Abstract
The native stem cell niche is a dynamic and complex microenvironment. Recapitulating this niche is a critical focus within the fields of stem cell biology, tissue engineering, and regenerative medicine and requires the development of well-defined, tunable materials. Recent biomaterial design strategies seek to create engineered matrices that interact with cells at the molecular scale and allow on-demand, cell-triggered matrix modifications. Peptide and protein engineering can accomplish these goals through the molecular-level design of bioinductive and bioresponsive materials. This brief review focuses on engineered peptide and protein materials suitable for use as in vitro neural stem cell niche mimics and in vivo central nervous system repair. A key hallmark of these materials is the immense design freedom to specify the exact amino acid sequence leading to multi-functional bulk materials with tunable properties. These advanced materials are engineered using rational design strategies to recapitulate key aspects of the native neural stem cell niche. The resulting materials often combine the advantages of biological matrices with the engineering control of synthetic polymers. Future design strategies are expected to endow these materials with multiple layers of bi-directional feedback between the cell and the matrix, which will lead to more advanced mimics of the highly dynamic neural stem cell niche.
Keywords: Engineered peptide, Engineered protein, Tissue engineering, Neural stem cell, Stem cell niche, Extracellular matrix
1. The neural stem cell niche: a complex and dynamic microenvironment
The native neural stem cell niche is still largely unknown and highly transient. While certain areas of the body are known to contain stem cells, and within the adult central nervous system (CNS) we are aware of neurogenic regions such as the subventricular zone [20] and dentate gyrus [27], the exact contacts these cells make and their immediate microenvironment is still a poorly defined idea. For neural stem cells (NSCs) specifically, it is proposed that they are highly concentrated around blood vessels where they reside in close contact with both endothelial cells (ECs) and astrocytes [90]. Both ECs and astrocytes produce molecules important to NSC self-renewal and differentiation [74,71]. Adding further complexity, the presence or absence of mature neurons can influence the fate of new NSCs through a feedback loop [19].
The NSC niche, like all tissues, is a constantly changing microenvironment with many factors at play. Soluble factors including cytokines, neurotrophic or growth factors, and differentiation cues are constantly synthesized, secreted, transported, and depleted. In addition, these diffusible, soluble factors may temporarily bind to components of the extracellular matrix (ECM) such as hyaluronin and laminin, thus further modifying the niche. Several components of the ECM present cell-adhesion ligands in different conformations and densities. The density of the matrix itself affects a cell’s ability to migrate, and matrix stiffness has been shown to impact the differentiation of NSCs [48,64,2]. The ECM is constantly being remodeled by NSC neighbors such as glial cells and capillary-lining ECs through matrix synthesis and degradation. Furthermore, the location of cells within these niches impacts their access to molecules even as small as oxygen. Deep tissue may be considered hypoxic in comparison to other tissues, and oxygen tension is a critical cue for stem cell maintenance and differentiation [58]. These dynamic niche processes create an environment in which cells must constantly sense and respond in real time. This series of dynamic feedback is hypothesized to be a critical ingredient to modulate NSC fate.
2. Engineering artificial neural stem cell niches
Recapitulating the stem cell niche is a critical goal of tissue engineers and biologists alike. Ideally, an engineered niche would include both spatial organization and dynamic modulation of cells, soluble factors, and matrix. While biomaterials technologies are being developed to address these needs [51], currently available matrices often lack this level of complexity. Nonetheless, even simplified models of the NSC niche are invaluable as reductionist models of cell–microenvironment interactions and as potential scaffolds for regenerative CNS therapies. This reductionist strategy asserts that not all aspects of the in vivo niche are required to create an in vitro mimic that guides specific cell responses, even while acknowledging that all of these cues are present and likely affecting cells in vivo. In order to study specific features of the NSC environment, many groups have attempted to engineer materials that allow isolation of individual variables, such as stiffness, without varying others, such as the density of ligands for integrin binding. For example, materials with tunable mechanical properties have been engineered to mediate NSC self-renewal and differentiation as well as astrocytic activation and glial scarring [48,64,2]. Such materials can be complex in their design and structure, but also elegantly simple.
In this review, we discuss engineered peptide and protein biomaterials that are designed at the molecular level to mimic critical aspects of the NSC niche. We explain the rationale for using peptide and protein design to create NSC niche mimics, illustrate design strategies to create controlled materials with tunable properties, and highlight recent advances in translating these materials to regenerative therapies. We conclude with a discussion of current efforts and future opportunities to move beyond reductionist approaches and to increase the complexity of peptide-engineered materials. In particular, efforts at making these materials more bio-responsive and bio-inductive by introducing dynamic feedback into the system will be instrumental in furthering fundamental studies and therapeutic applications of cell–niche interactions.
3. Designer peptides and proteins as ECM mimics
Engineered peptide and protein materials provide the advantages of a biological matrix with the control of a synthetic polymer. They could be considered a merger of the two, retaining the bioactivity of harvested protein materials, like collagen and fibrin, with the control and tunability of synthetic, chemically defined materials, such as poly(ethylene glycol) (PEG) and poly(lactic-co-glycolic acid) (PLGA).
Most cell–matrix studies to date have utilized native ECM molecules harvested from tissues, e.g., laminin, fibrin, collagen, and Matrigel. These bioactive materials are often highly cell adhesive; however, they suffer from large batch-to-batch variations in chemical makeup and mechanical properties. Although these materials can be used as either two-dimensional (2D) coatings or 3D hydrogels, they are ill suited for controlled studies of cell–matrix interactions. For example, decreasing the stiffness of a collagen gel can be achieved by decreasing the collagen concentration; however this also results in a decrease of adhesive ligand concentration and an increase in diffusivity, thus confounding the anticipated effects with additional variables. Furthermore, harvested materials, such as Matrigel, are often a mixture of multiple, ill-defined components. As a consequence, Matrigel is unlikely to meet FDA criteria for clinical applications and is a non-ideal culture choice for cells with clinical potential.
Engineered peptide and protein materials are uniquely positioned to meet the desire for a bioactive scaffold that is completely defined and tailorable. Designing these proteins from the molecule up gives users a great deal of flexibility and creativity while also enforcing strict reproducibility. Peptide and protein material design can not only provide specificity in structure, cellular interaction, neighboring protein binding and dynamic events, but can also create additional functionality such as reaching adhesive site densities not possible with native materials [73]. Such molecular-level design leads to new strategies and affords greater control over resulting matrices than using harvested or synthetic materials. Both peptide and protein engineered materials have a polymer backbone made of amino acid sequences that are inherently bioactive and bioresorbable. These sequences can be derived from nature or identified through computational design or high-throughput screening to achieve functionalities beyond what are capable with native materials. Typically these bioactive sequences are utilized as functional building blocks in the modular design of engineered peptide and protein materials (Fig. 1). This mix-and-match strategy enables enormous creativity in the design of matrices with tunable mechanical, chemical, and biological properties. In the next two sections, we discuss several examples of this bio-inspired design strategy to create NSC niche mimics from engineered peptide and protein materials.
Fig. 1.
