Abstract
AIM: To identify the radiological characteristics of focal autoimmune pancreatitis (f-AIP) useful for differentiation from pancreatic cancer (PC).
METHODS: Magnetic resonance imaging (MRI) and triple-phase computed tomography (CT) scans of 79 patients (19 with f-AIP, 30 with PC, and 30 with a normal pancreas) were evaluated retrospectively. A radiologist measured the CT attenuation of the pancreatic parenchyma, the f-AIP and PC lesions in triple phases. The mean CT attenuation values of the f-AIP lesions were compared with those of PC, and the mean CT attenuation values of pancreatic parenchyma in the three groups were compared. The diagnostic performance of CT attenuation changes from arterial phase to hepatic phase in the differentiation between f-AIP and PC was evaluated using receiver operating characteristic (ROC) curve analysis. We also investigated the incidence of previously reported radiological findings for differentiation between f-AIP and PC.
RESULTS: The mean CT attenuation values of f-AIP lesions in enhanced phases were significantly higher than those of PC (arterial phase: 60 ± 7 vs 48 ± 10, P < 0.05; pancreatic phase: 85 ± 6 vs 63 ± 15, P < 0.05; hepatic phase: 95 ± 7 vs 63 ± 13, P < 0.05). The mean CT attenuation values of f-AIP lesions were significantly lower those of uninvolved pancreas and normal pancreas in the arterial and pancreatic phase of CT (P < 0.001, P < 0.001), with no significant difference at the hepatic phase or unenhanced scanning (P = 0.4, P = 0.1). When the attenuation value increase was equal or more than 28 HU this was considered diagnostic for f-AIP, and a sensitivity of 87.5%, specificity of 100% and an area under the ROC curve of 0.974 (95%CI: 0.928-1.021) were achieved. Five findings were more frequently observed in f-AIP patients: (1) sausage-shaped enlargement; (2) delayed homogeneous enhancement; (3) hypoattenuating capsule-like rim; (4) irregular narrowing of the main pancreatic duct (MPD) and/or stricture of the common bile duct (CBD); and (5) MPD upstream dilation ≤ 5 mm.
CONCLUSION: Analysis of a combination of CT and MRI findings could improve the diagnostic accuracy of differentiating f-AIP from PC.
Keywords: Focal autoimmune pancreatitis, Pancreatic cancer, Computer tomography, Magnetic resonance imaging, Magnetic resonance cholangiopancreatography
Core tip: At present, focal autoimmune pancreatitis (f-AIP) is still very difficult to differentiate from pancreatic cancer (PC). In this study, we compared the incidence of radiological features, investigated the differences in the triple-phase enhancement pattern of f-AIP and PC, and found that the combination analysis contributed to improve the diagnostic accuracy of f-AIP thus avoiding unnecessary surgery.
INTRODUCTION
Autoimmune pancreatitis (AIP) is a rare form of immune-mediated chronic pancreatitis (CP) due to an autoimmune mechanism and is characterized by a marked infiltration of lymphocytes and plasma cells in pancreatic tissue (lymphoplasmacytic sclerosing pancreatitis) and was first described in 1961[1-5]. It can be classified into two radiological types: the diffuse form (the most frequent, 70% of cases) and the focal form (30% of cases), which is characterized by focal swelling of the pancreas, localized narrowing of the main pancreatic duct (MPD) with an irregular wall on imaging modalities[6-8]. Focal AIP (f-AIP) can be due to a mass formation or swollen pancreas located in one or two segments of the gland. AIP has a variety of manifestations, obstructive jaundice occurs in 76% and weight loss in 35% of patients, usually in combination with pancreatic enlargement, especially with focal pancreatic enlargement on imaging, making it very difficult to differentiate from pancreatic cancer[7,9,10].
Treatment for AIP and pancreatic cancer (PC) are completely different. Autoimmune pancreatitis is a benign disease and steroid therapy can rapidly resolve symptoms; for PC, however, surgical resection is preferred, and if necessary, PC patients might receive combined radiotherapy or chemotherapy. However, about 3%-5% of patients undergoing pancreatic resection for presumed PC in fact have AIP[11]. Kamisawa et al[12] reported that 7 of 37 (18.9%) AIP patients had surgery because they were misdiagnosed as having PC or bile duct cancer. In particular, it is very difficult to differentiate between f-AIP and PC. Chang et al[13] reported that 8 of 26 (31.8%) AIP patients had f-AIP who were frequently treated with surgery because differentiating f-AIP from PC was so difficult. It was reported that approximately 2.2%-35.2% of f-AIP patients had undergone surgery due to a presumed diagnosis of PC[12,14-16].
