Abstract
AIM: To investigate the effect of bone-marrow mesenchymal stem cells (BM MSCs) on the intestinal mucosa barrier in ischemia/reperfusion (I/R) injury.
METHODS: BM MSCs were isolated from male Sprague-Dawley rats by density gradient centrifugation, cultured, and analyzed by flow cytometry. I/R injury was induced by occlusion of the superior mesenteric artery for 30 min. Rats were treated with saline, BM MSCs (via intramucosal injection) or tumor necrosis factor (TNF)-α blocking antibodies (via the tail vein). I/R injury was assessed using transmission electron microscopy, hematoxylin and eosin (HE) staining, immunohistochemistry, western blotting and enzyme linked immunosorbent assay.
RESULTS: Intestinal permeability increased, tight junctions (TJs) were disrupted, and zona occludens 1 (ZO-1) was downregulated after I/R injury. BM MSCs reduced intestinal mucosal barrier destruction, ZO-1 downregulation, and TJ disruption. The morphological abnormalities after intestinal I/R injury positively correlated with serum TNF-α levels. Administration of anti-TNF-α IgG or anti-TNF-α receptor 1 antibodies attenuated the intestinal ultrastructural changes, ZO-1 downregulation, and TJ disruption.
CONCLUSION: Altered serum TNF-α levels play an important role in the ability of BM MSCs to protect against intestinal I/R injury.
Keywords: Bone marrow mesenchymal stem cells, Zona occludens 1, Ischemia-reperfusion injury, Intestinal mucosa, Tumor necrosis factor-α
Core tip: Intestinal ischemia/reperfusion (I/R) injury is clinically important. Bone-marrow mesenchymal stem cells (BM MSCs) can protect against I/R injury; however, the mechanism is unclear. This study demonstrates that submucosal infusion of BM MSCs decreased intestinal permeability and preserved intestinal mechanical barrier function after I/R injury in rats, via a mechanism linked to reduced serum tumor necrosis factor (TNF)-α levels and increased expression of the intestinal tight junction protein zona occludens 1. Altered serum TNF-α levels play an important role in the ability of BM MSCs to protect against intestinal I/R injury.
INTRODUCTION
Digestive organ transplantation and other abdominal surgical procedures can result in different degrees of intestinal ischemia/reperfusion (I/R) injury, which can delay patient recovery and lead to systemic organ failure. Therefore, intestinal I/R injury is an important clinical issue. The small intestine is composed of labile cells that are easily injured by I/R; however, the mechanisms responsible for intestinal I/R injury are unclear. Previous studies have reported that the serum level of tumor necrosis factor (TNF)-α is elevated in patients with severe intestinal I/R injury[1]. TNF-α is a cytokine with broad-spectrum physiological and pathological responsiveness, which is primarily secreted by monokaryons and macrophages. In addition to participating in the humoral and cellular immune responses, TNF-α also plays an important role in diseases such as severe hepatitis, septic shock and inflammatory bowel disease[2-6]; however, it is not known whether TNF-α affects the intestinal barrier function during I/R injury.
Bone-marrow mesenchymal stem cells (BM MSCs) are fibroblast-like, pluripotent adult stem cells. BM MSCs can adhere to plastic and grow readily in the laboratory. BM MSCs give rise to mesoderm cells[7,8], and have been reported to differentiate into all three germ cell lines[9], liver and neural cells[10,11], which have potential to be used for the treatment of various diseases. Allogeneic MSCs were transplanted into primates via an intravenous route and distributed to the gastrointestinal tract where they proliferated[12]. MSCs have also been shown to have immunomodulatory capabilities due to the secretion of several growth factors[13,14]. BM MSCs reduce intestinal I/R injury in rats[15]. Studies in I/R rodent models have demonstrated that MSCs can beneficially produce paracrine growth factors and anti-inflammatory cytokines[16]. It should be noted that MSCs respond to TNF-α, but do not produce TNF-α[17].
The intestinal mucosa is the physical and metabolic barrier against toxins and pathogens in the gut lumen. Tight junctions (TJs) are the main structures responsible for restricting the paracellular movement of compounds across the intestinal mucosa. Structurally, TJs are composed of cytoplasmic proteins, including the zona occludens proteins, ZO-1-3[18,19] and two distinct transmembrane proteins, occludin and claudin[20,21], which are linked to the actin-based cytoskeleton[22]. TJs function as occlusion barriers by maintaining cellular polarity and homeostasis, and by regulating the permeability of paracellular spaces in the epithelium[23]. ZO-1, a member of the membrane-associated guanylate kinase family of proteins, acts as a scaffold for the organization of transmembrane TJ proteins, and also recruits various signaling molecules and the actin cytoskeleton to TJs[24]. Although previous studies have provided an insight into the molecular structure of TJs, much less is known about TJ functionality under physiological or pathophysiological conditions. Few studies have described the intestinal mucosa ultrastructure or changes in TJs during I/R injury.
In this study, we used a rat model of intestinal I/R injury to investigate the effect of BM MSCs on intestinal mucosa ultrastructure, with an emphasis on the mechanisms of intestinal barrier dysfunction.
MATERIALS AND METHODS
Animals and I/R injury model
Male Sprague-Dawley rats (180-200 g) were obtained from the Military Medical Science Academy of China People’s Liberation Army (PLA; Beijing, China), housed at a constant temperature and humidity, and provided with food and water ad libitum. All animal experimental procedures were approved by the Ethics Committee of the Military Medical Science Academy of the PLA before commencement of the study.
One-hundred and eight male rats were fasted for 12 h with free access to water before surgery and randomly assigned to five experimental groups. The operative procedures were performed using standard sterile technique under general anesthesia using 5% chloral hydrate (10 mL/kg). All rats were subjected to laparotomy using a midline incision that was approximately 3 cm, and the principal branches of the superior mesenteric artery (SMA) were identified. In the Sham group, the SMA was isolated using blunt dissection, without clamping the vessel. In the BM MSCs + I/R injury group, the SMA was occluded for 30 min using an atraumatic microvascular clamp. Immediately after the clamp was released, 1 × 107 male rat BM MSCs suspended in 0.5 mL serum-free Dulbecco’s Modified Eagle’s Medium (DMEM) were injected into the intestinal submucosa at five different locations. Animals in the normal saline (NS) + I/R injury group underwent I/R followed by the injection of 0.5 mL normal saline into the intestinal submucosa at 10 different locations. The anti-TNF-α + I/R injury group and the anti-TNF-αR1-IgG + I/R injury group were administered with anti-TNF-α IgG (1000 μg per rat; United States Biological, Swampscott, MA, United States) or anti-TNF-α R1 antibody (1000 μg per rat; R and D Systems, Minneapolis, MN, United States), respectively. Injections were given via the tail vein after induction of I/R injury.
The abdomen was closed and the animals were allowed to recover with free access to tap water and standard pellet rat chow. Rats in the I/R injury, BMSCs + I/R injury and Sham groups were euthanized at 2, 6, 24, 72 and 144 h after I/R injury (n = 6 at each time point). Rats in the anti-TNF-α IgG + I/R and anti-TNF-α R1 antibody + I/R injury groups were euthanized at 6 h after I/R injury (n = 6 each). Blood samples and approximately 5 cm of the ileum were collected from each rat. The plasma was separated by centrifugation and stored at -80 °C until analysis. The intestinal samples were fixed for histopathological analysis and transmission electron microscopy.
