Abstract
AIM: To study whether alterations in the glycosylation of immunoglobulin G (IgG) specific to the Thomsen-Friedenreich glycotope (TF) have diagnostic and prognostic potential in gastric cancer.
METHODS: Serum samples were obtained from patients with histologically verified gastric carcinoma (n = 89), healthy blood donors (n = 40), and patients with benign stomach diseases (n = 22). The lectin-enzyme-linked immunosorbent assay-based glycoprofiling of TF-specific IgG (anti-TF IgG) was performed using synthetic TF-polyacrylamide conjugate as antigen, total IgG purified by affinity chromatography on protein G sepharose, and lectins of various sugar specificities: mannose-specific concanavalin A (ConA), fucose-specific Aleuria aurantia lectin (AAL) and sialic acid-specific Sambucus nigra agglutinin (SNA). The sensitivity and specificity of the differences between cancer patients and controls were evaluated by receiver operator characteristic (ROC) curve analysis. Overall survival was analyzed by the Kaplan-Meier method. Time-dependent ROC curve statistics were applied to determine cut-off values for survival analysis. All calculations and comparisons were performed using the GraphPad Prism 5 and SPSS 15.0 software.
RESULTS: The level of TF-specific IgG was significantly increased in cancer patients compared with non-cancer controls (P < 0.001). This increase was pronounced mostly in stage 1 of the disease. Cancer patients showed a higher level of ConA binding to anti-TF-IgG (P < 0.05) and a very low level of SNA lectin binding (P = 0.0001). No appreciable stage-dependency of the binding of any lectin to anti-TF IgG was found. A strong positive correlation between the binding of AAL and SNA was found in all groups studied (r = 0.71-0.72; P < 0.0001). The changes in ConA reactivity were not related to those of the fucose- or sialic acid-specific lectin. Changes in the SNA binding index and the ConA/SNA binding ratio demonstrated good sensitivity and specificity for stomach cancer: sensitivity 78.79% (95%CI: 61.09-91.02) and 72.73% (95%CI: 57.21-85.04); specificity 79.17 (95%CI: 65.01-89.53) and 88.64% (95%CI: 71.8-96.6), for the SNA binding index and the ConA/SNA binding ratio, respectively. The other combinations of lectins did not improve the accuracy of the assay. The low level of ConA-positive anti-TF IgG was associated with a survival benefit in cancer patients (HR = 1.56; 95%CI: 0.78-3.09; P = 0.19), especially in stages 3-4 of the disease (HR = 2.17; 95%CI: 0.98-4.79; P = 0.048). A significantly better survival rate was found in all cancer patients with a low reactivity of anti-TF IgG to the fucose-specific AAL lectin (HR = 2.39; 95%CI: 1.0-5.7; P = 0.038).
CONCLUSION: The changes in the TF-specific IgG glycosylation pattern can be used as a biomarker for stomach cancer detection, and to predict patient survival.
Keywords: Thomsen-Friedenreich antigen, Anticarbohydrate antibodies, Stomach cancer, IgG glycosylation, Survival, Lectins
INTRODUCTION
The aberrant glycosylation often observed in cancer cells leads to the expression of tumor-associated carbohydrate antigens (TACA) which may be autoimmunogenic and recognized by autoantibodies[1-5]. This makes TACA a promising target for cancer immunotherapy. In cancer patients, an abnormal glycosylation pattern has also been observed for many circulating glycoconjugates, such as transferrin, MUC1 mucin, alpha1-acid glycoprotein, and immunoglobulins[6-10]. This suggests a systemic impact of malignancy on glycosylation machinery or possibly represents a specific feature of the host metabolism. In both cases, such changes might be considered as a biomarker of cancer, a premalignant state, or the disposition of the host to cancer (risk factors).
The Thomsen-Friedenreich antigen (TF, CD176, core-1) (Galβ1, 3GalNAcα/β-O-Ser/Thr) is expressed in many carcinomas and results from incomplete synthesis of O-linked glycans on glycoproteins and glycolipids[1,2]. The TF glycotope is known as a pancarcinoma antigen which is expressed in approximately 90% of all human cancers and in premalignant conditions[2,11]. TF expression is associated with more aggressive tumors and is related to the induction of invasion, metastasis and cancer surveillance mechanisms[12-16]. The TF antigen seems to play a crucial role in the adhesion of cancer cells to the endothelium through interaction with galectin-3, thereby promoting metastasis[17,18].
Naturally-occurring TF antigen-specific immunoglobulin G (anti-TF IgG) autoantibodies are present in human serum in health and disease[3,19,20]. In cancer patients, their level is related to tumor progression and prognosis, being higher in patients with the early stages of the disease, in those with more differentiated tumors (G1-2), and in those with better survival[12,21,22]. This suggests an immediate impact of the humoral immune response on malignancy via direct or antibody-dependent cell-mediated effector pathways. However, the mechanisms behind these associations remain to be further elucidated.
Human serum IgG contains N-linked glycans attached to Asn297 on the fragment crystallizable (Fc) region. The Fc glycan structures are highly variable and differ in the level of terminal sialic acid, galactose (G0, G1, G2), core fucose and bisecting GlcNAc[23]. Changes in IgG Fc glycosylation strongly influence the Fc-receptor-mediated activities of antibody[23-25] and are associated with various pathologies, including cancer. However, little attention has been paid yet to the glycosylation of antibodies specific to tumor-associated antigens[26]. During the last decade, the diversity of IgG glycans has been thoroughly studied by the interaction of IgG with lectins[26-29], as well as by mass spectrometry-based methodology[9,10,30]. Our recent studies demonstrated an increase in the level of the ConA lectin-positive glycoform of both total serum IgG and TF antigen-specific IgG in patients with cancer[8,31]. Moreover, the low level of this IgG glycoform was associated with an overall survival benefit in patients with gastric cancer[8], indicating its functional relevance, as well as its potential clinical value. Similar changes in ConA reactivity have been reported for tumor-reactive IgG in patients with ovarian cancer[26]. However, the antigenic specificity of these antibodies remains unknown.
