials comparing zoledronic acid vs. placebo in the treatment of more than 1600 patients with metastases to bone from solid tumors (>500 from lung cancer), and showed similar survival in the two treatment groups in the intention-to-treat analysis (RR for zoledronic acid =0.94; p=1.1) [48]. Similarly, in a retrospective cohort study, 50 consecutive patients with stage IV NSCLC with bone metastases who had received zoledronic acid plus chemotherapy were compared with patients who received chemotherapy alone. Patients receiving chemotherapy in combination with zoledronic acid showed a statistically significant increase in survival (238 days vs 133 days, respectively; p=0.03) [18].
We found no differences in the time to disease progression between zoledronic acid + chemotherapy and chemotherapy alone. This was also observed by the Associazione Italiana Pneumologi Ospedalieri chest oncology group. The authors reported similar control over bone disease progression between patients treated with pamidronate and those who received radiation therapy [50].
Overall, bisphosphonates were well tolerated. However, among the studies included in our analysis, Zarogoulidis et al., reported up to a 5% incidence of osteonecrosis of the jaw in patients treated with zoledronic acid [31]. This adverse event has also occurred in studies of zoledronic acid for bone metastases from other cancers. A phase III, multi-center, randomized controlled trial examining zoledronic acid as an adjuvant therapy in patients with stage II or III breast cancer reported 11 (0.7%, 95%CI 0.3;1.1%) cases of osteonecrosis of the jaw in the zoledronic acid group [51].
Zoledronic acid has been extensively studied and has shown superior efficacy compared to other bisphosphonates or placebo in several trials for the treatment of bone metastases from various solid tumors [34, 55-58]. In various reviews by Coleman [59-61], zoledronic acid demonstrated the broadest clinical activity in patients with bone metastases from a wide variety of tumor types. The reported adverse events related to zoledronic acid were generally mild and infrequent, suggesting that the benefits of treatment will typically outweigh the risks [60].
In an economic evaluation from five European countries based on a randomized control trial by Rosen et al., 4 mg (or 8 mg) intravenous zoledronic acid every 3 weeks was shown to be cost-effective when compared to placebo in patients with lung cancer [63, 64]. Outcomes and assumptions about benefits were the reported cost-drivers, while adverse events and administration costs did not influence cost-effectiveness estimates [65]. Zoledronic acid has also received the broadest regulatory approval to be used to treat hypercalcemia of malignancy or bone lesions secondary to multiple myeloma and other solid tumors. In addition to patient preferences for shorter infusion times, the 15-minute intravenous infusion of zoledronic acid was felt to be efficient for infusion centers by increasing patient turnover [66]. Studies are ongoing to examine the use of zoledronic acid as a treatment for cancer with potential antitumoral effects other than the reduction of SREs and bone pain [67-69].
Our meta-analysis had limitations. The quality of the trials was generally poor, often because of barriers to effective blinding which cannot be easily resolved for concomitant therapies such as chemotherapy or radiation which may vary among patients. Furthermore, although a trend in improved survival was observed in patients receiving bisphosphonates, the sample sizes of the studies were small and some results did not reach statistical significance. There were 4 studies reporting combined data on both small cell and NSCLC and separate data could not be extracted. Small cell lung cancer is considered distinct from NSCLCs. It exhibit more aggressive behavior, with rapid growth, early spread to distant sites, and great responsiveness to chemotherapy and radiation. Although, bisphosphonates, in particular zolendronic acid, are associated with improved outcomes; this effect may differ between both types of lung cancer. Larger, well-powered, high quality randomized clinical trials could establish the effect of bisphosphonates on the each disease subtype. There were no controlled trials providing data on the efficacy of newer RANKL inhibitors such as denosumab in patients with lung cancer only, therefore, no trials comparing denosumab to bisphosphonates were included in this review. Further studies should evaluate the efficacy of this agent compared to bisphosphonates in patients with lung cancer.
In summary, bisphosphonates (zolendronic acid, pamidronate, and clodronate) reduced SREs and when added to other treatment modalities (e.g., chemotherapy, radiation therapy, radioisotope therapy) resulted in better pain control, quality of life, and less progression of bone lesions than the other therapies alone. Our findings suggest that bisphosphonate therapy is indicated in the treatment of patients with lung cancer and metastatic bone disease.
Acknowledgements
We would like to thank Ruili Luo, PhD, Yimin Geng, MS, and Hong Zhang, PhD, for their assistance translating the Chinese language articles. We are grateful to Eduardo Bruera, MD, oncologist at the Department of Palliative Care and Rehabilitation Medicine, The University of Texas MD Anderson Cancer Center for his invaluable feedback.