Schematic of design strategies (left) and assembled structures (right) for selected engineered peptide and protein materials. (A) Four repeats of the RADA amino acid sequence form peptides that self-assemble based on electrostatic and hydrophilic/hydrophobic interactions to form a 3D hydrogel [57], copyright 2006, with permission from Elsevier. (B) Peptide amphiphiles self-assemble to align their hydrophilic, cell-adhesive IKVAV sequences and their hydrophobic alkyl tails to create long nanofibers that branch and entangle into a 3D network [73], reprinted with permission from AAAS. (C) Resilin-like polypeptides have a modular design that incorporates the highly resilient resilin structural block with domains that enable cell binding, proteolytic degradation, and protein/polysaccharide binding. The resilin-like domains include amino acids suitable for covalent cross-linking to form a 3D hydrogel [13], with permission of The Royal Society of Chemistry. (D) Mixing-induced, two-component hydrogels (MITCH) are formed from two recombinant engineered proteins incorporating WW and polyproline domains that hetero-assemble to form a cell-adhesive, 3D hydrogel [25], copyright C.T.S. Wong Po Foo, J.S. Lee, W. Mulyasasmita, A. Parisi-Amon, S.C. Heilshorn and National Academy of Sciences of the United States of America.
4. Molecular-level design of engineered peptide materials
Synthetic approaches for peptide materials, i.e., short chains of amino acids, are quite different from protein materials, i.e., long chains of amino acids; however, both strategies allow researchers the unique ability to program the exact monomer sequence within the biopolymer. Utilization of synthetic peptides as a component within engineered biomaterials is now a standard technique. For example, peptides have been grafted to a variety of synthetic polymers to endow the material with cell-adhesive, enzymatically degradable, and growth factor-binding properties [89,17,66,68,37,39]. Common amino acid sequences employed to replicate the in vivo niche include cell-adhesive domains derived from collagen (DGEA, RGD), laminin (IKVAV, RGD, YIGSR), and fibronectin (REDV, RGDS).
More recently, groups have started using peptides not only as biofunctional modules that decorate synthetic polymers, but also as structural components that dictate the underlying network of the matrix. These structural components are often based on totally new peptide domains conceived through rational design based on predictable amino acid interactions. As with all polymeric materials, molecular interactions dictate the nanoscale architecture and the resulting bulk material properties. Through choice of amino acid monomer sequence, the peptides can predictably fold into secondary structures, such as α-helices and β-sheets, that further self-assemble through complementary electrostatic and/or hydrophilic/hydrophobic interactions. As a consequence, these peptides are programmed to organize into hierarchical forms like tubes, fibers, or micelles by controlling the processing conditions during self-assembly [16]. Microscopic structures such as fibers are a hallmark of native ECM, but have been difficult to reproduce with synthetic materials, which are typically amorphous hydrogels. In contrast, peptide engineered materials are commonly characterized by fibrous strands, providing a simple method to replicate this key aspect of ECM structure [16,35,94,59].
Typically, peptide hydrogels created through rational design are programmed to self-assemble into fibrous hydrogels under physiological conditions without the need for chemical crosslinkers. Often the individual peptide units are amphiphilic; they contain two distinct regions that are hydrophilic and hydrophobic. Under specific temperature, pH, and salt conditions, the hydrophobic domains self-associate to exclude water leaving the hydrophilic domains exposed on the surface of a fibril-like strand. As an example, peptide amphiphiles that utilize an increasingly hydrophobic sequence of four alanines, three glycines, and 16 alkyl groups have been designed to display hydrophilic, cell-adhesive IKVAV peptides (Fig. 1B) [36]. In aqueous conditions at pH 7.4, the molecules organize to form transparent 3D hydrogels with a composition of only 0.5 wt% matrix material, the remainder being aqueous cell culture medium. As a 3D culture matrix, these hydrogels support encapsulated neural progenitor cell (NPC) growth and differentiation into neurons at higher efficiencies than standard laminin-coated tissue culture plates [73].
Another example of amphiphilic directed assembly is a 200-amino-acid-long peptide family designed with hydrophilic polyelectrolyte segments and hydrophobic helical domains [59]. Termed diblock copolypeptide hydrogels (DCH), the molecules self-assemble in aqueous solution to form long fibril nanostructures that branch and entangle to create a 3D hydrogel network. Through an iterative design process, the researchers varied the length of the hydrophobic and hydrophilic segments to develop a fundamental understanding of the resulting structural properties and to gain control over hydrogel properties such as stiffness, porosity, and stability.
Both of these peptide amphiphile hydrogels associate through non-covalent interactions, thus making them thixotropic, i.e., they can be shear-thinned into a flowing liquid by applying force. Upon removal of force, the molecules re-assemble into a hydrogel network with a characteristic time scale. This opens up the possibility of clinically applying these materials through direct injection, enabling minimally invasive patient delivery.
Perhaps the most familiar peptide hydrogel is PuraMatrix™, commercially available since 2001. Also called self-assembling peptide nanofiber scaffolds (SAPNS) and RADA peptides based on their repeating amino acid sequence, they too form nanostructured fibrillar hydrogels under physiological salt conditions [94]. The base of this class of peptides is a 16 amino acid sequence, RADA repeated four times end to end (Fig. 1A). RADA-based hydrogels form through ionic bonding between arginine (R) and aspartic acid (D) monomers on neighboring β-sheet molecules and through hydrophic interactions between the interspersed alanine (A) sites. The original PuraMatrix, consisting of the RADA 16-mer, does not contain any biologically active domains but is conducive to neuronal growth, synapse formation, and drug delivery [57,40]. Proliferative NPCs (BrdU- and nestin-positive) grew out of an organotypic hippocampal slice culture and into the adjacent RADA peptide matrix [69]. Human fetal NSCs also proliferated and underwent induced neuronal differentiation when cultured in 3D RADA hydrogels [83]. Recent modifications to this 16-mer peptide have expanded the library with bioactive sequences. In one example, a bone marrow homing peptide (BMHP1) was fused to the RADA sequence [28]. Interestingly, insertion of a short spacer between the bioactive sequence and the structural segment improved hydrogel stability and exposure of the bioactive motif, leading to greater NSC adhesion [79]. RADA peptides have also been fused with the laminin-derived IKVAV domain, resulting in increased NSC proliferation and more efficient neuronal differentiation [95].
Another peptide material that self-assembles via the β-sheet motif is the MAX family of peptides [32,45]. These amphiphilic molecules fold into two β-strands connected by a tetrapeptide β-hairpin turn. Through alternating lysine and valine amino acids, the resulting folded structure presents separate hydrophilic and hydrophobic faces enabling further assembly into a fibrillar, supramolecular structure. Through careful selection of the amino acid primary sequence in these 20-mer peptides, the kinetics of hierarchical self-assembly can be tuned. For example, replacement of one lysine residue in the MAX1 peptide with glutamic acid in the MAX8 peptide enables much faster assembly and a stiffer resultant hydrogel [32]. These materials have been well characterized for their drug release properties [7,8]. Mesenchymal stem cells demonstrated good viability upon encapsulation in MAX8 and after shear-thinning through a syringe needle [32]. Furthermore, recent in vitro studies demonstrated a lack of macrophage activation, encouraging future in vivo exploration of the MAX family of peptides for drug and cell delivery applications [33].