Here, we report the radiological features of 19 cases of f-AIP presenting as PC to: (1) increase the awareness and recognition of f-AIP; and (2) improve the diagnostic accuracy and avoid unnecessary surgery due to misdiagnosis.
MATERIALS AND METHODS
Patient sample
This study was approved by the hospital ethical committees. Consecutive patients with focal autoimmune pancreatitis (f-AIP, n = 19) were recruited between November 2009 and October 2012. Patients with AIP were diagnosed according to the Asian Diagnostic Criteria for Autoimmune Pancreatitis (2008)[17]. A diagnosis of AIP was established when the criterion of (1) narrowing of the MPD with enlargement of the pancreas, as determined by a review of an imaging study, is met together with the criterion of (2) an increase in serum markers (g-globulin, IgG, or IgG4) and/or the criterion of (3) pathology. In this study, the diagnosis of AIP was established by the presence of criteria 1 and 2 in 4 patients; criteria 1 and 3 in 3 patients; and criteria 1, 2, and 3 in 12 patients.
The f-AIP group included 19 patients (14 men and 5 women; age range, 41-75 years; mean age, 54 years) with 21 lesions who had undergone contrast-enhanced computed tomography (CE-CT) (n = 19), CE magnetic resonance imaging (MRI) (n = 11), and magnetic resonance cholangiopancreatography (MRCP) (n = 16). One patient had 3 lesions which were located at the pancreatic head, body and tail.
Identification of patients with focal pancreatic carcinoma was performed by reviewing patient records between March 2012 and December 2012 obtained from the hospital’s pathology database. All 30 patients (21 men, 9 women; age range, 43-79 years; mean age, 58.2 years) with pathologically (histopathological examination of the surgically resected or biopsied tumor specimen) confirmed pancreatic ductal carcinoma (n = 30) were included in this study. All 30 patients had undergone CE-CT, 26 had undergone MRCP, and 12 had undergone CE MRI using extracellular MR contrast agents (Gd-DTPA).
Thirty patients (20 men, 10 women; age range, 41–68 years; mean age, 53 years) with normal pancreas were also recruited. They were confirmed by reviewing the radiological images and followed-up for more than 6 months. All 30 patients had undergone CE-CT.
CE-CT
In this study, all 79 patients underwent the triple-phase pancreatic CT protocol (Brilliance 16; PHILIPS Medical System) which included an unenhanced scan followed by triple-phase contrast-enhanced scans of the abdomen. The tube voltage was 120 kV, the tube current was 250 mA, and the rotation period was 0.75 s, A total of 90 mL of IV contrast material (iohexol, Omnipaque 300, GE Healthcare) containing 300 mg/mL iodine was intravenously administered as a bolus via a power injector. The injection rate was 3.5 mL/s. Images were obtained during the arterial, pancreatic and hepatic phases at 20, 40 and 80 s, respectively, after contrast medium injection. The median slice thickness for contrast-enhanced images was 3 mm.
MRI and MRCP
MR imaging and MR changiopancreatography were performed using a 1.5-T MR imaging system (AVANTO; SIEMENS Medical Systems) and a pre-contrast coil. Pre-contrast T1-weighted MR imaging [repetition time msec/echo time msec, 150 (R) 200/2.1, 4.2] with and without fat saturation and respiratory-triggered T2-weighted MR imaging (5000-8000/80-135) were performed, followed by dynamic fat-suppressed T1-weighted MR imaging (150-200/2.1) after administration of a gadolinium-based contrast agent. MRCP was performed with a single-shot fast spin-echo thick-slab technique (25000-30000/800-1000).
Imaging analysis
Three board-certified abdominal radiologists (with 9, 10 and 15 years of experience) reviewed all CT and MRI images retrospectively using a picture archiving and communication system (PACS) work station (General Electric Medical System). During analysis of the CT and MRI findings, all cases were randomly intermixed. The radiologists were blinded to the patients’ clinical data, official reports, radiological examinations on other dates, and histopathological findings. Decisions were made by consensus.
For each patient, the radiologists were asked to make judgments on the following signs: (1) focal pancreatic enlargement and the location of the lesion; (2) capsule-like rim of the lesion; (3) localized irregular narrowing of MPD; (4) stricture of the distal common bile duct; and (5) other associated findings such as calcification, peripancreatic lymphadenopathy and vascular invasion.