Funding/Conflicts of interest: Supported in part by a Cancer Center Support Grant (CA016672) from the National Institutes of Health. Dr. Suarez-Almazor has a K24 career award from the National Institute for Arthritis, Musculoskeletal and Skin Disorders (NIAMS; K24 AR53593).
Appendix 1.
Search strategy
| 1 | exp DIPHOSPHONATES/ |
| 2 | (Bisphosphon* or Bifosfonatos* or Biphosphon* or Difosfonatos* or Diphosphon*).mp. |
| 3 | (alendronate* or fosamax* or adrovance* or fosavance* or adronat* or arendal* or alendros* or onclast* or “alendronic acid*”).mp. |
| 4 | (clodronate* or bonefos* or ostac* or loron* or clodron* or “clodronic acid*”).mp. |
| 5 | (etidronate* or didronel* or didrocal* or “etidronic acid*”).mp. |
| 6 | (ibandronate* or boniva* or bondronat* or “ibandronic acid*”).mp. |
| 7 | (incadronate* or bisphonal* or “incadronic acid*”).mp. |
| 8 | (minodronate* or recalbon* or bonoteo* or “minodronic acid*”).mp. |
| 9 | (neridronate* or “neridronic acid*”).mp. |
| 10 | (olpadronate* or “olpadronic acid*”).mp. |
| 11 | (pamidronate* or aredia* or pamifos* or pamisol* or “pamidronic acid*”).mp. |
| 12 | (risedronate* or actonel* or optinate* or “risedronic acid*”).mp. |
| 13 | (tiludronate* or skelid* or “tiludronic acid*”).mp. |
| 14 | (zoledronate* or aclasta* or zometa* or “zoledronic acid*”).mp. |
| 15 | or/1-14 |
| 16 | exp LUNG NEOPLASMS/ |
| 17 | ((lung*1 or pulmonary*) adj10 (cancer* or neoplas* or carcinoma* or tumor* or tumour*)).mp. |
| 18 | ((NSCLC or SCLC) and (lung*1 or pulmonary* or cancer* or neoplas* or carcinoma* or tumor* or tumour*)).mp. |
| 19 | (pancoast adj10 (syndrom* or cancer* or neoplas* or carcinoma* or tumor* or tumour*)).mp. |
| 20 | (exp CARCINOMA, SQUAMOUS CELL/ or (squamous and scc).mp.) and (exp LUNG/ or (lung*1 or pulmonary*).mp.) |
| 21 | (exp ADENOCARCINOMA/ or adenocarcinoma*.mp. or (malignan* adj3 adenoma*).mp.) and (exp LUNG/ or (lung*1 or pulmonary*).mp.) |
| 22 | or/16-21 |
| 23 | 15 and 22 |
| 24 | exp NEOPLASM METASTASIS/ and exp “BONE AND BONES”/ |
| 25 | ((prevent* or inhibit*) adj10 metasta*).mp. and (exp “BONE AND BONES”/ or exp BONE NEOPLASMS/) |
| 26 | (bone*1 adj10 (metasta* or antimetasta* or anti-metasta*)).mp. |
| 27 | exp BONE NEOPLASMS/sc |
| 28 | or/24-27 |
| 29 | 23 and 28 |
| 30 | (animals not (humans and animals)).sh. |
| 31 | 29 not 30 |
| 32 | randomized controlled trial.pt. |
| 33 | controlled clinical trial.pt. |
| 34 | random allocation.sh. |
| 35 | double blind method.sh. |
| 36 | single blind method.sh. |
| 37 | (randomized or randomised).ti,ab. |
| 38 | or/32-37 |
| 39 | 31 and 38 |
| 40 | clinical trial.pt. |
| 41 | CLINICAL TRIALS AS TOPIC/ |
| 42 | (clinical* adj10 trial*).ti,ab. |
| 43 | ((singl* or doubl* or tripl* or trebl*) adj10 (blind* or mask*)).ti,ab. |
| 44 | placebo*.ti,ab. |
| 45 | randomly.ti,ab. |
| 46 | trial.ti,ab. |
| 47 | groups.ab. |
| 48 | drug therapy.fs. |
| 49 | RESEARCH DESIGN/ |
| 50 | CONTROL GROUPS/ |
| 51 | or/40-50 |
| 52 | 31 and 51 |
| 53 | 52 not 39 |
Appendix 2.
