Abstract
BACKGROUND
Persistent pain is measured by means of self-report, the sole reliance on which hampers diagnosis and treatment. Functional magnetic resonance imaging (fMRI) holds promise for identifying objective measures of pain, but brain measures that are sensitive and specific to physical pain have not yet been identified.
METHODS
In four studies involving a total of 114 participants, we developed an fMRI-based measure that predicts pain intensity at the level of the individual person. In study 1, we used machine-learning analyses to identify a pattern of fMRI activity across brain regions — a neurologic signature — that was associated with heat-induced pain. The pattern included the thalamus, the posterior and anterior insulae, the secondary somatosensory cortex, the anterior cingulate cortex, the periaqueductal gray matter, and other regions. In study 2, we tested the sensitivity and specificity of the signature to pain versus warmth in a new sample. In study 3, we assessed specificity relative to social pain, which activates many of the same brain regions as physical pain. In study 4, we assessed the responsiveness of the measure to the analgesic agent remifentanil.
RESULTS
In study 1, the neurologic signature showed sensitivity and specificity of 94% or more (95% confidence interval [CI], 89 to 98) in discriminating painful heat from nonpainful warmth, pain anticipation, and pain recall. In study 2, the signature discriminated between painful heat and nonpainful warmth with 93% sensitivity and specificity (95% CI, 84 to 100). In study 3, it discriminated between physical pain and social pain with 85% sensitivity (95% CI, 76 to 94) and 73% specificity (95% CI, 61 to 84) and with 95% sensitivity and specificity in a forced-choice test of which of two conditions was more painful. In study 4, the strength of the signature response was substantially reduced when remifentanil was administered.
CONCLUSIONS
It is possible to use fMRI to assess pain elicited by noxious heat in healthy persons. Future studies are needed to assess whether the signature predicts clinical pain. (Funded by the National Institute on Drug Abuse and others.)
ALTHOUGH BIOMARKERS FOR MEDICAL conditions have proliferated over the past 50 years, objective assessments related to mental health have lagged behind. Physical pain is an affliction associated with enormous cognitive, social, and economic costs,1 but pain is not easy to ascertain. It is primarily assessed by means of self-report, an imperfect measure of subjective experience. The capacity to effectively report pain is limited in many vulnerable populations (e.g., the very old or very young, persons with cognitive impairment, and those who are minimally conscious). Moreover, self-report provides a limited basis for understanding the neurophysiological processes underlying different types of pain and thus a limited basis for targeting treatments to the underlying neuropathologic conditions. As a result, current approaches to pain assessment focus on a convergence of biologic, behavioral, and self-report measures.2
It is plausible that neurologic signatures (patterns of activity across brain regions) derived from brain imaging could provide direct measures of pain intensity and be used to compare analgesic treatments.3 We combined the use of functional magnetic resonance imaging (fMRI) with machine learning 4,5 to develop a brain-based neurologic signature for experimental thermal pain.
METHODS
PARTICIPANTS
The studies included a total of 114 healthy participants. Study 1 included 20 participants, 8 of whom were women; the mean (±SD) age was 28.8±7.5 years. Study 2 included 33 participants, 22 of whom were women; the mean age was 27.9±9.0 years. Study 3 included 40 participants, 21 of whom were women; the mean age was 20.8±2.6 years.6 Study 4 included 21 participants, 11 of whom were women; the mean age was 24.7±4.2 years.7 The Columbia University institutional review board approved all the studies, and all participants provided written informed consent. All the authors vouch for the accuracy and completeness of the data and analyses reported and the fidelity of the studies to the protocols. See the Supplementary Appendix, available with the full text of this article at NEJM.org, for additional details.
STUDY DESIGN
In all four studies, we applied thermal stimuli in randomized sequences of varying intensity (trials) to the left forearm of each participant during fMRI scanning. For imaging, we used a 1.5-T General Electric scanner in studies 1, 3, and 4 and a 3-T Phillips scanner in study 2.