5. Molecular-level design of engineered protein materials
Similar to peptide-engineered materials, ECM-mimetic proteins are also created through rational design, with modular peptide units fused together to form a multi-functional protein chain. While peptide materials are typically produced using synthetic chemistry methods, engineered proteins are more commonly produced using recombinant technology for large-scale production [10,11]. By employing a cell’s molecular machinery, researchers can produce high fidelity protein polymers with specified amino acid content according to the pre-programmed DNA code.
One example of this strategy is the recombinant production of collagen based on a native human sequence [92]. These biopolymers address the need for defined bioactive polymers, avoiding potential contaminants and the potential immunogenicity of proteins harvested from xenogenic tissue. This product has been explored primarily for bone, cartilage, and skin applications due to collagen’s prevalence in those native tissues. In addition, recombinant collagen can deliver protein drugs via controlled release [81]. Similarly, recombinant silk proteins have been fashioned into drug delivery particles [46] and used for artificial ECM because of their remarkable mechanical strength [42]. These materials address the need for defined, controllable biopolymers by attempting to explicitly reproduce native amino acid sequences.
To take greater advantage of the recombinant protein engineering strategy, many ECM-mimetic proteins utilize different types of peptide domains that confer structural, bioinstructive, and bioresponsive properties into the biomaterial. These modules can be mixed, matched, and repeated multiple times within a full-length protein to create a multifunctional material with several desired characteristics. A common goal for niche-mimic applications is a material with the structural integrity to maintain encapsulated stem cells in a 3D environment. Sequences inspired from natural materials such as silk [4], elastin [18], and resilin [22] are commonly chosen for these applications, as each module has a unique combination of strength, elasticity, and resilience (the ability to recover after deformation under stress). While only elastin is native to the mammalian body, silk and resilin have unique strength and resilience properties, respectively, and all three have served as elements of successful cell scaffolds [4,77,6,41].
Along with these structural building blocks, other modules can instill a host of additional functionalities into the protein. In the creation of NSC niche mimics, well-known cell-adhesive domains RGD, IKVAV, and YIGSR amino acid sequences are frequently integrated into the material. Another type of commonly used biofunctional domain is preotolytically degradable sequences that enable cell-triggered degradation of the material via matrix metalloproteinases (MMPs) or other protease enzymes. Varying the amino acid sequence in the protease degradation site will control which enzymes trigger proteolysis, and hence may confer cell-specificity in the material [55]. Further modification of the sequence can alter the kinetics of the target enzyme, leading to biomaterials with widely tunable degradation properties [77]. As an example, a family of elastin-like proteins has been created with both interchangeable cell-adhesive domains and enzyme-degradable domains. By mixing cell-adhesive proteins with a non-adhesive elastin-like variant, the adhesion site density can be controlled without modifying protein concentration or cross-linking, resulting in tunable cell adhesivity and neurite extension [77]. Further demonstrating their utility in creating stem cell niches, elastin-like proteins also have been engineered with Notch receptor ligands [50]. Signaling through the Notch pathway is critical during tissue development and plays a role in gliogenesis and neurite extension [56,70,26]. Having manipulatable control over this pathway via the immediate cellular microenvironment may help provide understanding of both the NSC niche and nervous system repair.
One advantage of protein design is the ability to incorporate multiple functionalities into a signal molecule. One specific resilin polypeptide has been engineered with a cell-adhesive RGD peptide, an MMP-cleavable sequence to enable proteolytic degradation, and a heparin-binding domain for non-covalent attachment and release of growth factors (Fig. 1C) [13]. The tight control over the amino acid code can be used to modify any sequence to alter protein folding, improve yields, or simply make single amino acid substitutions. Individual amino acids such as lysine, tyrosine, or phenylalanine [77,13] can be edited in for post-production chemical modifications. This may include the addition of pendant chemical functionalities for drug delivery, or they may serve as sites for protein cross-linking and matrix formation.
While many engineered proteins are processed into hydrogels through chemical cross-linking, a variety of other strategies can also be utilized. For example, electrospinning [61], self-assembly [54], and photo-crosslinking [12] can lead to formation of a bulk material. A recently described hydrogel strategy involves the simple mixing of two different multi-domain proteins, which is termed Mixing-Induced Two-Component Hydrogel (MITCH) [25]. One protein includes proline-rich domains separated by hydrophilic spaces with cell-adhesive RGD sequences dispersed throughout (Fig. 1D). The second protein consists of WW domains separated by hydrophilic spacers of a different length. Upon mixing, the proline-rich and WW domains hetero-assemble through hydrogen bonding to form a networked hydrogel. An entire family of native and computationally derived WW domains binds to proline-rich domains, allowing simple tuning of protein-protein binding affinity and the resulting hydrogel mechanics. One advantage of this gelation strategy is that cells can be encapsulated without exposing them to chemical crosslinkers or non-physiological temperature, pH, or ionic strength. Furthermore, the specific binding between the proline-rich and WW domains is undisturbed by the presence of other proteins, enabling the straight-forward encapsulation of growth factors and other bioactive molecules [25]. MITCH was used to create an in vitro artificial stem cell niche capable of supporting NSC growth and differentiation. This shear-thinning material also regains its structure after being ejected through a syringe needle, enabling future use as a stem cell-delivery vehicle in vivo [25].
6. In vivo utilizations and successes using molecularly designed materials
Neural cell transplantation into the injured CNS is hindered by low post-transplantation cell viability [75,1,9]. In several transplantation models, improved outcomes have been achieved by using bioresorptive implant materials [80,98,82,60]. Peptide and protein materials may be ideally suited for these applications, as they can be tuned to mimic the compositional and mechanical properties of the surrounding environment, thus promoting integration with host tissue. In the last decade, peptide hydrogels have approached therapeutic realization, with notable successes in treating models of a cut optical track, traumatic brain injury (TBI), and spinal cord injury (SCI) (Fig. 2). In pre-clinical studies, these shear-thinning materials are often delivered by direct injection through a small-gauge needle, mimicking a minimally invasive clinical procedure. This route of delivery is particularly attractive for transplantation to the brain, as a needle can access sensitive deep regions of the brain without extensively disrupting the blood–brain barrier.
