Abstract
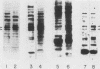
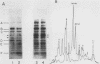
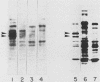
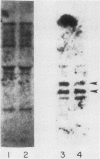
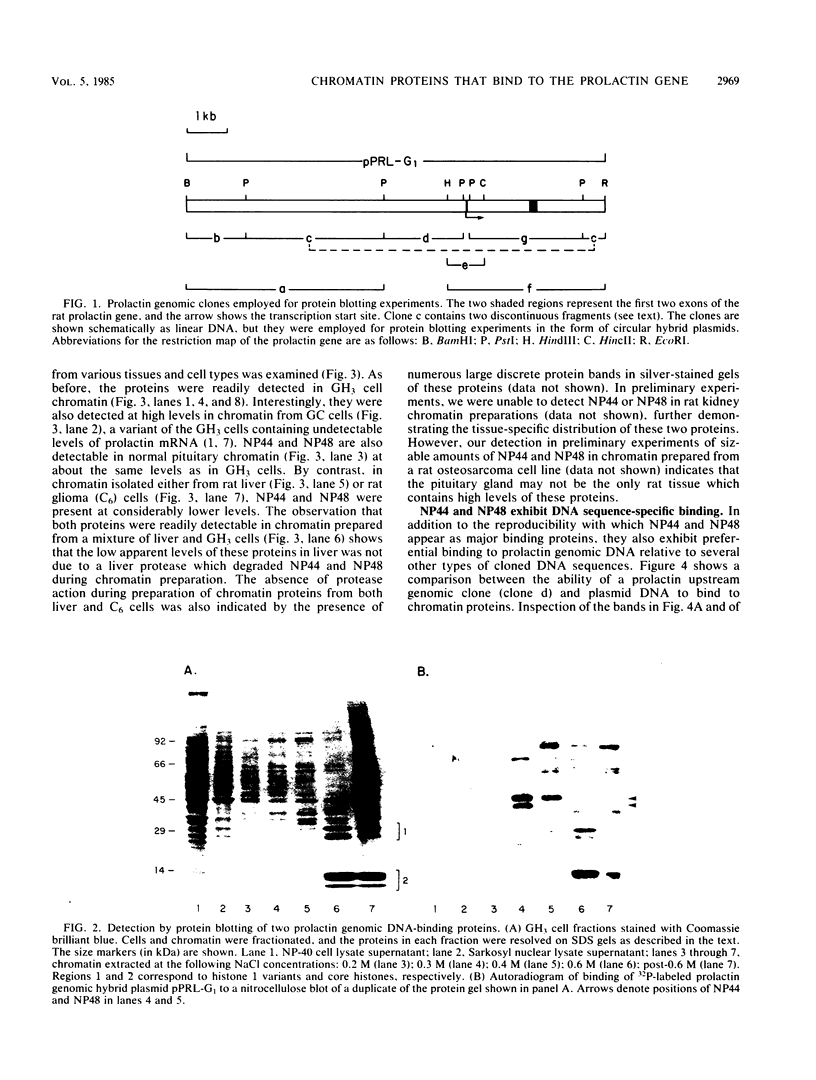
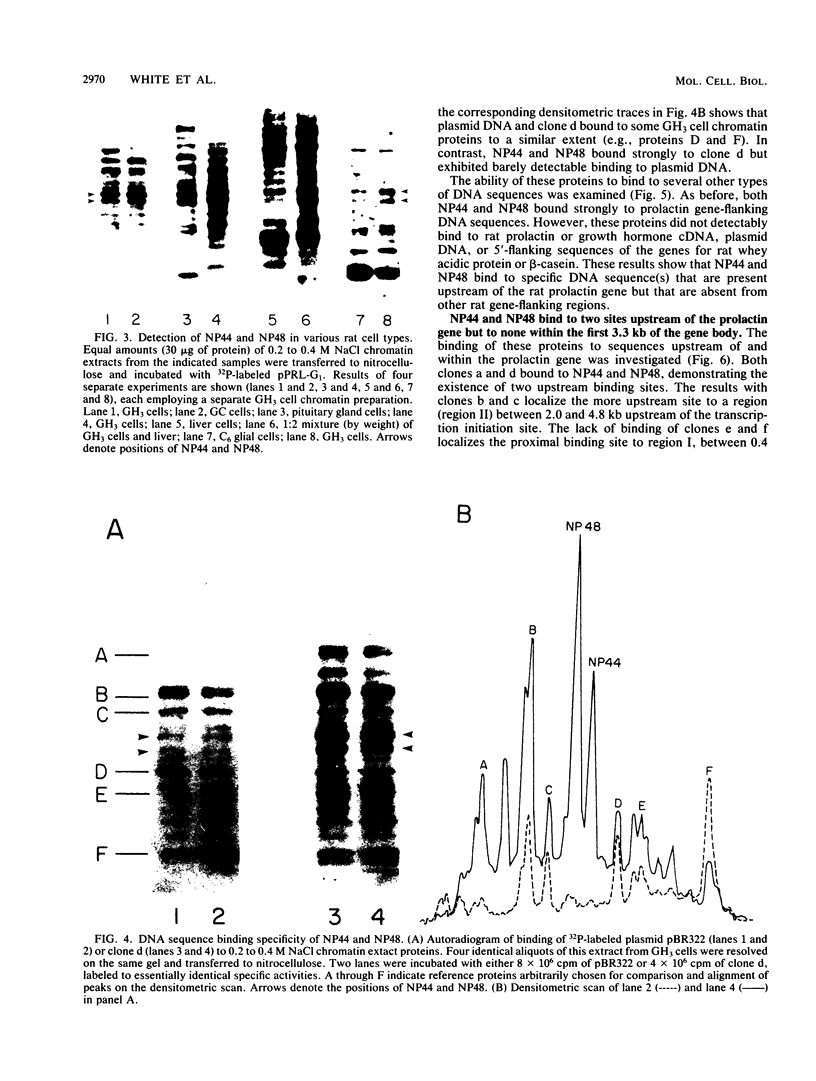
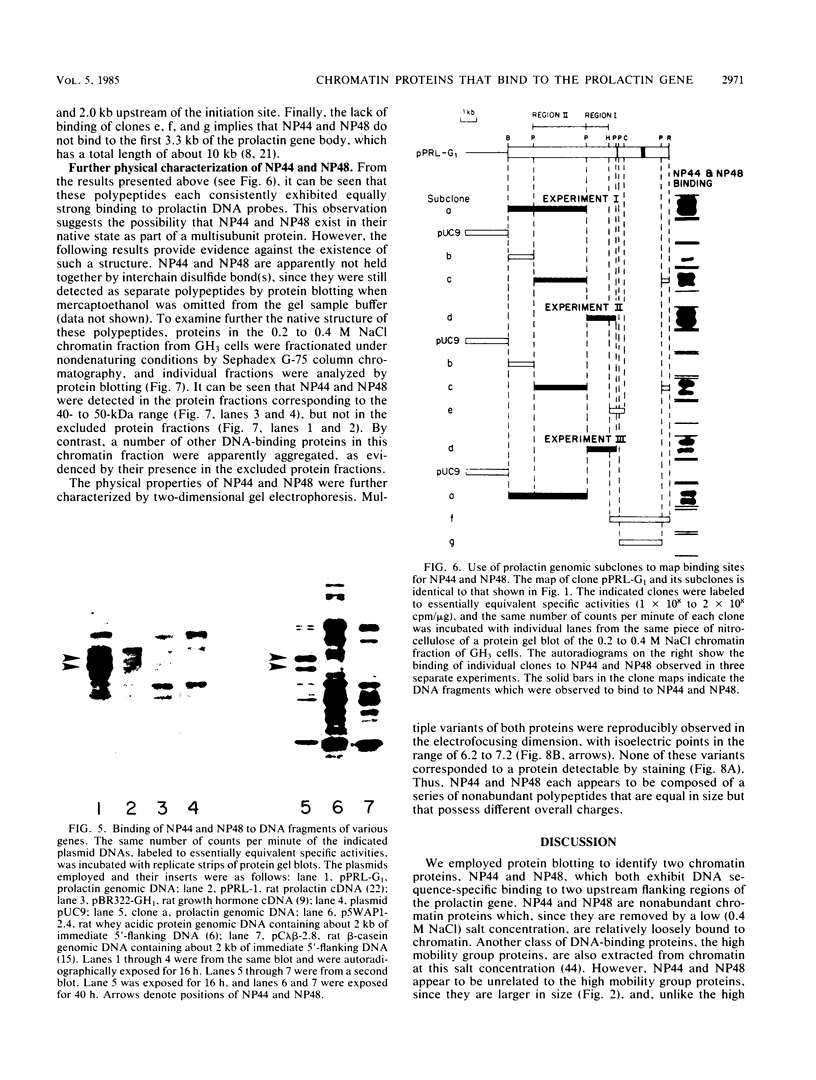
We employed a protein gel blotting procedure to search for nuclear proteins from rat pituitary cells that bind preferentially to the 5'-flanking region of the rat prolactin gene. By gel blots of chromatin proteins from GH3 rat pituitary tumor cells with a 32P-labeled prolactin genomic clone, we detected two major binding proteins with molecular weights of approximately 44,000 and 48,000, designated NP44 and NP48, respectively. Both NP44 and NP48 are minor chromatin proteins which are extracted at low salt concentrations (0.4 M NaCl) and exhibit a range of slightly acidic isoelectric variants. NP44 and NP48 were detected at similar levels in chromatin extracts of GH3 cells, the prolactin-negative GC cell variant of the GH3 cells, and normal rat pituitary tissue. Considerably lower levels of these two proteins were found in chromatin extracts from rat liver and rat C6 glial cells. NP44 and NP48 exhibit DNA sequence specificity, as evidenced by their strong binding to the upstream flanking region of the prolactin gene, but only very weak binding to plasmid DNA, rat prolactin or growth hormone cDNAs, or upstream flanking regions of two other rat genes. By analyzing subclones of a rat prolactin genomic clone, we established that NP44 and NP48 bind to at least two sites, which are located between 0.4 and 2.0 kilobases (region I) and between 2.0 and 4.8 kilobases (region II) upstream of the transcription initiation site. These findings are discussed in the context of a possible functional association between the strong binding of NP44 and NP48 to the prolactin 5'-flanking region and pituitary-specific expression of the prolactin gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Bodnar J. W., Jones C. J., Coombs D. H., Pearson G. D., Ward D. C. Proteins tightly bound to HeLa cell DNA at nuclear matrix attachment sites. Mol Cell Biol. 1983 Sep;3(9):1567–1579. doi: 10.1128/mcb.3.9.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. D. The role of stable complexes that repress and activate eucaryotic genes. Cell. 1984 Jun;37(2):359–365. doi: 10.1016/0092-8674(84)90366-0. [DOI] [PubMed] [Google Scholar]
