Abstract
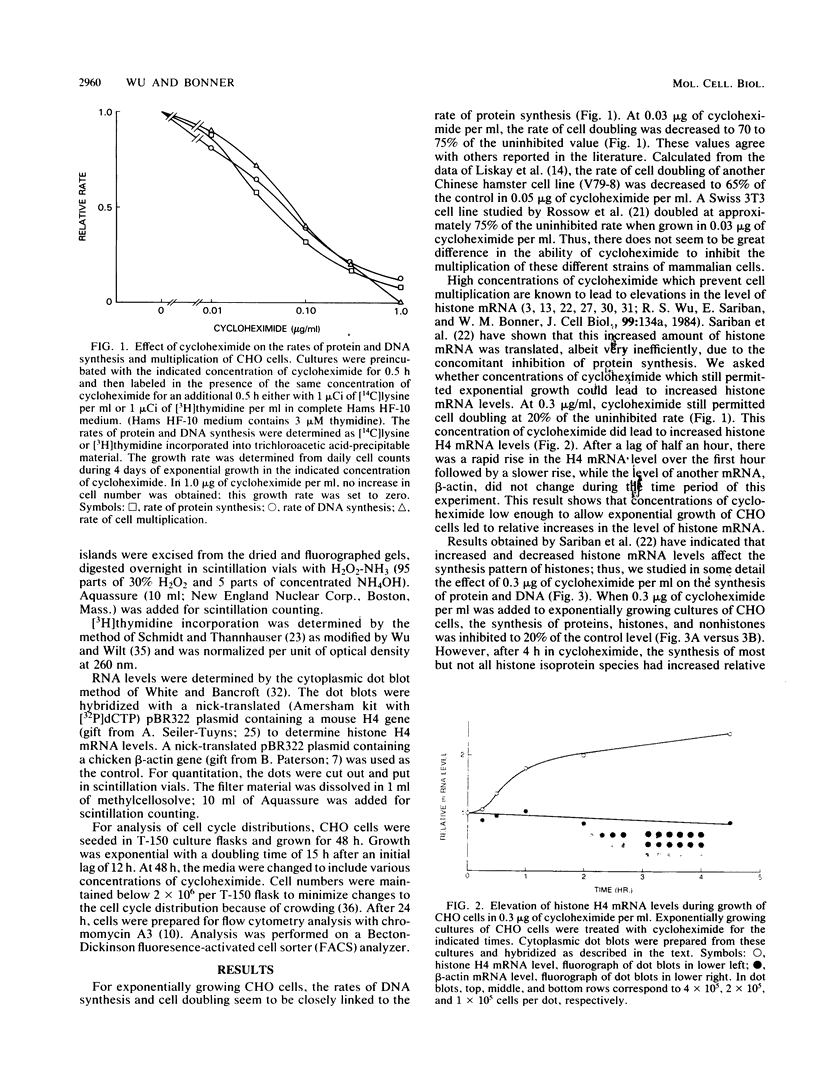
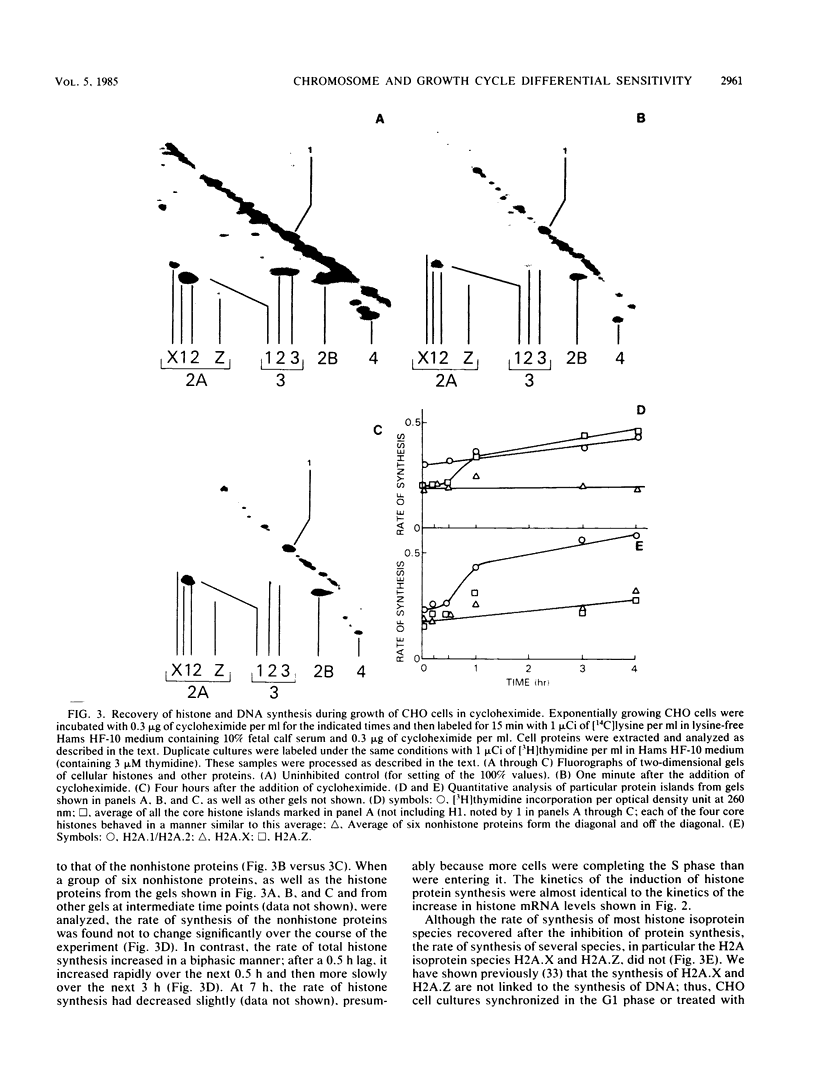
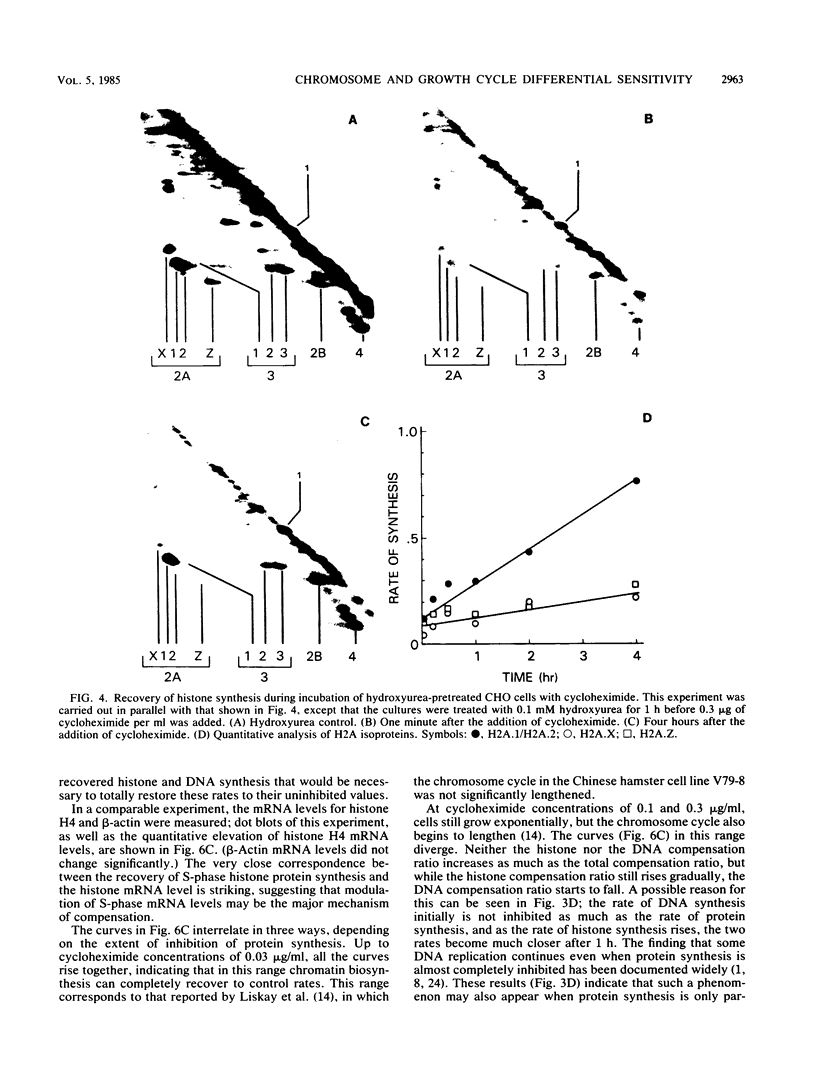
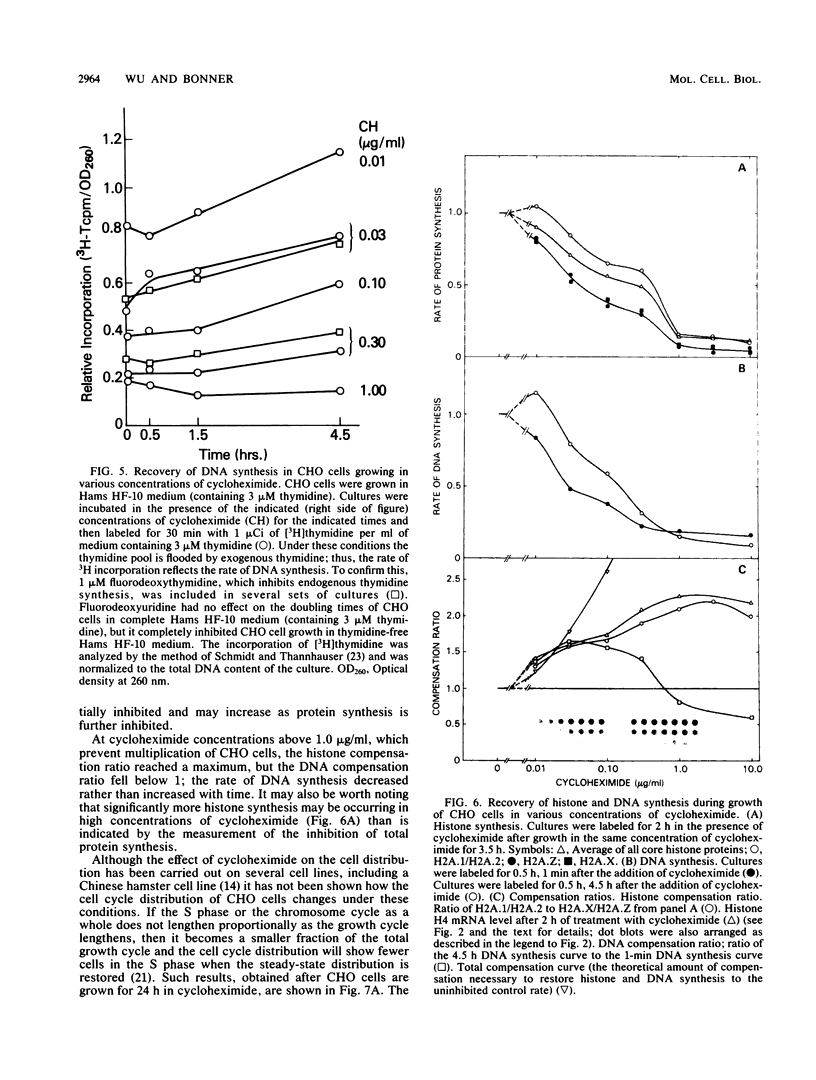
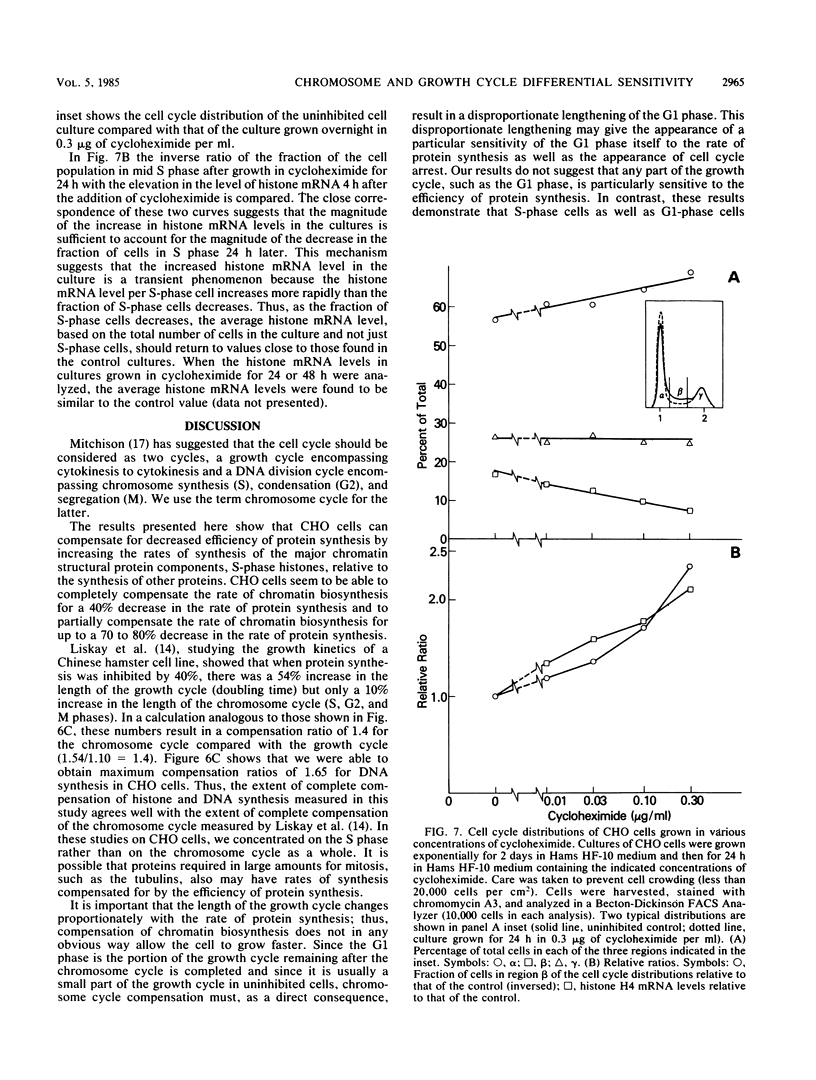
It has been documented widely that when the generation times of eucaryotic cells are lengthened by slowing the rate of protein synthesis, the duration of the chromosome cycle (S, G2, and M phases) remains relatively invariant. Paradoxically, when the growth of exponentially growing cultures of CHO cells is partially inhibited with inhibitors of protein synthesis, the immediate effect is a proportionate reduction in the rate of total protein, histone protein, and DNA synthesis. However, on further investigation it was found that over the next 2 h the rates of histone protein and DNA synthesis recover, in some cases completely to the uninhibited rate, while the synthesis rates of other proteins do not recover. We called this process chromosome cycle compensation. The amount of compensation seen in CHO cell cultures can account quantitatively for the relative invariance in the length of the chromosome cycle (S, G2, and M phases) reported for these cells. The mechanism for this compensation involves a specific increase in the levels of histone mRNAs. An invariant chromosome cycle coupled with a lengthening growth cycle must result in a disproportionate lengthening of the G1 phase. Thus, these