In an attempt to discover and evaluate potential biomarkers for stomach cancer diagnosis and patient prognosis, the TF antigen-specific IgG glycosylation profile was investigated using lectins of various sugar specificities. In this study, we demonstrate the aberrant glycosylation of anti-TF IgG in patients with stomach cancer, and the association of these changes with overall survival, indicating their potential clinical applicability.
MATERIALS AND METHODS
Subjects
Serum samples were obtained from healthy blood donors, patients with benign stomach diseases and patients with histologically verified gastric carcinoma (Table 1). The investigation was carried out in accordance with the ICH GCP Standards and approved by the Tallinn Medical Research Ethics Committee, Estonia. Written informed consent was obtained from each subject.
Table 1.
Characteristics of the subjects tested1
| Groups | n | Male | Female | M/F | Median age (range), yr |
| Donors | 40 | 12 | 28 | 0.43 | 52.2 (23–70) |
| Benign group2 | 22 | 19 | 3 | 6.3 | 62.0 (44–76) |
| Non-cancer3 | 62 | 31 | 31 | 1 | 59.5 (23–76) |
| Cancer patients | 89 | 53 | 36 | 1.47 | 66.0 (22–84) |
| Stages 1-4 | |||||
| Stage 1 | 18 | 8 | 10 | 0.86 | 65.0 (28–84) |
| Stage 2 | 19 | 14 | 5 | 1.67 | 66.5 (46–83) |
| Stage 3 | 42 | 22 | 20 | 0.83 | 65.0 (38–81) |
| Stage 4 | 10 | 9 | 1 | 9 | 65.0 (44–76) |
1All subjects were tested for anti-Thomsen-Friedenreich glycotope IgG levels and the concanavalin A binding. Aleuria aurantia lectin and Sambucus nigra agglutinin binding test was performed in all donors and patients with benign gastric disease, and in 33 patients with stomach cancer (stage 1, n = 4; stage 2, n = 5; stage 3, n = 19; stage 4, n = 5);
Peptic ulcer disease, n = 6; chronic gastritis, n = 7; atrophic gastritis, n = 9;
Combined group of donors and patients with benign stomach disease. M/F: Male/female.
Tumor staging was based on the histopathological (pTNM) classification of malignant tumors. Serum samples were stored in aliquots at -20 °C until use.
Serum IgG purification on protein G sepharose
To remove the anti-TF IgM and IgA isotype antibodies, preliminary purification of serum total IgG was performed on a Protein G HP Spin Trap column as described by the manufacturer (GE Healthcare, United States). The samples were immediately neutralized, dialyzed against phosphate buffered solution (PBS) - 0.1% NaN3 and stored at + 4 °C until tested. About 8.5 mg of IgG was obtained from 1 mL of serum applied onto the Protein G Sepharose column.
Anti-TF IgG antibody assay
The anti-TF IgG level was determined by enzyme-linked immunosorbent assay (ELISA) as described elsewhere[21], with minor modifications. Briefly, the plates (Maxisorp, Nunc, Roskilde, Denmark) were coated with a synthetic TF-polyacrylamide conjugate (Lectinity, Russia, 10 mol% of carbohydrate) in carbonate buffer, pH 9.6, 5 µg per well. After overnight incubation, triple washing and blocking with a Superblock solution (Pierce, United States) for 15 min at 25 °C, the purified IgG samples (50 μg/well) in PBS-0.05% Tween (Tw) were applied for 1.5 h at 25 °C. After subsequent washing with PBS-Tw, the bound anti-TF IgG was detected with alkaline phosphatase conjugated goat anti-human IgG (Dako, United States) and p-nitrophenylphosphate disodium hexahydrate (Sigma, United States). The absorbance values were read at 405 nm (Tecan Reader, Austria). The relatively high doses of total IgG were applied because of the low concentration of anti-TF IgG in the serum. These IgG doses correspond approximately to the 1:25-1:50 serum dilution used in our previous studies[21].
Lectin reactivity of the TF-specific IgG
The lectin reactivity of the TF glycotope-specific IgG was measured in a similar way, except that the binding of mannose-specific concanavalin A (ConA), fucose-specific Aleuria aurantia lectin (AAL) and neuraminic acid (sialic acid)-specific Sambucus nigra agglutinin (SNA) to the absorbed anti-TF IgG was measured as described by Kodar et al[8]. Biotinylated ConA (Sigma, United States) in the ConA binding buffer (0.05 mol/L Tris-HCl buffer, pH 7.2, containing 0.2 mol/L NaCl and 3 mmol/L CaCl2, MgCl2 and MnCl2 each), AAL (Vector Laboratories Inc., United States) in 10 mmol/L HEPES, 0.15 mol/L NaCl buffer, pH 7.5, and SNA (Vector Laboratories Inc., United States) in 10 mmol/L HEPES, 0.15 mol/L NaCl, 0.1 mmol/L CaCl2, pH 7.5, were each applied at a concentration of 5 μg/mL, for 1.5 h at 25 °C. The bound lectins were detected with a streptavidin-alkaline phosphatase conjugate (Dako, United States) and p-nitrophenylphosphate (Sigma, United States). The optical density value (A) of control wells (blank: the Superblock solution instead of TF-PAA for anti-TF or no sample for lectin binding testing) was subtracted from that of the IgG-coated wells to determine the binding of both IgG and lectin. Each sample was analyzed in duplicate.
To standardize the assay, standard IgG was included in each plate for IgG determination and lectin binding measurement. The interassay variations were minimized by using the correction factor (CF): CF = 1/(standard serum A values - blank) × 100. The results were expressed in relative units (RU): RU = sample A value × CF. The lectin reactivity of anti-TF IgG was calculated as the lectin index: (sample lectin binding RU)/(sample anti-TF IgG binding RU).