When the lesion was confirmed, CT attenuation values of the f-AIP, PC, the apparently unaffected pancreatic parenchyma, and the normal pancreas were measured by one radiologist using a workstation (Advantage Version 4.2, GE Healthcare). The CT attenuation values were measured using unenhanced images and images obtained from arterial, pancreatic and hepatic phases after contrast administration. Following the placement of a region of interest (ROI) in each segment of the pancreas (head, body and tail), CT attenuation values of the pancreatic parenchyma were measured. The mean value of the three segments was used as the CT attenuation value of the pancreatic parenchyma. In f-AIP and PC patients, ROIs were placed both above the lesion and in the unaffected segments of the pancreas. The largest possible spherical ROI was marked, ruling out the pancreatic duct and partial volume averaging from the extrapancreatic structures. The smallest ROI was approximately 3 mm in diameter when the pancreas was atrophic. Delayed enhancement of the AIP and PC lesions was defined as the change in CT attenuation of the lesion between the arterial phase and the hepatic phase. CT attenuation values of the liver and spleen were similarly measured on unenhanced images (when available) and on images obtained in the arterial, pancreatic and hepatic phases.
The MRCP images were reviewed to identify changes in the common bile duct (CBD) and MPD. The MPD and CBD observations were classified into 1 of 3 categories: as displaying (1) normal appearance; (2) stenosis; and (3) complete obstruction (nonvisualization of the obstructed segment). The MPD upstream diameter was evaluated by a review of MRCP images. The MPD upstream diameter and presence of distal pancreatic atrophy were not evaluated in patients with lesions in the pancreatic tail, while stenosis of CBD was not evaluated for patients with lesions in the pancreatic head.
Statistical analysis
Statistical analysis was performed using the Fisher’s exact test to compare the frequencies of imaging findings. The inter-reader agreement was evaluated by measurement of the kappa value. The mean CT attenuation value of the lesion in patients with f-AIP was compared with that of PC. Similarly, The mean CT attenuation values of unaffected segmental pancreas in f-AIP were compared with those of PC and normal pancreas. A comparison of the mean CT attenuation values of other organs (liver and spleen) was also performed in the three groups of patients.
Statistical analyses were performed with nonparametric tests due to the nongaussian distribution of the data and smaller sample sizes involved in some comparisons of interest. Wilcoxon’s rank sum test was used to compare the CT attenuation values. When comparing two groups, the KruskalWallis test was performed before Wilcoxon’s rank sum test. Fisher’s exact test was applied to compare frequencies of delayed enhancement of the masses and focal enlarged segments. The diagnostic performance of attenuation value increase between the arterial and hepatic phase in the differentiation between f-AIP and PC was evaluated using receiver operating characteristic (ROC) analysis. From the ROC curves, the appropriate cutoff values were determined by selecting the point at which the Yoden index (sum of sensitivity + specificity - 1) was largest. All tests were two sided, and P < 0.05 was considered statistically significant. Statistical analysis was performed with SPSS software (version 18.0, SPSS).
RESULTS
The imaging characteristics of f-AIP and PC identified by reviewing the CE-CT and MRI/MRCP results are summarized in Tables 1 and 2.
Table 1.
Comparison of imaging findings
| Imaging features | Data of assessable patients | Kappa | P | |
| f-AIP (n = 19) | f-PC (n = 30) | |||
| Sausage-shaped enlargement | 0.58 (11/19) | 0.03 (1/30) | 0.58 | < 0.001 |
| Delayed homogeneous enhancement | 1.00 (19/19) | 0.1 (3/30) | 0.88 | < 0.001 |
| Hypoattenuating capsule-like rim | 0.63 (12/19) | 0.03 (1/30) | 0.64 | < 0.001 |
| Distal pancreatic atrophy | 0.30 (3/10) | 0.95 (20/22) | -0.55 | < 0.001 |
| MPD | ||||
| Normal | 0 (0/19) | 0 (0/30) | NS | |
| Irregular narrowing | 0.58 (11/19) | 0.06 (2/30) | 0.05 | NS |
| Complete obstruction | 0.42 (8/19) | 0.93 (28/30) | -0.06 | NS |
| CBD | ||||
| Normal | 0.53 (10/19) | 0.47 (14/30) | -0.36 | 0.027 |
| Stenosis1 | 0.29 (4/14) | 0 (0/14) | 0.3 | 0.022 |
| Complete obstruction | 0 (3/19) | 0.53 (16/30) | -0.38 | < 0.01 |
| MPD upstream dilation ≤ 5 mm | 1.00 (10/10) | 0.14 (5/22) | 0.66 | < 0.001 |
| Affected location of pancreas | ||||
| Head | 0.26 (5/19) | 0.53 (16/30) | -0.08 | NS |
| Body | 0.26 (5/19) | 0.2 (6/30) | -0.27 | NS |
| Tail | 0.47 (9/19) | 0.27 (8/30) | 0.11 | NS |
| Other findings | ||||
| Vascular invasion | 0 (0/19) | 0.13 (4/30) | -0.17 | NS |
| Pancreatic lymph node | 0.16 (3/19) | 0.87 (26/30) | -0.65 | < 0.001 |
| Calcification | 0 (1/19) | 0 (0/30) | NS | |
Data are percentages and numbers in parentheses refer to numbers of lesions.