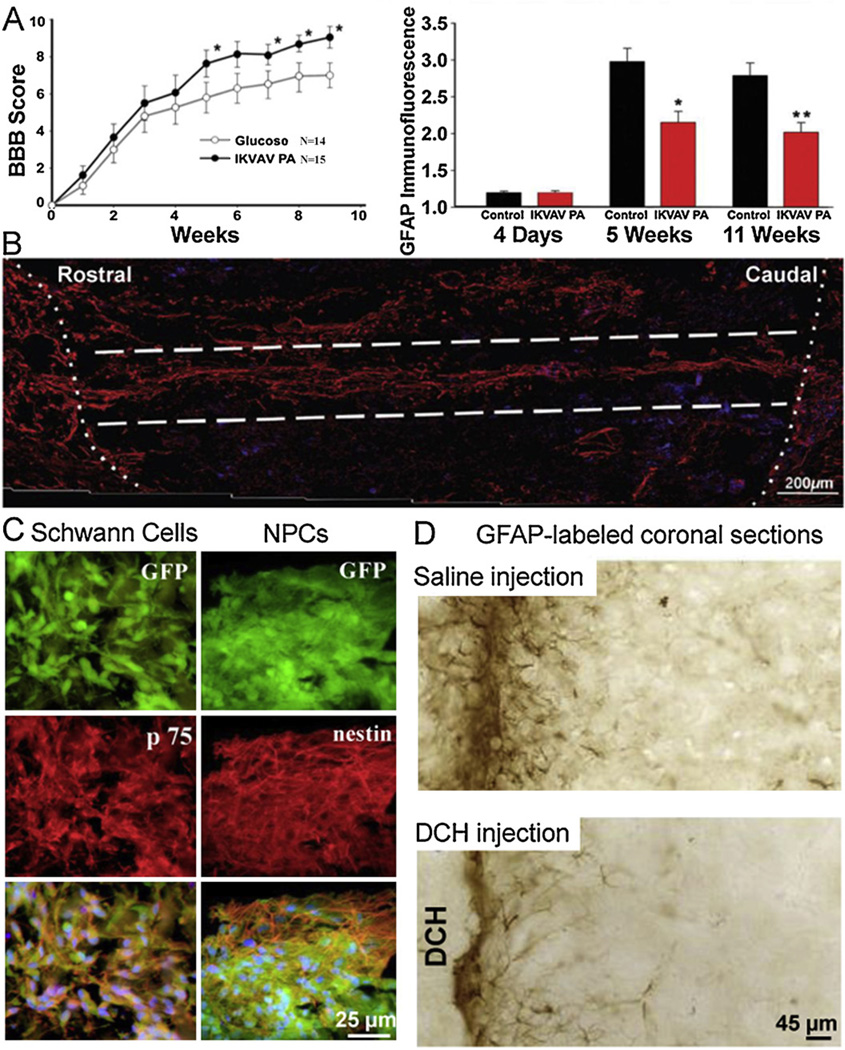
Fig. 2.
Selected in vitro and in vivo data demonstrating application of engineered peptide and protein materials in neural stem cell culture and CNS regeneration. (A) IKVAV peptide amphiphiles (PA) promote functional improvement after SCI. IKVAV PA-treated animals showed statistically greater improvement in mean locomotor BBB scores after 5 weeks. The hydrogel also attenuated astrogliosis, with less GFAP scarring observed at 5 and 11 weeks [85], with permission from the Society for Neuroscience. (B) Four weeks after SCI, scar tissue was replaced with degradable conduits filled with a modified RADA peptide. A longitudinal reconstruction of NF200-postive fibers shown in red indicates aligned neuronal growth through the hydrogel-filled conduit and crossing into both the rostral and the caudal host tissue [29]. Copyright (2011) American Chemical Society. (C) RADA peptide scaffolds support in vitro culture of GFP-positive Schwann cells and neural precursor cells (NPCs) for up to 4 weeks. Schwann cells continue to label for p75, and NPCs remain primarily nestin-positive, but also differentiate into neurons, astrocytes, and oligodendrocytes (not shown) [30], copyright 2007, with permission from Elsevier. (D) In a biocompatibility test, DCH injected into mouse forebrain showed astrocytosis comparable to saline up to 8 weeks. GFAP-labeled reactive astrocytes are observed around the needle track (top) and at the tissue interface with the DCH deposit (bottom) [93], copyright 2009, with permission from Elsevier.
In an initial biocompatibility study, dipeptide co-block hydrogels (DCH) composed of poly-lysine and poly-leucine were directly injected into the caudate putamen of rat brains. Over eight weeks, DCH-injected animals showed minimal astrogliosis, inflammation, and neuronal toxicity, similar to saline controls (Fig. 2D). Moreover, the DCH deposit was invaded by glia and a small number of neurofilament M- and tyrosine hydroxylase-positive nerve fibers, indicating functional neurite growth [93]. Another injectable peptide material, the 16-mer RADA peptide, has been injected into the damaged optical track [21] as well as TBI [31] and SCI models [30] with regenerative success. In the TBI treatment, the peptide nanofibers reduced glial activation and inflammation in the surrounding brain tissue, a critical step in limiting the subsequent damage often associated with this type of brain injury [31]. In the SCI treatment, the RADA peptide was used to deliver Schwann cells or NPCs into the dorsal column. Both cell types survived and migrated through the hydrogel matrix (Fig. 2C). In addition, invading host cells and blood vessel ingrowth were observed in the material [30].
As a demonstration of further molecular-level design, the RADA peptides were modified with a bone-marrow homing motif (BMHP1) that affects adhesion and differentiation of multiple stem cell types [14]. The hydrogel was injected 24 h after a contusion (impactor) SCI injury. Compared to non-treated and saline controls, the scaffold increased cellular infiltration, axon regeneration, and native ECM deposition including laminin and collagen IV. The Basso, Beattie, and Bresnahan (BBB) score, a common rating used to assess hind limb locomotor skills after SCI, demonstrated a slight, but statistically significant improvement in locomotor function relative to controls at eight weeks.
Further work combined this peptide gel with macroscopically structured tubes to physically orient regeneration longitudinally along the spinal cord [29]. In a unique surgical approach that mimics therapeutic intervention long after an injury, scar tissue was removed four weeks after the SCI. The resulting cyst was filled with PLGA/PCL conduits filled either with saline, the RADA-BMHP1 hydrogel alone, or the hydrogel with brain-derived and ciliary neurotrophic factors to support neuronal survival and outgrowth, vascular endothelial growth factor (VEGF) to promote angiogenesis, and chondroitinase ABC to promote remodeling of the glial scar. Over six months, the implantation site was remodeled with infiltrating astrocytes, oligodendrocytes, endothelial cells, and axon fibers with particularly high densities of neuronal fibers localized within the peptide-filled conduits. Superior function was shown in the treated groups by BBB and electrophysiology tests. Furthermore, nerve growth was continuous through the cyst and crossed both the rostral and caudal interfaces, indicating directed neural regeneration (Fig. 2B). While this directional regeneration is likely a consequence of the spatial orientation of the conduits, the hydrolytic degradation of the PLGA/PCL may also play a positive role. Lactic acid released during PLGA degradation has been shown to improve NSC survival in the presence of reactive oxygen species [47,49], an inflammatory component of damaged and regenerating neural tissues.
Shear-thinning IKVAV self-assembling peptide amphiphile nanofibers also have shown a remarkable ability to reduce astrogliosis and cell death when injected directly into the damaged spinal cord 24 h after compression injury (Fig. 2A) [85]. A notable achievement was an increase in the number of myelinating oligodendrocytes found at the injury site in the treatment group, with ascending sensory fibers and descending motor fibers passing through the injury site. Treated animals resulted in greater BBB behavioral improvement by 9 weeks (Fig. 2A). Again demonstrating the high level of design control, substituting the IKVAV sequence with a VEGF peptide led to enhanced blood vessel formation [86], a critical element of tissue regeneration. Alternatively, heparinbinding peptide domains can be used to deliver growth factors [76]. Recently these peptide amphiphiles were made into large fibers of aligned fibrils that can induce cell alignment [96] perhaps allowing completely peptide-based aligned conduits for SCI.
Similar to engineered peptide materials, the use of tailored, full-length recombinant proteins for in vivo applications is also promising. In recent clinical trials, recombinant human collagen was applied in 10 human subjects with corneal degeneration or scarring [23]. At two years, the treatment demonstrated endogenous epithelium colonization, recovery of nerve connections, and restored function [24]. Average nerve density continued to increase through 24 months, with 9 of 10 patients having nerves at the basal epithelium by 24 months.
