SUMMARY
Epidemiological and clinical studies have suggested that exercise is beneficial for patients with Parkinson’s disease (PD). Through research in normal (noninjured) animals, neuroscientists have begun to understand the mechanisms in the brain by which behavioral training and exercise facilitates improvement in motor behavior through modulation of neuronal function and structure, called experience-dependent neuroplasticity. Recent studies are beginning to reveal molecules and downstream signaling pathways that are regulated during exercise and motor learning in animal models of PD and that are important in driving protective and/or adaptive changes in neuronal connections of the basal ganglia and related circuitry. These molecules include the neurotransmitters dopamine and glutamate (and their respective receptors) as well as neurotrophic factors (brain-derived neurotrophic factor). In parallel, human exercise studies have been important in revealing ‘proof of concept’ including examining the types and parameters of exercise that are important for behavioral/functional improvements and brain changes; the feasibility of incorporating and maintaining an exercise program in individuals with motor disability; and, importantly, the translation and investigation of exercise effects observed in animal studies to exercise effects on brain and behavior in individuals with PD. In this article we highlight findings from both animal and human exercise studies that provide insight into brain changes of the basal ganglia and its related circuitry and that support potentially key parameters of exercise that may lead to long-term benefit and disease modification in PD. In addition, we discuss the current and future impact on patient care and point out gaps in our knowledge where continuing research is needed. Elucidation of exercise parameters important in driving neuroplasticity, as well as the accompanying mechanisms that underlie experience-dependent neuroplasticity may also provide insights towards new therapeutic targets, including neurorestorative and/or neuroprotective agents, for individuals with PD and related neurodegenerative disorders.
In the last decade, neuroscience research has supported the ability of the brain to respond to the environment and experiences through adaptive mechanisms called neuroplasticity, in which long-lasting alterations in neuronal circuitry result [1]. These processes include the ability of neurons to respond to experiences, such as exercise, through alterations in structure and function, most importantly at the level of the synapse (the point of communication between neurons) [2]. While these adaptations have largely been demonstrated to underlie learning and encoding of new behaviors in the healthy brain, they have also been postulated to be evoked in repair processes of the injured brain during exercise or rehabilitation training to relearn impaired or lost behaviors [2]. Exercise is defined as physical activity that is usually performed regularly and done with the intention of improving or maintaining physical fitness or health [3]. As such, there has been a general interest in understanding whether exercise and physical therapy may play a role in the repair of neurodegenerative disorders, such as Parkinson’s disease (PD), where dopaminergic neurons are reduced in number, glutamatergic-based neuronal connections within the basal ganglia are impaired or lost, and motor function such as gait and balance are significantly altered [4]. Although still largely unanswered, in this article we highlight exercise studies in both animal models of PD and in individuals with PD that support a potential role of exercise in providing long-term benefit and disease modification. Specifically, we review findings from studies that reveal exercise-induced processes of neuroplasticity as well as key parameters of behavioral training that may lead to: protection of ongoing dopamine neuronal loss; adaptation of remaining dopaminergic neurons; and alterations in downstream basal ganglia synaptic connections [5–7]. In addition, we discuss the current and future impact on patient care and point out gaps in our knowledge where continuing research is needed.
Parkinson’s disease is a chronic and progressive neurodegenerative disorder of the basal ganglia characterized by a 40% loss of substantia nigra pars compacta (SNpc) dopaminergic neurons, and 80% depletion of striatal dopamine [8]. Failure of normal dopamine neurotransmission in PD in turn leads to the consequential impairment and loss of downstream basal ganglion synaptic connections that are modulated through glutamate and its respective receptor families [9]. Loss of basal ganglia homeostasis occurs through increased activity of the indirect (dopamine receptor D2 pathway), and decreased activity of the direct (dopamine receptor D1 pathway) [10,11]. Classical features of PD include bradykinesia, gait dysfunction, balance impairment, rigidity and resting tremor. Additional characteristic aspects of PD, called non-motor features, include cognitive dysfunction (frontal executive), mood disorders (depression and anxiety) and autonomic dysfunction [12]. While dopamine-replacement therapy remains a very effective treatment for symptomatic control of motor aspects of the disease, its efficacy declines with disease progression. Currently there is no cure for this debilitating disease and no definitive agent available to modify disease course. Most recently, however, epidemiological studies have revealed that a high degree of physical activity, such as intensive and strenuous exercise throughout life, is associated with a lower risk of developing neurodegenerative disorders, including PD [13,14]. These studies, along with numerous exercise trials that have shown clinical benefit in individuals with PD, have served as a catalyst to further determine whether exercise is a critical therapeutic intervention in PD. More importantly there is a general interest in understanding whether exercise may provide a means to modify disease progression that may include neuroprotection of ongoing dopaminergic neuronal loss and/or driving neuroplastic or compensatory changes within the injured brain to restore basal ganglia homeostatic function and synaptic integrity.
Animal models & exercise
Much of our understanding of the neurobiological basis for the beneficial effects of exercise on the brain is derived from studies using normal (noninjured) animals where interventional experimentation can be performed. For example, within the normal animal brain exercise, both motor skill training and voluntary running, has been shown to be associated with changes in neuronal physiology, neurogenesis, angiogenesis, gene and protein expression of neurotrophic factors, and dendritic spine growth and density in a number of brain regions including the hippocampus, cerebral cortex and cerebellum [15–17]. While we classify these exercise effects as being categorically distinct, there is tremendous overlap with respect to their potential for modulating behavior and brain function and facilitating brain connections. More recently there has been a general interest to understand whether similar exercise-related mechanisms may be employed in recovery of the injured brain, in particular in the context of neurodegenerative disorders such as Alzheimer’s disease (AD) and PD.
Within the last decade neurotoxin-based animal models of disease have been utilized to understand the potential effects of exercise in PD. The two most common models used in these studies have been the 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine-(MPTP)- lesioned mouse models and 6-hydroxydopamine (6-OHDA) rat models [18–20]. Both toxins lead to the depletion of striatal dopamine and basal ganglia impairment, as well as behavioral motor deficits similar to those observed in the human condition. In addition, they provide valuable neurotranslational tools for examining exercise-induced mechanistic repair processes. Depending on the timing of neurotoxin administration and the initiation of the exercise paradigm, studies are designed to examine mechanisms of neuroprotection (exercise initiated either prior, during or shortly after neurotoxin delivery and within the window of toxin-induced cell death) or neurorestoration (exercise initiated after toxin-induced cell death is complete) [6,21,22]. Such study designs are relevant since they may provide insights regarding the effects of exercise in lowering the risk of PD and other related neurodegenerative processes in individuals not yet affected, and conversely address mechanisms potentially important in targeting new therapeutic approaches for individuals already diagnosed.
Changes in dopaminergic neurotransmission with exercise
Until recently studies in both normal animals and normal human subjects or patients with PD have been limited to examining the effects of exercise on peripheral blood levels of dopamine and its metabolites. In these studies exercise has been shown to elevate peripheral dopamine levels in the blood stream and improve the response to dopamine-replacement therapy in individuals with PD [23,24]. More recently, however, studies utilizing animal models of PD have demonstrated that the behavioral benefits of exercise may not necessarily be related to absolute changes in the total level of dopamine within the basal ganglia but rather dopamine handling and neurotransmission [6,7]. The following paragraphs outline these findings.