Definition of Outcome Measures
| Outcomes | Definition | Used by |
|---|---|---|
| Primary | ||
| Skeletal Related Eventsa | Radiation therapy or surgery to bone, spinal cord compression event or a pathologic bone fracture event Not specified |
Scagliotti[37-39], Francini[25], Rosen (Hirsh [6, 7, 27] Zaragoulidis[31, 35], Hirai[36], Kritikos[34] |
| Painb | ||
| Visual analogue scale (0-10) 4 categories: 0 = no pain, 1-3 mild, 4-6 moderate, 7-10= severe BPI |
Zaragoulidis[31, 35], Guo[26] |
|
| Verbal rating scale for analgesia effects 6-point McGill-Melzack pain questionnaire (reponses: no pain, mild pain, discomforting/moderate pain, distressing/severe pain, horrible/extremely severe pain, excruciating/life threatening pain) |
Rosen (Hirsh)[6, 7, 27] Zheng[32] Francini[25], Li[28] |
|
| Subjectively evaluated by patients and by analgesic needs (permanent pain, night- time pain, pain only occurring during movement) with 4 categories: no pain = no pain and no analgesics, mild pain = patient cannot rest, but needs no analgesics moderate pain = patient cannot rest and needs analgesics, severe pain = patient cannot sleep and needs nacotics. |
Su[30], Zhang[29] | |
| Pain control | ‘Significant improvement’ = pain decreased by 2 levels; ‘Effective control’ = pain decreased by 1 level; ‘No effect ‘= no change |
Francini[25], Guo[26], Zhang[29], Zheng[32] |
| Complete remission = 100% of pain alleviated; Partial remission = ≥ 50% of pain alleviated; No improvement = pain was not effectively controlled |
Su[30] | |
| No change = no pain improvement; Effective control = pain decreased by ≥1 level; Exacerbation = patients experience worse pain |
Li[28] | |
| Overall Survival | Days since beginning bisphosphonate therapy |
Scagliotti[37-39], Zaragoulidis[31, 35], Rosen (Hirsh)[6, 7, 27] |
| From the days of diagnosis to the date of death due to any cause (up to 1 year) Not Specified |
Pandya[33] Hirai[36] |
|
| Secondary | ||
| Time to the First Skeletal Related Event |
The time from randomization to the date of occurrence of the first SRE |
Scagliotti[37-39] |
| The time from the date of the first dose of study drug to the first documentation of bone metastasis |
Pandya[33] | |
| Not Specified | Hirai[36], Kritikos[34] | |
| Biomarkers | S-CTX and B-ALP NTx nM BCE |
Francini[22] Zaragoulidis[28, 32], Rosen (Hirsh)[5, 6, 24] |
| Bone lesion progression | CT scan (measurement of lesions approximately every 3 months) |
Rosen (Hirsh)[6, 7, 27] |
| Overall disease progression |
Response Evaluation Criteria in Solid Tumors (RECIST), CT scans and then new symptom occurrence |
Pandya[33], Francini[25] |
| Modified Southwest Oncology Group | Rosen (Hirsh)[6, 7, 27] |
|
| Time to disease progression |
The time from the date of the first dose of intervention to the date of first documented progression death due to underlying cancer, or date to loss of follow-up |
Pandya[33], Zaragoulidis[31, 35], |
| Not Specified | Hirai[36] | |
| Performance status | ECOG | Rosen (Hirsh)[6, 7, 27] |
| Quality of life | FACT-G | Rosen (Hirsh)[6, 7, 27] |
| Serious Adverse Events | Not specified | Zheng[32], Zaragoulidis[31, 35] |
Percentage of participants with >1event;
All studies used 3 levels for pain categorization, we therefore, defined “pain not controlled” for categories with no improvement, exacerbation or no effect and we defined “pain controlled” for categories defining effective control, significant improvement, complete or partial remission
Footnotes
Contributions: M. E. Suarez-Almazor had full access to all of the data in the study and takes responsibility for the integrity and the accuracy of the data analysis.
Study concept and design: M. E. Suarez-Almazor, N. Shah, M. A. Lopez-Olivo
Acquisition of data: G. Pratt, N. Shah, M. A. Lopez-Olivo
Analysis and interpretation of data: N. Shah, G. Pratt, M. A. Lopez-Olivo, M. E. Suarez-Almazor
Drafting of the manuscript: N. Shah, M. E. Suarez-Almazor, M.A. Lopez-Olivo
Critical revision of the manuscript for important intellectual content: M. E. Suarez-Almazor, N. Shah, M.A. Lopez-Olivo, G. Pratt
Statistical analysis: M. A. Lopez-Olivo, N. Shah
Obtained funding: M. E. Suarez-Almazor
Administrative, technical, or material support: M. E. Suarez-Almazor.