- Campbell S. M., Rosen J. M., Hennighausen L. G., Strech-Jurk U., Sippel A. E. Comparison of the whey acidic protein genes of the rat and mouse. Nucleic Acids Res. 1984 Nov 26;12(22):8685–8697. doi: 10.1093/nar/12.22.8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo A. J., Pool T. B., Sharp Z. D. Vasoactive intestinal peptide increases prolactin messenger ribonucleic acid content in GH3 cells. Endocrinology. 1985 Jan;116(1):202–206. doi: 10.1210/endo-116-1-202. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Thompson E. B. Genomic organization of rat prolactin and growth hormone genes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4583–4587. doi: 10.1073/pnas.77.8.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobner P. R., Kawasaki E. S., Yu L. Y., Bancroft F. C. Thyroid or glucocorticoid hormone induces pre-growth-hormone mRNA and its probable nuclear precursor in rat pituitary cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2230–2234. doi: 10.1073/pnas.78.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrin L. K., Weber J. L., Gorski J. Chromatin structure, transcription, and methylation of the prolactin gene domain in pituitary tumors of Fischer 344 rats. J Biol Chem. 1984 Jun 10;259(11):7086–7093. [PubMed] [Google Scholar]
- Emerson B. M., Felsenfeld G. Specific factor conferring nuclease hypersensitivity at the 5' end of the chicken adult beta-globin gene. Proc Natl Acad Sci U S A. 1984 Jan;81(1):95–99. doi: 10.1073/pnas.81.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gick G. G., Bancroft C. Regulation by calcium of prolactin and growth hormone mRNA sequences in primary cultures of rat pituitary cells. J Biol Chem. 1985 Jun 25;260(12):7614–7618. [PubMed] [Google Scholar]