To date, the majority of studies addressing the effects of exercise in PD have focused on evaluating neuroprotection from toxin-induced death of SNpc neurons. In these studies, a variety of exercise paradigms have been used ranging from voluntary wheel running, motorized treadmill and forced use. In general, these studies have demonstrated exercise-induced neuroprotection by evaluating the integrity of the SNpc neuron including: the expression of tyrosine hydroxylase, dopamine transporter (DAT) and vesicular transporter type-2 (VMAT-2); the survival of SNpc dopaminergic neurons through comparative cell counts; and/or analysis of the level of striatal dopamine and its metabolites. For example, exercise initiated 1 day prior to 6-OHDA or MPTP lesioning and continued for 1 week post-lesioning showed improved motor behavior, attenuation of striatal dopamine depletion, and a reduction in the loss of the dopaminergic markers including tyrosine hydroxylase, DAT and VMAT-2 when measured at completion of the exercise regimen [22]. In addition, intense motor practice through forced use of the impaired limb in the unilateral 6-OHDA-lesioned rat has been demonstrated to protect dopamine neuronal integrity and motor behavior when performed prior to lesioning or within the first week post-lesioning [5]. However, starting forced use at time points greater than 7 days post-lesioning showed no benefit, indicating that the window of neuroprotection may be limited. One hypothesis accounting for neuroprotection in these studies is thought to involve the elevation of neurotrophic factors such as gliaderived neurotrophic factor or brain-derived neurotrophic factor (BDNF) [21,25]. BDNF is the most widely distributed neurotrophic factor in the adult mammalian brain [1,26]. Increased expression of BDNF could provide protection from toxins by activating downstream signaling cascades including second messenger systems and protein kinases that may enhance neuronal survival and function [16,26,27]. An alternative hypothesis accounting for exercise-induced neuroprotection in these models may be due to reduced bioavailability or handling of the toxin itself through alterations in either the expression of DAT or VMAT-2. For example, both MPTP and 6-OHDA have prolonged periods of uptake into the dopaminergic neuron that range from 3 to 28 days, respectively [28,29]. Dopaminergic neurons may be protected owing to exercise-induced changes in the expression of DAT or VMAT-2, transporters important for uptake or storage of MPP+ (the toxic form of MPTP) and 6-OHDA in SNpc neurons [6,30–32]. In addition, exercise may induce either peripheral or central enzymes involved in metabolism or detoxification of these toxins [33–36]. For example, two studies using environmental enrichment that included an exercise component in the form of voluntary wheel running suggested that protection from MPTP lesioning was likely due to alterations in DAT and VMAT-2 protein expression in midbrain dopaminergic neurons [6,31]. Since environmental toxins have been proposed to contribute to the etiology of PD, and may share bio-activation and uptake mechanisms similar to MPTP or 6-OHDA, a possible neuroprotective benefit of exercise may be due to reduced bioavailability of toxicants capable of inducing dopaminergic neuronal cell death. Interestingly, studies from O’Dell and colleagues using pre-lesioning voluntary exercise with 4 weeks of post-lesion forced exercise showed improvement in motor performance but no protection of midbrain dopaminergic neurons from 6-OHDA-induced injury [37]. This study and similar reports suggest yet another alternative mechanism for exercise benefits including compensatory changes in remaining dopaminergic neurons and/or increased function of other basal ganglia pathways [38]. The implication of these neuroprotective studies is that exercise may be helpful in delaying or preventing PD in healthy individuals through dopaminergic and/or downstream compensatory mechanisms [39].
Because of our interest in examining the effects of exercise on neuroplastic mechanisms of the injured basal ganglia, we have focused our studies on examining the effects of intensive treadmill exercise initiated well after substantia nigra neuronal death is complete. In our experimental design, 30 days of intensive treadmill exercise was initiated 5 days after MPTP lesioning, a time point when toxin-induced cell death is complete [29]. Mice were subjected to exercise on a motorized treadmill for 30 days (5 days/week). Task-specific benefits were observed as improvements in both running velocity and endurance. Improvement was also observed on a learned motor task designed to assess balance [7]. These benefits were accompanied by increased dopamine availability, revealed as an increase in stimulus-evoked release and a decrease in extracellular decay (synaptic clearance), as measured by fast-scan cyclic voltammetry. This occurred despite the lack of any increase of total striatal dopamine to pre-lesioned levels. Interestingly, this exercise effect on dopamine release was significant within the dorsolateral striatum, an area involved in motor control. Additionally, we observed an increase in expression of dopamine D2 receptor mRNA and synaptic protein and downregulation of the DAT protein within the striatum, changes that are consistent with increased dopaminergic signaling [6]. PET imaging studies in our lab using [18F]-fallypride, a benzamide ligand with high affinity for the dopamine D2 receptor, have demonstrated an exercise-induced increase in binding potential in our mouse PD model, confirming our earlier findings that exercise facilitated dopaminergic handling and neurotransmission [40].
Changes in glutamatergic neurotransmission with exercise
Along with dopamine changes, animal studies have supported the suggestion that exercise may induce alterations in glutamate and glutamatergic receptor families, such as the N-methyl-d-aspartate (NMDA) or α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subtypes within the brain [41,42]. It is well established that both NMDA and AMPA receptors are critical components of neuroplasticity, for establishing normal synaptic function and for encoding information within the healthy brain, including long-term potentiation (LTP) and long-term depression (LTD) [43–45]. Alternatively, alterations in glutamate receptor expression within neurons and their connections may be the basis for many neurological disorders including schizophrenia, autism, addiction, AD and PD [46].
Focusing on the basal ganglia we have examined whether exercise induced any alterations in the synaptic expression of glutamate and its receptors at the level of the medium spiny neurons. In both PD and toxin-induced models of PD changes in glutamatergic neurotransmission have been well established and have been attributed to the loss of dopamine innervation and signaling [47]. These changes on the synaptic connections of medium spiny neurons include the loss of glutamatergic synapses (dendritic spine loss), changes in glutamate receptor expression and loss of synaptic plasticity (e.g., LTD) [48–51]. The medium spiny neurons comprise the majority of cells within the striatum (caudate and putamen). These neurons provide the major output projections from the striatum called the direct (dopamine D1 receptor) and indirect (dopamine D2 receptor) containing medium spiny neuron pathways [52]. Inputs to the basal ganglia and medium spiny neurons are glutamatergic, originating from either the cerebral cortex (corticostriatal) or thalamus (thalamostriatal). In PD, along with the suggestion of altered synchronization along the beta frequency band within the subthalamic nucleus of the basal ganglia, it has been postulated that aberrant and poorly modulated glutamatergic neurotransmission due to the loss of dopamine contributes to a hyperexcitability state within the striatal medium spiny neurons, and loss of synaptic connections and are thought to be responsible for mediating motor deficits [53]. The potential process by which exercise could restore synaptic