The stenosis of common bile duct (CBD) was not evaluated for focal autoimmune pancreatitis (f-AIP) and pancreatic cancer (PC) patients with lesions in the pancreatic head. When the Kappa value was negative, the corresponding item was not suitable for differentiation. f-AIP: Focal autoimmune pancreatitis; MPD: Main pancreatic duct; NS: Not significant.
Table 2.
Mean computed tomography attenuation values
| Group | n | Condition | Unenhanced scan (HU) | Arterial phase (HU) | Pancreatic phase (HU) | Hepatic phase (HU) |
| f-AIP | 19 | Lesions | 36 ± 4 | 60 ± 7 | 85 ± 6 | 95 ± 7 |
| Uninvolved pancreas | 42 ± 5 | 83 ± 10 | 105 ± 14 | 95 ± 7 | ||
| Liver | 57 ± 4 | 68 ± 8 | 119 ± 15 | 100 ± 10 | ||
| Spleen | 48 ± 3 | 111 ± 15 | 129 ± 15 | 96 ± 9 | ||
| PC | 30 | Lesion | 34 ± 5 | 48 ± 10 | 63 ± 15 | 63 ± 13 |
| Uninvolved pancreas | 39 ± 6 | 76 ± 14 | 103 ± 15 | 87 ± 12 | ||
| Liver | 58 ± 6 | 69 ± 9 | 108 ± 17 | 102 ± 10 | ||
| Spleen | 46 ± 4 | 102 ± 19 | 141 ± 18 | 101 ± 7 | ||
| Normal | 30 | Pancreas | 44 ± 7 | 87 ± 12 | 105 ± 20 | 93 ± 10 |
| Liver | 57 ± 7 | 65 ± 8 | 110 ± 18 | 99 ± 13 | ||
| Spleen | 47 ± 4 | 107 ± 17 | 133 ± 23 | 99 ± 11 |
Data are computed tomography attenuation values (mean ± SD). f-AIP: Focal autoimmune pancreatitis; PC: pancreatic cancer.
Focal pancreatic enlargement
Of the 49 f-AIP and PC cases, the affected segments of pancreas differed in the extent of enlargement. Of the f-AIP cases, the affected sites of the pancreas were the head in 5 patients, the body in 5 patients, and the tail in 9 patients. Of the PC cases, these values were 16, 6 and 8, respectively. The sausage-shaped enlargement of the affected segments of the pancreas was observed in 11 patients with f-AIP, but not in PC cases. Atrophy of the pancreas was observed in 3 f-AIP and 20 PC cases whose lesions were located in the head and body.
Density or signal abnormalities
CE-CT scans showed hypoattenuated or isoattenuated lesions in the involved segments of the pancreas in 4 and 15 f-AIP cases on precontrast scans, respectively. In all f-AIP patients, the affected pancreas appeared uniformly enlarged with the absence of pancreatic clefts and with a sharp outline (Figures 1-3). AIP and PC lesions all showed decreased enhancement on arterial phase and delayed enhancement on pancreatic and hepatic phases, however, the degree of delayed enhancement in f-AIP was greater than that in PC (Figures 2 and 4, Table 2). MRI imaging showed that the lesion was isointense or slightly hypointense in T1WI and slightly hyperintense in T2WI (Figure 3A and B). The MR enhancement patterns were similar to those of CE-CT.
Figure 1.
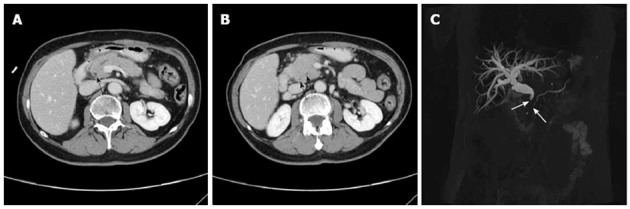
A 55-year-old woman with focal autoimmune pancreatitis of the pancreatic head. A, B: Contrast-enhanced computed tomography images obtained during enhanced phases showing enhanced thickening of the common bile duct (CBD) wall (A, black arrow), narrowing of the main pancreatic duct (MPD) and distal CBD (B, black arrows); C: Bile duct dilation and irregular narrowing of the MPD in the pancreatic head can be observed on the magnetic resonance cholangiopancreatography image (white arrows).
Figure 3.