Study supervision: M. E. Suarez-Almazor
REFERENCES
- 1.Decroisette C, Monnet I, Berard H, Quere G, Le Caer H, Bota S, Audigier-Valette C, Geriniere L, Vernejoux JM, Chouaid C. Epidemiology and treatment costs of bone metastases from lung cancer: a French prospective, observational, multicenter study (GFPC 0601) Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer. 2011;6:576–582. doi: 10.1097/JTO.0b013e318206a1e3. [DOI] [PubMed] [Google Scholar]
- 2.O’Donnell P. [Accessed on: May 29, 2012];Metastatic Cancer of Bone. Orthobullets. 2012 Available from: http://www.orthobullets.com/pathology/8045/metastatic-cancer-of-bone.
- 3.De Marinis F, Eberhardt W, Harper PG, Sureda BM, Nackaerts K, Soerensen JB, Syrigos K, Tredaniel J. Bisphosphonate use in patients with lung cancer and bone metastases: recommendations of a European expert panel. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer. 2009;4:1280–1288. doi: 10.1097/JTO.0b013e3181b68e5a. [DOI] [PubMed] [Google Scholar]
- 4.Hirsh V, Tchekmedyian NS, Rosen LS, Zheng M, Hei YJ. Clinical benefit of zoledronic acid in patients with lung cancer and other solid tumors: analysis based on history of skeletal complications. Clinical Lung Cancer. 2004;6:170–174. doi: 10.3816/CLC.2004.n.030. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RE. Bisphosphonates: clinical experience. Oncologist. 2004;9(Suppl 4):14–27. doi: 10.1634/theoncologist.9-90004-14. [DOI] [PubMed] [Google Scholar]
- 6.Rosen LS, Gordon D, Tchekmedyian NS, Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, De Souza P, Zheng M, Urbanowitz G, Reitsma D, Seaman J. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 7.Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, de Souza P, Zheng M, Urbanowitz G, Reitsma D, Seaman JJ. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial--the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. Journal of Clinical Oncology. 2003;21:3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 8.Yuen KK, Shelley M, Sze WM, Wilt T, Mason MD. Bisphosphonates for advanced prostate cancer. Cochrane Database Syst Rev. 2006;4:CD006250. doi: 10.1002/14651858.CD006250. [DOI] [PubMed] [Google Scholar]
- 9.Piga A, Bracci R, Ferretti B, Sandri P, Nortilli R, Acito L, Pancotti A, Di Furia L, Carle F, Cellerino R. A double blind randomized study of oral clodronate in the treatment of bone metastases from tumors poorly responsive to chemotherapy. J Exp Clin Cancer Res. 1998;17:213–217. [PubMed] [Google Scholar]
- 10.Oefelein MG, Ricchiuti V, Conrad W, Resnick MI. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol. 2002;168:1005–1007. doi: 10.1016/S0022-5347(05)64561-2. [DOI] [PubMed] [Google Scholar]
- 11.Norgaard M, Jensen AO, Jacobsen JB, Cetin K, Fryzek JP, Sorensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007) J Urol. 2010;184:162–167. doi: 10.1016/j.juro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Bloomfield DJ. Should bisphosphonates be part of the standard therapy of patients with multiple myeloma or bone metastases from other cancers? An evidence-based review. Journal of Clinical Oncology. 1998;16:1218–1225. doi: 10.1200/JCO.1998.16.3.1218. [DOI] [PubMed] [Google Scholar]
- 13.Bonjour JP, Rizzoli R. Treatment of hypercalcaemia of malignancy with clodronate. Bone. 1991;12(Suppl 1):S19–23. doi: 10.1016/8756-3282(91)90062-n. [DOI] [PubMed] [Google Scholar]
- 14.Hitron A, Adams V. The pharmacological management of skeletal-related events from metastatic tumors. Orthopedics. 2009;32:188. doi: 10.3928/01477447-20090301-13. [DOI] [PubMed] [Google Scholar]
- 15.Price N. Bisphosphonates to prevent skeletal morbidity in patients with lung cancer with bone metastases. Clinical Lung Cancer. 2004;5:267–269. doi: 10.1016/S1525-7304(11)70347-3. [DOI] [PubMed] [Google Scholar]
