Abstract
Merkel cell carcinomas (MCC) are rare but highly malignant skin cancers associated with a novel polyomavirus. MCC tumors were infiltrated by T cells, including effector, central memory and regulatory T cells. Infiltrating T cells showed markedly reduced activation as evidenced by reduced expression of CD69 and CD25. Treatment of MCC tumors in vitro with IL-2 and IL-15 led to T cell activation, proliferation, enhanced cytokine production and loss of viable tumor cells from cultures. Expanded tumor-infiltrating lymphocytes showed TCR repertoire skewing and upregulation of CD137. MCC tumors implanted into immunodeficient mice failed to grow unless human T cells in the tumor grafts were depleted with denileukin diftitox, suggesting tumor-specific T cells capable of controlling tumor growth were present in MCC. Both CD4+ and CD8+ FOXP3+ regulatory T cells were frequent in MCC. 50% of non-activated T cells in MCC expressed PD-1, a marker of T-cell exhaustion, and PD-L1 and PD-L2 were expressed by a subset of tumor dendritic cells and macrophages. In summary, we observed tumor-specific T cells with suppressed activity in MCC tumors. Agents that stimulate T cell activity, block Treg function or inhibit PD-1 signaling may be effective in the treatment of this highly malignant skin cancer.
Keywords: Merkel cell carcinoma, immune evasion, T cell dysfunction, PD-1
Introduction
Merkel cell carcinoma (MCC) is a rare and highly malignant neuroendocrine cancer that arises in the skin. MCC has a mortality rate of 30%, making it a more deadly cancer than malignant melanoma (Agelli and Clegg, 2003) and incidence has tripled in the last 20 years (Agelli et al., 2010). MCC is more frequent in immunosuppressed individuals, and the recently described Merkel cell polyomavirus (MCPyV) has been implicated in its etiology (Becker et al., 2008; Feng et al., 2008; Rodig et al., 2012). T cells specific for MCPyV oncoproteins are present in the blood and tumors of patients with MCC and these patients have levels of circulating antibodies specific for MCPyV oncoproteins that fluctuate with disease activity (Iyer et al., 2011; Paulson et al., 2010). However, these immune responses are insufficient in most cases to control growth of the cancer, suggesting that MCC tumors have potent immune evasion strategies.
This report details our studies of the local tumor microenvironment in MCC tumors. We find these tumors contain T cells capable of restraining tumor growth but that their activation is suppressed. We present findings that tumor resident regulatory T cells and T cell exhaustion may be two strategies used by MCC to evade immune destruction.
Results
MCCs are infiltrated by a mixed population of skin-homing effector memory, central memory and regulatory T cells
Immunostaining of MCC cryosections demonstrated the presence of tumor infiltrating lymphocytes (TILs) in MCC (Figure 1a), which in some cases infiltrated into tumor nests (Figure 1b). In other tumors, T cells surrounded the tumor but did not penetrate into it, similar to the peritumoral pattern previously described (Figure 1c) (Paulson et al., 2011). Cells isolated from primary MCC tumors (Figure 1d) included both CD4+ and CD8+ CD45RO+ memory T cells (Figure 1e).
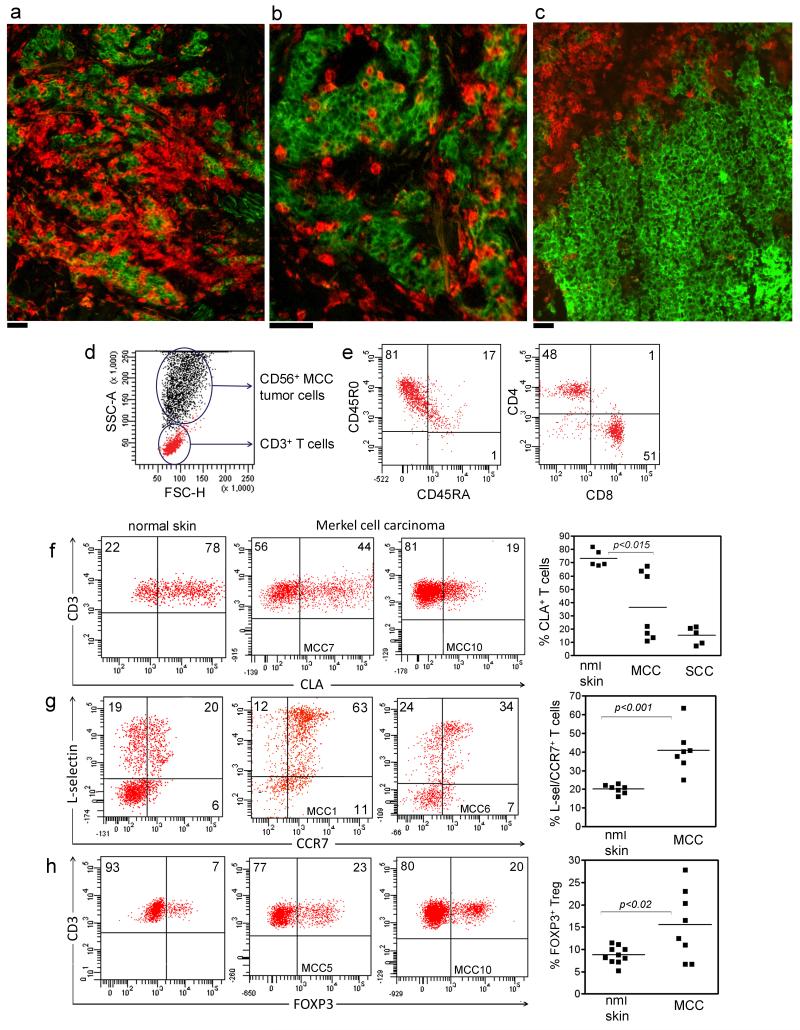
Figure 1. Merkel cell carcinomas are infiltrated by a mixed population of effector memory, central memory, and regulatory T cells.
(a) MCC cryosection immunostained for T cells (αCD3, red) and MCC tumor cells (αCD56, green) demonstrates numerous infiltrating T cells. (b) Higher power view shows T cells infiltrating within tumor nests. (c) In some tumors, T cells were located in a peritumoral distribution. (d) Short term cultures of MCC tumors allowed isolation of both T cells and tumor cells. The presence of T cells was studied by direct T cell isolation or immunostaining in 12 primary MCC tumors and three metastatic lesions. (e) In 8/8 MCC, TILs were CD45RO+ memory T cells with both CD4+ and CD8+ T cells. (f) MCC tumors had varied recruitment of CLA+ skin-homing T cells. The percentage of CLA+ T cells in normal skin (nml skin), MCC, and SCC are shown. A subset of MCC was infiltrated by CLA+ T cells whereas a second group of tumors excluded these T cells. The homing defect in this second subset was as pronounced as that observed in SCC, a tumor known to evade immune responses at least in part by excluding CLA+ T cells. (g) MCC were infiltrated by higher percentages of L-selectin/CCR7+ TCM and (h) FOXP3+ Tregs compared to normal skin. All histograms are gated to show CD3+ T cells. Scale bar = 50 μm.