Statistical analysis
Comparisons between the groups were performed using the nonparametric Mann-Whitney U test for unpaired data or regression analysis with the Spearman test. Survival analysis was carried out using the Kaplan-Meier method. The receiver operator characteristic (ROC) curve analysis as generated in SPSS 15.0 was used to evaluate the sensitivity and specificity of the changes found for stomach cancer. The area under the ROC curve and the P value of the ROC curve were calculated. The time-dependent ROC curve statistics were applied to determine cut-off values for survival analysis. The difference between the groups was considered to be significant when P ≤ 0.05. All calculations were performed using the GraphPad Prism 5 and SPSS 15.0 software.
RESULTS
TF antigen-specific IgG antibody level in total IgG preparations
A significantly higher level of TF-specific IgG was found in purified total IgG preparations from the serum of patients with stomach cancer compared with that in controls: P = 0.002, 0.0003 and 0.00004 for donors, benign and combined non-cancer groups, respectively (Figure 1). This increase was mostly pronounced in stage 1 of the disease (P = 0.02, P = 0.0006, P = 0.01 compared with stages 2, 3 and 4, respectively). Up to 10-fold interindividual variations in anti-TF IgG antibody levels were observed in all the groups and especially in cancer patients.
Figure 1.
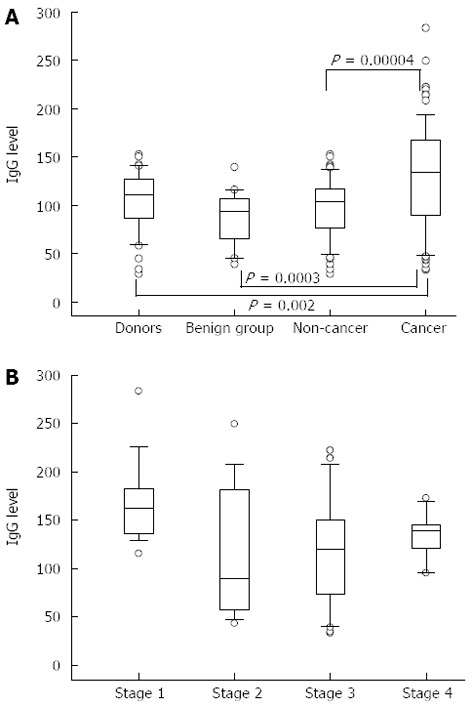
Thomsen-Friedenreich glycotope-IgG level in patients with stomach cancer and controls. A: Box plots of anti-TF IgG levels (medians, ranges and quartiles) in controls and cancer patients; B: Anti-TF IgG levels in cancer patients by stage. Significantly higher in stage 1 compared with stages 2, 3 and 4 (P = 0.02, 0.001 and 0.01, respectively). P values were calculated by the Mann-Whitney U test.
Lectin binding profile of TF-specific IgG
The anti-TF IgG of patients with cancer showed a significantly higher level of ConA-positive IgG glycoform than that of both controls: P = 0.013, 0.05 and 0.005 for donors, benign and non-cancer groups, respectively (Figures 2 and 3). In contrast, the binding of SNA was significantly lower in cancer patients compared with that of blood donors and patients with benign gastric diseases (P < 0.0001). In cancer patients, the binding of AAL did not differ from that of the donors (P = 0.64), but was significantly lower than that of the benign group (P = 0.000008) or non-cancer group (P = 0.005). A group of patients with chronic gastritis (n = 7, one with atrophic gastritis), who showed very high AAL index values, accounted for this difference. This was the only exception in this study when the benign group differed significantly from the donors (P = 0.01). Two of these patients also showed a high level of SNA binding. All patients with peptic ulcer disease (n = 6) demonstrated a similar level of binding for all three lectins compared with that of blood donors.
Figure 2.
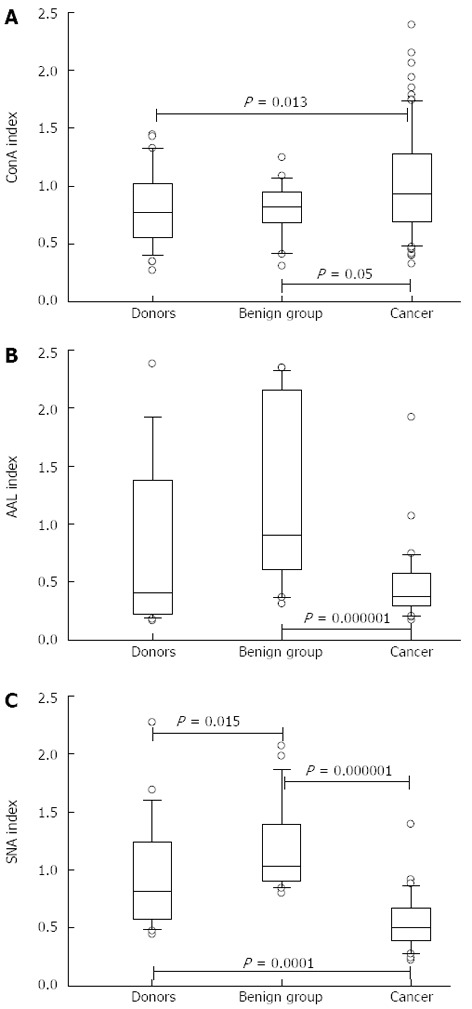
Binding of lectins to the Thomsen-Friedenreich glycotope-specific IgG in gastric cancer patients and controls. A: Concanavalin A (ConA); B: Aleuria aurantia lectin (AAL); C: Sambucus nigra agglutinin (SNA). Box plots of lectin index values (medians, ranges and quartiles) in patients with stomach cancer, healthy blood donors and patients with benign stomach disease. P values were calculated by the Mann-Whitney U test and are shown for significant differences.