- Hamada H., Seidman M., Howard B. H., Gorman C. M. Enhanced gene expression by the poly(dT-dG).poly(dC-dA) sequence. Mol Cell Biol. 1984 Dec;4(12):2622–2630. doi: 10.1128/mcb.4.12.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack R. S., Gehring W. J., Brack C. Protein component from Drosophila larval nuclei showing sequence specificity for a short region near a major heat-shock protein gene. Cell. 1981 May;24(2):321–331. doi: 10.1016/0092-8674(81)90322-6. [DOI] [PubMed] [Google Scholar]
- Jones W. K., Yu-Lee L. Y., Clift S. M., Brown T. L., Rosen J. M. The rat casein multigene family. Fine structure and evolution of the beta-casein gene. J Biol Chem. 1985 Jun 10;260(11):7042–7050. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Estradiol regulates the transcription of the prolactin gene. J Biol Chem. 1982 Mar 10;257(5):2133–2136. [PubMed] [Google Scholar]
- Maurer R. A., Gubbins E. J., Erwin C. R., Donelson J. E. Comparison of potential nuclear precursors for prolactin and growth hormone messenger RNA. J Biol Chem. 1980 Mar 25;255(6):2243–2246. [PubMed] [Google Scholar]
- Maurer R. A. Selective binding of the estradiol receptor to a region at least one kilobase upstream from the rat prolactin gene. DNA. 1985 Feb;4(1):1–9. doi: 10.1089/dna.1985.4.1. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Thyroid hormone specifically inhibits prolactin synthesis and decreases prolactin messenger ribonucleic acid levels in cultured pituitary cells. Endocrinology. 1982 May;110(5):1507–1514. doi: 10.1210/endo-110-5-1507. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Transcriptional regulation of the prolactin gene by ergocryptine and cyclic AMP. Nature. 1981 Nov 5;294(5836):94–97. doi: 10.1038/294094a0. [DOI] [PubMed] [Google Scholar]
- Mirkovitch J., Mirault M. E., Laemmli U. K. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984 Nov;39(1):223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Murdoch G. H., Franco R., Evans R. M., Rosenfeld M. G. Polypeptide hormone regulation of gene expression. Thyrotropin-releasing hormone rapidly stimulates both transcription of the prolactin gene and the phosphorylation of a specific nuclear protein. J Biol Chem. 1983 Dec 25;258(24):15329–15335. [PubMed] [Google Scholar]