Human squamous cell carcinomas of the skin evade immune responses by excluding CLA+ skin-homing effector memory T cells (TEM) and by recruiting FOXP3+ regulatory T cells (Tregs) with a L-selectin/CCR7+ central memory T cell (TCM) phenotype (Clark et al., 2008). A subset of MCC lacked CLA+ T cells, suggesting skin-homing T cells were excluded (Figure 1f). Immunostaining of MCC tumors demonstrated that CLA expression correlated with the pattern of T cell infiltration into tumors. 3/3 MCC with higher levels of T cell CLA expression were infiltrated by increased number of T cells and T cells were present within the present within the tumor nests themselves (Figure 1a,b) whereas in 4/4 tumors with decreased T cell CLA expression, T cells surrounded that did not penetrate into the tumor, the peritumoral pattern that has been correlated previously with poorer survival (Figure 1c) (Paulson et al., 2011). However, the presence of CLA+ skin-homing TILs in tumors was not entirely protective. Of the three patients with >50% CLA+ TILs, only one remained free of disease following primary excision and local radiotherapy; one had recurrent disease that responded to combination chemotherapy and brachytherapy, and one died from disease. L-selectin/CCR7+ TCM were significantly enhanced in MCC compared to normal skin, suggesting the preferential recruitment of TCM to these tumors (Figure 1g). FOXP3+ Treg were also increased in MCC tumors as compared to normal skin (Figure 1h) but this did not correlate with decreased survival. Of the three patients with >20% tumor Tregs, only one died; one had recurrence that responded to chemotherapy and brachytherapy, and one remained free of disease following primary excision and radiotherapy.
T cells infiltrating MCC show decreased activation
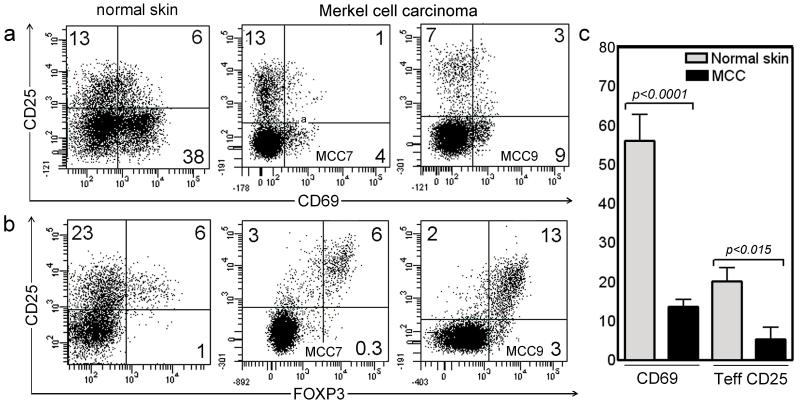
CD69 is a marker of early T cell activation that is expressed by roughly half of the T cells isolated from non-inflamed human tissues including skin and gut (Bos et al., 1987; Clark et al., 2006a; Kunkel et al., 2002). The CD69 expression of MCC TILs was much lower than T cells isolated from normal, non-inflamed human skin (Figure 2a, c), suggesting decreased activation. CD25 is a marker for later stages of T cell activation that is expressed by both activated T cells and FOXP3+ Tregs. Normal human skin contains a significant population of CD25+ FOXP3− activated T cells and a smaller population of CD25+ FOXP3+ Tregs (Figure 2b)(Clark et al., 2006a). Although normal numbers of MCC TILs expressed CD25, the vast majority of these were FOXP3+ Tregs (Figure 2a-c); the CD25+ FOXP3− activated effector T cells present in normal skin were absent. Taken together, these results demonstrate a marked inhibition of activation in the MCC TILs.
Figure 2. T cells infiltrating MCC show decreased activation.
(a) MCC TILs showed markedly reduced expression of the early activation antigen CD69. (b) Expression of the activation marker CD25 was largely restricted to FOXP3+ Treg. CD69 and CD25 expression from two tumors are shown; similar findings were observed in six additional tumors. The CD25+ FOXP3− activated T cell population observed in normal skin was absent from MCC tumors. (c) The mean and SEM of CD69+ and CD25+FOXP3− T cells from 8 MCC tumors are shown, compared to 4 samples of normal skin. All histograms are gated to show CD3+ T cells.
Culture of MCC tumors in IL-2 and IL-15 leads to T cell proliferation, activation, and expansion of CD8 T cells
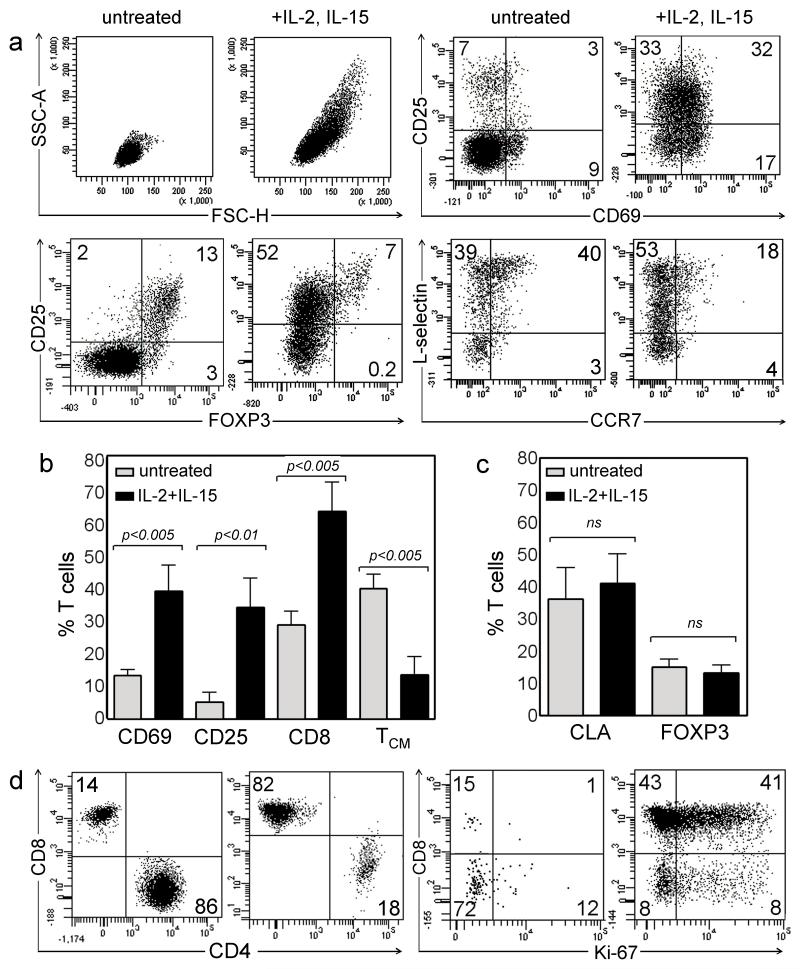
Immunostimulatory cytokines including IL-2 and IL-15 have been used in vitro to expand TILs with antitumor activity from malignant melanoma (Mueller et al., 2008; Rosenberg et al., 2011). We expanded T cells from MCC tumors with IL-2 and IL-15 for one week and observed marked increases in cell size and granularity, enhanced activation as evidenced by increased expression of both CD69 and CD25, increased numbers of CD25+ FOXP3− activated effector T cells, and reduced percentages of CCR7+/L-selectin+ TCM (Figure 3a, b). After three weeks of stimulation, we observed T cell proliferation, particularly CD8 T cells, as indicated by the proliferation marker Ki-67 (Figure 3d). In contrast to these population shifts, we found no changes in the percentages of CLA+ skin-homing or FOXP3+ Tregs (Figure 3c). Further studies demonstrated that IL-15 was critical for enhancing T cell activation and proliferation, as these were mostly unchanged by IL-2 treatment alone (Suppl. Figure 1).
