Abstract
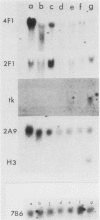
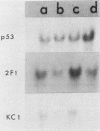
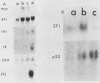
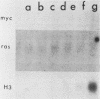
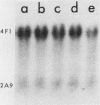
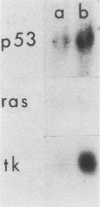
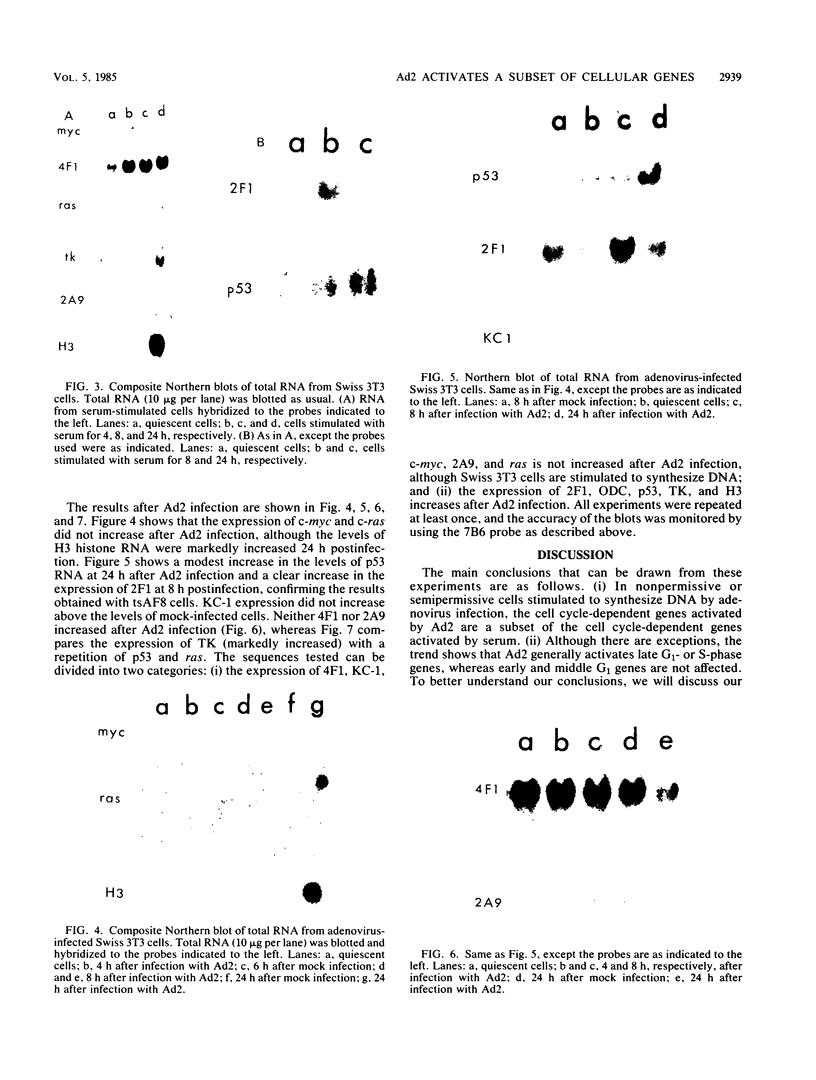
We have studied a panel of 10 genes and cDNA sequences that are expressed in a cell cycle-dependent manner in different types of cells from different species and that are inducible by different mitogens. These include five sequences (c-myc, 4F1, 2F1, 2A9, and KC-1) that are preferentially expressed in the early part of the G1 phase, three genes (ornithine decarboxylase, p53, and c-rasHa) preferentially expressed in middle or late G1, and two genes (thymidine kinase and histone H3) preferentially expressed in the S phase of the cell cycle. We have studied the expression of these genes in nonpermissive (tsAF8) and semipermissive (Swiss 3T3) cells infected with adenovirus type 2. Under the conditions of these experiments, adenovirus type 2 infection stimulates cellular DNA synthesis in both tsAF8 and 3T3 cells. However, four of the five early G1 genes (c-myc, 4F1, KC-1, and 2A9) and one of the late G1 genes (c-ras) are not induced by adenovirus infection, although they are strongly induced by serum. The other sequences (2F1, ornithine decarboxylase, p53, thymidine kinase, and histone H3) are activated by both adenovirus and serum. We conclude that the cell cycle-dependent genes activated by adenovirus 2 are a subset of the cell cycle-dependent genes activated by serum. The data suggest that the mechanisms by which serum and adenovirus induce cellular DNA synthesis are not identical.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Artishevsky A., Delegeane A. M., Lee A. S. Use of a cell cycle mutant to delineate the critical period for the control of histone mRNA levels in the mammalian cell cycle. Mol Cell Biol. 1984 Nov;4(11):2364–2369. doi: 10.1128/mcb.4.11.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashihara T., Chang S. D., Baserga R. Constancy of the shift-up point in two temperature-sensitive mammalian cell lines that arrest in G1. J Cell Physiol. 1978 Jul;96(1):15–22. doi: 10.1002/jcp.1040960103. [DOI] [PubMed] [Google Scholar]
- Babich A., Feldman L. T., Nevins J. R., Darnell J. E., Jr, Weinberger C. Effect of adenovirus on metabolism of specific host mRNAs: transport control and specific translational discrimination. Mol Cell Biol. 1983 Jul;3(7):1212–1221. doi: 10.1128/mcb.3.7.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga R. Growth in size and cell DNA replication. Exp Cell Res. 1984 Mar;151(1):1–5. doi: 10.1007/978-3-642-67986-5_1. [DOI] [PubMed] [Google Scholar]
- Basilico C., Matsuya Y., Green H. Origin of the thymidine kinase induced by polyoma virus in productively infected cells. J Virol. 1969 Feb;3(2):140–145. doi: 10.1128/jvi.3.2.140-145.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz G. A., Flint S. J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979 Jun 25;131(2):353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Berger F. G., Szymanski P., Read E., Watson G. Androgen-regulated ornithine decarboxylase mRNAs of mouse kidney. J Biol Chem. 1984 Jun 25;259(12):7941–7946. [PubMed] [Google Scholar]