integrity within the basal ganglia and restore behavioral function may include a decrease in glutamatergic hyperexcitability through a general reduction in glutamatergic neurotransmission and diminished synaptic strength (i.e., LTD) at the level of the medium spiny neuron. As such, studies in our laboratory support the theory that exercise-induced neuroplasticity alters glutamate release, glutamate receptor expression as well as the neurophysiological properties of medium spiny neurons in the MPTP mouse model, which could lead to the attenuation of glutamatergic hyperexcitability through a decrease in glutamate-based synaptic strength [6,54]. Specifically, immuno-electron microscopy studies show that intensive treadmill exercise decreases presynaptic glutamate within striatal terminals, thereby reducing glutamate release [6]. Exercise also has an effect on the expression of postsynaptic glutamate receptors [41]. AMPA receptors are: abundant in the basal ganglia; responsible for fast excitatory neurotransmission including modulating NMDA receptor activity; and are involved in experience-dependent synaptic plasticity in many regions of the brain including the visual system and hippocampus [55–58]. The AMPA receptor is an ionotropic channel that converts the chemical signal of presynaptic release of glutamate into a postsynaptic electrical signal through the mobilization of cations such as Na2+ and Ca2+ [59]. These receptors exist as a heteromeric tetramer consisting of four subunits, GluR1 through to GluR4, with GluR1 and GluR2 being the most abundant in the striatum [60,61]. The subunit GluR2 plays a special role in glutamate neurotransmission in that it acts to regulate calcium influx due to its restrictive channel properties and is a key component in receptor trafficking, a primary mechanism regulating synaptic neurotransmission [59,62,63]. GluR2 has also been suggested to be important in maintaining the integrity of the dendritic spine and its synapse [64]. Our published and ongoing studies show that intensive treadmill exercise leads to an increase in the synaptic occupancy of the GluR2 subunit and its phosphorylated state at the amino acid serine880 [54]. Alterations in GluR2 expression and its phosphorylation have been associated with diminished synaptic strength (i.e., LTD) [61,65–67]. Increased expression of the GluR2 subunit within the tetrameric complex of the AMPA receptor, as seen in our exercised mice, creates an additional positive charge within the channel pore, which impedes cation flow, lowers calcium conductance and thus diminishes synaptic strength [59,62]. Another means of regulating AMPA receptor transmission occurs via trafficking and its removal from the postsynaptic membrane. This may be regulated through phosphorylation of AMPA receptor subunits, including GluR2, leading to internalization of the entire receptor complex and decreased synaptic strength (i.e., LTD) [68,69]. Additional electrophysiological whole cell recordings in acute striatal slices of the MPTP mouse model indicate that exercise alters the synaptic expression of the GluR2 subunit and decreases excitability in the medium spiny neurons, as demonstrated by reduced polyamine sensitivity and loss of rectification in AMPA receptor conductance at depolarized membrane potentials, and reduced excitatory postsynaptic conductances generated by corticostriatal stimulation within medium spiny neurons [54]. These findings provide strong evidence that changes in GluR2 expression may play an important role in exercise-induced reduction in the excitatory postsynaptic conductances of medium spiny neurons and contribute to the return of normal synaptic function within the basal ganglia [59,62,63]. Research is ongoing in an attempt to demonstrate that exercise can restore synaptic connections (increased dendritic spine density) and synaptic function, as measured through LTD, in the striatum of MPTP mice [Walsh JP, Jakowec MW, Pers. Comm.]. Collectively, these data suggest that exercise through alterations in glutamatergic neurotransmission may help to re-establish normal synaptic plasticity of the injured basal ganglia resulting in the restoration of motor behavior. Within the normal brain, exercise has also been demonstrated to modulate glutamate receptor synaptic expression and LTP. Specifically, voluntary wheel running has been shown to lead to alterations in the mRNA expression of the NR2B subunit of the NMDA receptor within the dentate gyrus of the adult rat, to enhance synaptic efficiency (lower tetanic threshold to induce LTP) and to improve reference-based learning using the Morris water maze [41]. In addition, 1 month of voluntary exercise has also been shown to increase the level of protein expression of the AMPA receptor subunits, GluR1 and GluR2/3, within the frontal cortex of albino mice [42].
In summary, these findings suggest that alterations in both dopaminergic and glutamatergic neurotransmission through experience-dependent processes modulate cortical hyperexcitability of the basal ganglia. Thus modulation of corticostriatal hyperexcitability may underlie exercise-induced behavioral improvement. The next step is to translate these findings to humans, and to investigate whether high intensity exercise has similar benefits in patients with PD.
Neurotrophic factors
Studies in both humans and animals have shown that physical activity (primarily running) can increase a wide range of neurotrophic factors including BDNF, glia-derived neurotrophic factor, NGF, FGF and IGF-1 [70–74]. Exercise-induced induction of neurotrophic factors occurs in a number of brain regions involved in learning, memory, mental processing, mood and motor control, including the hippocampus, cerebellum, cerebral cortex and striatum [26,70,71]. The precise mechanism that triggers neurotrophic expression and the cells in which this occurs with exercise is not fully elucidated. Metabolic demand, neurotransmitter release and other released factors may serve as intrinsic signals to induce neurotrophic activation. In addition, the peripheral signals and sources may also play a role and may include components of the immune system (activated macrophages) or musculature and other tissues (sources of leptins or insulin signaling to the brain) [75–77]. One of the most studied factors is BDNF whose upregulation is closely linked to exercise and may be an important player in exercise-dependent benefits [17–20]. BDNF is widely distributed throughout the brain and provides both neurotrophic and neuroprotective support to many subpopulations of neurons throughout development and adulthood. Exercise modulates the induction of BDNF in a time-dependent manner within the hippocampus and cerebral cortex, is a key mediator of synaptic efficacy and experience-dependent neuroplasticity, and is associated with both learning and memory [1,16]. In rodents, BDNF and its receptors (TrkB and p75) appear quickly (days) when they are undergoing voluntary wheel running and induced levels are sustained for several weeks after completion of the exercise regimen. In addition, exercise primes a molecular memory for BDNF induction where a brief subsequent session after cessation of exercise can reinduce neurotrophic expression [27]. While many of the precise molecular details of neurotrophic factors, including the action of BDNF, are not yet fully elucidated it is proposed that they act to lower the threshold for conversion of experiences into strengthening of neuronal connections. For example, BDNF can lower the threshold for induction of LTP within the hippocampus, therefore promoting memory and learning [1]. In addition, neurotrophic factors can promote a wide range of effects including: synaptogenesis at dendritic sites (local translation effects); activation of kinases, including the MAPK cascades; induction of gene expression at the level of the cell nucleus; the regulation of presynaptic neurotransmitter release; and increased neurogenesis. These neurotrophic-mediated mechanisms may play a key role in exercise-induced neuroplasticity and repair processes in PD [17].