Participants in study 1 underwent 12 trials at each of four intensities, which were calibrated for each person: innocuous warmth (defined with the use of self-report by the participant as level 1 on a 9-point visual-analogue scale [VAS], with a mean [±SD] temperature of 41.0±1.9°C) and three levels of painful heat (participant-defined levels 3, 5, and 7, with mean temperatures of 43.3±2.1°C, 45.4±1.71°C, and 47.1±0.98°C, respectively). Each trial consisted of a warning cue and anticipation period (8 seconds), stimulation (10 seconds), and a pain-recall and rating period (4 seconds), with periods of rest before and after recall.
Participants in study 2 underwent a total of 75 trials across six temperatures (44.3 to 49.3°C in 1°C increments). After each trial, participants judged whether the stimulus was painful. They subsequently judged nonpainful warmth on a 100-point VAS and pain intensity on a 100-point VAS. Ratings were coded from 0 to 99 for nonpain-ful events and from 100 to 200 for painful events.
Participants in study 3 underwent 32 trials, consisting of 8 trials with each of four stimulus types. We delivered noxious heat (46.6±1.7°C, denoted “painful”) and warmth that was near the pain threshold (39.9±2.8°C, denoted “warm”) at temperatures calibrated for each person. Each participant had recently experienced a romantic breakup and continued to feel intensely rejected. During scanning, participants viewed an image of their ex-partner (denoted as “rejecter” trials, which elicit social pain8) and an image of a close friend (denoted as “friend” trials).
Participants in study 4 received two intravenous infusions of remifentanil, a potent μ-opioid agonist, during fMRI scanning in two series of trials. In the open-infusion series, participants knew they received remifentanil, and in the hidden-infusion series, they were told that no drug was delivered, even though it had been administered. Remifentanil doses (mean dose, 0.043±0.01 μg per kilogram of body weight per minute) were individually calibrated before the session to elicit analgesia without sedation, and we estimated the brain concentration of the drug over time using a pharmacokinetic model.9 We conducted 36 trials — 18 involving pain (mean temperature, 47.1±1.7°C) and 18 involving warmth (mean temperature, 41.2±2.6°C) — during each of the two infusion series. Drug infusion began partway through each series, after 6 trials, and ended after 24 trials. This design resulted in a continuously varying concentration of the drug over time during each infusion series.
DERIVING THE SIGNATURE
In study 1, we used a machine-learning–based regression technique, LASSO-PCR (least absolute shrinkage and selection operator-regularized principal components regression),10 to predict pain reports from the fMRI activity. We selected relevant brain areas a priori using the NeuroSynth meta-analytic databasei11 (see the Supplementary Appendix) and averaged the brain activity for each intensity level within each participant.12–14 We used the signal values from the voxels, each of which measured 3 mm3, in the a priori map to predict continuous pain ratings, using leave-one-participant-out cross-validation4 (see the Supplementary Appendix). The result was a spatial pattern of regression weights across brain regions, which was prospectively applied to fMRI activity maps obtained from new participants. Application of the signature to an activity map (e.g., a map obtained during thermal stimulation) yielded a scalar response value, which constituted the predicted pain for that condition.
We used permutation tests to obtain unbiased estimates of accuracy and bootstrap tests to determine which brain areas made reliable contributions to prediction (Fig. 1). Stimulation did not elicit head movement, and head-movement estimates did not predict pain (for a description of head-movement analyses, see the Supplementary Appendix).
Figure 1. Prediction of Physical Pain on the Basis of Normative Data from Other Participants in Study 1.