Isolation and characterization of BM MSCs
BM MSCs were isolated from the femur and tibia of male Sprague-Dawley rats (100-120 g). Red blood cells were lysed using 0.1 mol/L NH4Cl, and the remaining cells were washed, resuspended, and cultured for 4 wk in DMEM/F12 (Gibco, Carlsbad, CA, United States) containing 100 U/mL penicillin, 100 mg/mL streptomycin, and 15% fetal bovine serum. BM MSCs were cultured in an incubator at 37 °C, 5% CO2 with saturated humidity. The medium was changed every 72 h.
When the third-passage cells reached 80% confluence, the cells were trypsinized, washed, centrifuged, and resuspended at 1 × 107 cells/mL in phosphate-buffered saline (PBS). BM MSCs were stained using antibodies against CD29, CD90, RT1A, CD45 and RT1B (Biolegend, San Diego, CA, United States) and CD34 (Santa Cruz Biotechnology, Santa Cruz, CA, United States). They were analyzed by flow cytometry (FACSCalibur; BD, Alaska, MN, United States). The proportion of CD29-, CD90- and RT1A-positive cells, and CD34-, CD45- and RT1B-negative cells was > 98% (Figure 1A). BM MSCs were also confirmed as plastic-adherent cells with a spindle-shaped morphology under standard culture conditions by microscopy (Figure 1B). The purity of BM MSCs was > 95%.
Figure 1.
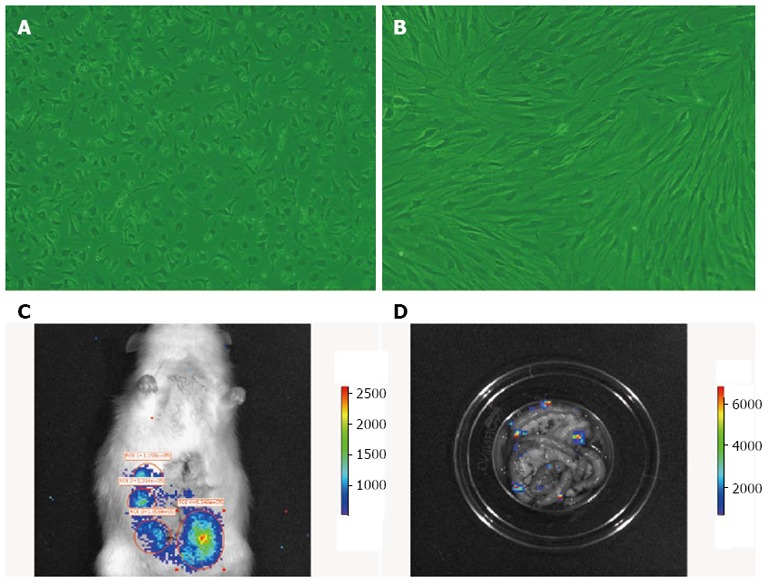
Morphology of bone-marrow mesenchymal stem cells in vitro, and in vivo cell tracing of bone-marrow mesenchymal stem cells colonization in rat intestine. A: First-passage bone-marrow mesenchymal stem cells (BM MSCs); B: Third-passage BM MSCs (× 200); C: Homing of fluorescently labeled BM MSCs (B16-F10-Luc-G5) to the rat intestine 6 h after transplantation; D: After the intestine was removed and washed repeatedly, fluorescently labeled BM MSCs were still observed, confirming the cells homed to the intestine and survived.
Detection of donor BM MSCs in recipient intestines
BM MSCs (1 × 107 cells) were incubated with 3.5 μg/mL 1,1’-dioctadecyl-3,3,3’,3’-tetramethyl indoctricarbocyanine lodide (DIR) in 10 mL PBS containing 0.5% ethanol for 30 min at 37 °C, and washed twice with PBS. Sprague-Dawley rats weighing 180-200 g were anesthetized using 5% chloral hydrate, subjected to a midline laparotomy, and 1 × 107 labeled BM MSCs suspended in 1 mL PBS were injected into the intestinal submucosa at five different points. At 2, 6, 24, 72 and 144 h later, luciferin was injected abdominally using a 25-gauge needle and, 7-8 min later, the animals were anesthetized and imaged using a high-sensitivity optical molecular imaging and high-resolution digital X-ray system (IVIS Lumina II, Alameda, CA, United States).
Histological measurement of intestinal mucosal injury
Serial 2-cm samples were taken from the terminal ileum and fixed with 10% neutral formalin. Tissues were processed, embedded, and stained with hematoxylin and eosin. Three paraffin sections were prepared from each tissue sample. Two pathologists who were blinded to the source of the slides analyzed. The degree of histopathological changes was graded semiquantitatively using the histological injury scale previously described by Chiu et al[25], as follows: 0, normal mucosal villi; 1, development of a subepithelial space, usually at the apex of the villi with capillary congestion; 2, extension of the subepithelial space with moderate lifting of the epithelial layer from the lamina propria; 3, massive epithelial lifting down the sides of the villi and ulceration at the villous tips; 4, denuded villi with dilated capillaries and increased cellularity of the lamina propria; and 5, degradation and disintegration of the lamina propria, hemorrhage, and ulceration. A minimum of six randomly chosen fields from each rat were evaluated and averaged to determine the degree of mucosal damage.
Serum D-lactate, diamine oxidase and TNF-α assay
The serum levels of TNF-α, D-lactate and diamine oxidase (DAO) were determined using enzyme linked immunosorbent assay kits (R and D Systems) according to the manufacturer’s protocol.
Detection and observation of intestinal mucosal ultrastructure
Ultrathin (70-nm) intestinal sections were prepared using standard techniques and examined using a transmission electron microscope (Hitachi H-600, Tokyo, Japan).
Immunohistochemical detection of ZO-1 in frozen tissue sections
Frozen intestinal tissue sections (5 μm) were fixed on glass slides by incubation in acetone for 10 min at 4 °C, and then incubated with 3% H2O2 for 20 min at room temperature, blocked in goat serum for 30 min at 37 °C, and then indirectly immunolabeled with a rabbit anti-mouse polyclonal ZO-1 antibody (1:50; Santa Cruz Biotechnology) using an ABC kit at 4 °C overnight (Takara, Dalian, China), according to the manufacturer’s instructions. For the negative controls, the primary antibody was replaced with PBS. The sections were then incubated in biotinylated goat anti-rabbit IgG (1:300 in PBS; Histostain-Plus kit, Zymed Laboratories, South San Francisco, CA, United States) for 2 h at room temperature, rinsed in PBS, rinsed in distilled water, then the staining was developed using 3,3’-diaminobenzidine and the sections were counterstained using hematoxylin.