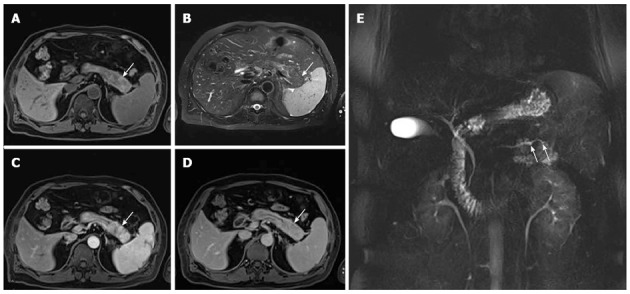
A 53-year-old man with focal autoimmune pancreatitis of the pancreatic tail. A-D: The lesion showed heterogenous T1WI hypointensity and heterogenous T2WI hyperintensity, which was delayed enhanced. The capsule-like rim appearing as a T1WI iso- or slight hyperintensity and a T2WI hypointensity area surrounding the pancreas (white arrow), which were delayed and moderately enhanced; E: Magnetic resonance cholangiopancreatography image shows the irregular narrowing main pancreatic duct in the pancreatic tail (white arrows).
Figure 2.
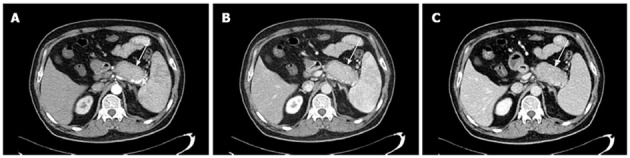
A 59-year-old man with focal autoimmune pancreatitis of the pancreatic tail. Contrast-enhanced computed tomography image obtained during the arterial phase (A, white arrow) showing that a hypoattenuating capsule-like rim can be observed around the pancreatic tail, which manifested delayed enhancement during the pancreatic phase (B, white arrow) and hepatic phase (C, white arrow). Attenuation values of the autoimmune pancreatitis lesion were 36, 51, 76 and 89 HU during the pre-contrast and enhanced phases, respectively.
Figure 4.
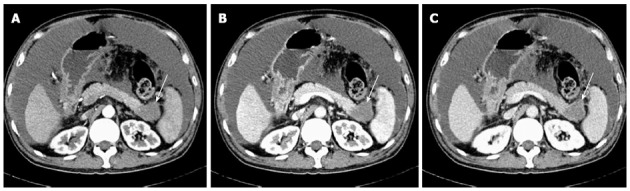
A 54-year-old man with pancreatic cancer of the pancreatic tail. Triple-phase computed tomography images show irregular enlargement of the pancreatic tail. Enhancement of the lesion is decreased (A, 39 HU) during arterial phase, and slightly increased enhancement during pancreatic phase (B, 48 HU) and hepatic phase (C, 62 HU), respectively (white arrows).
CBD and MPD Abnormalities
Of the AIP lesions (n = 21), irregular narrowing of the MPD was observed in 13 lesions (Figure 3E), and the remaining 8 lesions did not display this MPD abnormality. Bile duct dilatation was observed in 5 cases which included 4 patients who had lesions at the pancreatic head and 1 at the tail. The range of dilation was 9-14 mm (mean, 11 mm). The CBD showed a beak-like stricture (Figure 1C). In 3 patients with lesions in the pancreatic head, the upstream MPD was slightly dilated (less than 5 mm), distal pancreatic atrophy was not observed in 14 patients with lesions in the pancreatic body and tail.
Of the PC lesions (n = 30), no irregular narrowing of the MPD was observed. Bile duct dilatation was observed in all 8 patients with lesions located in the pancreatic head, and the distal CBD showed an abrupt interruption sign.
Capsule-like rim
Capsule-like rims were observed in f-AIP cases (n = 11), which were shown continuously or discontinuously as hypodense peripancreatic strands on the precontrast CT and delayed enhanced on the delayed phase of dynamic CT (Figure 2). On MR imaging, the capsule-like rim appeared as a T1WI isointense and T2WI hypointense area surrounding the pancreas (Figure 3A and B).
Other associated findings
Other associated findings included calcification (f-AIP, n = 1; PC, n = 0), peripancreatic lymphadenopathy (f-AIP, n = 3; PC, n = 26) and vascular invasion (f-AIP, n = 0; PC, n = 4).
Comparison of CT attenuation values
The results of the mean CT attenuation values of the f-AIP lesions and uninvolved segments in f-AIP patients and normal pancreas are shown in Table 2. The mean CT attenuation values of f-AIP lesions in enhanced phases were significantly higher than those of PC (P < 0.05, P < 0.05, P < 0.05) (Figure 5). The mean CT attenuation values of the f-AIP lesions were significantly lower than those of uninvolved pancreas and normal pancreas in the arterial and pancreatic phase of CT (P < 0.001, P < 0.001), however, there were no significant differences in the hepatic phase or unenhanced scanning (P = 0.4, P = 0.1). The mean CT attenuation values of normal and unaffected pancreatic parenchyma in the three groups showed no significant differences in the arterial, pancreatic, and hepatic phases (P = 0.1, P = 0.8, P = 0.2). The mean CT attenuation values of the liver and spleen were not significantly different between the three groups. When the attenuation value increase was equal or more than 28 HU this was considered diagnostic for f-AIP, and a sensitivity of 87.5%, specificity of 100% and an area under the ROC curve of 0.974 (95%CI: 0.928-1.021) were achieved (Figure 6).