Figure 3.
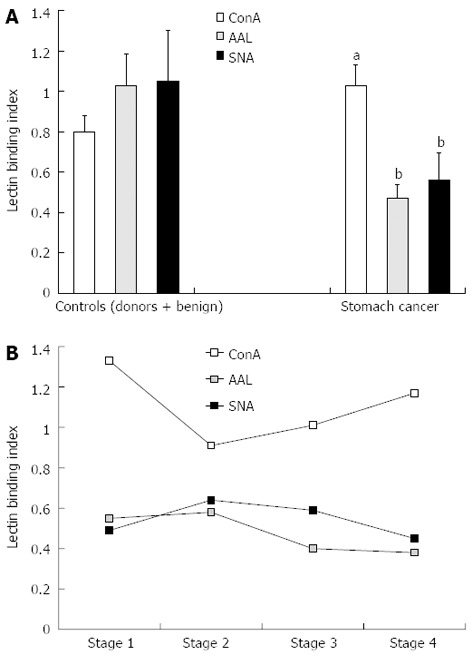
Anti-Thomsen-Friedenreich glycotope-specific IgG glycosylation profile as defined by concanavalin A, Aleuria aurantia lectin and Sambucus nigra agglutinin binding. A: Non-cancer group and cancer patients; B: Relation to the stage of cancer. The results are depicted as mean with error bars representing SEM. P values were calculated by the Mann-Whitney U test: aP < 0.05, bP < 0.01 vs controls for all lectins. ConA: Concanavalin A; AAL: Aleuria aurantia lectin SNA: Sambucus nigra agglutinin.
No appreciable stage-dependency of the binding of any lectin to the anti-TF IgG was found (Figure 3), though a slight trend towards higher ConA index values in stage 1 cancer patients was observed (P = 0.19 compared with stage 3 patients).
A strong positive correlation between the reactivities of AAL and SNA was demonstrated in all groups: cancer patients (r = 0.72; P < 0.0001); non-cancer group (r = 0.71; P < 0.0001) as well as the combined group of all tested subjects (r = 0.72; P < 0.0001). No significant correlation between the reactivities of ConA and the two other lectins was observed. Thus, the changes in ConA reactivity were not related to the modification of anti-TF IgG binding sites for the fucose- or sialic acid-specific lectins (AAL and SNA).
Anti-TF IgG level and lectin binding profile: Sensitivity and specificity in stomach cancer
ROC curve analysis was used to evaluate changes in the level and glycosylation profile of anti-TF-IgG as possible biomarkers. The diagnostic accuracy and ROC curve statistics are presented in Figure 4 and Table 2. In the absence of a correlation between the binding of ConA and the two other lectins, we investigated a possible interactive effect of lectin combinations using the ratios of ConA/SNA, ConA/AAL and AAL/SNA in the ROC analysis.
Figure 4.
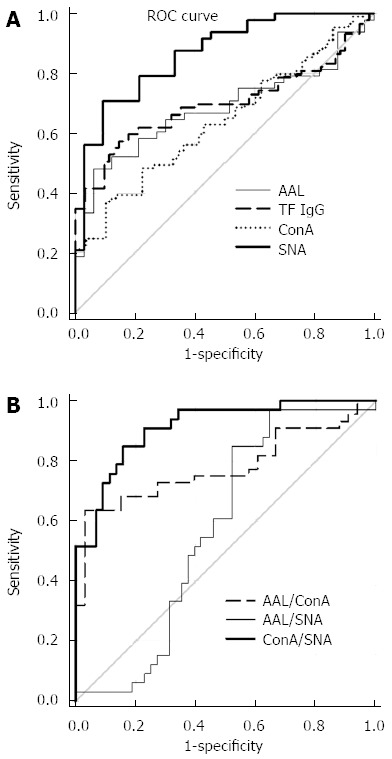
Sensitivity and specificity of anti-Thomsen-Friedenreich glycotope-specific IgG glycosylation changes in stomach cancer. A: Receiver operator characteristic (ROC) curve analysis by lectin binding index values; B: ROC curve analysis by lectin combinations using the ratios of concanavalin A (ConA)/Sambucus nigra agglutinin (SNA), ConA/Aleuria aurantia lectin (AAL) and AAL/SNA.
Table 2.
Anti-TF IgG level and lectin binding profile: Sensitivity and specificity for gastric cancer
| Parameter | Sensitivity % (95%CI) | Specificity % (95%CI) |
ROC statistics |
Sensitivity at specificity 90% | |
| Area under curve | P value | ||||
| Anti-TF IgG | 65.17 (51.34-76.26) | 67.74 (56.66-76.98) | 0.70 | < 0.0001 | 50.56 |
| ConA | 62.92 (48.37-74.49) | 56.90 (46.37-67.74) | 0.64 | 0.004 | 24.72 |
| SNA | 78.79 (61.09-91.02) | 79.17 (65.01-89.53) | 0.87 | < 0.0001 | 57.58 |
| AAL | 69.70 (51.29-84.41) | 64.58 (49.46-77.84) | 0.68 | 0.006 | 12.12 |
| ConA/SNA | 72.73 (57.21-85.04) | 88.64 (71.80-96.60) | 0.91 | < 0.0001 | 63.64 |
| AAL/ConA | 84.85 (68.10-94.89) | 68.18 (52.42-81.39) | 0.78 | < 0.0001 | 33.33 |
| AAL/SNA | 84.85 (72.24-93.93) | 47.92 (30.80-66.46) | 0.68 | 0.006 | 3.03 |
ConA: Concanavalin A; AAL: Aleuria aurantia lectin; SNA: Sambucus nigra agglutinin; Anti-TF IgG: Anti-Thomsen-Friedenreich glycotope immunoglobulin G; ROC: Receiver operator characteristic.