Western blotting of tissue ZO-1 content
Intestinal tissue samples were homogenized in lysis buffer [20 mmol/L Tris-HCl (pH 7.5), 1% Triton × 100, 0.2 mol/L NaCl, 2 mmol/L EDTA, 2 mmol/L EGTA, 1 mol/L DTT and 2 mol/L aprotinin]. The protein samples (50 μg) were electrophoresed on 8% SDS-PAGE gels, transferred to nitrocellulose membranes, blocked with non-fat dried milk in TBS containing 0.05% Tween-20 (TTBS) for 1 h at room temperature, and incubated with a rabbit anti-mouse polyclonal ZO-1 antibody (1:400; Santa Cruz Biotechnology) at 4 °C overnight. After three washes in TTBS, the membranes were incubated with alkaline-phosphatase-labeled goat anti-rabbit IgG (1:2000; Santa Cruz Biotechnology) for 2 h at room temperature. The bands were visualized using α-dianisidine and β-naphthyl acid phosphate (Sigma, St Louis, MO, United States).
Statistical analysis
SPSS version 10.0 (SPSS, Chicago, IL, United States) was used for the statistical analysis. Normally distributed data were shown as the mean ± SD. Different groups of data were compared by analysis of variance (ANOVA). The degree of relationship between TNF-α and the Chiu risk score was evaluated by a bivariate correlation. The results was statistically significant when P < 0.05, and was highly significant when P < 0.01.
RESULTS
Culture of BM MSCs
The cells were confirmed as BM MSCs based on their spindle-shaped morphology, adherence to plastic (Figure 1A and 1B), ability to differentiate hepatocytes in vitro (data not shown), and flow cytometry results (Figure 2). Most of the third-passage adherent cells were positive for CD90, CD29 and RT1A, and negative for the MSC markers, CD45, CD34 and RT1B. Furthermore, over the first three passages, the percentage of CD90+ and CD45- cells rapidly increased from 80% to > 98% (Figure 2), which was in agreement with a previous study[26].
Figure 2.
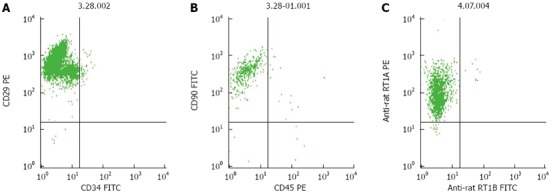
Flow cytometric analysis of third-passage bone-marrow mesenchymal stem cells. A: The proportion of CD29-positive and CD34-negative cells was approximately 96%; B: The proportion of CD90-positive and CD450-negative was approximately 98%; C: The proportion of RT1A-positive and RT1B-negative cells was > 98%.
Confirmation of donor-derived BM MSCs
Labeled BM MSCs homing to the intestine were visible 2, 6, 24, 72 and 144 h after transplantation (Figure 1C). After the intestine was washed repeatedly with PBS, the labeled BM MSCs were still visible (Figure 1D), which indicated that the transplanted BM MSCs could home to the intestine and survive long term.
Histopathological examination
The histopathological findings showed intact villi with no epithelial disruption in the Sham groups. In the NS + I/R injury group, massive destruction of the villi and inflammatory cell infiltration into the lamina propria were evident. In contrast, intestinal samples in the BM MSCs + I/R injury group (BM MSCs group) had significantly less damage in the small intestine. Major pathological changes observed were slight hyperemia, edema, and inflammatory cell infiltration in the mucosa and submucosa, with most of the intestinal villi intact (Figures 3 and 4). Chiu’s grade scores of the three groups are shown in Table 1.
Figure 3.
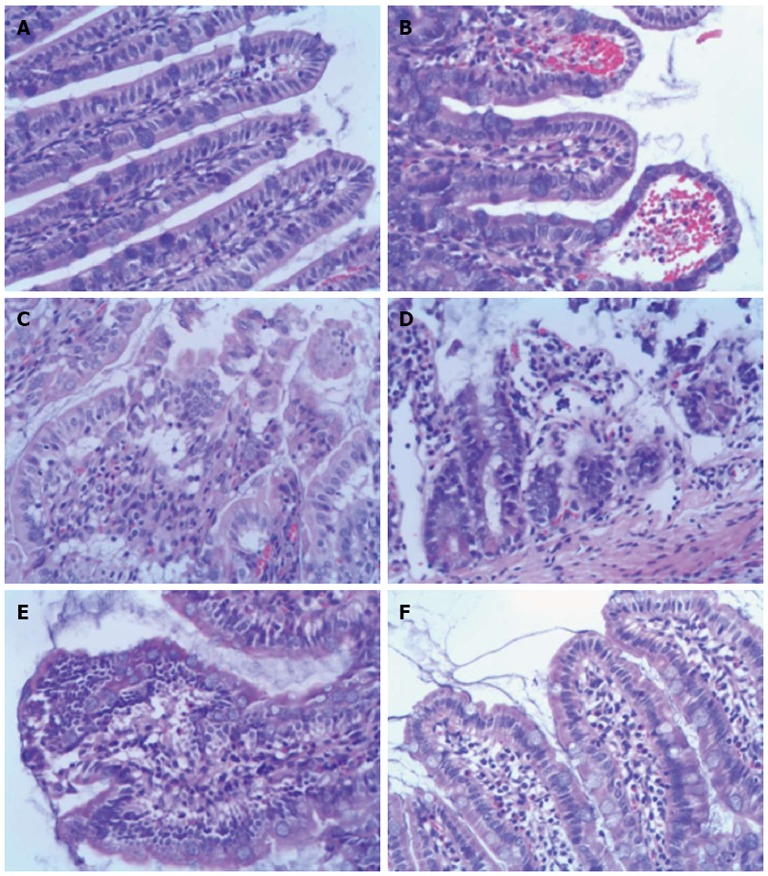
Histopathology of ileum sections at different time points after intestinal ischemia/reperfusion injury (hematoxylin and eosin, × 200). A: Sham group; the intestine showed normal villous architecture and glands, with no vascular congestion; B: In the ischemia/reperfusion (I/R) injury 2 h group, the degree of intestinal mucosa injury was marked with massive epithelial lifting down the sides of the villi and ulceration at the villous tips; C: At 6 h, there was intestinal mucosa degradation and disintegration of the lamina propria, hemorrhage, and ulceration; D: At 24 h, the damaged mucosa showed denuded villi with dilated capillaries and increased cellularity of the lamina propria; E: In the I/R injury group at 72 h, there was massive epithelial lifting down the sides of the villi and ulceration at the villous tips; F: However, at 144 h, the damaged mucosa had recovered.
Figure 4.
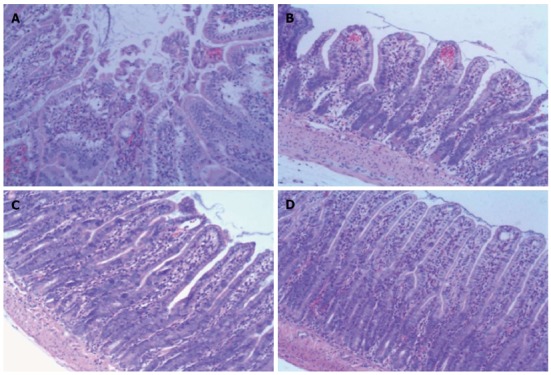
Histopathology of ileum sections of different groups at 6 h after ischemia/reperfusion injury (hematoxylin and eosin, × 100). A: In the ischemia/reperfusion (I/R) injury group there was marked intestinal mucosa injury at 6 h, with intestinal mucosa degradation and disintegration of the lamina propria, hemorrhage, and ulceration. B: In the bone-marrow mesenchymal stem cells + I/R injury group at 6 h, the damaged mucosa had recovered and there was extension of the subepithelial space with moderate lifting of the epithelial layer from the lamina propria, massive epithelial lifting down the sides of the villi, and ulceration at the villous tips. C and D: In the anti-tumor necrosis factor (TNF)-α + I/R injury group and the anti-TNF-αR1-IgG + I/R injury group at 6 h, the damaged mucosa had almost recovered to resemble that in the Sham control group.