Figure 5.
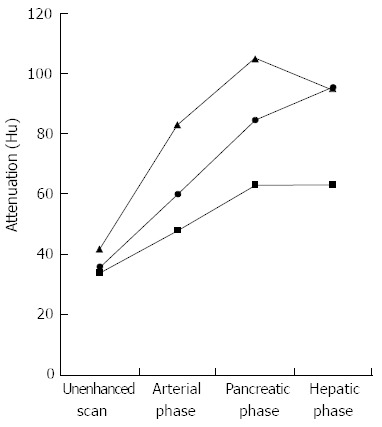
Graph shows mean computed tomography attenuation values of normal pancreatic parenchyma (triangle) and lesions in patients with focal autoimmune pancreatitis (circle) and pancreatic cancer (square) in relation to phase of contrast enhancement.
Figure 6.
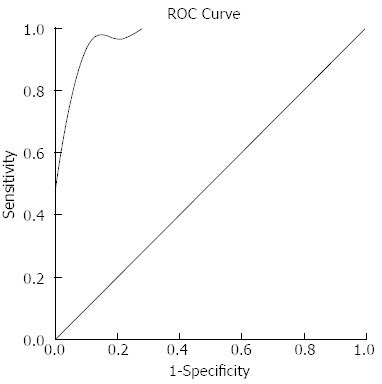
Receiver operating characteristic curve analysis result. Receiver operating characteristic (ROC) curve analysis revealed that when values were more than or equal 28 HU they were considered diagnostic for focal autoimmune pancreatitis, and a sensitivity of 87.5%, a specificity of 100%, and an area under the ROC curve of 0.974 (95%CI: 0.928-1.021) were achieved.
DISCUSSION
Although the diagnosis of AIP has improved due to a growing awareness of the condition and proposed diagnostic criteria[18], there is no practical strategy to differentiate PC from f-AIP. One must distinguish between the two disorders to prevent unnecessary surgery or delayed initiation of corticosteroid therapy. A review of the CE-CT and MRI data indicated five imaging features of AIP: (1) delayed homogeneous enhancement; (2) hypoattenuateing capsule-like rim; (3) the absence of distal pancreatic atrophy; (4) irregular narrowing of the MPD; and (5) stenosis of the CBD in patients with lesions in the body or/and tail. The analysis also indicated that those imaging features could be used to differentiate AIP from PC with high accuracy.
Some studies have reported that the head of the pancreas is involved in most AIP cases[19,20], which is consistent with the study by Woo Ik Chang who showed that the affected site was the pancreatic head in 5 (62.5%) of 8 patients[13]. However, in our study, the affected site was the pancreatic head in only 4 patients (21.1%). This difference may be due to the following reasons: (1) the pancreatic head may not be the most commonly involved site in f-AIP; and (2) the number of patients studied may have been too small.
In our study, we identified the homogeneous good enhancement during the delayed phases as useful in differentiating between f-AIP and PC. Similarly, in the study by Kamisawa[12], delayed enhancement was observed in 17 of 17 (100%) AIP patients, while Wakabayashi et al[20] described delayed homogeneous enhancement in 9 of 9 (100%) AIP patients. In addition, Chang et al[13] described homogeneous enhancement during the hepatic phase in six of seven AIP patients. The consistency of delayed homogenous enhancement in this and previous studies shows one of the common imaging features of AIP, but the absence of a further quantitative analysis.
We found that the attenuation values of AIP were significantly higher than those of PC in the enhanced phases, lower than those of unaffected pancreatic parenchyma in the arterial and pancreatic phase, however, no significant differences were observed in the hepatic phase (95 ± 7 HU vs 95 ± 7 HU, 75 s). Takahashi found significantly higher CT attenuation values for AIP (90 ± 19 HU) than for pancreatic carcinoma (64 ± 19 HU) during the hepatic phase (60-70 s)[21]. They also found greater enhancement during the pancreatic phase (71 ± 22 HU vs 59 ± 20 HU), although the difference between the two groups was not statistically significant[10]. The difference between the CT attenuation values obtained in this study and those by Takahashi may be related to differences in the timing of scan acquisition, contrast injection rates and CT scanners.