Despite the significant difference in anti-TF IgG levels between cancer patients and controls these changes showed a low sensitivity and specificity for cancer, possibly due to great variations within each group. The same was true for the ConA and AAL lectin index values. In contrast, changes in the SNA binding index and, especially, ConA/SNA ratio values demonstrated rather good sensitivity and specificity reaching 78.8%-88.6% (Table 2). Since no notable cancer stage dependency of lectin binding was observed, the sensitivity and specificity values are presented for the combined cancer group and non-cancer controls (Figure 4 and Table 2). Using the combination of ConA/AAL and AAL/SNA lectin ratios did not improve the accuracy of the assay and showed lower sensitivity and specificity values, usually below 70% (Table 2).
Lectin binding of TF-specific IgG and survival of cancer patients
The subgroups of cancer patients with high and low levels of lectin binding to anti-TF IgG were compared. The cut-off levels were calculated using time-dependent ROC curve analysis for each lectin. Despite the opposite changes in the binding of ConA and AAL or SNA lectin in cancer patients (increase vs decrease) a similar association with survival was demonstrated for all three lectins tested, with a common trend in the early and advanced stages of the disease (Figure 5).
Figure 5.
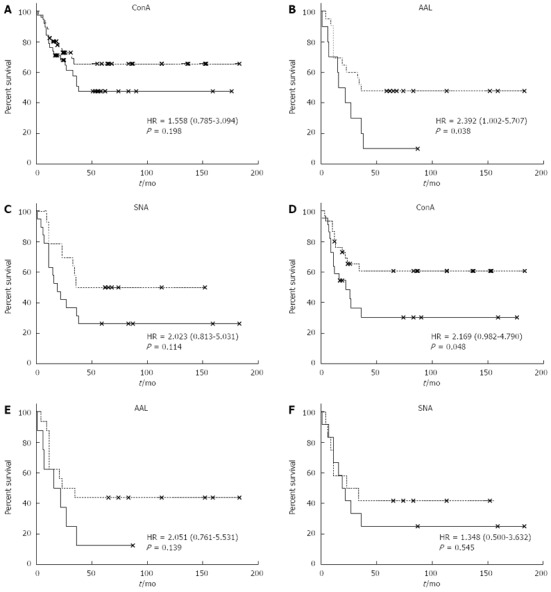
Binding of lectins to Thomsen-Friedenreich glycotope-specific IgG and the survival of patients with stomach cancer. The probability of survival (Kaplan-Meier method) of stomach cancer patients in relation to lectin binding to Thomsen-Friedenreich glycotope (TF)-specific IgG. A: Concanavalin A (ConA) lectin binding of all cancer patients; B: Aleuria aurantia lectin (AAL) binding in all cancer patients; C: Sambucus nigra agglutinin (SNA) binding in all cancer patients; D: ConA binding in patients with stage 3-4 cancer; E: AAL binding in patients with stage 3-4 cancer; F: SNA binding of patients with stage 3-4 cancer. Patient lectin index values, which were either lower, equal to (dashed line) or higher (solid line) than the cut-off are compared. The cut-off levels were calculated using time-dependent receiver operator characteristic curve analysis.
The low level of ConA-positive anti-TF IgG was associated with a survival benefit in cancer patients (HR = 1.56; 95%CI: 0.78-3.09; P = 0.19), especially in those with stages 3-4 of the disease (HR = 2.17; 95%CI: 0.98-4.79; P = 0.048). A significantly better survival rate was found in all cancer patients with low reactivity of anti-TF IgG to the fucose-specific AAL lectin (HR = 2.39; 95%CI: 1.0-5.7; P = 0.038). The association of SNA lectin reactivity with survival showed a similar trend. Considering that no correlation between the binding of ConA and the two other lectins was found, a possible interactive effect of the combination of the two lectins was investigated using the ratios of ConA/SNA, ConA/AAL and AAL/SNA. However, no additional information regarding association with survival was obtained (data not shown).
DISCUSSION
The role of autoantibodies against tumor-associated glycans in cancer surveillance has been mostly considered for IgM[2,4]. These antibodies are not affinity-matured which argues in favor of their inherent natural origin. In contrast, the presence of IgG anti-glycan antibodies suggests an adaptive immune response. The origin of anti-glycan autoantibodies of both isotypes is still unclear though the available evidence suggests that at least anti-TF and anti-alpha Gal antigen-specific antibodies may be induced by bacterial glycans or, possibly, by cross-reactivity with these antigens[3,32]. In any case, large and unexplained interindividual variations in their level in health and disease exist[21,22], possibly reflecting the distinct immunological histories of each individual. Moreover, the anti-TF IgG level is rather stable over time at an individual level in both patients and controls[22,33].
In this study, a significantly higher level of TF-specific IgG in purified total IgG preparations from the serum of patients with stomach cancer than in both control groups was observed. This increase was mostly pronounced in stage 1 of the disease, suggesting that an adaptive immune response cannot be excluded in the early stages of tumor with a subsequent decrease in the anti-TF IgG level in advanced cancer as a result of tumor-induced immunodepression. If this is the case, the population of anti-TF IgG should be expected to be heterogeneous and to include both naturally-occurring TF glycotope-specific antibodies, whose presence precedes tumor development, and those triggered by disease, i.e., induced by the tumor-derived TF glycotope. Because of large interindividual variations in anti-TF IgG level, the human population may be divided into low and strong responders to the TF glycotope. Notably, donors and the benign group showed a more compact distribution. Further characterization of anti-TF IgG subpopulations is needed to determine their structural and functional diversities, and clinical significance.
A significantly higher binding of ConA (P = 0.005) and a highly significant (P = 0.00000003) decrease in SNA lectin binding was characteristic of the anti-TF IgG from the samples of cancer patients compared with those of the non-cancer group (Figure 3). The ConA binding index was higher in stage 1 patients, whereas the SNA and AAL lectin binding index values were low irrespective of the disease stage. We previously found similar changes in the binding of ConA to the total IgG from the serum of patients with gastric cancer.