Table 1.
Grade of intestinal mucosal injury after intestinal ischemia/reperfusion in the different groups
| Group |
Chiu’s score |
||||
| 2 h | 6 h | 24 h | 72 h | 144 h | |
| Sham group | 0.5 ± 0.8 | 0.5 ± 0.5 | 0.7 ± 0.8 | 0.3 ± 0.8 | 0.7 ± 0.8 |
| NS + I/R injury | 18.0 ± 3.9b | 27.8 ± 2.3b | 23.7 ± 5.2b | 19.0 ± 3.8b | 11.0 ± 3.0b |
| BM MSCs + I/R injury | 13.7 ± 3.3bd | 15.2 ± 2.9bd | 13.7 ± 1.5bd | 8.3 ± 2.3bd | 5.8 ± 2.6bd |
| Anti-TNF-α + I/R injury | 6.5 ± 1.2df | ||||
| Anti-TNF-αR1-IgG + I/R injury | 7.7 ± 1.2df | ||||
All values are mean ± SD (n = 6, three paraffin sections were prepared from each tissue sample. Two pathologists who were blinded to the source of the slides analyzed each slide);
P < 0.01 vs the Sham group,
P < 0.01 vs the saline (NS) + ischemia/reperfusion (I/R) injury group,
P < 0.01 vs bone-marrow mesenchymal stem cells (BM MSCs) + I/R injury group. TNF-α: Tumor necrosis factor-α.
Serum D-lactate and DAO
The levels of D-lactate and DAO significantly increased, reaching a peak at 6 h in the NS + I/R injury and BM MSCs + I/R injury groups, compared to the Sham group. This confirmed that I/R injury increased intestinal permeability.
The serum D-lactate and DAO levels in the NS + I/R injury group increased more than twofold compared to the Sham group at 2, 6 and 24 h after I/R (P < 0.01). However, the serum DAO levels in the BM MSCs + I/R injury group were significantly lower than in the NS + I/R group at 2, 6 and 24 h, and the serum D-lactate levels in the BM MSCs + I/R injury group were significantly lower than in the NS + I/R group at 6 and 24 h. At 6 h, the serum D-lactate and DAO levels in the anti-TNF-α + I/R injury and anti-TNF-αR-IgG + I/R injury groups were lower than in the NS + I/R injury group (P < 0.01; Table 2). At 72 and 144 h, the serum DAO levels in the NS + I/R and BM MSCs + I/R injury groups had reduced, but remained higher than in the Sham group, whereas D-lactate levels were not significantly different in the NS + I/R, BM MSCs + I/R and Sham groups at 144 h.
Table 2.
Serum levels of diamine oxidase and D-lactate in a rat model of ischemia/reperfusion injury
| Group |
DAO (IU/mL) |
D-lactate (μg/mL) |
||||||||
| 2 h | 6 h | 24 h | 72 h | 144 h | 2 h | 6 h | 24 h | 72 h | 144 h | |
| Sham group | 2.08 ± 0.16 | 2.08 ± 0.75 | 2.03 ± 0.46 | 1.95 ± 0.36 | 2.24 ± 0.62 | 5.11 ± 0.24 | 5.30 ± 0.0.44 | 5.38 ± 0.45 | 5.46 ± 0.42 | 5.54 ± 0.69 |
| NS + I/R injury | 11.04 ± 0.59b | 14.58 ± 2.01b | 7.36 ± 1.28b | 5.12 ± 0.66b | 3.91 ± 0.59b | 14.73 ± 1.37b | 17.85 ± 1.86b | 12.73 ± 0.56b | 8.22 ± 1.78 | 6.54 ± 1.04 |
| BM MSCs + I/R injury | 8.16 ± 0.71bd | 11.36 ± 1.89bd | 5.04 ± 1.04bd | 4.93 ± 0.69b | 3.55 ± 0.59a | 12.62 ± 2.24b | 13.40 ± 1.53ab | 9.80 ± 1.20bd | 6.82 ± 0.80b | 6.44 ± 0.83 |
| Anti-TNF-α + I/R injury | - | 7.99 ± 1.70d | - | - | - | - | 12.77 ± 1.44d | - | - | - |
| Anti-TNF-αR1-IgG + I/R injury | - | 7.83 ± 1.28d | - | - | - | - | 12.16 ± 1.47d | - | - | - |
All values are mean ± SD (n = 6).
P < 0.05,
P < 0.01 vs Sham group;
P < 0.01 vs the saline (NS) + ischemia/reperfusion (I/R) injury group.
These data indicate that serum DAO is a more sensitive marker of intestinal permeability than D-lactate, and also that the administration BM MSCs or TNF-α blockade reduced the permeability of the small intestine and accelerated the recovery of intestinal barrier function after I/R injury in rats.
Ultrastructural characteristics of the intestinal mucosa
Compared to the Sham group, we observed obvious ultrastructural changes in the intestinal mucosa after I/R injury in the rats from the NS + I/R group. Epithelial cell microvilli were sparsely distributed, disarranged and distorted, and the epithelial cells were swollen or shrunken. The mitochondrial matrices were swollen, cristae were broken, and numerous TJs were disrupted. There was no disruption of TJs in the BM MSCs + I/R injury group, and only swelling of the epithelial cells was observed. The ultrastructural pathological changes in the groups treated with anti-TNF-α and anti-TNF-αR-IgG were also less severe than in the NS + I/R injury group (Figure 5).
Figure 5.
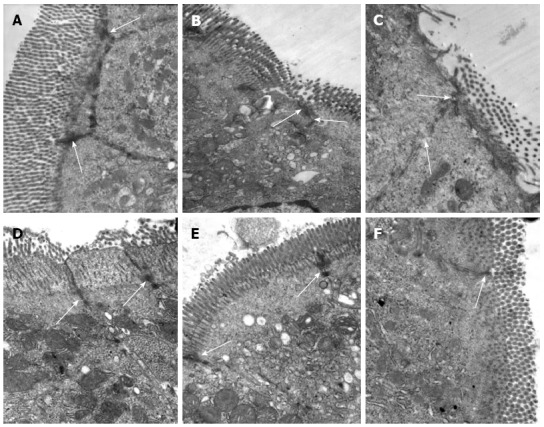
Bone-marrow mesenchymal stem cells and tumor necrosis factor-α blockade prevent ultrastructural pathological damage after intestinal ischemia/reperfusion injury. Transmission electron microscopy of the rat intestine after ischemia/reperfusion (I/R) injury. A: Epithelial cells and tight junctions (TJs) (arrows) were intact in the Sham group, × 30000; B: At 2 h after I/R injury, epithelial cells were swollen and shrunken, microvilli and organelles were normal, and TJs (arrows) were disrupted in the saline (NS) + I/R injury group, × 25000; C: At 6 h after I/R injury in the NS + I/R injury group, some microvilli were loose, TJs (arrows) were disrupted, and organelles were swollen with reduced electron density, × 30000; D: At 6 h after I/R injury and administration of bone-marrow mesenchymal stem cells, the microvilli and mitochondria of the endothelial cells were almost normal and TJs (arrows) were not disrupted, × 30000; E and F: TJs (arrows) between endothelial cells were intact 6 h after I/R injury in rats that received anti-tumor necrosis factor (TNF)-α IgG + I/R antibody (E, × 25000) or anti-TNF-α R1 antibody; (F, × 30000) before I/R injury.