- 16.Djulbegovic B, Wheatley K, Ross J, Clark O, Bos G, Goldschmidt H, Cremer F, Alsina M, Glasmacher A. Bisphosphonates in multiple myeloma. Cochrane Database of Systematic Reviews. 2002;3:CD003188. doi: 10.1002/14651858.CD003188. [DOI] [PubMed] [Google Scholar]
- 17.Pavlakis N, Schmidt R, Stockler M. Bisphosphonates for breast cancer. Cochrane Database of Systematic Reviews. 2005;3:CD003474. doi: 10.1002/14651858.CD003474.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Calderone R, Nimako K, Leary A, Popat S, O’Brien ME. Under usage of zoledronic acid in non-small cell lung cancer patients with metastatic bone disease - a short communication. European Journal of Cancer. 2011;47:1603–1605. doi: 10.1016/j.ejca.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis J, Clarke M, Devereaux P, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Furlan AD, Pennick V, Bombardier C, van Tulder M. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976) 2009;34:1929–1941. doi: 10.1097/BRS.0b013e3181b1c99f. [DOI] [PubMed] [Google Scholar]
- 21.van der Velde G, van Tulder M, Cote P, Hogg-Johnson S, Aker P, Cassidy JD, Carragee E, Carroll L, Guzman J, Haldeman S, Holm L, Hurwitz E, Nordin M, Peloso P. The sensitivity of review results to methods used to appraise and incorporate trial quality into data synthesis. Spine (Phila Pa 1976) 2007;32:796–806. doi: 10.1097/01.brs.0000258903.67718.d5. [DOI] [PubMed] [Google Scholar]
- 22.Bradburn MJ, Deeks JJ, Altman DG. Metan- a command for meta-analysis in STATA. STB. 1998;44:4–15. [Google Scholar]
- 23.Harris RJ, Bradburn MJ, Deeks JJ, Harbord RM, Altman DG, JAC S. Metan: Fixed- and random-effects metaanalysis. The Stata Journal. 2008;8:3–28. [Google Scholar]
- 24.Borenstein M, Hedges L, Higgins J, H. R. Book Comprehensive Meta-Analysis. Version 2 Biostat, City: 2005. Comprehensive Meta-Analysis Version 2. [Google Scholar]
- 25.Francini F, Pascucci A, Bargagli G, Francini E, Conca R, Miano ST, Martellucci I, Migali C, Gotti G, Fiaschi AI, Cozzolino A, Petrioli R. Effects of intravenous zoledronic acid and oral ibandronate on early changes in markers of bone turnover in patients with bone metastases from non-small cell lung cancer. International Journal of Clinical Oncology. 2011;16:264–269. doi: 10.1007/s10147-010-0179-x. [DOI] [PubMed] [Google Scholar]
- 26.Guo C, Guo Q, Qi J, Liu Q, Wu N. Comparison of the effects of Ibandronate and Bonefos on non-small cell lung cancer with bone metastases. Chinese Journal of Clinical Oncology. 2008;35:310–312. [Google Scholar]
- 27.Hirsh V, Major PP, Lipton A, Cook RJ, Langer CJ, Smith MR, Brown JE, Coleman RE. Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclast activity. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer. 2008;3:228–236. doi: 10.1097/JTO.0b013e3181651c0e. [DOI] [PubMed] [Google Scholar]
- 28.Li XX, Zhou TC, Zhao J. Clinical evaluation of combined-modality therapy for bone metastasis of non-small-cell lung cancer. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:752–753. [PubMed] [Google Scholar]
- 29.Zhang S, Liu G, Hu F. A clinical trial of Bonin in the bone metastases of lung cancer. Chinese Journal of Clinical Oncology. 1999;26:445–447. [Google Scholar]
- 30.Su J, You C, Cai S, Meng Y. 89SrCl2 and/or bonefos in the treatment of bone metastasis from pulmonary carcinoma. Chinese Journal of Lung Cancer. 2002;5:357–359. doi: 10.3779/j.issn.1009-3419.2002.05.11. [DOI] [PubMed] [Google Scholar]
- 31.Zarogoulidis K, Boutsikou E, Zarogoulidis P, Eleftheriadou E, Kontakiotis T, Lithoxopoulou H, Tzanakakis G, Kanakis I, Karamanos NK. The impact of zoledronic acid therapy in survival of lung cancer patients with bone metastasis. Int J Cancer. 2009;125:1705–1709. doi: 10.1002/ijc.24470. [DOI] [PubMed] [Google Scholar]
- 32.Zheng T, Chen W. Evaluation on the effect of acesodyne treatment in patient with lung cancer bone metastasis. Chinese Journal of Clinical Rehabilitation. 2004;8:5714–5715. [Google Scholar]