- Myers R. M., Rio D. C., Robbins A. K., Tjian R. SV40 gene expression is modulated by the cooperative binding of T antigen to DNA. Cell. 1981 Aug;25(2):373–384. doi: 10.1016/0092-8674(81)90056-8. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Tesser P., Azorin F., Kwon Y. H., Möller A., Rich A. Isolation of Drosophila proteins that bind selectively to left-handed Z-DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7729–7733. doi: 10.1073/pnas.79.24.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowock J., Sippel A. E. Specific protein-DNA interaction at four sites flanking the chicken lysozyme gene. Cell. 1982 Sep;30(2):607–615. doi: 10.1016/0092-8674(82)90257-4. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Prior C. P., Cantor C. R., Johnson E. M., Littau V. C., Allfrey V. G. Reversible changes in nucleosome structure and histone H3 accessibility in transcriptionally active and inactive states of rDNA chromatin. Cell. 1983 Oct;34(3):1033–1042. doi: 10.1016/0092-8674(83)90561-5. [DOI] [PubMed] [Google Scholar]
- Ryan R., Shupnik M. A., Gorski J. Effect of estrogen on preprolactin messenger ribonucleic acid sequences. Biochemistry. 1979 May 15;18(10):2044–2048. doi: 10.1021/bi00577a031. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Scheidereit C., Beato M. Contacts between hormone receptor and DNA double helix within a glucocorticoid regulatory element of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1984 May;81(10):3029–3033. doi: 10.1073/pnas.81.10.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss F., Varshavsky A. A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell. 1984 Jul;37(3):889–901. doi: 10.1016/0092-8674(84)90424-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triadou P., Crepin M., Gros F., Lelong J. C. Tissue-specific binding of total and beta-globin genomic deoxyribonucleic acid to non-histone chromosomal proteins from mouse erythropoietic cells. Biochemistry. 1982 Nov 23;21(24):6060–6065. doi: 10.1021/bi00267a006. [DOI] [PubMed] [Google Scholar]
- Ucker D. S., Yamamoto K. R. Early events in the stimulation of mammary tumor virus RNA synthesis by glucocorticoids. Novel assays of transcription rates. J Biol Chem. 1984 Jun 25;259(12):7416–7420. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wark J. D., Tashjian A. H., Jr Regulation of prolactin mRNA by 1,25-dihydroxyvitamin D3 in GH4C1 cells. J Biol Chem. 1983 Oct 25;258(20):12118–12121. [PubMed] [Google Scholar]