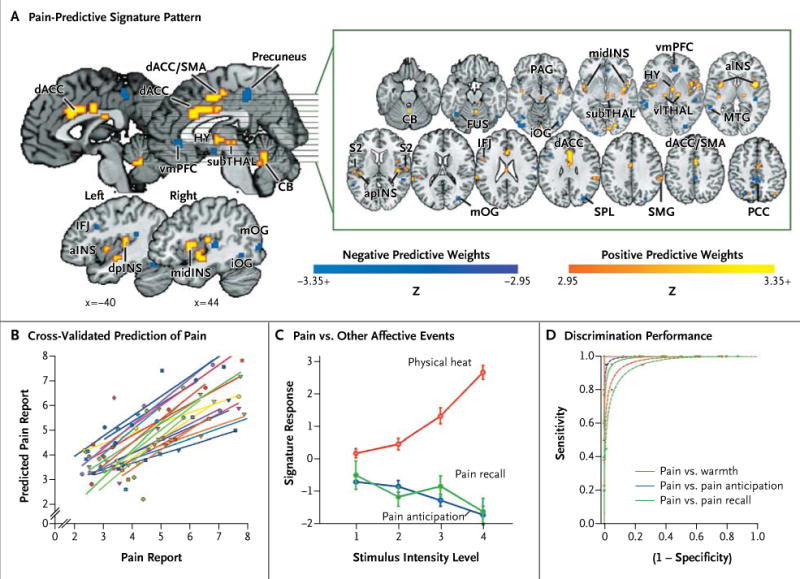
Panel A shows the signature map, consisting of voxels in which activity reliably predicted pain. The map shows weights that exceed a threshold (a false discovery rate of q < 0.05) for display only; all weights were used in prediction. ACC denotes anterior cingulate cortex, CB cerebellum, FUS fusiform, HY hypothalamus, IFJ inferior frontal junction, INS insula, MTG middle temporal gyrus, OG occipital gyrus, PAG periaqueductal gray matter, PCC posterior cingulate cortex, PFC prefrontal cortex, S2 secondary somatosensory cortex, SMA supplementary motor area, SMG supramarginal gyrus, SPL superior parietal lobule, TG temporal gyrus, and THAL thalamus. Direction is indicated with preceding lowercase letters as follows: a denotes anterior, d dorsal, i inferior, l lateral, m middle, mid mid-insula, p posterior, and v ventral. Panel B shows reported pain versus cross-validated predicted pain. Each colored line or symbol represents an individual participant. Panel C shows the signature response versus the pain intensity for heat, pain-anticipation, and pain-recall conditions. Signatureresponse values were calculated by taking the dot product of the signature-pattern weights and parameter estimates from a standard, single-participant general linear model, with regressors for each condition. The estimates shown are derived from cross-validation, so that signature weights and test data are independent. I bars indicate standard errors. The receiver-operating-characteristic plots in Panel D show the tradeoff between specificity and sensitivity. Lines are fitted curves, assuming gaussian signal distributions. The test of pain versus no pain and the forced-choice test are shown by dashed lines and solid lines, respectively. Performance on the forced-choice test was at 100% for all conditions; thus, the lines are overlapping.
PREDICTING PAIN IN AN INDEPENDENT SAMPLE
In study 2, we tested the neurologic signature identified in study 1, with no further model fitting, for the prediction of pain in individual participants, using data from a different scanner. We also estimated activity maps and signature responses for individual trials, which allowed us to use mixed-effects regression models to test the relationship between neurologic signature responses and intensity judgments during trials involving painful and nonpainful stimuli.
TESTING FOR SPECIFICITY
In study 3, we applied the signature to activation maps that resulted from physical sensation (painful and warm conditions) and from viewing images related to social pain (rejecter and friend conditions).6
RESPONSE TO ANALGESIC TREATMENT
In study 4, we tested the effects of stimulus intensity (painful vs. warm), administration of remi-fentanil (drug concentration), and manner of drug administration (open vs. hidden) on the signature response. For each of the open and hidden trial series, we estimated activation maps for painful stimulation, warm stimulation, and the magnitude of changes in each that followed the a priori time course of drug concentration from the phar-macokinetic model (Fig. S5 in the Supplementary Appendix). Because the drug concentration was continuous over time, the binary classification of painful versus warm conditions was based on the averages of the results of three trials before drug administration and three trials performed at the peak drug concentration.