The changes in anti-TF IgG glycosylation showed rather a high level of sensitivity and specificity in cancer and non-cancer group discrimination (Table 2). It appears that the SNA binding index and, in particular, the ConA/SNA ratio are promising as diagnostic markers to differentiate stomach cancer from controls, including benign gastric diseases. As there are no reliable markers for gastric cancer yet, these findings may be of clinical value.
We reported recently that the level of agalactosylated IgG (G0 glycoform) in the total IgG of gastric cancer patients was significantly higher than that of controls[9]. Interestingly, this shift positively correlated with the binding of ConA to the total serum IgG, and was also observed in purified TF-specific IgG samples (unpublished data). This indicates that IgG asialylation/agalactosylation is associated with an increased ConA binding possibly due to a better accessibility of the d-mannose residue to the ConA because of conformational changes in the Fc G0 glycoform. The absence of a correlation between the binding of ConA and SNA may be a reason for a positive interactive effect of using the ConA/SNA binding ratios for cancer vs non-cancer group discrimination.
Despite the opposite changes in the binding of ConA and the two other lectins to anti-TF IgG in patients with cancer (Figure 2), a better survival rate was associated with lower reactivity of anti-TF IgG to AAL and ConA. The SNA reactivity revealed no significant association with survival, though a similar slight trend was observed. It seems that IgG desialylation alone is not sufficient for the impact on survival, and further degalactosylation is needed to attain this effect. In this study, the patients were subjected to follow-up for more than 10 years. The association of the binding of ConA and AAL with survival became evident after 2.5-3 years of observation, reaching a maximum after 5 years.
Several studies have demonstrated that agalactosylated IgGs show an increased inflammatory activity[34-37] that may promote tumor growth[38,39]. In addition, the IgG1 lacking the branching fucose or with an additional bisecting GlcNAc shows an enhanced ADCC activity through an increased interaction with Fc gamma receptors[36,40-42]. The association of the high level of the G0 IgG glycoform with a lower survival rate we reported recently[9], and the decreased anti-TF IgG fucosylation associated with better survival in the present findings may be related to these mechanisms.
It has been shown that sialylated glycans predominate in glycosylated antibody binding fragments (Fab) whereas the Fc fragment N-glycans are mostly monosialylated[30,43]. Our findings cannot answer the question of whether the Fc or Fab fragment sialylation is responsible for the changes observed in anti-TF IgG glycosylation.
In addition, it remains unclear whether changes in the sialylation and core fucosylation of anti-TF IgG glycans in both IgG fragments may be independent or concordant events, despite the positive correlation observed between the binding of SNA and AAL lectin to the whole molecule of the TF-specific IgG. Given that an active immunization reduces the sialylation of IgG, especially the antigen-driven IgG[36], we hypothesize that the decreased sialylation of anti-TF IgG observed in cancer patients may be an indicator of an adaptive immune response to tumor-derived TF antigen, in addition to naturally-occurring anti-TF IgG antibodies, which are present in every individual in different amounts.
In conclusion, the results show the glycosylation of TF-specific IgG antibodies in patients with gastric cancer to undergo significant changes when compared with that of controls. The appearance of these alterations already in the early stages of cancer and their association with survival suggest that they play a significant role in cancer development and progression. The lectin-based glycoprofiling ELISA assay is an informative and clinically applicable tool for the analysis of IgG glycans. The results imply that changes in the TF-specific IgG glycosylation have diagnostic and prognostic potential for stomach cancer. However, a further study is needed to support these findings on a larger scale using different control groups. Mass spectrometry-based methodology might help to further specify different subsets of anti-TF IgG of clinical importance.
COMMENTS
Background
Gastric cancer is the second leading cause of cancer deaths worldwide. Yet there are still no reliable serum biomarkers for gastric cancer diagnostics and prognostics. Previous studies have demonstrated that naturally-occurring antibodies (Ab) to tumor-associated glycans are involved in natural tumor immunity, being associated with tumor progression and cancer patients survival.
Research frontiers
Recent findings about the impact of Ab glycosylation on their effect or functions suggest that the evaluation of not just the level of antibodies but rather their structural diversity might improve the clinical potential of the antibody-based approach in the disease diagnostics and prognostics. With this purpose in mind, a research team from Estonia led by Kurtenkov O aimed to evaluate whether the glycosylation profile of immunoglobulin G (IgG) antibody to the so-called tumor-associated Thomsen-Friedenreich antigen (TF) could serve as a marker of gastric cancer and the disease outcome. The study is based on the analysis of a long-term (for more than 10 years) survival of cancer patients.
Innovations and breakthroughs
This study demonstrates for the first time that the glycosylation of anti-TF antibodies reveals gastric cancer-related changes with a diagnostic sensitivity and specificity of about 80%. It is striking that the stage of cancer had little effect on these parameters, thus allowing one to diagnose the disease in its early stages. Besides, the authors show that some Ab glycoforms may predict patient survival. Based on these results, the authors conclude that this serology-based approach may be of clinical importance for gastric cancer diagnostics and prognostics.
Applications
The results indicate that changes in the TF-specific IgG glycosylation have diagnostic and prognostic potential for stomach cancer. The lectin-based glycoprofiling of anti-TF IgG antibodies is an informative and clinically applicable tool to specify sets of tumor-associated antibody glycoforms which can be used as a biomarker for stomach cancer detection and follow-up. It is plausible that the elevated level of a specific antibody glycoform might serve as a marker for a stronger immune response, providing protection against cancer. The findings may eventually lead to the development of novel forms of cancer immunotherapy based on vaccination with TF as target and the manipulation of Ab glycosylation machinery. This will need further work, in particular performance of functional tests using tumor cells and different Ab glycoforms, to understand the biological relevance of the results.