Figure 3. Culture of MCC tumors in IL-2 and IL-15 leads to T cell activation, proliferation, and expansion of CD8 T cells.
(a) T cells from tumors treated for one week with IL-2 and IL-15 showed marked upregulation of CD69 and CD25, increased percentages of CD25+FOXP3− activated effector T cells and reduced percentages of CCR7+/L-selectin+ TCM. The mean and SEM from seven tumors of parameters that (b) changed or (c) remain unchanged after one week of IL-2 and IL-15 treatment are shown. The percentages of CLA+ skin-homing and FOXP3+ Tregs were not altered by cytokine treatment of tumors. (d) By three weeks of culture, CD8+ T cells predominated and marked T cell proliferation was evident, as indicated by the proliferation marker Ki-67. All histograms are gated to show CD3+ T cells.
In vitro treatment of MCC tumors with IL-2 and IL-15 enhances T cell cytokine production
We next studied T cell cytokine production in MCC tumors in the presence or absence of treatment with IL-2 and IL-15. In one patient, T cells showed markedly enhanced production of IFNγ and TNFα by both CD4 and CD8 T cells and enhanced CD4 IL-17 production after IL-2/IL-15 treatment (Suppl. Figure 2a,b). The patient was 90 years-old at diagnosis with a high-risk, 3-cm primary lesion and evidence of micrometastases. She was treated with wide excision and local radiation therapy and remains well despite the high-risk nature of her primary lesion. A second subset of patients demonstrated increased production of IFNγ and/or TNFα but little IL-17 production (Suppl. Figure 2c). A third group of patients showed significant production of Th2 cytokines (IL-4, IL-13) and IL-10 and no enhancement of IFNγ, TNFα, and IL-17 production after treatment with IL-2/IL-15 (Suppl. Figure 2d).
T cells isolated from patient MCC6 at the time of initial biopsy showed low levels of IFNγ and TNFα production even after IL-2/IL-15 treatment. However, T cells isolated from the same primary tumor 19 days later at the time of full excision showed enhanced production of IFNγ and TNFα, suggesting that the initial biopsy event triggered either migration of Th1 T cells into the tumor or allowed enhanced cytokine production (Suppl. Figure 2e).
MCC tumors contain CD8+ FOXP3+ Treg and plasmacytoid dendritic cells
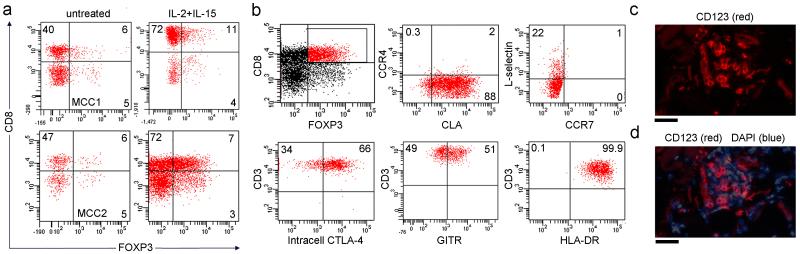
Classical FOXP3+ Tregs are CD4+ but suppressive CD8+FOXP3+ Tregs have been described in human transplant recipients, mouse models of autoimmune diseases, and the microenvironments of many tumors (Chang et al., 2002; Rifa’i et al., 2004; Xystrakis et al., 2004; Yang et al., 2010). We observed a discrete population of CD8+FOXP3+ T cells in MCC that expanded in concert with effector T cells during IL-2/IL-15 treatment (Figure 4a). These CD8 Tregs expressed the skin-homing addressin CLA, but lacked CCR4 and had a CCR7− non-TCM phenotype (Figure 4b). The majority expressed intracellular CTLA-4 and GITR, two immunomodulatory molecules also expressed by CD4+ Tregs (Sakaguchi, 2000), and nearly all expressed high levels of HLA-DR, a marker for high suppressive capacity in humans (Baecher-Allan et al., 2006). Expression of HLA-DR, GITR and intracellular CTLA-4 by T cells from normal human skin is also included (Suppl. Figure 3). Immunostained tumor cryosections revealed frequent CD123+ plasmacytoid dendritic cells (PDC) (Figure 4c,d), which have been shown to induce formation of CD8+FOXP3+ Tregs in the tumor microenvironment of ovarian carcinoma (Wei et al., 2005).
Figure 4. MCC tumors contain CD8+ FOXP3+ Treg and plasmacytoid dendritic cells (PDC).
(a) MCC tumors contained CD8+ FOXP3+ Tregs and similar or increased numbers of these cells were evident after two weeks of TIL expansion with IL-2/IL-15. Two representative tumors are shown. (b) Most CD8+ Treg expressed CLA and lacked the TCM markers L-selectin/CCR7, consistent with a skin-tropic effector T cell phenotype. The majority of CD8+ Tregs expressed intracellular CTLA-4 and GITR, and all expressed high levels of HLA-DR, a marker of highly suppressive Tregs. All histograms are gated to show CD3+ T cells. (c) MCC cryosections immunostained for CD123 demonstrated the presence of PDC in tumors, a cell type known to induce formation of CD8+ Tregs. (e) PDC were present within tumor nests. A superimposed nuclear stain demonstrates the presence of PDC in tumor nests. A representative tumor is shown; similar results were obtained in seven additional tumors.