Clinical studies of exercise in PD
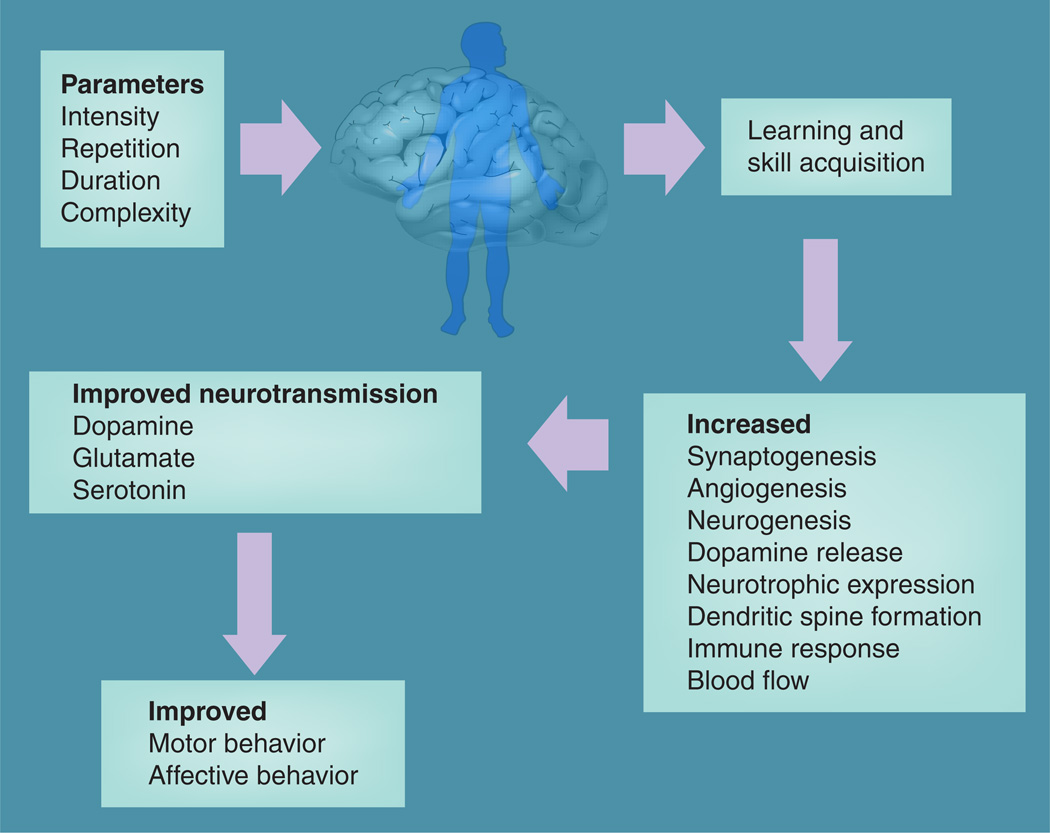
While in the last few decades there have been numerous studies demonstrating the beneficial effects of exercise in individuals with PD, only recently has there been a general interest in examining whether exercise may be able to restore brain function and/or modify disease progression [78]. Figure 1 highlights potential mechanisms of the effects of exercise on the brain. For example, an analysis of these older exercise studies show that while in general they were of low-to-moderate intensity, they could be grouped into six categories including: passive range of motion (ROM) and stretching; active ROM; balance activities; gait; resistance training; and practice of functional activities and transitional movements (i.e., sit-to-stand). These studies have shown that exercise may help walking ability and activities of daily living, as well as neurological symptoms such as slowness, stiffness and balance dysfunction [79–81]. More recently, a number of studies examining the effects of treadmill training have shown that individuals with PD can benefit from treadmill exercise as gait behavior is driven more automatically [82]. Improved motor performance has been reported and treadmill speeds have gradually increased from studies in which subjects trained at self-selected velocities for comfort to speeds above over-ground walking velocity [83–87]. In these later studies, individuals with PD undergoing treadmill training were compared with those undergoing standard physical therapy involving general conditioning and ROM, with treadmill subjects exhibiting significantly greater changes in disease severity ratings as determined by the Unified Parkinson’s Disease Rating Scale (UPDRS), and in gait and balance parameters. However, despite the increase in the level of intense and challenging exercise implanted in PD clinical trials, there remains a major gap in our knowledge regarding the exercise effects on the CNS and brain function in individuals with PD.
Figure 1.
Exercise and the brain in patients with Parkinson’s disease.
Given that PD is considered a disorder with limited capacity for repair or compensatory mechanisms, only recently has there been interest in examining exercise-induced changes specifically within the basal ganglia circuitry, a region most affected in PD. As such, we and others have initiated studies to address exercise-induced changes within the brain of individuals with PD [88]. Specifically, the behavioral benefits and exercise-induced changes in basal ganglia function observed in the rodent models of PD led us to ask the question: could exercise induce changes in basal ganglia function in individuals with PD that mirror those observed in the rodent model? To address this question we incorporated two parameters of exercise (high motor challenge and high repetition) that we, and others, consider essential for driving neuroplasticity [2]. Individuals with PD (no more than 3 years from initial diagnosis) underwent treadmill training three-times per week for 8 weeks. Subjects were challenged to walk at higher than self-selected gait speeds within the constraints of an observationally normal gait pattern. Subjects were progressed to running speeds of up to 8 miles/h. The intent of this challenging and intensive practice was to target the skill of gait and dynamic balance, motor functions that are commonly impaired in PD. In the course of this training subjects execute a high repetition of stepping, are actively engaged in the training and have the sensory experience of normal gait kinematics. Outcomes consisted of measures of motor performance, including gait kinematics, sit-to-stand and stair climbing. Unique to this human trial, and directly related to our animal findings, was the inclusion of measures of corticoexcitability using transcranial magnetic stimulation (TMS). TMS is a noninvasive method of stimulating the brain and provides a tool for assessment of excitability of the corticospinal motor system. Single TMS pulses are applied over the motor cortex while recording surface electromyography responses over the contralateral target muscle. If the target muscle is preactivated (contracted), the TMS pulse induces a characteristic transient period of electromyography silence called the cortical silent period (CSP). Importantly, TMS studies have shown systematic abnormalities of CSP and other corticoexcitability measures in individuals with PD [89,90]. In general, these abnormalities reflect cortical hyperexcitability in PD compared with non-PD control subjects [91,92]. As CSP represents inhibitory influences on corticoexcitability, higher excitability would be evident as a shortened CSP duration. In fact, shortened CSP durations are among the most consistent and widely reproduced TMS finding amongst PD patients [93]. Furthermore, symptomatic treatment of PD with surgical or pharmacological interventions is associated with lengthening of the CSP towards levels seen in control subjects [94,95]. Thus, increased CSP duration could underlie symptomatic improvement, such as improved motor performance. Not only is TMS an excellent tool to measure CSP duration and to examine possible exercise-induced changes in PD, but also, more importantly, TMS may be used to support the existence of CNS changes in response to different exercise parameters including intensity. After 24 sessions of treadmill training, subjects demonstrated improved postural control walking performance including increased gait velocity, stride length, step length, and hip and ankle joint excursion, and improved weight distribution during sit-to-stand. More importantly, these subjects also showed reversal of cortical hyperexcitability indicated by increased CSP. In fact, every subject undergoing intensive treadmill training showed exercise-induced lengthening of CSP. To our knowledge this was the first demonstration of exercise-induced cortical changes in the brain in individuals with PD.
Given that the animal studies demonstrated an exercise-induced change in dopamine signaling (increased expression of striatal dopamine D2 receptors), without an absolute change in striatal dopamine, we sought to investigate the effects of intensive treadmill exercise on D2 receptor expression using PET imaging and [18F]-fallypride. Our preliminary results show that 8 weeks of intensive treadmill exercise is accompanied by a 80–90% increase in dopamine D2 receptor expression (indicated by increased binding potential) in two individuals with PD compared with no change in two individuals with PD that did not exercise [Fisher BE, Petzinger GM, Pers. Comm.]. Although not directly examined, other investigators have shown improvements in behavior that imply exercise-induced central changes. For example, Ridgel and colleagues showed improved reaction time in bimanual dexterity after forced tandem bike training in individuals with PD [96]. Farley et al. showed changes in hand writing after total body high amplitude training [97]. Given that these behavioral outcomes were unrelated to the exercise task, it suggests a generalized motor benefit that could be attributed to central brain changes.
While these results are intriguing and high-light the need for further studies examining the role of exercise in modifying disease progression of PD, there are many questions that remain. First, it is important to determine whether exercise-induced alterations in basal ganglia function and brain circuitry directly link to improved behavioral performance. Another important question that remains is whether challenge, intensity and the principals of practice are necessary and sufficient ingredients for driving neuroplastic brain changes or whether aerobic exercise in combination with skill training or alone may be required to supply the needed metabolic demand of a compromised motor circuitry. Other challenging questions include the interactions between drugs and exercise, as well as deep brain stimulation and exercise, and finally stem cell or gene therapy and exercise.