- 33.Pandya KJ, Gajra A, Warsi GM, Argonza-Aviles E, Ericson SG, Wozniak AJ. Multicenter, randomized, phase 2 study of zoledronic acid in combination with docetaxel and carboplatin in patients with unresectable stage IIIB or stage IV non-small cell lung cancer. Lung Cancer. 2010;67:330–338. doi: 10.1016/j.lungcan.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Kritikos K, Heras P, Hatzopoulos A, Androutsos N. Efficacy and Safety of Intravenous Zoledronic Acid 4 mg Infused over 15 minutes: Results from a 2-year Study of Lung Cancer Patients with Metastatic Bone Disease. Ann Oncol. 2008;19:viii286–viii287. [Google Scholar]
- 35.Zarogoulidis K, Boutsikou E, Zarogoulidou V, Lithoxopoulou H, Tsiouda T, Ballasoulis G, Kontakiotis T. The impact of bisphosphonate therapy on survival of lung cancer patients with bone metastasis. Pneumon. 2009;22:25–37. [Google Scholar]
- 36.Hirai F, Murakami H, Yamamoto N, Yamanaka T, Okamoto I, Sawa T, Hirashima T, Takeda K, Fukuoka M, Nakagawa K. Randomized phase II trial of zoledronic acid in combination with docetaxel in previously treated non-small cell lung cancer (NSCLC) patients with bone metastases - Result of a west Japan oncology group study. European Journal of Cancer. 2011;47(Supplement):S628. [Google Scholar]
- 37.Scagliotti G, C. M, De Marinis F, Engel-Riedel W, Albert I, Sallo V, Chen YM, Perez JR, Kosmidis P. [accessed on December 00172012, 00172011];A Study to Evaluate the Safety and Efficacy of Zoledronic Acid in the Prevention or Delaying of Bone Metastasis in Patients With Stage IIIA and IIIB Non-small Cell Lung Cancer. 2011 Clinical Trialsgov NCT00172042: Available << http://clinicaltrials.gov/ct2/show/results/NCT00172042?sect=X00172543dcba00987601.
- 38.Scagliotti G, Manegold C, Kosmidis P, Kirner A. Efficacy of zoledronic acid for the prevention of bone metastases in patients with non-small cell lung cancer. Bone. 2006;38:S83–S83. [Google Scholar]
- 39.Scagliotti G, Manegold C, De Marinis F, Engel-Riedel W, Albert I, Sallo V, Chen YM, Perez JR, Kosmidis P. Evaluating the efficacy of zoledronic acid for the prevention of disease progression in patients with non-small cell lung cancer (NSCLC) European Journal of Cancer. 2009;7(Supplement):522. [Google Scholar]
- 40.Manas A, Casas F, Ciria JP, Lopez C, Saez J, Palacios A, de las Heras M, Porto C, Sanchez E, Martin C, Esco R, Veiras C, Martinez JC, Marquez M, Ramos A, Calvo F, Fuertes J, Andreu FJ, Contreras J, Perez L, Romero J, Vayreda J, Victoria C. Randomised study of single dose (8 Gy vs. 6 Gy) of analgesic radiotherapy plus zoledronic acid in patients with bone metastases. Clinical Translational Oncology. 2008;10:281–287. doi: 10.1007/s12094-008-0198-5. [DOI] [PubMed] [Google Scholar]
- 41.Roqué i Figuls M, Martinez-Zapata M, JoséAlonso-Coello P, Catalá E, Garcia Jose L, Ferrandiz M. Radioisotopes for metastatic bone pain. Cochrane Database of Systematic Reviews. 2003;6:CD003347. doi: 10.1002/14651858.CD003347. [DOI] [PubMed] [Google Scholar]
- 42.Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy. Cochrane Database of Systematic Reviews. 2004;2:CD004721. doi: 10.1002/14651858.CD004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong R, Wiffen PJ. Bisphosphonates for the relief of pain secondary to bone metastases. Cochrane Database of Systematic Reviews. 2002;2:CD002068. doi: 10.1002/14651858.CD002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosen L, Gordon D, Tchekmedyian S, Hirsh V, Yanagihara R, Coleman R. Long-term zoledronic acid therapy is effective and safe for reducing the risk of skeletal complications in patients with non-small cell lung cancer (NSCLC) and bone metastases. Bone. 2004;34:S89. [Google Scholar]