Expression of ZO-1 protein
Immunohistochemical analysis revealed strong ZO-1 expression in the intestinal tissue of the Sham group. In the intestinal tissue of the NS + I/R injury group, ZO-1 was expressed at low levels 2 h after injury, slightly increased at 6 and 24 h, and by 72 h, ZO-1-positive signals were detected throughout the entire intestine (Figure 6). Western blot analysis confirmed that ZO-1 expression decreased more significantly in the NS + I/R group than the BM MSCs + I/R injury group, particularly at 6 h (Figure 7). Consistent with the immunohistochemical results, western blotting indicated that ZO-1 expression was significantly higher in the BM MSCs + I/R injury group and the two antibody-treated groups at 6 h than in the NS + I/R injury group (Figure 8).
Figure 6.
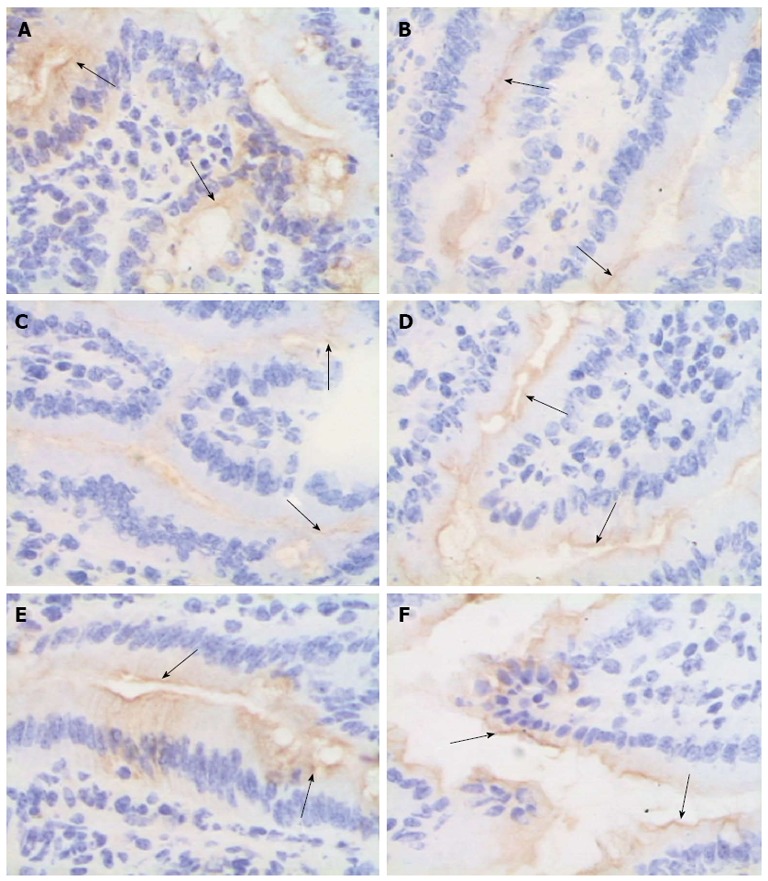
Bone-marrow mesenchymal stem cells and tumor necrosis factor-α blockade attenuate zona occludens 1 downregulation after intestinal ischemia/reperfusion injury. The mucosal tissue sections after ischemia/reperfusion (I/R) injury were immunohistochemically labeled for zona occludens 1 (ZO-1) (brown) and counterstained with hematoxylin (blue). A: Sham group; B and C: In the normal saline + I/R injury group, decreased ZO-1 staining (arrows) was observed in the epithelial cells 2 h (B) and 6 h (C) after I/R; D-F: ZO-1 was not obviously affected in the bone-marrow mesenchymal stem cells + I/R injury group (D), anti-tumor necrosis factor (TNF)-α + I/R injury group (E), or anti-TNF-α R1 antibody + I/R injury group (F) at 6 h; original magnification, × 400.
Figure 7.
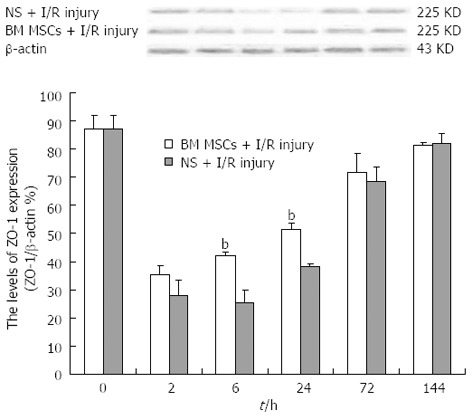
Bone-marrow mesenchymal stem cells attenuate zona occludens 1 downregulation after intestinal ischemia/reperfusion injury. Representative western blots and quantification of zona occludens 1 (ZO-1) protein expression in the intestinal mucosa. ZO-1 expression was significantly lower in the saline (NS) + ischemia/reperfusion (I/R) injury group than the bone-marrow mesenchymal stem cells (BM MSCs) + I/R injury group at 6 h (25.35 ± 4.58% vs 42.32 ± 1.26%; P < 0.01). Actin was used as a loading control. Values are shown as the mean ± SD (n = 3 rats per group); bP < 0.01 BM MSCs +I/R injury group vs the NS + I/R injury group [one-way ANOVA followed by the least significant difference (LSD) test].
Figure 8.
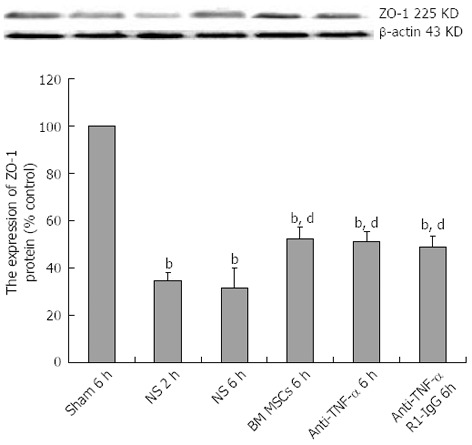
Bone-marrow mesenchymal stem cells and tumor necrosis factor-α blockade attenuate zona occludens 1 downregulation after intestinal ischemia/reperfusion injury. Representative western blots and quantification of zona occludens 1 (ZO-1) protein expression in the intestinal mucosa. Actin was used as a loading control. Values are the mean ± SD (n = 3 rats per group); bP < 0.01 vs Sham group; dP < 0.01 vs the saline (NS) + ischemia/reperfusion (I/R) injury group (one-way analysis of variance followed by the LSD test). BM MSCs: Bone-marrow mesenchymal stem cells; Anti-TNF: Anti-tumor necrosis factor.