While harvested silk proteins have been used in peripheral nerve surgery [42], recombinant silk has been limited by the ability to accurately reproduce the desired strength characteristics of the native protein. Recently the recombinant production of full-length silk was reported, which may now enable the synthesis of recombinant high tensile strength fibers and eventual application in nerve regeneration therapies [91]. In a different approach, silk modules were fused with elastin-like sequences to create a silk-elastin fusion protein that can be fabricated into any arbitrary shape. The mechanical strength and durability of the resulting recombinant protein matrix was designed to mimic the nucleus pulposus, the material found in the center of an intervertebral disc [5]. In a human trial of patients with herniated discs and leg pain unresponsive to conservative treatment, the engineered protein implant resulted in significant pain improvement with no observed re-herniations [3]. Elastin-like proteins have also been studied in pre-clinical drug delivery applications. Controlled drug release from a supramolecular, elastin-like protein assembly was achieved by adjusting the amino acid sequence and the total protein molecular weight [53]. These thermo-responsive molecules gelled in vivo and served as a localized source of model drug to nearby dorsal root ganglion with a seven-fold greater half-life compared to soluble protein alone.
7. Future perspective: designing bi-directional, cell-matrix feedback into niche mimics
The dynamic “give and take” of cell–ECM interactions is an increasingly recognized critical component of stem cell function. Stem cells and their surrounding support cells constantly remodel, degrade, and replace the niche matrix, which in turn results in altered stem cell behavior. As a mimic of this dynamic interplay, several biomaterial strategies have been developed to enable triggered matrix modification. One of the most common design elements for this purpose is the inclusion of proteolytic degradation sites. Depending on the location of the cleavable target sites, this strategy has been used to accomplish matrix softening [77], release of tethered bioactive factors [66,65], and selective degradation of internal channels [78]. These examples demonstrate how cell-secreted enzymes can be harnessed to initiate matrix modifications “on demand”.
In the future, it is expected that several individual cell–matrix interactions will be layered together in a single engineered material to create dynamic, bi-directional feedback systems (Fig. 3). For example, as stem cells proliferate in response to a growth-permissive matrix doped with time-released growth factors, they may secrete an accumulating amount of a specific protease. This stem-cell-specific protease may then trigger a modification of the material such as matrix softening. In response to the new microenvironment, the stem cells may be induced to follow a specific differentiation pathway. As the cells begin differentiation, their proteome is altered, leading to secretion of a different set of proteases. This second family of proteases can then be utilized to trigger a second type of matrix remodeling.
Fig. 3.
Future engineered stem cell niches may be designed to mimic the dynamic native niche environment through incorporation of bi-directional feedback between encapsulated cells and their matrix. In one potential realization, (1) stem cells initially adhere to a matrix that (2) releases factor 1 at a specific time. (3) Factor 1 could be a growth factor that leads to stem cell proliferation and the accumulation of protease 1. (4) Protease 1 degrades specific regions of the matrix, which may lead to a change in stiffness, thus inducing stem cell differentiation. (5) Upon differentiation, cells alter their proteome and begin secreting protease 2. (6) Protease 2 triggers the cleavage of cell binding ligands, (7) thereby inducing cell migration and extension of processes such as axons and dendrites into the surrounding material. Listed below are several potential bi-directional response elements that could be designed into future dynamic matrices.
While protease secretion is perhaps the most recognized and straight-forward route through which cells can remodel an engineered matrix, a variety of cell-responsive elements could be designed into these materials. For example, cells are known to remodel the native extracellular matrix through fiber bundling [97], stretching of proteins to reveal hidden “cryptic” domains and molecules [43,88], and deposition of new proteins and stiffening of the matrix [67]. Each of these native cell behaviors could be replicated in an engineered polypeptide material. In addition, polypeptide materials can be designed to respond to a variety of external stimuli such as pH [72], temperature [53], ion concentration [84], and exposure to ultraviolet light [38,87,63]. An external light stimulus was recently utilized to induce material remodeling of a synthetic polymeric hydrogel [17,44]. Based on several different clever design strategies, a common external stimulus (i.e., light exposure) could be used to open up channels within the hydrogel, to weaken the mechanical properties of the matrix, or to reduce or increase the concentration of cell binding ligands within the matrix [17,44]. Each of these matrix-remodeling processes was observed to initiate specific, distinct cellular responses. These results highlight the importance of dynamic matrix control when designing biomimetic materials to interface with cells.
Engineered peptides and proteins are ideal building blocks to fabricate these dynamic, biomimetic matrices for both in vitro and in vivo applications. As platforms for in vitro culture, these materials can be used both to help maintain stem cells in a more “native-like” environment and to probe mechanistic pathways of cell–matrix interactions. During prolonged culture, stem cells often begin to display reduced proliferative capacity and limited differentiation potential, which is hypothesized to be a consequence of the absence of normal microenvironmental cues [52]. A key aspect of the native stem cell niche, and hence a critical goal of engineered niche mimics, is dimensionality. Most cell types, including neural cells, display markedly different morphology in three-dimensional versus two-dimensional culture platforms [48,62]. In general, it is thought that three-dimensional culture materials induce organization of cytoskeletal structures that more accurately replicate in vivo cellular phenomena [15,34]. Several engineered peptide and protein materials have demonstrated success at supporting the three-dimensional culture of neuronal cell types, encouraging the further development of these materials as reproducible culture platforms [73,28,25]. As these materials become dynamic matrices capable of bi-directional interactions with cells, they will open up new avenues of stem cell biology research by allowing temporal control of matrix properties.
Similar to biomaterials used as culture matrices, biomaterials used for in vivo applications must be bioactive and should allow reproducible control of chemical and material properties. In addition, for clinical application these materials must be minimally immunogenic and be composed of defined FDA-approvable ingredients. Engineered peptide and protein materials fulfill these needs and enable the development of combination therapies that deliver matrix, cells, and soluble factors in a single construct [30,29,76]. As development of these combination therapies continues, materials must be designed to serve not just as cell- and growth factor-delivery depots, but as niche mimics that can guide cell fate and development. Recent in vivo data highlight the combined use of soluble factor delivery from a nanoscale matrix structure to enhance the regenerative ability of endogenous cells in an SCI model [29]. While several peptide hydrogels form nano-fibrous structures, typically the fibrils are randomly oriented. To overcome this limitation, peptide materials can be combined with novel processing methods to produce aligned nano-fibrils that physically guide cell growth [96]. Future engineered materials are likely to include additional levels of molecular-, nano-, and micro-scale design in order to mimic the multiple structural length scales observed in native tissue. Peptide and protein materials will further our understanding of the stem cell niche and allow researchers to control and perturb system elements in a defined, bioactive, and bioresponsive three-dimensional matrix. Furthermore, as strategies to create dynamic bioactive materials develop, they will be molecularly designed to meet the evolving requirements of transplanted cells during the multiple stages of cell engraftment and tissue regeneration.
Acknowledgements
KJL was supported by a Ruth L. Kirschstein NRSA Postdoctoral Fellowship. The authors acknowledge funding from CIRM RT2-01938, NIH DP2-OD-006477, and NSF DMR-0846363. Special thanks to Widya Mulyasasmita for editing assistance.