Effects of exercise on non-motor behaviors in PD
While the majority of studies concerning exercise in patients with PD and in animal models have focused on benefits to motor behavior, there is growing interest in the potential benefits of exercise on non-motor behaviors, including anxiety, depression and cognition. Studies have reported beneficial effects of exercise on cognition (frontal and hippocampal function), depression and anxiety in humans, including improvements in mood disturbances associated with chronic disease [98–102]. Rats exposed to exercise training exhibited more emotional stability in the open field compared with their pre-exercise level and showed resistance to behavioral deficits under stress-induced depression [103,104]. Furthermore, the beneficial effects of exercise on mood may also be sustained. For example, in a study by Motl and colleagues, which randomized older adults to 6 months of walking or resistance/flexibility training, depressive symptoms were reduced after the intervention and were sustained in the long term after intervention initiation [105]. Sustained effects of exercise (6 months postexercise) on depression have also been reported in adult volunteers with major depression [100,106]. The underlying mechanisms are currently unknown, but the beneficial effects of exercise may reflect increased levels of neurotrophic factors such as BDNF [16] or improvement in circadian rhythmicity [107,108]. Indeed, it seems likely that exercise has a variety of effects on neurotransmitters and neurotrophic factors. In light of the importance of serotonin and dopamine in affective behavior, and since we observe significant changes in dopamine neurotransmission in both the MPTP-lesioned mouse model and in patients with PD undergoing intensive treadmill exercise, it is likely that the serotonergic system plays a central role in mediating the benefits of exercise on non-motor behaviors. Exercise may increase CNS levels of serotonin indirectly through elevated uptake of tryptophan across the blood–brain barrier. In addition, animal studies suggest that exercise also enhances release of serotonin and/or alters the expression of serotonin receptors. The adaptive changes in serotonin receptor subtypes are similar to the changes induced by a variety of antidepressants after long-term treatment in animal models [109]. Our studies in MPTP-lesioned mouse models showed that either forced or voluntary exercise is able to reduce anxiety but levels of dopamine and serotonin are unaltered [110]. This suggests that receptors and their downstream pathways may mediate the benefits of exercise.
Potential parameters of exercise that drive neuroplasticity
While we are currently in the early stages of understanding the key ingredients of exercise that can lead to meaningful changes within the CNS, basic and clinical studies have identified various exercise parameters. There are both exercise-specific and patient-specific parameters to consider. For exercise to result in central changes it must involve skill acquisition. In other words, repair of the injured brain is a learning process. Exercise-specific parameters known to promote a skill acquisition process include, but are not limited to, repetition, complexity and intensity [2]. Repetition is critical for inducing neuroplastic changes. Animal studies have shown that low levels of repetition, while showing some signs of behavioral benefit, do not become consolidated in long-term plastic changes compared with high levels of repetition [111]. Simply engaging a neural circuit is not sufficient to drive neuroplasticity but a critical threshold must be reached to transfer exercise into central changes. As in any skill acquisition or learning paradigm the complexity and intensity of the task engages the learner, thereby recruiting and strengthening multiple neuronal circuits [112,113].
There are a number of important patient-specific parameters that must be taken into consideration in order for an intervention to have long-lasting benefits. As stated above, in order for task practice to result in permanent (or long-lasting) changes in synaptic connections, the individual patient must be engaged and attending to the task. This will involve motivation on the part of the patient; they must be aware of the potential benefits of exercise. Since neurotransmitter systems (especially dopamine projections to the ventral striatum) that involve motivation and reward are compromised in PD, patients may need to be actively engaged by a trained physical therapist or personal trainer to assist with bolstering motivation [114]. Additionally, since learning is a critical factor in driving the benefits of experience-dependent neuroplasticity, feedback from a physical therapist can also assist patients with problem-solving during exercise. Another patient-specific parameter to consider is age [115–118]. A younger patient whose brain may display more robust experience-dependent plasticity may manifest increased benefits from exercise, while older patients may require greater intensity or duration to gain a similar level of effects. Disease severity may also influence the impact of exercise on neuroplasticity, as well as dictate the mode of exercise delivery. In conclusion, the mode of exercise delivery may not be as important as incorporating the parameters of skill acquisition into any exercise regimen that is employed.
Exercise- and patient-specific parameters highlight some of the challenges to designing an exercise regimen for treating individuals with PD. Many other questions remain that will be addressed in further clinical studies and basic research in animal models. Does aerobic exercise that promotes angiogenesis lead to potential disease-modifying changes compared with skill acquisition that promotes synaptogenesis [119]? Can patients who engage in an exercise regimen in the late stage of the disease overcome synaptic loss? How does exercise interact with ongoing therapeutic treatments of PD such as dopamine-replacement therapy and deep brain stimulation?
Impact on patient care
We have described exercise-induced neuroplasticity in the injured basal ganglia through alterations in dopaminergic and glutamatergic neurotransmission. These alterations may underlie the exercise-induced attenuation of corticostriatal hyperexcitability, which may account for observed behavioral benefits in PD. Our animal studies have led to the employment of intensive and challenging treadmill training in individuals with early-stage PD. The improvements in behavioral and central changes support the concept that exercise could lead to disease-modifying neuroplastic change in individuals with PD.
Understanding the impact of exercise in PD has broad implications. Not only is there potential to develop new insights into mechanisms of neuroplasticity and motor recovery in PD, but the study of exercise may lead to the development of novel therapeutics aimed at facilitating synaptic connections in a variety of degenerative disorders including AD, multiple sclerosis, amyotrophic lateral sclerosis and Huntington’s disease.
Future perspective
It is now becoming evident that exercise is a critical component in the care of many neurodegenerative disorders, including PD, and should be part of the standard of care. Small clinical trials and studies in animal models of PD have shown the benefits of exercise and are beginning to reveal the underlying molecular mechanisms. However, there remain many major gaps in our knowledge that must be addressed by large double-blind clinical trials, as well as studies in animal models. For example, we do not yet know which parameters of exercise, including duration, intensity and repetition, are necessary to gain the most benefit. What are the delineating factors between aerobic exercise and skill acquisition required to improve both body and brain health in individuals with PD? Animal models will continue to provide valuable insights into the molecular mechanisms underlying the benefits of exercise, especially those studies focused on developing healthy synapses, and promoting factors that are important in protecting and promoting survival of neurons and their respective connections. An additional exciting aspect of animal models is the availability of transgenic strains targeting genes and proteins implicated in familial forms of PD, including α-Synuclein, DJ-1, Parkin and LRRK2, where the effects of exercise on disease progression can also be investigated. Taken together, studies in PD and animal models of PD continue to provide valuable insight regarding the potential for experience and aspects of exercise and a healthy lifestyle that may prove beneficial in the long-term health of the human brain.
Practice Points.
-
▪
Epidemiological studies have shown that exercise, especially high intensity exercise over the lifetime of a subject, reduces the risk for developing Parkinson’s disease (PD).
-
▪
While controlled clinical trials have been limited, they do show that exercise provides motor benefit to patients with PD and in aged subjects, and exercise provides cognitive benefits including improved executive function, memory and learning.
-
▪
The adult brain possesses a tremendous capacity for experience-dependent neuroplasticity, even in the context of aging and neurodegenerative disorders including PD, where the activation of neurotrophic factors may play a key role.
-
▪
Animal models of dopamine depletion are beginning to reveal the underlying mechanisms by which exercise can remodel the brain through alteration in neuronal synaptic connections, especially dopaminergic and glutamatergic neurotransmission within the basal ganglia.
-
▪
Exercise is beneficial and should be routinely prescribed in the management of PD.
-
▪
Studies are needed to better define the parameters of exercise that may lead to disease modification in individuals with PD, including the degree of aerobic versus skill training, duration, intensity and frequency.
Acknowledgments
This work was supported by the Parkinson’s Alliance, Team Parkinson LA, the Whittier Parkinson’s Disease Education Group, National Institute of Neurological Disorders and Stroke Grant R01 NS44327–1, and the United States Army Neurotoxin Exposure Treatment Research Program Grant W81XWH-04–1-0444. A special thanks to friends of the University of Southern California Parkinson’s Disease Research Group, including George and MaryLou Boone, Roberto Gonzales, and Walter and Susan Doniger for their financial support.