TILs in MCC show evidence of T cell exhaustion
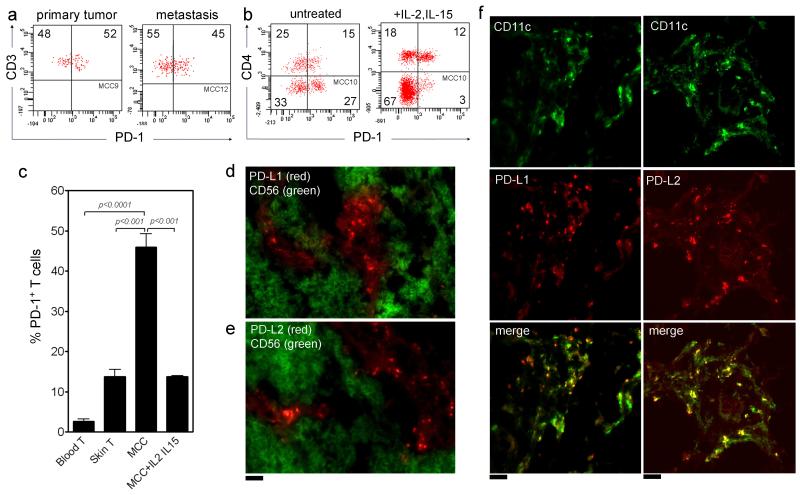
PD-1 expression by TILs in tumors in the presence of PD-1 ligands can indicate T cell exhaustion (Ahmadzadeh et al., 2009; Blank et al., 2006; Chapon et al., 2011; Jin et al.). Despite the low levels of activation observed in MCC TILs, a significant percentage of both CD4+ and CD8+ TILs expressed PD-1 (Figure 5a, b, c). PD-1 expression by TILs was reduced after treatment of tumors with IL2/IL-15, particularly among CD8+ T cells (Figure 5 b,c). TIL expression of PD-1 by tumor T cells was significantly higher than that of T cells from normal skin and blood (Figure 5c). Treatment of tumors with IL-2 and IL-15 reduced TIL expression of PD-1 to levels observed in normal skin T cells (Figure 5c). Two PD-1 ligands, PD-L1 and PD-L2, were we present in the tumor microenvironment but not expressed by the tumor cells themselves (Figure 6d,e). Instead, they were expressed by a population of CD11c+ dendritic cells (Figure 5f). In addition, a small subpopulation of CD163+ macrophages within the tumor also expressed PD-L1 and PD-L2 (data not shown).
Figure 5. PD-1 is expressed by T cells and PD-1 ligands are expressed within the tumor microenvironment in MCC.
(a) A significant proportion of MCC TILs expressed PD-1. T cells from two representative tumors are shown; similar results were observed in four additional tumors. (b) PD-1 was expressed by both CD4+ and CD8+ T cells in untreated MCC and PD-1 expression was reduced, especially on CD8+ T cells, after treatment of tumors with IL-2 and IL-15. (c) T cells from untreated MCC expressed PD-1 at markedly higher levels than those from normal human skin and blood. TIL expression of PD-1 was significantly reduced after treatment of tumors with IL-2 and IL-15. (d,e) MCC tumor cryosections immunostained for PD-L1 or PD-L2 (red) and CD56 (green, delineating tumor cells) show that although PD-L1 and PD-L2 expressing cells are frequent in tumors, they are not tumor cells. Representative cryosections are shown; comparable results were observed in 6 additional tumors. All histograms are gated to show CD3+ T cells. (f) PD-L1 and PD-L2 are expressed by dendritic cells in MCC. Cryosections immunostained for the dendritic cell marker CD11c and PD-L1 or PD-L2 are shown. PD-L1+ or PD-L2+ dendritic cells appear yellow in merged images. In addition to CD11c+ dendritic cells, PD-L1 and PD-L2 were also expressed by a small subset of CD163+ macrophages (data not shown). Representative cryosections are shown; similar results were obtained in 6 additional tumors. Scale bar = 50 μm.
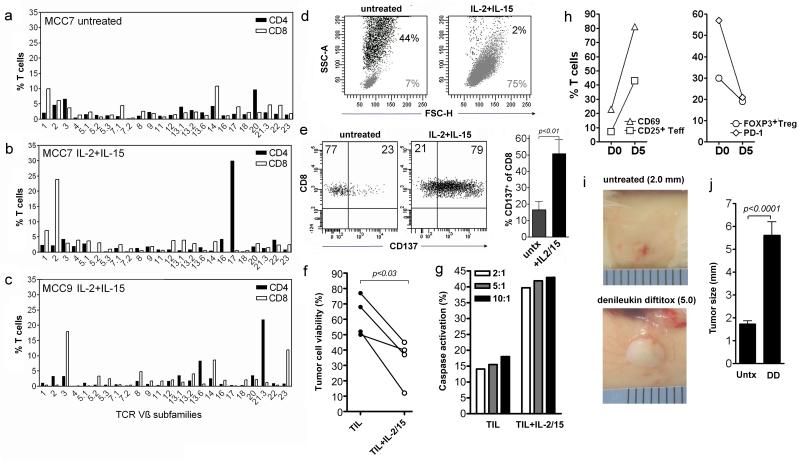
Figure 6. MCC tumors contain tumor-specific T cells capable of controlling tumor growth.
(a-c) Expansion of TIL by treatment of tumors with IL-2 and IL-15 led to marked skewing of the T cell repertoire. Two representative tumors are shown; similar results were observed in three additional tumors. Tumors were cultured for three weeks in IL-2 and IL-15 or control medium. (d) Tumors treated for three weeks with IL-2 and IL-15 showed marked expansion of CD3+ T cells (gray) and loss of viable CD56+ tumor cells (black) from the cultures. (e) CD8+ T cells tumors treated for three weeks showed upregulation of CD137, a marker of antigen-specific T cell activation. Representative histograms and the mean and SEM of CD137 expression by non-expanded and expanded CD8 T cells from five MCC are shown. Histograms are gated to show CD8+T cells. (f) Death of autologous tumor cells was observed after co-culture with autologous expanded T cells from IL-2 and IL-15 treated tumors and was greater than that observed after co-culture with non-expanded T cells from the same tumors. (g) A short term cytotoxicity assay measuring caspase activation demonstrated cytotoxic killing of autologous tumor cells by T cells isolated from IL-2 and IL-15 treated tumors. The ratios of T cells:tumor cells are shown. (h) T cells isolated from MCC before (D0) and five days after (D5) subcutaneous implantation into immunodeficient mice showed increased expression of activation antigens CD69 and CD25, reduced percentages of FOXP3+ Treg and reduced expression of PD-1. (i) MCC tumors implanted subcutaneously into immunodeficient mice did not grow unless activated T cells transferred with the graft were depleted with IP administration of denileukin diftitox. Grafts from the same original MCC tumor are shown eight weeks after implantation; the mean and SEM of tumor sizes are shown (n=28). Scale of ruler shown = 1 mm/division.