- 45.Rosen LS. Efficacy and safety of zoledronic acid in the treatment of bone metastases associated with lung cancer and other solid tumors. Semin Oncol. 2002;29:28–32. doi: 10.1053/sonc.2002.37416. [DOI] [PubMed] [Google Scholar]
- 46.Brown J, Cook R, Hirsh V, Major P. Risk factors for skeletal-related event in zoledronic acid-treated patients with bone metastases from non-small cell lung cancer. Ann Oncol. 2008;19:viii275. [Google Scholar]
- 47.Athanassiou E, Kyrgias G, Panoussaki E, Christodoulidou M, Pantelakos P, Skarlos DV, Karpasitis N. Response of patients with bone metastasis of breast, lung and prostate cancer to radiation therapy (RT) alone, versus radiation therapy and diphosphonate (pamidronate) Ann Oncol Suppl. 1994;8:200. [Google Scholar]
- 48.Coleman R, Cook R, Saad F, Hirsh V, Major P, Dias R, Lipton A. Effects of Zoledronic Acid on Survival in Patients with Metastatic Bone Disease and High Bone Turnover: Results from a Meta-Analysis. Ann Oncol. 2008;19:viii276. [Google Scholar]
- 49.Coleman RE, Major P, Lipton A, Brown JE, Lee KA, Smith M, Saad F, Zheng M, Hei YJ, Seaman J, Cook R. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. Journal of Clinical Oncology. 2005;23(22):4925–4935. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 50.Altieri AM, Portalone L, Alimonti A, Antilli A, Barbera S, Nunziati F, Perrone N, Signora M. Pamidronate versus radiotherapy in the treatment of bone metastases by lung cancer: A randomized AIPO Chest Oncology Study Group trial. Ann Oncol. 2000;11:672P. [Google Scholar]
- 51.Coleman R, Woodward E, Brown J, Cameron D, Bell R, Dodwell D, Keane M, Gil M, Davies C, Burkinshaw R, Houston SJ, Grieve RJ, Barrett-Lee PJ, Thorpe H. Safety of zoledronic acid and incidence of osteonecrosis of the jaw (ONJ) during adjuvant therapy in a randomised phase III trial (AZURE: BIG 01-04) for women with stage II/III breast cancer. Breast Cancer Research Treatment. 2011;127:429–438. doi: 10.1007/s10549-011-1429-y. [DOI] [PubMed] [Google Scholar]
- 52.Lopez-Carrizosa MC, Samper-Ots PM, Perez AR. Serum C-telopeptide levels predict the incidence of skeletal-related events in cancer patients with secondary bone metastases. Clinical Translational Oncology. 2010;12:568–573. doi: 10.1007/s12094-010-0555-z. [DOI] [PubMed] [Google Scholar]
- 53.Kaira R, Murakami H, Kaira K, Takahashi T, Tsuya A, Nakamura Y, Naito T, Endo M, Yamamoto N. N-telopeptide of type I collagen is useful for monitoring therapeutic response in non-small cell lung cancer patients with bone metastases. International Journal of Clinical Oncology. 2010;15:484–488. doi: 10.1007/s10147-010-0100-7. [DOI] [PubMed] [Google Scholar]
- 54.Lu S, Zhang L, Wu YL, Zhang Y, Zhou C, Chen G, Hou M, Song X, Wang J. An observational study on the efficacy and safety of zoledronic acid (ZA) in patients with non-small cell lung cancer (NSCLC) patients with bone metastases with high urine n-telopeptide (uNTX) at diagnosis (C-TONG 0801) Journal of Clinical Oncology. 2011;29:e19541. [Google Scholar]
- 55.Kotteas E, Alamara C, Kiagia M, Pantazopoulos K, Boufas A, Provata A, Charpidou A, Syrigos KN. Safety and efficacy of zoledronic acid rapid infusion in lung cancer patients with bone metastases: a single institution experience. Anticancer Res. 2008;28:529–533. [PubMed] [Google Scholar]
- 56.Kretzschmar A, Wiegel T, Al-Batran SE, Hinrichs HF, Kindler M, Steck T, Illiger HJ, Heinemann V, Schmidt K, Haus U, Kirner A, Ehninger G. Rapid and sustained influence of intravenous zoledronic acid on course of pain and analgesics consumption in patients with cancer with bone metastases: A multicenter open-label study over 1 year. Supportive Cancer Therapy. 2007;4:203–210. doi: 10.3816/SCT.2007.n.016. [DOI] [PubMed] [Google Scholar]
- 57.Shi X, Teng LS, Yu XM, Ma SL. Clinical comparative study of zoledronic acid versus pamidronate disodium in the treatment of pain of skeletal metastases lung cancer. Chinese Journal of Cancer Prevention and Treatment. 2008;15:707–708. [Google Scholar]