References
- 1.Bakshi A, CA Keck, Koshkin VS, LeBold DG, Siman R, Snyder EY, McIntosh TK. Caspase-mediated cell death predominates following engraftment of neural progenitor cells into traumatically injured rat brain. Brain Research. 2005;1065(1–2):8–19. doi: 10.1016/j.brainres.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee A, Arha M, Choudhary S, Ashton RS, Bhatia SR, Schaffer DV, Kane RS. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 2009;30(27):4695–4699. doi: 10.1016/j.biomaterials.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berlemann U, Schwarzenbach O. An injectable nucleus replacement as an adjunct to microdiscectomy: 2 year follow-up in a pilot clinical study. European Spine Journal. 2009;18(11):1706–1712. doi: 10.1007/s00586-009-1136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bini E, Foo CWP, Huang J, Karageorgiou V, Kitchel B, Kaplan DL. RGD-functionalized bioengineered spider dragline silk biomaterial. Biomacro-molecules. 2006;7(11):3139–3145. doi: 10.1021/bm0607877. [DOI] [PubMed] [Google Scholar]
- 5.Boyd LM, Carter AJ. Injectable biomaterials and vertebral endplate treatment for repair and regeneration of the intervertebral disc. European Spine Journal. 2006;15:S414–S421. doi: 10.1007/s00586-006-0172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bracalello A, Santopietro V, Vassalli M, Marletta G, Del Gaudio R, Bochicchio B, Pepe A. Design, production of a chimeric resilin-, elastin-, and collagen-like engineered polypeptide. Biomacromolecules. 2011;12(8):2957–2965. doi: 10.1021/bm2005388. [DOI] [PubMed] [Google Scholar]
- 7.Branco MC, Pochan DJ, Wagner NJ, Schneider JP. Macromolecular diffusion and release from self-assembled beta-hairpin peptide hydrogels. Biomaterials. 2009;30(7):1339–1347. doi: 10.1016/j.biomaterials.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Branco MC, Pochan DJ, Wagner NJ, Schneider JP. The effect of protein structure on their controlled release from an injectable peptide hydrogel. Biomaterials. 2010;31(36):9527–9534. doi: 10.1016/j.biomaterials.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns TC, Steinberg GK. Stem cells and stroke: opportunities, challenges and strategies. Expert Opinion on Biological Therapy. 2011;11(4):447–461. doi: 10.1517/14712598.2011.552883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cappello J, Crissman J, Dorman M, Mikolajczak M, Textor G, Marquet M, Ferrari F. Genetic-engineering of structural protein polymers. Biotechnology Progress. 1990;6(3):198–202. doi: 10.1021/bp00003a006. [DOI] [PubMed] [Google Scholar]
- 11.Cappello J, Crissman JW, Crissman M, Ferrari FA, Textor G, Wallis O, Whitledge JR, Zhou X, Burman D, L Aukerman, Stedronsky ER. In situ self-assembling protein polymer gel systems for administration, delivery, and release of drugs. Journal of Controlled Release. 1998;53(1–3):105–117. doi: 10.1016/s0168-3659(97)00243-5. [DOI] [PubMed] [Google Scholar]
- 12.Carrico IS, Maskarinec SA, Heilshorn SC, Mock ML, Liu JC, Nowatzki PJ, Franck C, Ravichandran G, Tirrell DA. Lithographic patterning of photoreactive cell-adhesive proteins. Journal of the American Chemical Society. 2007;129(16):4874. doi: 10.1021/ja070200b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charati MB, Ifkovits JL, Burdick JA, Linhardt JG, Kiick KL. Hydrophilic elastomeric biomaterials based on resilin-like polypeptides. Soft Matter. 2009;5(18):3412–3416. doi: 10.1039/b910980c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cigognini D, Satta A, Colleoni B, Silva D, Donega M, Antonini S, Gelain F. Evaluation of early and late effects into the acute spinal cord injury of an injectable functionalized self-assembling scaffold. Plos One. 2011;6(5) doi: 10.1371/journal.pone.0019782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell–matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 16.Dahlin RL, Kasper FK, Mikos AG. Polymeric nanofibers intissue engineering. Tissue Engineering Part B-Reviews. 2011;17(5):349–364. doi: 10.1089/ten.teb.2011.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nature Materials. 2009;8(8):659–664. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Zio K, Tirrell DA. Mechanical properties of artificial protein matrices engineered for control of cell and tissue behavior. Macromolecules. 2003;36(5):1553–1558. [Google Scholar]
- 19.Doetsch F. A niche for adult neural stem cells. Current Opinion in Genetics & Development. 2003;13(5):543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 21.Ellis-Behnke RG, Liang YX, You SW, Tay DKC, Zhang SG, So KF, Schneider GE. Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(13):5054–5059. doi: 10.1073/pnas.0600559103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, Liyou NE, CC Wong D, Merritt DJ, Dixon NE. Synthesis and properties of crosslinked recombinant pro-resilin. Nature. 2005;437(7061):999–1002. doi: 10.1038/nature04085. [DOI] [PubMed] [Google Scholar]
- 23.Fagerholm P, Lagali NS, Carlsson DJ, Merrett K, Griffith M. Corneal regeneration following implantation of a biomimetic tissue-engineered substitute. CTS-Clinical and Translational Science. 2009;2(2):162–164. doi: 10.1111/j.1752-8062.2008.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fagerholm P, Lagali NS, Merrett K, Jackson WB, Munger R, Liu Y, Polarek JW, Soderqvist M, Griffith M. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Science Translational Medicine. 2010;2(46):1–9. doi: 10.1126/scitranslmed.3001022. [DOI] [PubMed] [Google Scholar]
- 25.Foo CTSWP, Lee JS, Mulyasasmita W, Parisi-Amon A, Heilshorn SC. Two-component protein-engineered physical hydrogels for cell encapsulation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(52):22067–22072. doi: 10.1073/pnas.0904851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frisen J, Lendahl U. Oh no, notch again! Bioessays. 2001;23(1):3–7. doi: 10.1002/1521-1878(200101)23:1<3::AID-BIES1001>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 27.Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. Journal of Neurobiology. 1998;36(2):249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Gelain F, Bottai D, Vescovi A, Zhang S. Designer self-assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. Plos One. 2006;1(2) [Google Scholar]
- 29.Gelain F, Panseri S, Antonini S, C Cunha, Donega M, Lowery J, Taraballi F, Cerri G, Montagna M, Baldissera F, Vescovi A. Transplantation of nanostructured composite scaffolds results in the regeneration of chronically injured spinal cords. ACS Nano. 2011;5(1):227–236. doi: 10.1021/nn102461w. [DOI] [PubMed] [Google Scholar]