MCC tumors contain tumor-specific T cells capable of controlling tumor growth
T cells expanded from MCC treated in vitro with IL-2 and IL-15 showed skewing of the TCR repertoire among both CD4+ and CD8+ T cells, suggesting expansion may be antigen-specific (Figure 6a-c). The expansion of T cells and simultaneous loss of viable tumor cells suggested that viable tumor cells may be killed by expanding T cells (Figure 6d). Cytokine treated CD8+ TILs showed upregulation of CD137, a marker of antigen-specific T cell activation used to identify melanoma or virus-specific TIL after in vitro expansion (Figure 6e) (Hernandez-Chacon et al., 2011; Wehler et al., 2008). Significant killing of tumor cells was observed after culture of autologous tumor cells with IL-2/IL15 expanded tumor T cells as compared to that induced by non-expanded TILs (Figure 6f). A shorter term cytotoxicity assay utilizing a fluorogenic caspase substrate also demonstrated tumor cell killing by expanded TILs that was superior to that observed using non-expanded TILs from the same tumor (Figure 6g). 2 mm MCC tumor fragments implanted subcutaneously into NOD/SCID/IL2-receptor γ-chainnull (NSG) mice failed to grow in size (Figure 6i,j). Hypothesizing that T cells transferred with the tumor may be preventing tumor growth, we isolated T cells from tumors five days after implantation into mice. T cells from implanted tumors showed enhanced activation, as shown by increased expression of CD69 and CD25 (Figure 6h). T cell expression of PD-1 declined, as did the percentage of FOXP3+ Treg. We treated implanted mice systemically with denileukin diftitox, a recombinant fusion protein that selectively depletes human CD25-expressing T cells, including activated effector and regulatory cells (Ho et al., 2004; Morse et al., 2008). MCC implanted into denileukin diftitox treated mice grew and could be transferred to additional animals (Figure 6i,j).
Metastatic lesions of MCC also contain T cells that can be activated and expanded by treatment with IL-2 and IL-15
Similar to our findings in primary tumors, T cells from IL-2/IL-15 treated MCC metastases expanded, upregulated expression of activation markers CD69 and CD25, and CD8 expression of CD137 was upregulated (Suppl. Figure 4a,b). There was enhanced IFNγ production and skewing of the T cell repertoire and increased percentages of effector T cells (Suppl. Figure 4c,d). In brief, metastatic lesions also contain T cells, and their activation and cytokine production was enhanced by IL-2/IL-15 treatment.
Discussion
As with other virally associated cancers, T cell immunity plays a critical role in the susceptibility and immune responses to MCC. Incidence is markedly increased in immunosuppressed individuals and withdrawal of iatrogenic immunosuppression or biopsy itself has induced regression of MCC (Friedlaender et al., 2002; Heath et al., 2008; Muirhead and Ritchie, 2007; Val-Bernal et al., 2011; Wooff et al., 2010). Although 80% of MCCs have genomic integration of MCV and produce viral proteins including small and large T antigens, these tumors are still highly malignant in immunocompetent individuals (Becker et al., 2009; Feng et al., 2008; Foulongne et al., 2008; Nakamura et al., 2010; Paolini et al., 2011; Shuda et al., 2009; Shuda et al., 2008). 92% of MCC occur in immunocompetent individuals, and mortality is 30%, making MCC a more fatal cancer than malignant melanoma (Heath et al., 2008). The highly malignant nature of this virally mediated cancer suggests that MCC has potent strategies for evading immune response and that neutralization of these strategies may enhance anti-tumor immunity.
Cancer destruction requires not only the generation of tumor-specific T cells but also the ability of these T cells to access the tumor once they are generated (Gajewski, 2007). T cells are imprinted with expression of tissue specific addressins and preferentially migrate to the peripheral tissue in which they first encountered antigen (Campbell and Butcher, 2002; Kupper and Fuhlbrigge, 2004). MCC-specific T cells should express the skin-homing addressin CLA because MCC are cutaneous tumors and T cells will have first encountered antigen within the skin-draining lymph nodes. We observed decreased numbers of skin-homing CLA+ T cells in a subset of MCC, suggesting the presence of a T-cell homing defect in at least some tumors. Impaired T cell homing as a result of decreased vascular addressin expression has been reported in a number of human cancers, including malignant melanoma, cutaneous SCC, breast, gastric, cervical and lung cancers (Clark et al., 2008; Madhavan et al., 2002; Piali et al., 1995; Trimble et al.; Weishaupt et al., 2007). Indeed, prior studies have shown that the presence of CD8 T cells within the MCC tumor itself is correlated with better patient outcomes (Paulson et al., 2011). However, patients with tumors that showed infiltration with CLA+ skin-homing T cells did not have a markedly improved survival, suggesting that other immunosuppressive mechanisms were also at work.
Our results suggested that at least some MCC tumors contained tumor-specific T cells but that activation of these T cells was markedly suppressed. Expression of the activation markers CD69 and CD25 was severely decreased in MCC TIL, lower even than T cells from normal, non-inflamed human skin (Figure 2). In vitro culture of MCC with the T cell activating cytokines IL-2 and IL-15 led to marked activation and expansion of tumor T cells, loss of viable tumor cells from cultures and upregulation of CD137 on CD8 T cells, a marker of antigen-specific T cell activation that has been used to identify melanoma or virus-specific TIL after in vitro expansion (Figure 6) (Hernandez-Chacon et al., 2011; Wehler et al., 2008). T cells expanded from MCC tumors killed autologous tumor cells in vitro (Figure 6f,g). Significantly, MCC tumors implanted subcutaneously into NSG mice, an immunodeficient mouse strain that lacks T cells, B cells and NK cells, failed to grow unless their activated T cells were depleted from the transplanted tumor by treatment with denileukin diftitox (Figure 6h-j) (Ho et al., 2004; Morse et al., 2008). These experiments suggest that at least some MCC tumors contain T cells capable of controlling tumor growth.