- Watkins D., White B. A. Identification and characterization of calmodulin-binding proteins in islet secretion granules. J Biol Chem. 1985 Apr 25;260(8):5161–5165. [PubMed] [Google Scholar]
- Weber J. L., Durrin L. K., Gorski J. Repetitive DNA sequences within and around the rat prolactin gene. Mol Cell Biochem. 1985 Jan;65(2):171–179. doi: 10.1007/BF00221100. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Groudine M., Weintraub H. Interaction of HMG 14 and 17 with actively transcribed genes. Cell. 1980 Jan;19(1):289–301. doi: 10.1016/0092-8674(80)90410-9. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of a subclass of nuclear proteins responsible for conferring a DNase I-sensitive structure on globin chromatin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):630–634. doi: 10.1073/pnas.76.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Epidermal growth factor and thyrotropin-releasing hormone interact synergistically with calcium to regulate prolactin mRNA levels. J Biol Chem. 1983 Apr 10;258(7):4618–4622. [PubMed] [Google Scholar]
- White B. A., Bauerle L. R., Bancroft F. C. Calcium specifically stimulates prolactin synthesis and messenger RNA sequences in GH3 cells. J Biol Chem. 1981 Jun 25;256(12):5942–5945. [PubMed] [Google Scholar]
- White B. A. Evidence for a role of calmodulin in the regulation of prolactin gene expression. J Biol Chem. 1985 Jan 25;260(2):1213–1217. [PubMed] [Google Scholar]
- Wu C. Activating protein factor binds in vitro to upstream control sequences in heat shock gene chromatin. Nature. 1984 Sep 6;311(5981):81–84. doi: 10.1038/311081a0. [DOI] [PubMed] [Google Scholar]
- Wu C. Two protein-binding sites in chromatin implicated in the activation of heat-shock genes. Nature. 1984 May 17;309(5965):229–234. doi: 10.1038/309229a0. [DOI] [PubMed] [Google Scholar]
- Yu L. Y., Tushinski R. J., Bancroft F. C. Glucocorticoid induction of growth hormone synthesis in a strain of rat pituitary cells. J Biol Chem. 1977 Jun 10;252(11):3870–3875. [PubMed] [Google Scholar]