- 30.Guo J, Su H, Zeng Y, Liang Y-X, Wong WM, Ellis-Behnke RG, So K-F, Wu W. Reknitting the injured spinal cord by self-assembling peptide nanofiber scaffold. Nanomedicine-Nanotechnology Biology and Medicine. 2007;3(4):311–321. doi: 10.1016/j.nano.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Guo J, Leung KKG, Su H, Yuan Q, Wang L, Chu T-H, Zhang W, Pu JKS, Ng GKP, Wong WM, Dai X, Wu W. Self-assembling peptide nanofiber scaffold promotes the reconstruction of acutely injured brain. Nanomedicine-Nanotechnology Biology and Medicine. 2009;5(3):345–351. doi: 10.1016/j.nano.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, Pilarz M, Lamm MS, Pochan DJ, Schneider JP. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(19):7791–7796. doi: 10.1073/pnas.0701980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haines-Butterick LA, Salick DA, Pochan DJ, Schneider JP. In vitro assessment of the pro-inflammatory potential of beta-hairpin peptide hydrogels. Biomaterials. 2008;29(31):4164–4169. doi: 10.1016/j.biomaterials.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hakkinen KM, Harunaga JS, Doyle AD, Yamada KM. Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue Engineering Part A. 2011;17(5–6):713–724. doi: 10.1089/ten.tea.2010.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294(5547):1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 36.Hartgerink JD, Beniash E, Stupp SI. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(8):5133–5138. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.L Hern D, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. Journal of Biomedical Materials Research. 1998;39(2):266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 38.Higuchi M, Minoura N, Kinoshita T. Photo-responsive behavior of a monolayer composed of an azobenzene containing polypeptide in the main-chain. Colloid and Polymer Science. 1995;273(11):1022–1027. [Google Scholar]
- 39.Hoffmann JC, West JL. Three-dimensional photolithographic patterning of multiple bioactive ligands in poly(ethylene glycol) hydrogels. Soft Matter. 2010;6(20):5056–5063. [Google Scholar]
- 40.Holmes TC, de Lacalle S, Su X, Liu GS, Rich A, Zhang SG. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(12):6728–6733. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu XA, Wang XL, Rnjak J, Weiss AS, L Kaplan D. Biomaterials derived from silk-tropoelastin protein systems. Biomaterials. 2010;31(32):8121–8131. doi: 10.1016/j.biomaterials.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang J, Foo CWP, Kaplan DL. Biosynthesis and applications of silk-like and collagen-like proteins. Polymer Reviews. 2007;47(1):29–62. [Google Scholar]
- 43.Klotzsch E, Smith ML, Kubow KE, Muntwyler S, Little WC, Beyeler F, Gourdon D, Nelson BJ, Vogel V. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(43):18267–18272. doi: 10.1073/pnas.0907518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324(5923):59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kretsinger JK, Haines LA, Ozbas B, Pochan DJ, Schneider JP. Cytocompatibility of self-assembled ss-hairpin peptide hydrogel surfaces. Biomaterials. 2005;26(25):5177–5186. doi: 10.1016/j.biomaterials.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 46.Lammel A, Schwab M, Hofer M, Winter G, Scheibel T. Recombinant spider silk particles as drug delivery vehicles. Biomaterials. 2011;32(8):2233–2240. doi: 10.1016/j.biomaterials.2010.11.060. [DOI] [PubMed] [Google Scholar]
- 47.Lampe KJ, Namba RM, Silverman TR, Bjugstad KB, Mahoney MJ. Impact of lactic acid on cell proliferation and free radical-induced cell death in monolayer cultures of neural precursor cells. Biotechnology and Bioengineering. 2009;103(6):1214–1223. doi: 10.1002/bit.22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lampe KJ, Mooney RG, Bjugstad KB, Mahoney MJ. Effect of macromer weight percent on neural cell growth in 2D and 3D nondegradable PEG hydrogel culture. Journal of Biomedical Materials Research Part A. 2010;94A(4):1162–1171. doi: 10.1002/jbm.a.32787. [DOI] [PubMed] [Google Scholar]
- 49.Lampe KJ, Bjugstad KB, Mahoney MJ. Impact of degradable macromer content in a poly(ethylene glycol) hydrogel on neural cell metabolic activity, redox state, proliferation, and differentiation. Tissue Engineering Part A. 2010;16(6):1857–1866. doi: 10.1089/ten.tea.2009.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu CY, Apuzzo MLJ, Tirrell DA. Engineering of the extracellular matrix: working toward neural stem cell programming and neurorestoration—concept and progress report. Neurosurgery. 2003;52(5):1154–1165. [PubMed] [Google Scholar]
- 51.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnology. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 52.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462(7272):433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacKay JA, Chen M, McDaniel JR, Liu W, Simnick AJ, Chilkoti A. Self-assembling chimeric polypeptide–doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nature Materials. 2009;8(12):993–999. doi: 10.1038/nmat2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Megeed Z, Haider M, Li DQ, O’Malley BW, Cappello J, Ghandehari H. In vitro and in vivo evaluation of recombinant silk-elastinlike hydrogels for cancer gene therapy. Journal of Controlled Release. 2004;94(2–3):433–445. doi: 10.1016/j.jconrel.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 55.Miller JS, Shen CJ, Legant WR, Baranski JD, Blakely BL, Chen CS. Bioactive hydrogels made from step-growth derived PEG-peptide macromers. Biomaterials. 2010;31(13):3736–3743. doi: 10.1016/j.biomaterials.2010.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, Anderson DJ. Transient notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101(5):499–510. doi: 10.1016/s0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 57.Nagai Y, Unsworth LD, Koutsopoulos S, Zhang SG. Slow release of molecules in self-assembling peptide nanofiber scaffold. Journal of Controlled Release. 2006;115(1):18–25. doi: 10.1016/j.jconrel.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 58.Noble M, Smith J, Power J, Mayer-Proschel M. Redox state as a central modulator of precursor cell function, in: Parkinson’s disease: the life cycle of the dopamine neuron. Annals of the New York Academy of Sciences. 2003 doi: 10.1111/j.1749-6632.2003.tb07481.x. PMID:12846992. [DOI] [PubMed] [Google Scholar]
- 59.Nowak AP, Breedveld V, Pakstis L, Ozbas B, Pine DJ, Pochan D, Deming TJ. Rapidly recovering hydrogel scaffolds from self-assembling diblock copolypeptide amphiphiles. Nature. 2002;417(6887):424–428. doi: 10.1038/417424a. [DOI] [PubMed] [Google Scholar]
- 60.Piantino J, Burdick JA, Goldberg D, Langer R, Benowitz LI. An injectable: biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Experimental Neurology. 2006;201(2):359–367. doi: 10.1016/j.expneurol.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 61.Qiu WG, Huang YD, Teng WB, Cohn CM, Cappello J, Wu XY. Complete recombinant silk-elastinlike protein-based tissue scaffold. Biomacromolecules. 2010;11(12):3219–3227. doi: 10.1021/bm100469w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribeiro A, Vargo S, Powell EM, Leach JB. Substrate three-dimensionality induces elemental morphological transformation of sensory neurons on a physiologic timescale. Tissue Engineering Part A. 2011 doi: 10.1089/ten.tea.2011.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rughani RV, Branco MC, Pochan D, Schneider JP. De novo design of a shear-thin recoverable peptide-based hydrogel capable of intrafibrillar photopolymerization. Macromolecules. 2010;43(19):7924–7930. [Google Scholar]
- 64.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate modulus directs neural stem cell behavior. Biophysical Journal. 2008;95(9):4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakiyama-Elbert SE, Panitch A, Hubbell JA. Development of growth factor fusion proteins for cell-triggered drug delivery. FASEB Journal. 2001;15(7):1300–1302. doi: 10.1096/fj.00-0564fje. [DOI] [PubMed] [Google Scholar]