These findings suggest that potent mechanisms for the suppression of T cell activation exist within the MCC tumor microenvironment and that intratumoral administration of T cell activating agents may be capable of stimulating TILs, thereby enhancing immune responses. Indeed, intratumoral injection of interferon-beta was recently reported to induce MCC regression in four patients (Paulson, 2011). We found that the combination of IL-2 and IL-15 was critical for the expansion and activation of MCC TILs. Surprisingly, IL-2 alone had little effect on proliferation and cytokine production of MCC TILs (Suppl. Figure 1) whereas IL-15 alone enhanced cytokine production but resulted in less proliferation (data not shown). Both IL-2 and IL-15 induce antigen-independent proliferation of T cells and participate in bystander T cell proliferation during immune responses (Lodolce et al., 2001). Under normal conditions, IL-2 plays a crucial role in sustaining Tregs and maintaining peripheral tolerance, whereas IL-15 controls the survival and turnover of memory T cells (Sprent et al., 2008). In a mouse model of adoptive immunotherapy for melanoma, T cells expanded in vitro with IL-15 were longer-lived and more effective in vivo then those generated with IL-2 (Mueller et al., 2008). Our results suggest intratumoral therapy with potent activators of T-cell function, including the combination of IL-2 and IL-15, may be effective in enhancing local antitumor immunity.
Our studies have identified Treg inhibition and T cell exhaustion as two possible mechanisms for the inhibition of T cell activity we observe in MCC. MCC contained increased numbers of both CD4 and CD8 Tregs (Figure 1, 4). CD8 Tregs contribute to disease progression in prostate and colorectal cancer and have also been described in ovarian cancer and malignant melanoma (Chaput et al., 2009; Kaufman et al.; Kiniwa et al., 2007; Wei et al., 2005). CD8 Tregs were more efficient than CD4 Tregs in reducing CD4 T cell proliferation and Th1 cytokine production (Filaci et al.). MCC are also heavily infiltrated by PDC, a cell type known to induce the formation of CD8 Tregs (Wei et al., 2005). Our results suggest that therapies that inhibit Treg activity, such as ipilimumab, may be useful in MCC
PD-1 was expressed on approximately half of MCC T cells, despite their markedly suppressed activation status. PD-1, a CD28/CTLA-4 family member, is expressed by activated T cells but when expressed in tumors in the presence of its ligands PD-L1 or PD-L2, it can be a sign of T cell exhaustion (Jin et al.). PD-1 is upregulated on T cells in malignant melanoma (Blank et al., 2006; Chapon et al., 2011), and increased PD-1 expression correlates with an exhausted phenotype and impaired effector function (Ahmadzadeh et al., 2009). MCC TILs showed markedly suppressed activation and PD-1 expression was therefore unlikely to be the result of T cell activation and more likely reflected T cell exhaustion. Activation and expansion of TILs with IL-2 and IL-15 led to marked decreases in TIL PD-1 expression along with potent enhancement of cytokine production and anti-tumor cytotoxicity, consistent with recovery from the exhausted phenotype (Figures 5, 6 and Suppl. Figure 2). We observed a population of dendritic cells and macrophages within MCC that expressed PD-L1 and PD-L2 and may be responsible for the induction of PD-1 on tumor T cells. These findings suggest that medications such as MDX-1106 that target the PD-1 pathway may have a role in the treatment of patients with MCC (Brahmer et al., 2010).
MCC are virally associated cancers that should be eminently recognizable by the immune system. The fact that MCC are highly malignant in immunocompetent individuals suggests they must have formidable strategies for evading immune responses. We found that MCC tumors utilize a spectrum of immune evasion strategies, including impairment of T cell homing and local suppression of T cell activation. Impaired T cell activation likely results at least in part from locally high concentrations of both CD4 and CD8 Tregs and expression of PD-L1 and PD-L2 within the tumor microenvironment, leading to T-cell exhaustion. Our work suggests that adjuvant therapy of high risk patients with agents that stimulate T cell activity, block Treg function, and inhibit PD-1 signaling may enhance antitumor immunity in patients with MCC.
Materials and methods
Methods Patient samples
MCC tumors and squamous cell carcinomas (SCC) were obtained from the Department of Dermatology at Brigham and Women’s Hospital, the Cutaneous Oncology Program at the Dana Farber/Brigham and Women’s Cancer Center, and the Fred Hutchinson Cancer Research Center, University of Washington. Acquisition of tumor samples and all studies were approved by the Institutional Review Board of the Dana Farber Cancer Institute/Harvard Cancer Center and were performed in accordance with the Declaration of Helsinki. Written informed consent was received from participants prior to inclusion in the study. Twelve primary tumors and three metastases were studied. Six out of six tumors studied tested positive for the presence of the Merkel cell polyomavirus, four by RT-PCR and two by immunohistochemical staining for large T antigen as recently described (data not shown)(Rodig et al., 2012).
Normal human skin was obtained as discarded tissues following plastic surgery procedures.
Isolation of T cells from MCC tumors and normal skin
T cells were isolated from normal skin, biopsy-proven MCC tumors, and SCC tumors as previously described (Clark et al., 2006b).
Flow cytometry studies
Flow cytometry analysis of T cells was performed using directly conjugated monoclonal antibodies from: BD Biosciences (CD3, CD4, CD8, CD25, CD69, CD45RO, CD45RA, CD56 and CD137), BD PharMingen (CLA, CD73), Abcam (CD39), Beckman Coulter (L-selectin), R&D Systems (CCR7), and eBioscience (FOXP3, clone PCH101). IOtest Beta Mark kit (Beckman Coulter) was used for TCR repertoire analysis. For cytokine production analysis, T cells from MCC tumors were stimulated with either control medium or 50 ng/ml PMA and 750 ng/ml ionomycin for 6 h, with 10 μg/ml Brefeldin-A (Calbiochem) added after 1 h. Cells were stained for surface markers, fixed, permeabilized, and stained with directly conjugated anti-cytokine antibodies. Analysis of flow cytometry samples was performed on Becton Dickinson FACScan or FACSCanto instruments, and data were analyzed using FACSDiva software (V5.1).
Immunofluorescence studies
MCC tumors were embedded in OCT, frozen, and stored at −80°C. 5 μm cryosections were cut, air dried, fixed in acetone, rehydrated in PBS, and blocked with 20 μg/ml of human IgG (15 min, Jackson ImmunoResearch Laboratories). Sections were incubated with primary antibody (30 min), followed by three rinses in PBS/1% BSA. If necessary, secondary antibody was added (1:100 dilution, 30 min), followed by three rinses. Sections were mounted using Prolong Gold anti-fade mounting medium with DAPI (Invitrogen) and examined by a microscope (Eclipse 6600; Nikon) equipped with a [40×/0.75] objective lens (Plan Fluor; Nikon). Images were captured with a camera (SPOT RT model 2.3.1; Diagnostic Instruments) and acquired with SPOT [4.0.9] software (Diagnostic Instruments). Antibodies from: BD Biosciences (CD3, PD-1, PD-L1, PD-L2, HLA-DR), Abcam (CD163), and R&D Systems (CD11c), Biolegend (CD56, clone HCD56).