- 58.Pandey R, Dey S, Mukhopadhyay A. The better bisphosphonate in patients with bone metastasis: Zoledronic acid or ibandronic acid? A study from Eastern India. Journal of Clinical Oncology. 2009;27:e20524. [Google Scholar]
- 59.Coleman R, Burkinshaw R, Winter M, Neville-Webbe H, Lester J, Woodward E, Brown J. Zoledronic acid. Expert Opinion Drug Safety. 2010;10:133–145. doi: 10.1517/14740338.2011.540387. [DOI] [PubMed] [Google Scholar]
- 60.Coleman R, Cook R, Hirsh V, Major P, Lipton A. Zoledronic acid use in cancer patients: more than just supportive care? Cancer. 2011;117:11–23. doi: 10.1002/cncr.25529. [DOI] [PubMed] [Google Scholar]
- 61.Coleman RE, Seaman JJ. The role of zoledronic acid in cancer: clinical studies in the treatment and prevention of bone metastases. Semin Oncol. 2001;28:11–16. doi: 10.1016/s0093-7754(01)90260-x. [DOI] [PubMed] [Google Scholar]
- 62.Facchini G, Caraglia M, Santini D, Nasti G, Ottaiano A, Striano S, Maiolino P, Ruberto M, Fiore F, Tonini G, Budillon A, Iaffaioli RV, Zeppetella GL. The clinical response on bone metastasis from breast and lung cancer during treatment with zoledronic acid is inversely correlated to skeletal related events (SRE) J Exp Clin Cancer Res. 2007;26:307–312. [PubMed] [Google Scholar]
- 63.Botteman MF, Logman JFS, Kaura S. Cost-effectiveness assessment of zoledronic acid (ZOL) relative to placebo (PBO) in the treatment of lung cancer patients with skeletal metastases in five European countries. Value in Health. 2009;12:A271. [Google Scholar]
- 64.Joshi AD, Carter JA, Botteman MF, Kaura S. Cost-Effectiveness of Zoledronic Acid in the Management of Skeletal Metastases in Patients With Lung Cancer in France, Germany, Portugal, The Netherlands, and the United Kingdom. Clinical Therapeutics. 2011;33:291–304. doi: 10.1016/j.clinthera.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Carter JA, Joshi AD, Kaura S, Botteman MF. Pharmacoeconomics of bisphosphonates for skeletal-related event prevention in metastatic non-breast solid tumours. Pharmacoeconomics. 2012;30:373–386. doi: 10.2165/11631390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 66.Berenson J, Hirschberg R. Safety and convenience of a 15-minute infusion of zoledronic acid. Oncologist. 2004;9:319–329. doi: 10.1634/theoncologist.9-3-319. [DOI] [PubMed] [Google Scholar]
- 67.Di Salvatore M, Orlandi A, Bagala C, Quirino M, Cassano A, Astone A, Barone C. Anti-tumour and anti-angiogenetic effects of zoledronic acid on human non-small-cell lung cancer cell line. Cell Proliferation. 2011;44:139–146. doi: 10.1111/j.1365-2184.2011.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gnant M, Clezardin P. Direct and indirect anticancer activity of bisphosphonates: A brief review of published literature. Cancer Treatment Reviews. 2011 doi: 10.1016/j.ctrv.2011.09.003. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 69.Hirsh V. Bisphosphonates in Lung Cancer: Can They Provide Benefits Beyond Prevention of Skeletal Morbidity? Anti-cancer Agents Medicinal Chemistry. 2011 doi: 10.2174/187152012799014922. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 70.Matsumoto S, Kimura S, Segawa H, Kuroda J, Yuasa T, Sato K, Nogawa M, Tanaka F, Maekawa T, Wada H. Efficacy of the third-generation bisphosphonate, zoledronic acid alone and combined with anti-cancer agents against small cell lung cancer cell lines. Lung Cancer. 2005;47:31–39. doi: 10.1016/j.lungcan.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Koshimune R, Aoe M, Toyooka S, Hara F, Ouchida M, Tokumo M, Sano Y, Date H, Shimizu N. Anti-tumor effect of bisphosphonate (YM529) on non-small cell lung cancer cell lines. BMC Cancer. 2007;7:8. doi: 10.1186/1471-2407-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]