Terminology
Glycans: The chains of sugars that coat the outer surface of cells or are attached to other molecules (proteins, lipids). Glycosylation is the enzymatic process in which sugars are attached to other molecules.
Peer review
This important study extends findings from studies of the same authors published previously and make a great contribution to the understanding of the molecular mechanisms that underline aberrant glycosylation in gastric carcinogenesis, and to the evaluation of potential biomarkers for gastric cancer diagnosis and patient prognosis.
Footnotes
Supported by The Estonian Science foundation, No. 7317 and 8399
P- Reviewer Vorobjova T S- Editor Gou SX L- Editor Cant MR E- Editor Zhang DN
References
- 1.Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- 2.Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224:1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 3.Springer GF, Desai PR, Spencer BD, Tegtmeyer H, Carlstedt SC, Scanlon EF. T/Tn antigen vaccine is effective and safe in preventing recurrence of advanced breast carcinoma. Cancer Detect Prev. 1995;19:374–380. [PubMed] [Google Scholar]
- 4.Vollmers HP, Brändlein S. Natural antibodies and cancer. J Autoimmun. 2007;29:295–302. doi: 10.1016/j.jaut.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Wandall HH, Blixt O, Tarp MA, Pedersen JW, Bennett EP, Mandel U, Ragupathi G, Livingston PO, Hollingsworth MA, Taylor-Papadimitriou J, et al. Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res. 2010;70:1306–1313. doi: 10.1158/0008-5472.CAN-09-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanoh Y, Mashiko T, Danbara M, Takayama Y, Ohtani S, Egawa S, Baba S, Akahoshi T. Changes in serum IgG oligosaccharide chains with prostate cancer progression. Anticancer Res. 2004;24:3135–3139. [PubMed] [Google Scholar]
- 7.Abd Hamid UM, Royle L, Saldova R, Radcliffe CM, Harvey DJ, Storr SJ, Pardo M, Antrobus R, Chapman CJ, Zitzmann N, et al. A strategy to reveal potential glycan markers from serum glycoproteins associated with breast cancer progression. Glycobiology. 2008;18:1105–1118. doi: 10.1093/glycob/cwn095. [DOI] [PubMed] [Google Scholar]
- 8.Kodar K, Kurtenkov O, Klaamas K. The Thomsen-Friedenreich antigen and alphaGal-specific human IgG glycoforms: concanavalin A reactivity and relation to survival of cancer patients. Immunol Invest. 2009;38:704–717. doi: 10.3109/08820130903147193. [DOI] [PubMed] [Google Scholar]
- 9.Kodar K, Stadlmann J, Klaamas K, Sergeyev B, Kurtenkov O. Immunoglobulin G Fc N-glycan profiling in patients with gastric cancer by LC-ESI-MS: relation to tumor progression and survival. Glycoconj J. 2012;29:57–66. doi: 10.1007/s10719-011-9364-z. [DOI] [PubMed] [Google Scholar]
- 10.Bones J, Byrne JC, O’Donoghue N, McManus C, Scaife C, Boissin H, Nastase A, Rudd PM. Glycomic and glycoproteomic analysis of serum from patients with stomach cancer reveals potential markers arising from host defense response mechanisms. J Proteome Res. 2011;10:1246–1265. doi: 10.1021/pr101036b. [DOI] [PubMed] [Google Scholar]
- 11.Springer GF, Desai PR, Tegtmeyer H, Spencer BD, Scanlon EF. Pancarcinoma T/Tn antigen detects human carcinoma long before biopsy does and its vaccine prevents breast carcinoma recurrence. Ann N Y Acad Sci. 1993;690:355–357. doi: 10.1111/j.1749-6632.1993.tb44029.x. [DOI] [PubMed] [Google Scholar]
- 12.Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med (Berl) 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 13.Baldus SE, Zirbes TK, Hanisch FG, Kunze D, Shafizadeh ST, Nolden S, Mönig SP, Schneider PM, Karsten U, Thiele J, et al. Thomsen-Friedenreich antigen presents as a prognostic factor in colorectal carcinoma: A clinicopathologic study of 264 patients. Cancer. 2000;88:1536–1543. [PubMed] [Google Scholar]
- 14.Desai PR. Immunoreactive T and Tn antigens in malignancy: role in carcinoma diagnosis, prognosis, and immunotherapy. Transfus Med Rev. 2000;14:312–325. doi: 10.1053/tmrv.2000.16229. [DOI] [PubMed] [Google Scholar]
- 15.Yu LG. The oncofetal Thomsen-Friedenreich carbohydrate antigen in cancer progression. Glycoconj J. 2007;24:411–420. doi: 10.1007/s10719-007-9034-3. [DOI] [PubMed] [Google Scholar]
- 16.Schindlbeck C, Jeschke U, Schulze S, Karsten U, Janni W, Rack B, Krajewski S, Sommer H, Friese K. Prognostic impact of Thomsen-Friedenreich tumor antigen and disseminated tumor cells in the bone marrow of breast cancer patients. Breast Cancer Res Treat. 2007;101:17–25. doi: 10.1007/s10549-006-9271-3. [DOI] [PubMed] [Google Scholar]
- 17.Glinsky VV, Glinsky GV, Rittenhouse-Olson K, Huflejt ME, Glinskii OV, Deutscher SL, Quinn TP. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001;61:4851–4857. [PubMed] [Google Scholar]
- 18.Heimburg J, Yan J, Morey S, Glinskii OV, Huxley VH, Wild L, Klick R, Roy R, Glinsky VV, Rittenhouse-Olson K. Inhibition of spontaneous breast cancer metastasis by anti-Thomsen-Friedenreich antigen monoclonal antibody JAA-F11. Neoplasia. 2006;8:939–948. doi: 10.1593/neo.06493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Springer GF, Desai PR, Ghazizadeh M, Tegtmeyer H. T/Tn pancarcinoma autoantigens: fundamental, diagnostic, and prognostic aspects. Cancer Detect Prev. 1995;19:173–182. [PubMed] [Google Scholar]