Irregular narrowing of the MPD is one of the most important features of AIP. In our study, 79% of AIP patients (15 of 19) showed segmental irregular narrowing of the MPD, while the number was 6% (2 of 30) in PC patients. Moreover, no or minimal upstream dilatation can also be helpful to differentiate AIP from PC[10,22]. In the current study, upstream MPD dilatation (≥ 5 mm) was observed in only 5% of patients (1 of 19), while the number was 86% in PC patients. Some studies reported that stricture of the distal CBD was often observed in AIP, and this occurred due to the combined effect of extrinsic compression by the inflamed pancreatic head and inflammatory changes in the CBD[10]. The f-AIP patients showed a smooth pattern, whereas the patients with PC showed an irregular pattern. In our study, CBD stricture of smooth pattern was seen in 8 of 19 patients, that is 4 of 5 patients with pancreatic head lesions and four with pancreatic tail lesions. Therefore, we suggest that the inflammatory changes in the CBD and AIP may be independent or have occurred one after another, and this might be useful for the diagnosis of f-AIP.
In the current study, the small number of patients may not represent all the characteristics of f-AIP according to radiological features only. Thus, we tried to identify radiological findings to diagnosis f-AIP, however, these findings were insufficient to exclude PC. Therefore, we recommend that IgG4 should be tested and histological findings should be obtained where possible to discriminate f-AIP from PC. We believe that future studies with more patients will clarify and validate the radiological features of f-AIP.
In conclusion, this study found that the analysis of a combination of imaging findings, either of delayed enhancement with more than 28 HU, or of three imaging findings (1) focal pancreatic enlargement with a capsule-like rim; (2) irregular narrowing of the MPD; and (3) stricture of the CBD in patients with lesions (not located in the pancreatic head) contribute to improve the diagnostic accuracy of f-AIP thus avoiding unnecessary surgery.
COMMENTS
Background
Autoimmune pancreatitis (AIP), a rare form of chronic pancreatitis (CP), can be classified as the diffuse form and the focal form. Although AIP is well-known among radiologists, focal AIP (f-AIP) is still very difficult to differentiate from pancreatic cancer (PC).
Research frontiers
F-AIP has a variety of manifestations and is very difficult to differentiate from PC. Improving the diagnostic accuracy can help avoid unnecessary surgery in f-AIP patients.
Related publications
The analysis of combined imaging with computed tomography and magnetic resonance to improve the diagnostic accuracy of differentiating f-AIP from PC has rarely been reported.
Innovations and breakthroughs
This is the first study to report that the triple-phase enhancement pattern of f-AIP is different from that of PC. Three imaging features are more frequently found in f-AIP, including focal pancreatic enlargement with a capsule-like rim, irregular narrowing of the main pancreatic duct and stricture of the common bile duct in patients with lesions not located in the pancreatic head. The combination of these findings could further improve the diagnostic accuracy of f-AIP and avoid unnecessary surgery.
Applications
The study results suggest that a combination of imaging findings will help improve the diagnostic accuracy of f-AIP and avoid unnecessary surgery.
Peer review
It is a good clinical study in which the authors analyzed the radiological characteristics of f-AIP. The results are interesting and suggest that the combination of triple-phase enhancement pattern and imaging features of f-AIP can help for the differential diagnosis of f-AIP from PC.
Footnotes
Supported by National Nature Science Foundation of China No. 30970801; National Nature Science Foundation of China, No. 81170435; the China Post-doctoral Science Foundation, No. 20100480545; and the Shanghai Leading Talent Team Construction Special Funds, No. 2011-036
P- Reviewer Shigeru BHK S- Editor Zhai HH L- Editor Webster JR E- Editor Lu YJ
References
- 1.Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas--an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688–698. doi: 10.1007/BF02232341. [DOI] [PubMed] [Google Scholar]