Effect of I/R injury on serum TNF-α levels
Compared to the Sham group, the serum TNF-α levels increased significantly, peaking at 6 h, in the NS + I/R injury group. The serum level of TNF-α was significantly lower in the BM MSCs+ I/R injury group at 6 and 24 h than the NS + I/R injury group (P < 0.05, Table 3). The morphological abnormalities after intestinal I/R injury were positively correlated with serum TNF-α levels (Table 4).
Table 3.
Serum levels of tumor necrosis factor-α in a rat model of ischemia/reperfusion injury
| Group |
Tumor necrosis factor-α (pg/mL) |
||||
| 2 h | 6 h | 24 h | 72 h | 144 h | |
| Sham group | 87.07 ± 6.47 | 84.45 ± 4.18 | 87.60 ± 6.25 | 83.91 ± 6.67 | 86.54 ± 5.62 |
| NS + I/R injury | 226.32 ± 11.94b | 332.95 ± 49.03d | 221.80 ± 16.06d | 180.87 ± 8.63b | 134.84 ± 7.18b |
| BM MSCs + I/R injury | 214.21 ± 17.77b | 236.76 ± 20.66bd | 190.39 ± 4.24a,d | 177.00 ± 2.52b | 91.67 ± 3.84b |
All values are mean ± SD (n = 6).
P < 0.01 vs Sham group;
P < 0.01 vs the saline (NS) + ischemia/reperfusion (I/R) injury group. BM MSCs: Bone-marrow mesenchymal stem cells.
Table 4.
Serum levels of tumor necrosis factor-α and intestinal mucosal injury grade after intestinal ischemia/reperfusion
| Group |
TNF-α (pg/mL) |
Chiu’s grade scores |
||||||||
| 2 h | 6 h | 24 h | 72 h | 144 h | 2 h | 6 h | 24 h | 72 h | 144 h | |
| Saline + I/R injury group | 243.27 | 290.41 | 210.51 | 186.58 | 132.55 | 19 | 28 | 18 | 14 | 10 |
| 212.21 | 286.35 | 241.42 | 189.44 | 125.39 | 14 | 27 | 21 | 14 | 8 | |
| 221.76 | 283.66 | 203.19 | 174.82 | 137.45 | 17 | 30 | 17 | 13 | 10 | |
| 235.42 | 295.87 | 210.37 | 175.76 | 129.57 | 18 | 28 | 19 | 13 | 9 | |
| 229.54 | 345.78 | 226.96 | 189.26 | 145.57 | 18 | 29 | 20 | 14 | 12 | |
| 215.74 | 395.61 | 238.32 | 169.34 | 138.51 | 15 | 30 | 21 | 12 | 10 | |
| Spearman | 0.947 | 0.931 | 0.961 | 0.971 | 0.956 | |||||
| P | 0.004 | 0.011 | 0.002 | 0.001 | 0.003 | |||||
The degree of relationship between tumor necrosis factor (TNF)-α and the Chiu risk score was evaluated by bivariate correlation. The results were statistically significant when P < 0.05, and was highly significant when P < 0.01. I/R: Ischemia/reperfusion.
DISCUSSION
I/R injury to the gut is a common event in a variety of clinical conditions, such as trauma, burn injuries, septic shock, heart and aortic surgery, and liver and small bowel transplantation[27,28]. Intestinal I/R results in edema, apoptosis, necrosis of epithelial cells, disruption of mucosal integrity and small intestine function, which in turn increases mucosal and vascular permeability, bacterial translocation, as well as the risk of systemic inflammation response syndrome, multiple organ dysfunction and death[29,30]. Until recently, no effective treatments existed for intestinal I/R injury. Research has suggested that BM MSCs could possibly play a role in the treatment of I/R injury in the heart, kidney and brain[31-33]; however, studies of the effects of BM MSCs in intestinal disorders are scarce. In the current study, the therapeutic potential of BM MSCs was evaluated in an experimental rat model of I/R injury, which led to disruption of intestinal mechanical barrier function. The results of this study suggest that BM MSCs can effectively reduce both the intestinal permeability and pathological damage associated with I/R injury. BM MSCs have the potential for multidirectional differentiation. They participate in colonic mucosal regeneration[34]. In this study, intestinal I/R injury lead to necrosis and the loss of a large number of intestinal epithelial cells. BM MSCs could reduce I/R injury and protect the intestine.
Stem cell homing processes are thought to play a crucial role in the success of cell therapy for organ function disorders. Intravenous or intra-arterial infusions of BM MSCs often result in the entrapment of the administered cells in organ capillary beds, especially in the lung and the liver[35]. The transplantation of BM MSCs by intravenous or intra-arterial routes usually results in a low engraftment rate; therefore, increasing the number of MSCs within the injured area would improve the efficacy of cell therapy. Zhang et al[36] used gene-modified MSCs to enhance the homing rate of BM MSCs to the irradiated intestine by 20% using an intravenous delivery route. However, using viral vectors to transfect MSCs may decrease the viability of MSCs. In this study, we directly injected MSCs into the wall of the intestine after I/R injury, which significantly increased the homing of MSCs into the I/R damaged intestinal mucosa. This indicated that the direct injection of BM MSCs into the intestine may provide a better method to enhance the homing rate.
The intestinal mucosal barrier is composed of mucosal fluid, microvilli, epithelial mucosal cell TJs, and other special structures. TJs are the most important structures in the mucosal barrier. The mechanisms responsible for intestinal I/R injury include cytotoxic effects and alterations in the structure of the intestinal mucosa[15]. However, few studies have examined the intestinal mucosa and TJ ultrastructure during I/R injury, and the role and mechanism of action of BM MSCs in intestinal I/R injury are unclear. In the present study, we found that severe intestinal mucosal damage occurred 2, 6 and 24 h after I/R injury. The morphological alterations to the intestinal mucosa included the shedding of epithelial cells, fracturing of villi, fusion of adjacent villi, mucosal atrophy and edema. Disruption of TJs between enterocytes, and damage to the mitochondria and endoplasm were also observed. Although damage to the intestinal mucosa plays a significant role in the permeability of the intestine, the mechanisms which cause this damage are poorly characterized. Moreover, we observed that the intestinal permeability increased 2, 6 and 24 h after I/R injury, with simultaneous disruption in TJ integrity. Additionally, the administration of BM MSCs significantly attenuated the histological damage due to I/R injury (Figure 4) and reduced intestinal permeability (Table 2), compared with the NS + I/R injury group. Therefore, we hypothesized that changes in intestinal permeability may occur due to the disruption of TJs between intestinal mucosa epithelial cells.
To understand the mechanism of TJ disruption, we investigated the expression of ZO-1. ZO-1 was the first TJ-related protein to be identified[37], and it connects the actin cytoskeleton to the transmembrane occludin proteins[38]. ZO-1 plays a vital role in the maintenance of intestinal mucosal barrier integrity and TJs during pathological insults[39]. In this study, ZO-1 expression in the intestinal mucosa significantly decreased after I/R injury; thus, we concluded that decreased ZO-1 expression lead to TJ disruption and possibly increased gut permeability.