Footnotes
Disclosure
All authors have provided substantial contributions to the primary data presented in this manuscript.
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167(3):588–597. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 2008;51(1):S225–S239. doi: 10.1044/1092-4388(2008/018). ▪▪ Excellent review of field of neuroplasticity and the parameters of exercise for repair.
- 3.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 4.Fahn S, Przedborski S. Parkinsonism. In: Rowland LP, editor. Merritt’s Neurology. Philadelphia, PA, USA: Lippincott Williams and Wilkins; 2000. pp. 679–693. [Google Scholar]
- 5. Tillerson JL, Cohen AD, Philhower J, et al. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J. Neurosci. 2001;21(12):4427–4435. doi: 10.1523/JNEUROSCI.21-12-04427.2001. ▪▪ One of first papers to investigate the role of exercise in a rodent model of Parkinson’s disease.
- 6.Fisher BE, Petzinger GM, Nixon K, et al. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J. Neurosci. Res. 2004;77(3):378–390. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- 7. Petzinger GM, Walsh JP, Akopian G, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J. Neurosci. 2007;27(20):5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. ▪▪ Demonstrates the effects of exercise on dopamine neurotransmission in a rodent model of Parkinson’s disease.
- 8.Hornykiewicz O, Kish SJ. Biochemical pathophysiology of Parkinson’s disease. Adv. Neurol. 1987:4519–4534. [PubMed] [Google Scholar]
- 9.Garcia BG, Neely MD, Deutch AY. Cortical regulation of striatal medium spiny neuron dendritic remodeling in parkinsonism: modulation of glutamate release reverses dopamine depletion-induced dendritic spine loss. Cereb. Cortex. 2010;20(10):2423–2432. doi: 10.1093/cercor/bhp317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc. Natl Acad. Sci. USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obeso JA, Marin C, Rodriguez-Oroz C, et al. The basal ganglia in Parkinson’s disease: current concepts and unexplained observations. Ann. Neurol. 2008;64(Suppl. 2):S30–S46. doi: 10.1002/ana.21481. [DOI] [PubMed] [Google Scholar]
- 12.Poewe W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008;15(Suppl.):114–120. doi: 10.1111/j.1468-1331.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 13. Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64(4):664–669. doi: 10.1212/01.WNL.0000151960.28687.93. ▪▪ Epidemiological study supporting the theory that intensive exercise can be protective against Parkinson’s disease.
- 14.Xu Q, Park Y, Huang X, et al. Physical activities and future risk of Parkinson disease. Neurology. 2010;75(4):341–348. doi: 10.1212/WNL.0b013e3181ea1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122(3):647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 16. Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci. Lett. 2004;363(1):43–48. doi: 10.1016/j.neulet.2004.03.058. ▪▪ Implicates elevated expression of brain-derived neurotrophic factor by exercise and its role in neuroplasticity.
- 17.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 19.Jakowec MW, Petzinger GM. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned model of parkinson’s disease, with emphasis on mice and nonhuman primates. Comp. Med. 2004;54(5):497–513. [PubMed] [Google Scholar]
- 20.Simola N, Morelli M, Carta AR. The 6-hydroxydopamine model of Parkinson’s disease. Neurotox. Res. 2007;11(3–4):151–167. doi: 10.1007/BF03033565. [DOI] [PubMed] [Google Scholar]
- 21.Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J. Neurochem. 2003;85(2):299–305. doi: 10.1046/j.1471-4159.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- 22.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience. 2003;119(3):899–911. doi: 10.1016/s0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 23.Carter JH, Nutt JG, Woodward WR. The effect of exercise on levodopa absorption. Neurology. 1992;42(10):2042–2045. doi: 10.1212/wnl.42.10.2042. [DOI] [PubMed] [Google Scholar]
- 24.Muhlack S, Welnic J, Woitalla D, Muller T. Exercise improves efficacy of levodopa in patients with Parkinson’s disease. Mov. Disord. 2007;22(3):427–430. doi: 10.1002/mds.21346. [DOI] [PubMed] [Google Scholar]
- 25.Wu SY, Wang TF, Yu L, et al. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav. Immun. 2010;25(1):135–146. doi: 10.1016/j.bbi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 26. Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726(1–2):49–56. ▪ An early report of elevated brain-derived neurotrophic factor expression with exercise.
- 27. Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–861. doi: 10.1016/j.neuroscience.2005.03.026. ▪▪ Demonstrates that exercise may have long-term effects in providing a molecular memory.
- 28.Sauer H, Oertel W. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59(2):401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- 29.Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4(3):257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 30.Gainetdinov RR, Fumagalli F, Jones SR, Caron MG. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J. Neurochem. 1997;69(3):1322–1325. doi: 10.1046/j.1471-4159.1997.69031322.x. [DOI] [PubMed] [Google Scholar]
- 31.Bezard E, Dovero S, Belin D, et al. Enriched environment confers resistance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and cocaine: involvement of dopamine transporter and trophic factors. J. Neurosci. 2003;23(5):10999–11007. doi: 10.1523/JNEUROSCI.23-35-10999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guillot TS, Miller GW. Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol. Neurobiol. 2009;39(2):149–170. doi: 10.1007/s12035-009-8059-y. [DOI] [PubMed] [Google Scholar]
- 33.Hattori S, Naoi M, Nishino H. Striatal dopamine turnover during treadmill running in the rat: relation to the speed of running. Brain Res. Bull. 1994;35(1):41–49. doi: 10.1016/0361-9230(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 34.Tumer N, Demirel HA, Serova L, et al. Gene expression of catecholamine biosynthetic enzymes following exercise: modulation by age. Neuroscience. 2001;103(3):703–711. doi: 10.1016/s0306-4522(01)00020-3. [DOI] [PubMed] [Google Scholar]
- 35.Erdem SR, Demirel HA, Broxson CS, et al. Effect of exercise on mRNA expression of select adrenal medullary catecholamine biosynthetic enzymes. J. Appl. Physiol. 2002;93(2):463–468. doi: 10.1152/japplphysiol.00627.2001. [DOI] [PubMed] [Google Scholar]
- 36.Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic. Biol. Med. 2008;44(2):153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 37.O’Dell SJ, Gross NB, Fricks AN, et al. Running wheel exercise enhances recovery from nigrostriatal dopamine injury without inducing neuroprotection. Neuroscience. 2007;144(3):1141–1151. doi: 10.1016/j.neuroscience.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 38.Pothakos K, Kurz MJ, Lau YS. Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson’s disease with severe neurodegeneration. BMC Neurosci. 2009;10(1):6. doi: 10.1186/1471-2202-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasco AJ, Paffenbarger RSJ, Gendre I, Wing AL. The role of physical exercise in the occurrence of Parkinson’s disease. Arch. Neurol. 1992;49(4):360–365. doi: 10.1001/archneur.1992.00530280040020. [DOI] [PubMed] [Google Scholar]
- 40. Vuckovic MG, Li Q, Fisher B, et al. Exercise elevates dopamine D2 receptor in a mouse model of Parkinson’s disease: in vivo imaging with [18F]fallypride. Mov. Disord. 2010;25(16):2777–2784. doi: 10.1002/mds.23407. ▪ Demonstrates that exercise can influence postsynaptic sites involved in dopamine neurotransmission.
- 41.Farmer J, Zhao X, van Praag H, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124(1):71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 42.Dietrich MO, Mantese CE, Porciuncula LO, et al. Exercise affects glutamate receptors in postsynaptic densities from cortical mice brain. Brain Res. 2005;1065(1–2):20–25. doi: 10.1016/j.brainres.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 43.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 44. Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543–554. doi: 10.1016/j.neuron.2008.11.005. ▪ Excellent review of the pathophysiology of basal ganglia function.