results suggest that chromosome cycle invariance may be due more to specific cellular compensation mechanisms rather than to the more usual interpretation involving a rate-limiting step for cell cycle progression in the G1 phase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annunziato A. T., Seale R. L. Chromatin replication, reconstitution and assembly. Mol Cell Biochem. 1983;55(2):99–112. doi: 10.1007/BF00673705. [DOI] [PubMed] [Google Scholar]
- Baumbach L. L., Marashi F., Plumb M., Stein G., Stein J. Inhibition of DNA replication coordinately reduces cellular levels of core and H1 histone mRNAs: requirement for protein synthesis. Biochemistry. 1984 Apr 10;23(8):1618–1625. doi: 10.1021/bi00303a006. [DOI] [PubMed] [Google Scholar]
- Brooks R. F. Continuous protein synthesis is required to maintain the probability of entry into S phase. Cell. 1977 Sep;12(1):311–317. doi: 10.1016/0092-8674(77)90209-4. [DOI] [PubMed] [Google Scholar]
- Butler W. B., Mueller G. C. Control of histone synthesis in HeLa cells. Biochim Biophys Acta. 1973 Feb 4;294(1):481–496. doi: 10.1016/0005-2787(73)90104-4. [DOI] [PubMed] [Google Scholar]
- Gautschi J. R., Kern R. M. DNA replication in mammalian cells in the presence of cycloheximide. Exp Cell Res. 1973 Jul;80(1):15–26. doi: 10.1016/0014-4827(73)90270-x. [DOI] [PubMed] [Google Scholar]