- 66.Salinas CN, Anseth KS. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials. 2008;29(15):2370–2377. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schedin P, Keely PJ. Mammary gland ECM remodeling stiffness and mechanosignaling in normal development and tumor progression. Cold Spring Harbor Perspectives in Biology. 2011;3(1) doi: 10.1101/cshperspect.a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schense JC, Bloch J, Aebischer P, Hubbell JA. Enzymatic incorporation of bioactive peptides into fibrin matrices enhances neurite extension. Nature Biotechnology. 2000;18(4):415–419. doi: 10.1038/74473. [DOI] [PubMed] [Google Scholar]
- 69.Semino CE, Kasahara J, Hayashi Y, Zhang SG. Entrapment of migrating hippocampal neural cells in three-dimensional peptide nanofiber scaffold. Tissue Engineering. 2004;10(3–4):643–655. doi: 10.1089/107632704323061997. [DOI] [PubMed] [Google Scholar]
- 70.Sestan N, Artavanis-Tsakonas S, Rakic P. Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science. 1999;286(5440):741–746. doi: 10.1126/science.286.5440.741. [DOI] [PubMed] [Google Scholar]
- 71.Shen Q, Goderie SK, L Jin, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304(5675):1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 72.Shen W, Kornfield JA, Tirrell DA. Structure and mechanical properties of artificial protein hydrogels assembled through aggregation of leucine zipper peptide domains. Soft Matter. 2007;3(1):99–107. doi: 10.1039/b610986a. [DOI] [PubMed] [Google Scholar]
- 73.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303(5662):1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 74.Song H, Stevens CF, Gage FH. Nature. 6884. Vol. 417. London: 2002. Astroglia induce neurogenesis from adult neural stem cells; pp. 39–44. [DOI] [PubMed] [Google Scholar]
- 75.Sortwell CE. Strategies for the augmentation of grafted dopamine neuron survival. Frontiers in Bioscience. 2003;8:S522–S532. doi: 10.2741/1096. [DOI] [PubMed] [Google Scholar]
- 76.Stendahl JC, Wang L-J, Chow LW, Kaufman DB, Stupp SI. Growth factor delivery from self-assembling nanofibers to facilitate islet transplantation. Transplantation. 2008;86(3):478–481. doi: 10.1097/TP.0b013e3181806d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Straley KS, Heilshorn SC. Independent tuning of multiple biomaterial properties using protein engineering. Soft Matter. 2009;5(1):114–124. [Google Scholar]
- 78.Straley KS, Heilshorn SC. Dynamic, 3D-pattern formation within enzyme-responsive hydrogels. Advanced Materials. 2009;21(41):4148–4152. [Google Scholar]
- 79.Taraballi F, Natalello A, Campione M, Villa O, Doglia SM, Paleari A, Gelain F. Glycine-spacers influence functional motifs exposure and self-assembling propensity of functionalized substrates tailored for neural stem cell cultures. Frontiers in Neuroengineering. 2010;3:1–9. doi: 10.3389/neuro.16.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tate CC, Shear DA, Tate MC, Archer DR, Stein DG, LaPlaca MC. Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain. Journal of Tissue Engineering and Regenerative Medicine. 2009;3(3):208–217. doi: 10.1002/term.154. [DOI] [PubMed] [Google Scholar]
- 81.Teles H, Vermonden T, Eggink G, Hennink WE, de Wolf FA. Hydrogels of collagen-inspired telechelic triblock copolymers for the sustained release of proteins. Journal of Controlled Release. 2010;147(2):298–303. doi: 10.1016/j.jconrel.2010.07.098. [DOI] [PubMed] [Google Scholar]
- 82.Teng YD, Lavik EB, Qu XL, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(5):3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thonhoff JR, Lou DI, Jordan PM, Zhao X, Wu P. Compatibility of human fetal neural stem cells with hydrogel biomaterials in vitro. Brain Research. 2008;1187:42–51. doi: 10.1016/j.brainres.2007.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Topp S, Prasad V, Cianci GC, Weeks ER, Gallivan JP. A genetic toolbox for creating reversible Ca2+-sensitive materials. Journal of the American Chemical Society. 2006;128(43):13994–13995. doi: 10.1021/ja064546i. [DOI] [PubMed] [Google Scholar]
- 85.Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, Stupp SI, Kessler JA. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. Journal of Neuroscience. 2008;28(14):3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Webber MJ, Tongers J, Newcomb CJ, Marquardt K-T, Bauersachs J, Losordo DW, Stupp SI. Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(33):13438–13443. doi: 10.1073/pnas.1016546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams AJ, Gupta VK. Incorporation of a photochromic hinge in a rodlike polypeptide and its influence on dielectric and optical properties. Journal of Polymer Science Part B-Polymer Physics. 2001;39(22):2759–2773. [Google Scholar]
- 88.Wipff P-J, Rifkin DB, Meister J-J, Hinz B. Myofibroblast contraction activates latent TGF-beta 1 from the extracellular matrix. Journal of Cell Biology. 2007;179(6):1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wosnick JH, Shoichet MS. Three-dimensional chemical patterning of transparent hydrogels. Chemistry of Materials. 2008;20(1):55–60. [Google Scholar]
- 90.Wurmser AE, Palmer TD, Gage FH. Cellular interactions in the stem cell niche. Science. 2004;304(5675):1253. doi: 10.1126/science.1099344. [DOI] [PubMed] [Google Scholar]
- 91.Xia X-X, Qian Z-G, Ki CS, Park YH, Kaplan DL, Lee SY. Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(32):14059–14063. doi: 10.1073/pnas.1003366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang CL, Hillas PJ, Baez JA, Nokelainen M, Balan J, Tang J, Spiro R, Polarek JW. The application of recombinant human collagen in tissue engineering. Biodrugs. 2004;18(2):103–119. doi: 10.2165/00063030-200418020-00004. [DOI] [PubMed] [Google Scholar]
- 93.Yang CY, Song B, Ao Y, Nowak AP, Abelowitz RB, Korsak RA, Havton LA, Deming TJ, Sofroniew MV. Biocompatibility of amphiphilic diblock copolypeptide hydrogels in the central nervous system. Biomaterials. 2009;30(15):2881–2898. doi: 10.1016/j.biomaterials.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 94.Zhang SG, Holmes T, Lockshin C, Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(8):3334–3338. doi: 10.1073/pnas.90.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang ZX, Zheng QX, Wu YC, Hao DJ. Compatibility of neural stem cells with functionalized self-assembling peptide scaffold in vitro. Biotechnology and Bioprocess Engineering. 2010;15(4):545–551. [Google Scholar]
- 96.Zhang S, Greenfield MA, Mata A, Palmer LC, Bitton R, Mantei JR, Aparicio C, de la Cruz MO, Stupp SI. A self-assembly pathway to aligned monodomain gels. Nature Materials. 2010;9(7):594–601. doi: 10.1038/nmat2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhong CL, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. Journal of Cell Biology. 1998;141(2):539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhong J, Chan A, Morad L, Kornblum HI, Fan G, Carmichael ST. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabilitation and Neural Repair. 2010;24(7):636–644. doi: 10.1177/1545968310361958. [DOI] [PMC free article] [PubMed] [Google Scholar]