- Braithwaite A. W., Cheetham B. F., Li P., Parish C. R., Waldron-Stevens L. K., Bellett A. J. Adenovirus-induced alterations of the cell growth cycle: a requirement for expression of E1A but not of E1B. J Virol. 1983 Jan;45(1):192–199. doi: 10.1128/jvi.45.1.192-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite A. W., Murray J. D., Bellett A. J. Alterations to controls of cellular DNA synthesis by adenovirus infection. J Virol. 1981 Aug;39(2):331–340. doi: 10.1128/jvi.39.2.331-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstin S. J., Meiss H. K., Basilico C. A temperature-sensitive cell cycle mutant of the BHK cell line. J Cell Physiol. 1974 Dec;84(3):397–408. doi: 10.1002/jcp.1040840308. [DOI] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Maryak J. M., Wei C. M., Shih T. Y., Shober R., Cheung H. L., Ellis R. W., Hager G. L., Scolnick E. M., Lowy D. R. Functional organization of the Harvey murine sarcoma virus genome. J Virol. 1980 Jul;35(1):76–92. doi: 10.1128/jvi.35.1.76-92.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham B. F., Bellett A. J. A biochemical investigation of the adenovirus-induced G1 to S phase progression: thymidine kinase, ornithine decarboxylase, and inhibitors of polyamine biosynthesis. J Cell Physiol. 1982 Feb;110(2):114–122. doi: 10.1002/jcp.1041100203. [DOI] [PubMed] [Google Scholar]
- Cheetham B. F., Shaw D. C., Bellett A. J. Adenovirus type 5 induces progression of quiescent rat cells into S phase without polyamine accumulation. Mol Cell Biol. 1982 Oct;2(10):1295–1298. doi: 10.1128/mcb.2.10.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Zullo J., Verma I. M., Stiles C. D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science. 1984 Nov 30;226(4678):1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Pim D. C., Gurney E. G., Goodfellow P., Taylor-Papadimitriou J. Detection of a common feature in several human tumor cell lines--a 53,000-dalton protein. Proc Natl Acad Sci U S A. 1981 Jan;78(1):41–45. doi: 10.1073/pnas.78.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Bregni M., Erikson J., Patterson D., Gallo R. C., Croce C. M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle A. J., Graves R. A., Marzluff W. F., Johnson L. F. Regulation of histone mRNA production and stability in serum-stimulated mouse 3T6 fibroblasts. Mol Cell Biol. 1983 Nov;3(11):1920–1929. doi: 10.1128/mcb.3.11.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehlinger P. J., Litt M. Ornithine decarboxylase induction and nucleolar RNA synthesis in Friend leukemia cells. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1054–1057. doi: 10.1016/0006-291x(78)91457-2. [DOI] [PubMed] [Google Scholar]
- Estes J. E., Huang E. S. Stimulation of cellular thymidine kinases by human cytomegalovirus. J Virol. 1977 Oct;24(1):13–21. doi: 10.1128/jvi.24.1.13-21.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Plumb M. A., Yang U. C., Stein G. S., Stein J. L. Effect of adenovirus infection on expression of human histone genes. Mol Cell Biol. 1984 Jul;4(7):1363–1371. doi: 10.1128/mcb.4.7.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floros J., Jonak G., Galanti N., Baserga R. Induction of cell DNA replication in G1-specific ts mutants by microinjection of SV40 DNA. Exp Cell Res. 1981 Mar;132(1):215–223. doi: 10.1016/0014-4827(81)90097-5. [DOI] [PubMed] [Google Scholar]
- Fuller D. J., Gerner E. W., Russell D. H. Polyamine biosynthesis and accumulation during the G1 to S phase transition. J Cell Physiol. 1977 Oct;93(1):81–88. doi: 10.1002/jcp.1040930111. [DOI] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Expression of a cellular oncogene during liver regeneration. Science. 1983 Feb 4;219(4584):510–512. doi: 10.1126/science.6297003. [DOI] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Regulated transcription of c-Ki-ras and c-myc during compensatory growth of rat liver. Mol Cell Biol. 1984 Aug;4(8):1493–1498. doi: 10.1128/mcb.4.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R. R., Aller P., Yuan Z. A., Gibson C. W., Baserga R. Cell-cycle-specific cDNAs from mammalian cells temperature sensitive for growth. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6004–6008. doi: 10.1073/pnas.81.19.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R. R., Marashi F., Baserga R., Stein J., Stein G. Expression of histone genes in a G1-specific temperature-sensitive mutant of the cell cycle. Biochemistry. 1984 Jul 31;23(16):3731–3735. doi: 10.1021/bi00311a025. [DOI] [PubMed] [Google Scholar]