Next, we examined the mechanism of TJ disruption and reduced ZO-1 protein expression during I/R injury. We observed that TNF-α increased at 2, 6 and 24 h after I/R injury, and correlated with ZO-1 downregulation and TJ disruption. The pathophysiological processes of I/R injury in vivo are complex, and it is thought that TNF-α may play an important role. Inflammation involves the sequential activation of signaling pathways which result in the production of pro- and anti-inflammatory mediators during I/R injury. Amongst the proinflammatory mediators, the TNF-α and TNF-αR1 systems play central roles in the physiological regulation of intestinal barrier function[40,41], and both TNF-α and interferon (IFN)-γ can induce intestinal epithelial barrier dysfunction[42]. Some cytokines can induce endocytosis[43] and internalization of epithelial TJ proteins[44]. In mice with fulminant hepatic failure, reduced expression of occludin in intestinal epithelial cells was linked to increased TNF-α production[4]. TNF-α can also induce an increase in Caco-2 cell TJ permeability via nuclear factor-κB activation, leading to downregulation of ZO-1 protein expression and altered junctional localization[38,45]. We hypothesize that TNF-α acts as an initiator, which can induce expression of other cytokines such as IL-6 and IFN-γ, which then initiate and aggravate the development of I/R injury, and disrupt intestinal TJs.
After the transplantation of BM MSCs, the serum TNF-α level significantly decreased, the damaged mucosa recovered, ZO-1 expression increased and intestinal permeability significantly improved. TNF-α is known to inhibit the expression of ZO-1[44], and if the TJs are damaged, intestinal barrier dysfunction will occur. Research has confirmed that BM MSCs can inhibit the generation of TNF-α in dendritic cells in vitro[46,47], and therefore we hypothesized that BM MSCs could repair intestinal I/R injury by inhibiting the release of TNF-α. In order to further study the role of TNF-α, we used anti-TNF-α and anti-TNFR antibodies. The TNF-α antibody neutralizes TNF-α, whereas the anti-TNFR antibody blocks the binding of TNF-α to the TNF-α receptor. TNF-α blockade significantly decreased the severity of I/R injury, which indicates that TNF-α is an important mediator of intestinal mucosa damage during I/R injury. These findings suggest that I/R injury increases TNF-α, leading to downregulation of ZO-1 protein expression, whereas BM MSCs can inhibit production of TNF-α, leading to increased expression of ZO-1 and reduced intestinal mucosa damage. These effects were observed over a relatively short observation period, and long-term studies are required to elucidate if TNF-α exerts long-lasting effects during I/R injury.
In summary, this study demonstrates that the submucosal infusion of BM MSCs decreased intestinal permeability and preserved intestinal mechanical barrier function after I/R injury in rats, in a mechanism linked to reduced serum TNF-α levels and the increased expression of the intestinal TJ protein ZO-1. Future studies using exogenous or autologous BM MSCs to prevent or modulate intestinal I/R injuries are required to assess the clinical potential of BM MSCs. The mechanisms by which BM MSCs and TNF-α blockade protect against I/R-induced disruption of intestinal barrier function remain to be further investigated.
Disruption of the intestinal mucosa and the consequent increase in permeability after I/R injury may be due to reduced levels of the TJ-associated protein, ZO-1. BM MSCs restored the epithelial structure, promoted the recovery of intestinal permeability, increased ZO-1 protein expression and protected against intestinal I/R injury. TNF-α plays an important role in the ability of BM MSCs to protect against intestinal I/R injury, as the epithelial structure remained normal, and changes in intestinal permeability and ZO-1 protein expression were reduced when rats were treated with anti-TNF-α IgG antibody or anti-TNF-α R1 antibodies before I/R injury. This study confirms that high levels of TNF-α damage TJs and downregulate ZO-1 protein expression in vivo. The mechanism of TNF-α-induced change during I/R injury is complex and requires further study.
COMMENTS
Background
Digestive organ transplantation and other abdominal surgical procedures can result in different degrees of intestinal ischemia/reperfusion (I/R) injury, which can delay patient recovery and lead to systemic organ failure. Therefore, intestinal I/R injury is an important clinical issue. Bone-marrow mesenchymal stem cells (BM MSCs) can protect against I/R injury; however, the mechanism is unclear. Although previous studies have provided an insight into the molecular structure of tight junctions (TJs), much less is known about TJ functionality under physiological or pathophysiological conditions. Few studies have described the intestinal mucosa ultrastructure or changes in TJs during intestinal I/R injury. In this study, the authors used a rat model of intestinal I/R injury to investigate the effect of BM MSCs on the intestinal mucosa ultrastructure, with an emphasis on the mechanisms of intestinal barrier dysfunction.
Research frontiers
In this study, the authors demonstrated that the submucosal infusion of BM MSCs decreased intestinal permeability and preserved intestinal mechanical barrier function after I/R injury in rats, in a mechanism linked to reduced serum tumor necrosis factor (TNF)-α levels and the increased expression of the intestinal TJ protein zona occludens (ZO)-1. Altered serum TNF-α levels play an important role in the ability of BM MSCs to protect against intestinal I/R injury.
Innovations and breakthroughs
Recent reports have highlighted the importance of BM MSCs reducing intestinal I/R injury in rats. Although previous studies have provided an insight into the molecular structure of TJs, much less is known about TJ functionality under physiological or pathophysiological conditions. Few studies have described the intestinal mucosa ultrastructure or changes in TJs during intestinal I/R injury. This is believed to be the first study to report that BM MSCs reduce rat intestinal I/R injury, ZO-1 downregulation, and TJ disruption via a TNF-α-regulated mechanism.
Applications
By understanding how BM MSCs reduce rat intestinal I/R injury, this study may represent a future strategy for therapeutic intervention in the treatment of patients with digestive organ transplantation and other abdominal surgical procedures that result in different degrees of intestinal I/R injury, which can delay patient recovery and lead to systemic organ failure.
Terminology
TJs are the main structures responsible for restricting the paracellular movement of compounds across the intestinal mucosa. Structurally, TJs are composed of cytoplasmic proteins, including ZO-1-3 and two distinct transmembrane proteins, occludin and claudin, which are linked to the actin-based cytoskeleton. ZO-1, as a scaffold for the organization of transmembrane TJ proteins, also recruits various signaling molecules and the actin cytoskeleton to TJs.
Peer review
This paper shows the impact of BM MSCs on rat intestinal I/R injury. This study will be of interest and the paper is clearly written.