Cell viability and cytotoxicity studies
Autologous tumor cells present in explant cultures were co-cultured with nonexpanded or expanded T cells from three week explant cultures and tumor cell viability was assessed by flow cytometry after 18 hours by cell scatter and/or exclusion of 7-AAD viability stain (BD Biosciences). A second, shorter term cytotoxicity assay was carried out by incubating autologous T cells at the indicated T cell to tumor cell ratio for two hours in the presence of a fluorogenic caspase substrate; cytotoxic tumor cell death was assayed by flow cytometry as per manufacturer’s instructions (CyToxiLux Kit, OncoImmunin).
Mice xenografted with MCC tumors
Freshly excised MCC tumors were divided into four equal portions at least 3 mm in each dimension and implanted subcutaneously on the dorsal flank of NOD/SCID/IL2-receptor γ-chainnull mice (Jackson Laboratories). Mice received 50 uL intraperitoneal injections on days 0, 2, 6, 7 and 9 of either saline or denileukin diftitox (7.2 mcg/mL). Tumor size was monitored by palpation for 8 weeks, at which point they were harvested and measured. The Harvard Medical Area (HMA) Standing Committee on Animals approved all described studies
Supplementary Material
Acknowledgments and grant support
The authors would like to thank the patients for their donation of tissues that made these studies possible. Normal skin was kindly provided by Drs. Bohdan Pomahac, Simon Talbot and Elof Eriksson of BWH and by Dr. Thomas Cochran of the Boston Center. Dr. Jessica Scanlon assisted with patient outcomes. Dr. Thomas S. Kupper provided helpful advice and support. This research was supported by grants NIH/NIAMS R01 AR056720 (to RAC), NIH/NIMH R03 MH095529 (to RAC), NIH R01AI097128 (to RAC/TSK), a Damon Runyon Cancer Research Foundation Clinical Investigator Award (to RAC), a Scleroderma Foundation New Investigator Award (to RAC), an American Skin Association Medical Student Grant (to MD), a Skin Cancer Foundation Joseph A. Stirrup Research Award (to VH), Dermatology Foundation Dermatologist Investigator Research Fellowship (to VH), a Women’s Dermatologic Society Academic Research Grant Award (to VH) and NIH/NIAMS T32-AR07098-33 (to TSK, supporting VH).
Abbreviations used
- MCC
Merkel cell carcinoma
- CLA
cutaneous lymphocyte antigen
- SCC
squamous cell carcinoma
- NO
nitric oxide
- TILs
tumor infiltrating lymphocytes
Footnotes
Conflicts of Interest None to report.
Conflict of interest statement: The authors declare no conflicts of interest.
References
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832–41. doi: 10.1016/s0190-9622(03)02108-x. [DOI] [PubMed] [Google Scholar]
- Agelli M, Clegg LX, Becker JC, Rollison DE. The etiology and epidemiology of merkel cell carcinoma. Curr Probl Cancer. 2010;34:14–37. doi: 10.1016/j.currproblcancer.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–31. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- Becker JC, Houben R, Ugurel S, Trefzer U, Pfohler C, Schrama D. MC polyomavirus is frequently present in Merkel cell carcinoma of European patients. The Journal of investigative dermatology. 2009;129:248–50. doi: 10.1038/jid.2008.198. [DOI] [PubMed] [Google Scholar]
- Becker JC, Schrama D, Houben R. Merkel cell carcinoma. Cell Mol Life Sci. 2008 doi: 10.1007/s00018-008-8483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank C, Kuball J, Voelkl S, Wiendl H, Becker B, Walter B, et al. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119:317–27. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- Bos JD, Zonneveld I, Das PK, Krieg SR, van der Loos CM, Kapsenberg ML. The skin immune system (SIS): distribution and immunophenotype of lymphocyte subpopulations in normal human skin. The Journal of investigative dermatology. 1987;88:569–73. doi: 10.1111/1523-1747.ep12470172. [DOI] [PubMed] [Google Scholar]
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. The Journal of experimental medicine. 2002;195:135–41. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nature immunology. 2002;3:237–43. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- Chapon M, Randriamampita C, Maubec E, Badoual C, Fouquet S, Wang SF, et al. Progressive upregulation of PD-1 in primary and metastatic melanomas associated with blunted TCR signaling in infiltrating T lymphocytes. J Invest Dermatol. 2011;131:1300–7. doi: 10.1038/jid.2011.30. [DOI] [PubMed] [Google Scholar]
- Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Menegaux F, et al. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58:520–9. doi: 10.1136/gut.2008.158824. [DOI] [PubMed] [Google Scholar]
- Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006a;176:4431–9. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- Clark RA, Chong BF, Mirchandani N, Yamanaka K, Murphy GF, Dowgiert RK, et al. A novel method for the isolation of skin resident T cells from normal and diseased human skin. The Journal of investigative dermatology. 2006b;126:1059–70. doi: 10.1038/sj.jid.5700199. [DOI] [PubMed] [Google Scholar]
- Clark RA, Huang SJ, Murphy GF, Mollet IG, Hijnen D, Muthukuru M, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. The Journal of experimental medicine. 2008;205:2221–34. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filaci G, Fenoglio D, Indiveri F. CD8+ T regulatory/suppressor cells and their relationships with autoreactivity and autoimmunity. Autoimmunity. 2011;44:51–7. doi: 10.3109/08916931003782171. [DOI] [PubMed] [Google Scholar]
- Foulongne V, Kluger N, Dereure O, Brieu N, Guillot B, Segondy M. Merkel cell polyomavirus and Merkel cell carcinoma, France. Emerg Infect Dis. 2008;14:1491–3. doi: 10.3201/eid1409.080651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlaender MM, Rubinger D, Rosenbaum E, Amir G, Siguencia E. Temporary regression of Merkel cell carcinoma metastases after cessation of cyclosporine. Transplantation. 2002;73:1849–50. doi: 10.1097/00007890-200206150-00028. [DOI] [PubMed] [Google Scholar]
- Gajewski TF. Failure at the Effector Phase: Immune Barriers at the Level of the Melanoma Tumor Microenvironment. 2007:5256–61. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Penas PF, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375–81. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Chacon JA, Li Y, Wu RC, Bernatchez C, Wang Y, Weber JS, et al. Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. J Immunother. 2011;34:236–50. doi: 10.1097/CJI.0b013e318209e7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho VT, Zahrieh D, Hochberg E, Micale E, Levin J, Reynolds C, et al. Safety and efficacy of denileukin diftitox in patients with steroid-refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:1224–6. doi: 10.1182/blood-2004-01-0028. [DOI] [PubMed] [Google Scholar]