- Hyland J. K., Rogers C. M., Scolnick E. M., Stein R. B., Ellis R., Baserga R. Microinjected ras family oncogenes stimulate DNA synthesis in quiescent mammalian cells. Virology. 1985 Mar;141(2):333–336. doi: 10.1016/0042-6822(85)90268-5. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Khalili K., Weinmann R. Actin mRNAs in HeLa cells. Stabilization after adenovirus infection. J Mol Biol. 1984 Dec 25;180(4):1007–1021. doi: 10.1016/0022-2836(84)90268-7. [DOI] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M., Melnick J. L. Enzyme induction in SV40-infected green monkey kidney cultures. Virology. 1966 May;29(1):69–83. doi: 10.1016/0042-6822(66)90197-8. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Laughlin C., Strohl W. A. Factors regulating cellular DNA synthesis induced by adenovirus infection. II. The effects of actinomycin D on productive virus-cell systems. Virology. 1976 Oct 1;74(1):44–56. doi: 10.1016/0042-6822(76)90126-4. [DOI] [PubMed] [Google Scholar]
- Ledinko N. Stimulation of DNA synthesis and thymidine kinase activity in human embryonic kidney cells infected by Adenovirus 2 or 12. Cancer Res. 1967 Aug;27(8):1459–1469. [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Growth-related changes in specific mRNAs of cultured mouse cells. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4271–4275. doi: 10.1073/pnas.80.14.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. T., Gibson C. W., Hirschhorn R. R., Rittling S., Baserga R., Mercer W. E. Expression of thymidine kinase and dihydrofolate reductase genes in mammalian ts mutants of the cell cycle. J Biol Chem. 1985 Mar 25;260(6):3269–3274. [PubMed] [Google Scholar]
- Makino R., Hayashi K., Sugimura T. C-myc transcript is induced in rat liver at a very early stage of regeneration or by cycloheximide treatment. Nature. 1984 Aug 23;310(5979):697–698. doi: 10.1038/310697a0. [DOI] [PubMed] [Google Scholar]
- McCairns E., Fahey D., Muscat G. E., Murray M., Rowe P. B. Changes in levels of actin and tubulin mRNAs upon the lectin activation of lymphocytes. Mol Cell Biol. 1984 Sep;4(9):1754–1760. doi: 10.1128/mcb.4.9.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Nelson D., DeLeo A. B., Old L. J., Baserga R. Microinjection of monoclonal antibody to protein p53 inhibits serum-induced DNA synthesis in 3T3 cells. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6309–6312. doi: 10.1073/pnas.79.20.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J., Milner S. SV40-53K antigen: a possible role for 53K in normal cells. Virology. 1981 Jul 30;112(2):785–788. doi: 10.1016/0042-6822(81)90327-5. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Nishimoto T., Okubo C. K., Raskas H. J. Abortive replication of adenovirus 2 in the temperature-sensitive hamster cell mutant tsAF8. Virology. 1977 May 1;78(1):1–10. doi: 10.1016/0042-6822(77)90074-5. [DOI] [PubMed] [Google Scholar]