Footnotes
Supported by Natural Science Foundation of China, No. 81270528; the Natural Science Foundation of Tianjin, No. 08JCYBJC08400, No. 11JCZDJC27800 and No. 12JCZDJC25200; the Technology Foundation of Health Bureau in Tianjin, No. 2011KY11
P- Reviewer Fujino Y S- Editor Zhai HH L- Editor Kerr C E- Editor Zhang DN
References
- 1.Muto Y, Nouri-Aria KT, Meager A, Alexander GJ, Eddleston AL, Williams R. Enhanced tumour necrosis factor and interleukin-1 in fulminant hepatic failure. Lancet. 1988;2:72–74. doi: 10.1016/s0140-6736(88)90006-2. [DOI] [PubMed] [Google Scholar]
- 2.Wang JH, Redmond HP, Watson RW, Bouchier-Hayes D. Role of lipopolysaccharide and tumor necrosis factor-alpha in induction of hepatocyte necrosis. Am J Physiol. 1995;269:G297–G304. doi: 10.1152/ajpgi.1995.269.2.G297. [DOI] [PubMed] [Google Scholar]
- 3.Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol. 1995;146:1220–1234. [PMC free article] [PubMed] [Google Scholar]
- 4.Song HL, Lv S, Liu P. The roles of tumor necrosis factor-alpha in colon tight junction protein expression and intestinal mucosa structure in a mouse model of acute liver failure. BMC Gastroenterol. 2009;9:70. doi: 10.1186/1471-230X-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corredor J, Yan F, Shen CC, Tong W, John SK, Wilson G, Whitehead R, Polk DB. Tumor necrosis factor regulates intestinal epithelial cell migration by receptor-dependent mechanisms. Am J Physiol Cell Physiol. 2003;284:C953–C961. doi: 10.1152/ajpcell.00309.2002. [DOI] [PubMed] [Google Scholar]
- 6.Poddar U, Thapa BR, Prasad A, Sharma AK, Singh K. Natural history and risk factors in fulminant hepatic failure. Arch Dis Child. 2002;87:54–56. doi: 10.1136/adc.87.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 8.Kassem M, Kristiansen M, Abdallah BM. Mesenchymal stem cells: cell biology and potential use in therapy. Basic Clin Pharmacol Toxicol. 2004;95:209–214. doi: 10.1111/j.1742-7843.2004.pto950502.x. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 10.Snykers S, De Kock J, Tamara V, Rogiers V. Hepatic differentiation of mesenchymal stem cells: in vitro strategies. Methods Mol Biol. 2011;698:305–314. doi: 10.1007/978-1-60761-999-4_23. [DOI] [PubMed] [Google Scholar]
- 11.Ni WF, Yin LH, Lu J, Xu HZ, Chi YL, Wu JB, Zhang N. In vitro neural differentiation of bone marrow stromal cells induced by cocultured olfactory ensheathing cells. Neurosci Lett. 2010;475:99–103. doi: 10.1016/j.neulet.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 12.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 13.Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lönnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 14.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 15.Jiang H, Qu L, Li Y, Gu L, Shi Y, Zhang J, Zhu W, Li J. Bone marrow mesenchymal stem cells reduce intestinal ischemia/reperfusion injuries in rats. J Surg Res. 2011;168:127–134. doi: 10.1016/j.jss.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 17.van den Berk LC, Jansen BJ, Siebers-Vermeulen KG, Roelofs H, Figdor CG, Adema GJ, Torensma R. Mesenchymal stem cells respond to TNF but do not produce TNF. J Leukoc Biol. 2010;87:283–289. doi: 10.1189/jlb.0709467. [DOI] [PubMed] [Google Scholar]
- 18.Morita K, Itoh M, Saitou M, Ando-Akatsuka Y, Furuse M, Yoneda K, Imamura S, Fujimoto K, Tsukita S. Subcellular distribution of tight junction-associated proteins (occludin, ZO-1, ZO-2) in rodent skin. J Invest Dermatol. 1998;110:862–866. doi: 10.1046/j.1523-1747.1998.00209.x. [DOI] [PubMed] [Google Scholar]
- 19.Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279:G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 24.González-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol. 2000;11:315–324. doi: 10.1006/scdb.2000.0178. [DOI] [PubMed] [Google Scholar]
- 25.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 26.Harting M, Jimenez F, Pati S, Baumgartner J, Cox C. Immunophenotype characterization of rat mesenchymal stromal cells. Cytotherapy. 2008;10:243–253. doi: 10.1080/14653240801950000. [DOI] [PubMed] [Google Scholar]
- 27.Nowicki PT, Nankervis CA. The role of the circulation in the pathogenesis of necrotizing enterocolitis. Clin Perinatol. 1994;21:219–234. [PubMed] [Google Scholar]
- 28.Grant D, Wall W, Mimeault R, Zhong R, Ghent C, Garcia B, Stiller C, Duff J. Successful small-bowel/liver transplantation. Lancet. 1990;335:181–184. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 29.Deitch EA, Morrison J, Berg R, Specian RD. Effect of hemorrhagic shock on bacterial translocation, intestinal morphology, and intestinal permeability in conventional and antibiotic-decontaminated rats. Crit Care Med. 1990;18:529–536. doi: 10.1097/00003246-199005000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Deitch EA, Bridges W, Berg R, Specian RD, Granger DN. Hemorrhagic shock-induced bacterial translocation: the role of neutrophils and hydroxyl radicals. J Trauma. 1990;30:942–951; discussion 951-952. doi: 10.1097/00005373-199008000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 32.Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, Igarashi P. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003;14:1188–1199. doi: 10.1097/01.asn.0000061595.28546.a0. [DOI] [PubMed] [Google Scholar]
- 33.Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112:42–49. doi: 10.1172/JCI17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valcz G, Krenács T, Sipos F, Leiszter K, Tóth K, Balogh Z, Csizmadia A, Muzes G, Molnár B, Tulassay Z. The role of the bone marrow derived mesenchymal stem cells in colonic epithelial regeneration. Pathol Oncol Res. 2011;17:11–16. doi: 10.1007/s12253-010-9262-x. [DOI] [PubMed] [Google Scholar]
- 35.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Gong JF, Zhang W, Zhu WM, Li JS. Effects of transplanted bone marrow mesenchymal stem cells on the irradiated intestine of mice. J Biomed Sci. 2008;15:585–594. doi: 10.1007/s11373-008-9256-9. [DOI] [PubMed] [Google Scholar]
- 37.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112(Pt 1):137–146. doi: 10.1242/jcs.112.1.137. [DOI] [PubMed] [Google Scholar]
- 39.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 40.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiegs G, Wolter M, Wendel A. Tumor necrosis factor is a terminal mediator in galactosamine/endotoxin-induced hepatitis in mice. Biochem Pharmacol. 1989;38:627–631. doi: 10.1016/0006-2952(89)90208-6. [DOI] [PubMed] [Google Scholar]
- 42.Nakama T, Hirono S, Moriuchi A, Hasuike S, Nagata K, Hori T, Ido A, Hayashi K, Tsubouchi H. Etoposide prevents apoptosis in mouse liver with D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure resulting in reduction of lethality. Hepatology. 2001;33:1441–1450. doi: 10.1053/jhep.2001.24561. [DOI] [PubMed] [Google Scholar]
- 43.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–933. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 45.Song HL, Lu S, Ma L, Li Y, Liu P. Effect of TNF-alpha on tight junctions between the epithelial cells of intestinal mucosal barrier. World Chin J Digestol. 2004;12:1303–1306. [Google Scholar]
- 46.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 47.Weil BR, Markel TA, Herrmann JL, Abarbanell AM, Meldrum DR. Mesenchymal stem cells enhance the viability and proliferation of human fetal intestinal epithelial cells following hypoxic injury via paracrine mechanisms. Surgery. 2009;146:190–197. doi: 10.1016/j.surg.2009.03.031. [DOI] [PubMed] [Google Scholar]