- 45.Keifer J, Zheng Z. AMPA receptor trafficking and learning. Eur. J. Neurosci. 2010;32(2):269–277. doi: 10.1111/j.1460-9568.2010.07339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr. Neurol. Neurosci. Rep. 2010;10(3):207–214. doi: 10.1007/s11910-010-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pisani A, Centonze D, Bernardi G, Calabresi P. Striatal synaptic plasticity: implications for motor learning and Parkinson’s disease. Mov. Disord. 2005;20(4):395–402. doi: 10.1002/mds.20394. [DOI] [PubMed] [Google Scholar]
- 48. Day M, Wang Z, Ding J, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat. Neurosci. 2006;9(2):251–259. doi: 10.1038/nn1632. ▪▪ Demonstrates the loss of dendritic synapses in models of Parkinson’s disease.
- 49. Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30(5):211–219. doi: 10.1016/j.tins.2007.03.001. ▪▪ Reviews the physiological role of dopamine in the basal ganglia.
- 50.Calabresi P, Mercuri NB, Di Filippo M. Synaptic plasticity, dopamine and Parkinson’s disease: one step ahead. Brain. 2009;132(Pt 2):285–287. doi: 10.1093/brain/awn340. [DOI] [PubMed] [Google Scholar]
- 51. Villalba RM, Smith Y. Striatal spine plasticity in Parkinson’s disease. Front. Neuroanat. 2010;4:133. doi: 10.3389/fnana.2010.00133. ▪▪ Review of spine structure in Parkinson’s disease.
- 52.Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr. Opin. Neurobiol. 1996;6(6):751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- 53.Calabresi P, Pisani A, Mercuri NB, Bernardi G. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci. 1996;19(1):19–24. doi: 10.1016/0166-2236(96)81862-5. [DOI] [PubMed] [Google Scholar]
- 54.VanLeeuwen JE, Petzinger GM, Walsh JP, et al. Altered AMPA receptor expression with treadmill exercise in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J. Neurosci. Res. 2010;88(3):650–668. doi: 10.1002/jnr.22216. [DOI] [PubMed] [Google Scholar]
- 55.Groc L, Gustafsson B, Hanse E. AMPA signalling in nascent glutamatergic synapses: there and not there! Trends Neurosci. 2006;29(3):132–139. doi: 10.1016/j.tins.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 56.Hanley JG. AMPA receptor trafficking pathways and links to dendritic spine morphogenesis. Cell Adh. Migr. 2008;2(4):276–282. doi: 10.4161/cam.2.4.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61(3):340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKinney RA. Excitatory amino acid involvement in dendritic spine formation, maintenance and remodelling. J. Physiol. 2010;588(Pt 1):107–116. doi: 10.1113/jphysiol.2009.178905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science. 1991;252(5007):851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- 60.Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J. Neurosci. 1997;17(2):819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54(6):859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Geiger JR, Melcher T, Koh DS, et al. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15(1):193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 63.Pellegrini-Giampietro DE, Gorter JA, Bennett MV, Zukin RS. The GluR2 (GluR-B) hypothesis: Ca2+-permeable AMPA receptors in neurological disorders. Trends Neurosci. 1997;20(10):464–470. doi: 10.1016/s0166-2236(97)01100-4. [DOI] [PubMed] [Google Scholar]
- 64.Passafaro M, Nakagawa T, Sala C, Sheng M. Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2. Nature. 2003;424(6949):677–681. doi: 10.1038/nature01781. [DOI] [PubMed] [Google Scholar]
- 65.Liu SJ, Cull-Candy SG. Activity-dependent change in AMPA receptor properties in cerebellar stellate cells. J. Neurosci. 2002;22(10):3881–3889. doi: 10.1523/JNEUROSCI.22-10-03881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seidenman KJ, Steinberg JP, Huganir R, Malinow R. Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J. Neurosci. 2003;23(27):9220–9228. doi: 10.1523/JNEUROSCI.23-27-09220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gardner SM, Takamiya K, Xia J, et al. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45(6):903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 68.Esteban JA. Intracellular machinery for the transport of AMPA receptors. Br. J. Pharmacol. 2008;153(Suppl. 1):S35–S43. doi: 10.1038/sj.bjp.0707525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang L, Role LW. Facilitation of corticoamygdala synapses by nicotine: activity-dependent modulation of glutamatergic transmission. J. Neurophysiol. 2008;99(4):1988–1999. doi: 10.1152/jn.00933.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gomez-Pinilla F, Dao L, So V. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain Res. 1997;764(1–2):1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- 71.Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J. Neurosci. 2001;21(15):5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ang ET, Wong PT, Moochhala S, Ng YK. Neuroprotection associated with running: is it a result of increased endogenous neurotrophic factors? Neuroscience. 2003;118(2):335–345. doi: 10.1016/s0306-4522(02)00989-2. [DOI] [PubMed] [Google Scholar]
- 73.Zoladz JA, Pilc A, Majerczak J, et al. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J. Physiol. Pharmacol. 2008;59(Suppl. 7):119–132. [PubMed] [Google Scholar]
- 74.Llorens-Martin M, Torres-Aleman I, Trejo JL. Exercise modulates insulin-like growth factor 1-dependent and -independent effects on adult hippocampal neurogenesis and behaviour. Mol. Cell Neurosci. 2010;44(2):109–117. doi: 10.1016/j.mcn.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 75.Pedersen BK, Bruunsgaard H, Jensen M, Krzywkowski K, Ostrowski K. Exercise and immune function: effect of ageing and nutrition. Proc. Nutr. Soc. 1999;58(3):733–742. doi: 10.1017/s0029665199000968. [DOI] [PubMed] [Google Scholar]
- 76.Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 2001;21(5):1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Archer T, Fredriksson A, Schupsilontz E, Kostrzewa RM. Influence of physical exercise on neuroimmunological functioning and health: aging and stress. Neurotox. Res. 2010 doi: 10.1007/s12640-010-9224-9. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 78. Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 2008;23(5):631–640. doi: 10.1002/mds.21922. ▪▪ Critical review of clinical trials of exercise in individuals with Parkinson’s disease.
- 79.Hass CJ, Waddell DE, Fleming RP, Juncos JL, Gregor RJ. Gait initiation and dynamic balance control in Parkinson’s disease. Arch. Phys. Med. Rehabil. 2005;86(11):2172–2176. doi: 10.1016/j.apmr.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 80.Hackney ME, Earhart GM. Tai Chi improves balance and mobility in people with Parkinson disease. Gait Posture. 2008;28(3):456–460. doi: 10.1016/j.gaitpost.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schenkman M, Hall D, Kumar R, Kohrt WM. Endurance exercise training to improve economy of movement of people with Parkinson disease: three case reports. Phys. Ther. 2008;88(1):63–76. doi: 10.2522/ptj.20060351. [DOI] [PubMed] [Google Scholar]
- 82. Mehrholz J, Friis R, Kugler J, et al. Treadmill training for patients with Parkinson’s disease. Cochrane Database Syst. Rev. 2010;1 doi: 10.1002/14651858.CD007830.pub2. CD007830. ▪▪ A critical review of clinical trials of exercise in individuals with Parkinson’s disease.