- Nishimoto T., Raskas H. J., Basilico C. Temperature-sensitive cell mutations that inhibit adenovirus 2 replication. Proc Natl Acad Sci U S A. 1975 Jan;72(1):328–332. doi: 10.1073/pnas.72.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. G., Lewis M. A., Diamond L. Ornithine decarboxylase activity and DNA synthesis after treatment of cells in culture with 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1979 Nov;39(11):4477–4480. [PubMed] [Google Scholar]
- Oren M., Bienz B., Givol D., Rechavi G., Zakut R. Analysis of recombinant DNA clones specific for the murine p53 cellular tumor antigen. EMBO J. 1983;2(10):1633–1639. doi: 10.1002/j.1460-2075.1983.tb01637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D., Brown K. D., Rupniak H. T., Ristow H. J. Cell cycle regulation by growth factors and nutrients in normal and transformed cells. In Vitro. 1978 Jan;14(1):76–84. doi: 10.1007/BF02618176. [DOI] [PubMed] [Google Scholar]
- Piña M., Green M. Biochemical studies on adenovirus multiplication. XIV. Macromolecule and enzyme synthesis in cells replicating oncogenic and nononcogenic human adenovirus. Virology. 1969 Aug;38(4):573–586. doi: 10.1016/0042-6822(69)90178-0. [DOI] [PubMed] [Google Scholar]
- Plumb M., Stein J., Stein G. Coordinate regulation of multiple histone mRNAs during the cell cycle in HeLa cells. Nucleic Acids Res. 1983 Apr 25;11(8):2391–2410. doi: 10.1093/nar/11.8.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochron S., Rossini M., Darzynkiewicz Z., Traganos F., Baserga R. Failure of accumulation of cellular RNA in hamster cells stimulated to synthesize DNA by infection with adenovirus 2. J Biol Chem. 1980 May 25;255(10):4411–4413. [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rossini M., Jonak G. J., Baserga R. Identification of adenovirus 2 early genes required for induction of cellular DNA synthesis in resting hamster cells. J Virol. 1981 Jun;38(3):982–986. doi: 10.1128/jvi.38.3.982-986.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini M., Weinmann R., Baserga R. DNA synthesis in temperature-sensitive mutants of the cell cycle infected by polyoma virus and adenovirus. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4441–4445. doi: 10.1073/pnas.76.9.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutzbank T., Robinson R., Oren M., Levine A. J. SV40 large tumor antigen can regulate some cellular transcripts in a positive fashion. Cell. 1982 Sep;30(2):481–490. doi: 10.1016/0092-8674(82)90245-8. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Eng C. Y., Berk A. J. An adenovirus early region 1A protein is required for maximal viral DNA replication in growth-arrested human cells. J Virol. 1985 Mar;53(3):742–750. doi: 10.1128/jvi.53.3.742-750.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Kung H. F. Transformation of NIH 3T3 cells by microinjection of Ha-ras p21 protein. Nature. 1984 Aug 9;310(5977):508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- Stoscheck C. M., Florini J. R., Richman R. A. The relationship of ornithine decarboxylase activity to proliferation and differentiation of L6 muscle cells. J Cell Physiol. 1980 Jan;102(1):11–18. doi: 10.1002/jcp.1041020103. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt R., Stanton L. W., Marcu K. B., Gallo R. C., Croce C. M., Rovera G. Nucleotide sequence of cloned cDNA of human c-myc oncogene. Nature. 1983 Jun 23;303(5919):725–728. doi: 10.1038/303725a0. [DOI] [PubMed] [Google Scholar]
- Yoder S. S., Robberson B. L., Leys E. J., Hook A. G., Al-Ubaidi M., Yeung C. Y., Kellems R. E., Berget S. M. Control of cellular gene expression during adenovirus infection: induction and shut-off of dihydrofolate reductase gene expression by adenovirus type 2. Mol Cell Biol. 1983 May;3(5):819–828. doi: 10.1128/mcb.3.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]