- 20.Kurtenkov O, Miljukhina L, Smorodin J, Klaamas K, Bovin N, Ellamaa M, Chuzmarov V. Natural IgM and IgG antibodies to Thomsen-Friedenreich (T) antigen in serum of patients with gastric cancer and blood donors--relation to Lewis (a,b) histo-blood group phenotype. Acta Oncol. 1999;38:939–943. doi: 10.1080/028418699432626. [DOI] [PubMed] [Google Scholar]
- 21.Kurtenkov O, Klaamas K, Mensdorff-Pouilly S, Miljukhina L, Shljapnikova L, Chuzmarov V. Humoral immune response to MUC1 and to the Thomsen-Friedenreich (TF) glycotope in patients with gastric cancer: relation to survival. Acta Oncol. 2007;46:316–323. doi: 10.1080/02841860601055441. [DOI] [PubMed] [Google Scholar]
- 22.Smorodin EP, Kurtenkov OA, Sergeyev BL, Kodar KE, Chuzmarov VI, Afanasyev VP. Postoperative change of anti-Thomsen-Friedenreich and Tn IgG level: the follow-up study of gastrointestinal cancer patients. World J Gastroenterol. 2008;14:4352–4358. doi: 10.3748/wjg.14.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 24.Scallon BJ, Tam SH, McCarthy SG, Cai AN, Raju TS. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol Immunol. 2007;44:1524–1534. doi: 10.1016/j.molimm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol. 2008;20:471–478. doi: 10.1016/j.coi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Gerçel-Taylor C, Bazzett LB, Taylor DD. Presence of aberrant tumor-reactive immunoglobulins in the circulation of patients with ovarian cancer. Gynecol Oncol. 2001;81:71–76. doi: 10.1006/gyno.2000.6102. [DOI] [PubMed] [Google Scholar]
- 27.Sumar N, Bodman KB, Rademacher TW, Dwek RA, Williams P, Parekh RB, Edge J, Rook GA, Isenberg DA, Hay FC. Analysis of glycosylation changes in IgG using lectins. J Immunol Methods. 1990;131:127–136. doi: 10.1016/0022-1759(90)90242-n. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto S. Clinical applications of glycomic approaches for the detection of cancer and other diseases. Curr Opin Mol Ther. 2006;8:507–513. [PubMed] [Google Scholar]
- 29.Pasek M, Duk M, Podbielska M, Sokolik R, Szechiński J, Lisowska E, Krotkiewski H. Galactosylation of IgG from rheumatoid arthritis (RA) patients--changes during therapy. Glycoconj J. 2006;23:463–471. doi: 10.1007/s10719-006-5409-0. [DOI] [PubMed] [Google Scholar]
- 30.Stadlmann J, Weber A, Pabst M, Anderle H, Kunert R, Ehrlich HJ, Peter Schwarz H, Altmann F. A close look at human IgG sialylation and subclass distribution after lectin fractionation. Proteomics. 2009;9:4143–4153. doi: 10.1002/pmic.200800931. [DOI] [PubMed] [Google Scholar]
- 31.Klaamas K, Kodar K, Kurtenkov O. An increased level of the Concanavalin A-positive IgG in the serum of patients with gastric cancer as evaluated by a lectin enzyme-linked immunosorbent assay (LELISA) Neoplasma. 2008;55:143–150. [PubMed] [Google Scholar]
- 32.Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffiss JM. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988;56:1730–1737. doi: 10.1128/iai.56.7.1730-1737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurtenkov O, Klaamas K, Miljukhina L. The lower level of natural anti-Thomsen-Friedenreich antigen (TFA) agglutinins in sera of patients with gastric cancer related to ABO(H) blood-group phenotype. Int J Cancer. 1995;60:781–785. doi: 10.1002/ijc.2910600609. [DOI] [PubMed] [Google Scholar]
- 34.Burton DR, Dwek RA. Immunology. Sugar determines antibody activity. Science. 2006;313:627–628. doi: 10.1126/science.1131712. [DOI] [PubMed] [Google Scholar]
- 35.Jefferis R. A sugar switch for anti-inflammatory antibodies. Nat Biotechnol. 2006;24:1230–1231. doi: 10.1038/nbt1006-1230. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 37.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreiber H, Wu TH, Nachman J, Rowley DA. Immunological enhancement of primary tumor development and its prevention. Semin Cancer Biol. 2000;10:351–357. doi: 10.1006/scbi.2000.0331. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Curr Drug Targets. 2010;11:1133–1146. doi: 10.2174/138945010792006799. [DOI] [PubMed] [Google Scholar]
- 40.Satoh M, Iida S, Shitara K. Non-fucosylated therapeutic antibodies as next-generation therapeutic antibodies. Expert Opin Biol Ther. 2006;6:1161–1173. doi: 10.1517/14712598.6.11.1161. [DOI] [PubMed] [Google Scholar]
- 41.Iida S, Kuni-Kamochi R, Mori K, Misaka H, Inoue M, Okazaki A, Shitara K, Satoh M. Two mechanisms of the enhanced antibody-dependent cellular cytotoxicity (ADCC) efficacy of non-fucosylated therapeutic antibodies in human blood. BMC Cancer. 2009;9:58. doi: 10.1186/1471-2407-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizushima T, Yagi H, Takemoto E, Shibata-Koyama M, Isoda Y, Iida S, Masuda K, Satoh M, Kato K. Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes Cells. 2011;16:1071–1080. doi: 10.1111/j.1365-2443.2011.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stadlmann J, Pabst M, Altmann F. Analytical and Functional Aspects of Antibody Sialylation. J Clin Immunol. 2010:Epub ahead of print. doi: 10.1007/s10875-010-9409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