- Iyer JG, Afanasiev OK, McClurkan C, Paulson K, Nagase K, Jing L, et al. Merkel cell polyomavirus-specific CD8(+) and CD4(+) T-cell responses identified in Merkel cell carcinomas and blood. Clin Cancer Res. 2011;17:6671–80. doi: 10.1158/1078-0432.CCR-11-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol. 2011;350:17–37. doi: 10.1007/82_2010_116. [DOI] [PubMed] [Google Scholar]
- Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17:718–30. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–58. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Boisvert J, Murphy K, Vierra MA, Genovese MC, Wardlaw AJ, et al. Expression of the Chemokine Receptors CCR4, CCR5, and CXCR3 by Human Tissue-Infiltrating Lymphocytes. Am J Pathol. 2002;160:347–55. doi: 10.1016/S0002-9440(10)64378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nature reviews. 2004;4:211–22. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15Ralpha signals are required for bystander proliferation. The Journal of experimental medicine. 2001;194:1187–94. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan M, Srinivas P, Abraham E, Ahmed I, Vijayalekshmi NR, Balaram P. Down regulation of endothelial adhesion molecules in node positive breast cancer: possible failure of host defence mechanism. Pathol Oncol Res. 2002;8:125–8. doi: 10.1007/BF03033721. [DOI] [PubMed] [Google Scholar]
- Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, et al. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112:610–8. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, Schweier O, Pircher H. Efficacy of IL-2-versus IL-15-stimulated CD8 T cells in adoptive immunotherapy. European journal of immunology. 2008;38:2874–85. doi: 10.1002/eji.200838426. [DOI] [PubMed] [Google Scholar]
- Muirhead R, Ritchie DM. Partial regression of Merkel cell carcinoma in response to withdrawal of azathioprine in an immunosuppression-induced case of metastatic Merkel cell carcinoma. Clin Oncol (R Coll Radiol) 2007;19:96. doi: 10.1016/j.clon.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sato Y, Watanabe D, Ito H, Shimonohara N, Tsuji T, et al. Nuclear localization of Merkel cell polyomavirus large T antigen in Merkel cell carcinoma. Virology. 2010;398:273–9. doi: 10.1016/j.virol.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Paolini F, Donati P, Amantea A, Bucher S, Migliano E, Venuti A. Merkel cell polyomavirus in Merkel cell carcinoma of Italian patients. Virol J. 2011;8:103. doi: 10.1186/1743-422X-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson K, Tegeder A, Willmes C, Iyer J, Schrama D, Koelle D, Cleary M, Bhatia S, Becker J, Nghiem P. Reversal of local immune evasion mechanisms and regression of human Merkel cell carcinoma by intralesional injection of interferon-beta. The Journal of investigative dermatology. 2011;131:S92. [Google Scholar]
- Paulson KG, Carter JJ, Johnson LG, Cahill KW, Iyer JG, Schrama D, et al. Antibodies to merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in merkel cell carcinoma patients. Cancer research. 2010;70:8388–97. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson KG, Iyer JG, Tegeder AR, Thibodeau R, Schelter J, Koba S, et al. Transcriptome-Wide Studies of Merkel Cell Carcinoma and Validation of Intratumoral CD8+ Lymphocyte Invasion As an Independent Predictor of Survival. J Clin Oncol. 2011;29:1539–46. doi: 10.1200/JCO.2010.30.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piali L, Fichtel A, Terpe HJ, Imhof BA, Gisler RH. Endothelial vascular cell adhesion molecule 1 expression is suppressed by melanoma and carcinoma. The Journal of experimental medicine. 1995;181:811–6. doi: 10.1084/jem.181.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifa’i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. The Journal of experimental medicine. 2004;200:1123–34. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodig SJ, Cheng J, Wardzala J, Dorosario A, Scanlon JJ, Laga AC, et al. Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. The Journal of clinical investigation. 2012;122:4645–53. doi: 10.1172/JCI64116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- Shuda M, Arora R, Kwun HJ, Feng H, Sarid R, Fernandez-Figueras MT, et al. Human Merkel cell polyomavirus infection I. MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. Int J Cancer. 2009;125:1243–9. doi: 10.1002/ijc.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuda M, Feng H, Kwun HJ, Rosen ST, Gjoerup O, Moore PS, et al. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci U S A. 2008;105:16272–7. doi: 10.1073/pnas.0806526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J, Cho JH, Boyman O, Surh CD. T cell homeostasis. Immunol Cell Biol. 2008;86:312–9. doi: 10.1038/icb.2008.12. [DOI] [PubMed] [Google Scholar]
- Trimble CL, Clark RA, Thoburn C, Hanson NC, Tassello J, Frosina D, et al. Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J Immunol. 2010;185:7107–14. doi: 10.4049/jimmunol.1002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Val-Bernal JF, Garcia-Castano A, Garcia-Barredo R, Landeras R, De Juan A, Garijo MF. Spontaneous complete regression in merkel cell carcinoma after biopsy. Adv Anat Pathol. 2011;18:174–7. doi: 10.1097/PAP.0b013e31820ce11f. author reply 7. [DOI] [PubMed] [Google Scholar]
- Wehler TC, Karg M, Distler E, Konur A, Nonn M, Meyer RG, et al. Rapid identification and sorting of viable virus-reactive CD4(+) and CD8(+) T cells based on antigen-triggered CD137 expression. J Immunol Methods. 2008;339:23–37. doi: 10.1016/j.jim.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer research. 2005;65:5020–6. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- Weishaupt C, Munoz KN, Buzney E, Kupper TS, Fuhlbrigge RC. T-cell distribution and adhesion receptor expression in metastatic melanoma. Clin Cancer Res. 2007;13:2549–56. doi: 10.1158/1078-0432.CCR-06-2450. [DOI] [PubMed] [Google Scholar]
- Wooff JC, Trites JR, Walsh NM, Bullock MJ. Complete spontaneous regression of metastatic merkel cell carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:614–7. doi: 10.1097/DAD.0b013e3181cd3158. [DOI] [PubMed] [Google Scholar]
- Xystrakis E, Dejean AS, Bernard I, Druet P, Liblau R, Gonzalez-Dunia D, et al. Identification of a novel natural regulatory CD8 T-cell subset and analysis of its mechanism of regulation. Blood. 2004;104:3294–301. doi: 10.1182/blood-2004-03-1214. [DOI] [PubMed] [Google Scholar]
- Yang ZQ, Yang ZY, Zhang LD, Ping B, Wang SG, Ma KS, et al. Increased liver-infiltrating CD8+FoxP3+ regulatory T cells are associated with tumor stage in hepatocellular carcinoma patients. Hum Immunol. 2010;71:1180–6. doi: 10.1016/j.humimm.2010.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.