- 83.Miyai I, Fujimoto Y, Ueda Y, et al. Treadmill training with body weight support: its effect on Parkinson’s disease. Arch. Phys. Med. Rehabil. 2000;1(7):849–852. doi: 10.1053/apmr.2000.4439. [DOI] [PubMed] [Google Scholar]
- 84.Miyai I, Fujimoto Y, Yamamoto H, et al. Long-term effect of body weight-supported treadmill training in Parkinson’s disease: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2002;83(10):1370–1373. doi: 10.1053/apmr.2002.34603. [DOI] [PubMed] [Google Scholar]
- 85.Pohl M, Rockstroh G, Ruckriem S, Mrass G, Mehrholz J. Immediate effects of speed-dependent treadmill training on gait parameters in early Parkinson’s disease. Arch. Phys. Med. Rehabil. 2003;84(12):1760–1766. doi: 10.1016/s0003-9993(03)00433-7. [DOI] [PubMed] [Google Scholar]
- 86.Toole T, Maitland CG, Warren E, Hubmann MF, Panton L. The effects of loading and unloading treadmill walking on balance, gait, fall risk, and daily function in Parkinsonism. NeuroRehabilitation. 2005;20(4):307–322. [PubMed] [Google Scholar]
- 87.Herman T, Giladi N, Gruendlinger L, Hausdorff JM. Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson’s disease: a pilot study. Arch. Phys. Med. Rehabil. 2007;88(9):1154–1158. doi: 10.1016/j.apmr.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 88.Fisher BE, Wu AD, Salem GJ, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch. Phys. Med. Rehabil. 2008;89(7):1221–1229. doi: 10.1016/j.apmr.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kleine BU, Praamstra P, Stegeman DF, Zwarts MJ. Impaired motor cortical inhibition in Parkinson’s disease: motor unit responses to transcranial magnetic stimulation. Exp. Brain Res. 2001;138(4):477–483. doi: 10.1007/s002210100731. [DOI] [PubMed] [Google Scholar]
- 90.Cantello R, Tarletti R, Civardi C. Transcranial magnetic stimulation and Parkinson’s disease. Brain Res. Brain Res. Rev. 2002;38(3):309–327. doi: 10.1016/s0165-0173(01)00158-8. [DOI] [PubMed] [Google Scholar]
- 91.Cantello R, Gianelli M, Bettucci D, et al. Parkinson’s disease rigidity: magnetic motor evoked potentials in a small hand muscle. Neurology. 1991;41(9):1449–1456. doi: 10.1212/wnl.41.9.1449. [DOI] [PubMed] [Google Scholar]
- 92.Valls-Sole J, Pascual-Leone A, Brasil-Neto JP, et al. Abnormal facilitation of the response to transcranial magnetic stimulation in patients with Parkinson’s disease. Neurology. 1994;44(4):735–741. doi: 10.1212/wnl.44.4.735. [DOI] [PubMed] [Google Scholar]
- 93.Cantello R, Gianelli M, Civardi C, Mutani R. Magnetic brain stimulation: the silent period after the motor evoked potential. Neurology. 1992;42(10):1951–1959. doi: 10.1212/wnl.42.10.1951. [DOI] [PubMed] [Google Scholar]
- 94.Young MS, Triggs WJ, Bowers D, Greer M, Friedman WA. Stereotactic pallidotomy lengthens the transcranial magnetic cortical stimulation silent period in Parkinson’s disease. Neurology. 1997;49(5):1278–1283. doi: 10.1212/wnl.49.5.1278. [DOI] [PubMed] [Google Scholar]
- 95.Strafella AP, Valzania F, Nassetti SA, et al. Effects of chronic levodopa and pergolide treatment on cortical excitability in patients with Parkinson’s disease: a transcranial magnetic stimulation study. Clin. Neurophysiol. 2000;111(7):1198–1202. doi: 10.1016/s1388-2457(00)00316-3. [DOI] [PubMed] [Google Scholar]
- 96.Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabil. Neural Repair. 2009;23(6):600–608. doi: 10.1177/1545968308328726. [DOI] [PubMed] [Google Scholar]
- 97.Farley BG, Koshland GF. Training BIG to move faster: the application of the speed-amplitude relation as a rehabilitation strategy for people with Parkinson’s disease. Exp. Brain Res. 2005;167(3):462–467. doi: 10.1007/s00221-005-0179-7. [DOI] [PubMed] [Google Scholar]
- 98.Barbour KA, Blumenthal JA. Exercise training and depression in older adults. Neurobiol. Aging. 2005;26(Suppl. 1):119–123. doi: 10.1016/j.neurobiolaging.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 99.De Moor MH, Beem AL, Stubbe JH, Boomsma DI, De Geus EJ. Regular exercise, anxiety, depression and personality: a population-based study. Prev. Med. 2006;42(4):273–279. doi: 10.1016/j.ypmed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 100.Brene S, Bjornebekk A, Aberg E, et al. Running is rewarding and antidepressive. Physiol. Behav. 2007;92(1–2):136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn. Sci. 2007;11(8):342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 102.Tanaka K, Quadros AC, Jr, Santos RF, et al. Benefits of physical exercise on executive functions in older people with Parkinson’s disease. Brain Cogn. 2009;69(2):435–441. doi: 10.1016/j.bandc.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 103.Fulk LJ, Stock HS, Lynn A, et al. Chronic physical exercise reduces anxiety-like behavior in rats. Int. J. Sports Med. 2004;25(1):78–82. doi: 10.1055/s-2003-45235. [DOI] [PubMed] [Google Scholar]
- 104.Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156(3):456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 105.Motl RW, Konopack JF, McAuley E, et al. Depressive symptoms among older adults: long-term reduction after a physical activity intervention. J. Behav. Med. 2005;28(4):385–394. doi: 10.1007/s10865-005-9005-5. [DOI] [PubMed] [Google Scholar]
- 106.Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom. Med. 2000;62(5):633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 107.Solberg LC, Horton TH, Turek FW. Circadian rhythms and depression: effects of exercise in an animal model. Am. J. Physiol. 1999;276(1 Pt 2):R152–R161. doi: 10.1152/ajpregu.1999.276.1.R152. [DOI] [PubMed] [Google Scholar]
- 108.Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J. Neurosci. Res. 2004;76(2):216–222. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- 109.Dey S. Physical exercise as a novel antidepressant agent: possible role of serotonin receptor subtypes. Physiol. Behav. 1994;55(2):323–329. doi: 10.1016/0031-9384(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 110.Gorton LM, Vuckovic MG, Vertelkina N, et al. Exercise effects on motor and affective behavior and catecholamine neurochemistry in the MPTP-lesioned mouse. Behav. Brain Res. 2010;213(2):253–262. doi: 10.1016/j.bbr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kleim JA, Hogg TM, VandenBerg PM, et al. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J. Neurosci. 2004;24(3):628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J. Neurosci. 1999;19(22):10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liepert J, Bauder H, Wolfgang HR, et al. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31(6):1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 114.Green CS, Bavelier D. Exercising your brain: a review of human brain plasticity and training-induced learning. Psychol. Aging. 2008;23(4):692–701. doi: 10.1037/a0014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006;7(1):30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 116.Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog. Brain Res. 2006:15781–15109. doi: 10.1016/S0079-6123(06)57006-2. [DOI] [PubMed] [Google Scholar]
- 117.Mora F, Segovia G, Del Arco A. Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res. Rev. 2007;55(1):78–88. doi: 10.1016/j.brainresrev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y. Differential effect of aging on synaptic plasticity in the ventral and dorsal striatum. Neurobiol. Learn. Mem. 2008;89(1):70–75. doi: 10.1016/j.nlm.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 119. Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc. Natl Acad. Sci. USA. 1990;87(14):5568–5572. doi: 10.1073/pnas.87.14.5568. ▪▪ Delineates synaptic formation and blood flow in learning and aerobic exercise.