- 2.Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991;22:387–395. doi: 10.1016/0046-8177(91)90087-6. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 4.Ectors N, Maillet B, Aerts R, Geboes K, Donner A, Borchard F, Lankisch P, Stolte M, Lüttges J, Kremer B, et al. Non-alcoholic duct destructive chronic pancreatitis. Gut. 1997;41:263–268. doi: 10.1136/gut.41.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klöppel G, Sipos B, Zamboni G, Kojima M, Morohoshi T. Autoimmune pancreatitis: histo- and immunopathological features. J Gastroenterol. 2007;42 Suppl 18:28–31. doi: 10.1007/s00535-007-2048-6. [DOI] [PubMed] [Google Scholar]
- 6.Nahon Uzan K, Lévy P, O’Toole D, Belmatoug N, Vullierme MP, Couvelard A, Ponsot P, Palazzo L, Abbas A, Hammel P, et al. Is idiopathic chronic pancreatitis an autoimmune disease? Clin Gastroenterol Hepatol. 2005;3:903–909. doi: 10.1016/s1542-3565(05)00540-9. [DOI] [PubMed] [Google Scholar]
- 7.Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670–2676. doi: 10.1056/NEJMra061200. [DOI] [PubMed] [Google Scholar]
- 8.Lévy P, Hammel P, Ruszniewski P. [Autoimmune pancreatitis] Presse Med. 2007;36:1925–1934. doi: 10.1016/j.lpm.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 10.Kim KP, Kim MH, Song MH, Lee SS, Seo DW, Lee SK. Autoimmune chronic pancreatitis. Am J Gastroenterol. 2004;99:1605–1616. doi: 10.1111/j.1572-0241.2004.30336.x. [DOI] [PubMed] [Google Scholar]
- 11.Wolfson D, Barkin JS, Chari ST, Clain JE, Bell RH, Alexakis N, Neoptolemos JP. Management of pancreatic masses. Pancreas. 2005;31:203–217. doi: 10.1097/01.mpa.0000180613.07948.ca. [DOI] [PubMed] [Google Scholar]
- 12.Kamisawa T, Imai M, Yui Chen P, Tu Y, Egawa N, Tsuruta K, Okamoto A, Suzuki M, Kamata N. Strategy for differentiating autoimmune pancreatitis from pancreatic cancer. Pancreas. 2008;37:e62–e67. doi: 10.1097/MPA.0b013e318175e3a0. [DOI] [PubMed] [Google Scholar]
- 13.Chang WI, Kim BJ, Lee JK, Kang P, Lee KH, Lee KT, Rhee JC, Jang KT, Choi SH, Choi DW, et al. The clinical and radiological characteristics of focal mass-forming autoimmune pancreatitis: comparison with chronic pancreatitis and pancreatic cancer. Pancreas. 2009;38:401–408. doi: 10.1097/MPA.0b013e31818d92c0. [DOI] [PubMed] [Google Scholar]
- 14.Abraham SC, Wilentz RE, Yeo CJ, Sohn TA, Cameron JL, Boitnott JK, Hruban RH. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: are they all ‘chronic pancreatitis’? Am J Surg Pathol. 2003;27:110–120. doi: 10.1097/00000478-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Hardacre JM, Iacobuzio-Donahue CA, Sohn TA, Abraham SC, Yeo CJ, Lillemoe KD, Choti MA, Campbell KA, Schulick RD, Hruban RH, et al. Results of pancreaticoduodenectomy for lymphoplasmacytic sclerosing pancreatitis. Ann Surg. 2003;237:853–858; discussion 853-858;. doi: 10.1097/01.SLA.0000071516.54864.C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber SM, Cubukcu-Dimopulo O, Palesty JA, Suriawinata A, Klimstra D, Brennan MF, Conlon K. Lymphoplasmacytic sclerosing pancreatitis: inflammatory mimic of pancreatic carcinoma. J Gastrointest Surg. 2003;7:129–137; discussion 137-139. doi: 10.1016/s1091-255x(02)00148-8. [DOI] [PubMed] [Google Scholar]
- 17.Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, Park SW, Shimosegawa T, Lee K, Ito T, et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008;43:403–408. doi: 10.1007/s00535-008-2205-6. [DOI] [PubMed] [Google Scholar]
- 18.Okazaki K, Kawa S, Kamisawa T, Naruse S, Tanaka S, Nishimori I, Ohara H, Ito T, Kiriyama S, Inui K, et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006;41:626–631. doi: 10.1007/s00535-006-1868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klöppel G, Lüttges J, Löhr M, Zamboni G, Longnecker D. Autoimmune pancreatitis: pathological, clinical, and immunological features. Pancreas. 2003;27:14–19. doi: 10.1097/00006676-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Wakabayashi T, Kawaura Y, Satomura Y, Watanabe H, Motoo Y, Okai T, Sawabu N. Clinical and imaging features of autoimmune pancreatitis with focal pancreatic swelling or mass formation: comparison with so-called tumor-forming pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2679–2687. doi: 10.1111/j.1572-0241.2003.08727.x. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi N, Fletcher JG, Hough DM, Fidler JL, Kawashima A, Mandrekar JN, Chari ST. Autoimmune pancreatitis: differentiation from pancreatic carcinoma and normal pancreas on the basis of enhancement characteristics at dual-phase CT. AJR Am J Roentgenol. 2009;193:479–484. doi: 10.2214/AJR.08.1883. [DOI] [PubMed] [Google Scholar]
- 22.Horiuchi A, Kawa S, Hamano H, Hayama M, Ota H, Kiyosawa K. ERCP features in 27 patients with autoimmune pancreatitis. Gastrointest Endosc. 2002;55:494–499. doi: 10.1067/mge.2002.122653. [DOI] [PubMed] [Google Scholar]