- Graves R. A., Marzluff W. F. Rapid reversible changes in the rate of histone gene transcription and histone mRNA levels in mouse myeloma cells. Mol Cell Biol. 1984 Feb;4(2):351–357. doi: 10.1128/mcb.4.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. W., Coffino P. Cell cycle analysis by flow cytometry. Methods Enzymol. 1979;58:233–248. doi: 10.1016/s0076-6879(79)58140-3. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Unger M. W. Unequal division in Saccharomyces cerevisiae and its implications for the control of cell division. J Cell Biol. 1977 Nov;75(2 Pt 1):422–435. doi: 10.1083/jcb.75.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms S., Baumbach L., Stein G., Stein J. Requirement of protein synthesis for the coupling of histone mRNA levels and DNA replication. FEBS Lett. 1984 Mar 12;168(1):65–69. doi: 10.1016/0014-5793(84)80207-0. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Kornfeld B., Fullerton P., Evans R. Protein synthesis and the presence of absence of a measurable G1 in cultured Chinese hamster cells. J Cell Physiol. 1980 Sep;104(3):461–467. doi: 10.1002/jcp.1041040318. [DOI] [PubMed] [Google Scholar]
- Martegani E., Levi M., Trezzi F., Alberghina L. Nuclear division cycle in Neurospora crassa hyphae under different growth conditions. J Bacteriol. 1980 Apr;142(1):268–275. doi: 10.1128/jb.142.1.268-275.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Rossow P. W., Riddle V. G., Pardee A. B. Synthesis of labile, serum-dependent protein in early G1 controls animal cell growth. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4446–4450. doi: 10.1073/pnas.76.9.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariban E., Wu R. S., Erickson L. C., Bonner W. M. Interrelationships of protein and DNA syntheses during replication of mammalian cells. Mol Cell Biol. 1985 Jun;5(6):1279–1286. doi: 10.1128/mcb.5.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale R. L., Simpson R. T. Effects of cycloheximide on chromatin biosynthesis. J Mol Biol. 1975 May 25;94(3):479–501. doi: 10.1016/0022-2836(75)90216-8. [DOI] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Birnstiel M. L. Structure and expression in L-cells of a cloned H4 histone gene of the mouse. J Mol Biol. 1981 Oct 5;151(4):607–625. doi: 10.1016/0022-2836(81)90426-5. [DOI] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive H. L., Heintz N., Roeder R. G. Regulation of human histone gene expression during the HeLa cell cycle requires protein synthesis. Mol Cell Biol. 1984 Dec;4(12):2723–2734. doi: 10.1128/mcb.4.12.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Martin L. Do cells cycle? Proc Natl Acad Sci U S A. 1973 Apr;70(4):1263–1267. doi: 10.1073/pnas.70.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancel G. M., Prescott D. M., Liskay R. M. Most of the G1 period in hamster cells is eliminated by lengthening the S period. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6295–6298. doi: 10.1073/pnas.78.10.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimac E., Groppi V. E., Coffino P. Increased histone mRNA levels during inhibition of protein synthesis. Biochem Biophys Res Commun. 1983 Jul 18;114(1):131–137. doi: 10.1016/0006-291x(83)91604-2. [DOI] [PubMed] [Google Scholar]
- Stimac E., Groppi V. E., Jr, Coffino P. Inhibition of protein synthesis stabilizes histone mRNA. Mol Cell Biol. 1984 Oct;4(10):2082–2090. doi: 10.1128/mcb.4.10.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Wu R. S., Bonner W. M. Separation of basal histone synthesis from S-phase histone synthesis in dividing cells. Cell. 1981 Dec;27(2 Pt 1):321–330. doi: 10.1016/0092-8674(81)90415-3. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Tsai S., Bonner W. M. Patterns of histone variant synthesis can distinguish G0 from G1 cells. Cell. 1982 Dec;31(2 Pt 1):367–374. doi: 10.1016/0092-8674(82)90130-1. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Wilt F. H. The synthesis and degradation of RNA containing polyriboadenylate during sea urchin embryogeny. Dev Biol. 1974 Dec;41(2):352–370. doi: 10.1016/0012-1606(74)90312-1. [DOI] [PubMed] [Google Scholar]
- Yen A., Pardee A. B. Exponential 3T3 cells escape in mid-G1 from their high serum requirement. Exp Cell Res. 1978 Oct 1;116(1):103–113. doi: 10.1016/0014-4827(78)90068-x. [DOI] [PubMed] [Google Scholar]