STATISTICAL ANALYSIS
We assessed the sensitivity and specificity of the signature for two kinds of decisions. In one test, the discrimination of pain from no pain, we compared the signature-response value (i.e., the strength of expression of the signature pattern) for one condition with a threshold, with a response over the threshold being classified as a pain response. Receiver-operating-characteristic plots traced the tradeoff of sensitivity and specificity at different thresholds (Fig. 1D), and the threshold that minimized overall classification errors is reported (Table 1).
Table 1.
Pain-Classification Performance, According to Study.*
| Study | Discrimination between Pain and No Pain† | Effect Size‡ | P Value | Performance on Forced-Choice Test§ | ||||
|---|---|---|---|---|---|---|---|---|
| Signature-Response Threshold | Sensitivity | Specificity percent (95% CI) |
Positive Predictive Value | AUC | Discriminability | (percent 95% CI) | ||
|
| ||||||||
| Study 1 | ||||||||
|
| ||||||||
| Painful vs. warm¶ | 1.40 | 95 (86–100) | 95 (86–100) | 95 (85–100) | 0.95 | 2.69 | < 0.001 | 100 (100–100) |
|
| ||||||||
| Pain vs. pain anticipation | 0.36 | 100 (100–100) | 99 (96–100) | 95 (86–100) | 0.99 | 3.69 | < 0.001 | 100 (100–100) |
|
| ||||||||
| Pain vs. pain recall | 0.54 | 95 (85–100) | 94 (89–98) | 79 (64–92) | 0.96 | 2.35 | < 0.001 | 100 (100–100) |
|
| ||||||||
| Study 2 | ||||||||
|
| ||||||||
| Painful vs. warm ||** | 1.32 | 93 (84–100) | 93 (84–100) | 93 (84–100) | 0.92 | 1.54 | < 0.001 | 100 (100–100) |
|
| ||||||||
| Painful vs. near pain threshold†† | 2.50 | 88 (77–97) | 85 (72–95) | 85 (73–96) | 0.88 | 1.74 | < 0.001 | 100 (100–100) |
|
| ||||||||
| High vs. low warmth | 1.00 | 56 (36–75) | 100 (100–100) | 100 (100–100) | 0.79 | 1.31 | 0.001 | 100 (100–100) |
|
| ||||||||
| Study 3 | ||||||||
|
| ||||||||
| Painful vs. warm | 1.40‡‡ | 85 (76–94) | 78 (67–89) | 80 (68–89) | 0.86 | 1.64 | < 0.001 | 93 (86–98) |
|
| ||||||||
| Painful vs. rejecter | 1.40‡‡ | 85 (76–94) | 73 (61–84) | 76 (65–86) | 0.88 | 1.83 | < 0.001 | 95 (89–100) |
|
| ||||||||
| Rejecter vs. friend | 1.40‡‡ | 27 (16–38) | 88 (79–95) | 69 (50–88) | 0.57 | 0.31 | 0.22 | 56 (43–69) |
|
| ||||||||
| Study 4 | ||||||||
|
| ||||||||
| Painful vs. warm, before drug treatment | 1.40‡‡ | 90 (79–100) | 81 (65–95) | 83 (67–95) | 0.89 | 1.61 | < 0.001 | 90 (79–100) |
|
| ||||||||
| Painful vs. warm, during drug treatment | 1.61 | 86 (73–96) | 62 (42–80) | 69 (52–84) | 0.74 | 1.01 | 0.003 | 76 (61–90) |
|
| ||||||||
| Painful before vs. during drug treatment | 1.61 | 86 (72–96) | 62 (43–79) | 69 (54–83) | 0.74 | 1.01 | 0.003 | 76 (60–92) |
Study 1 included 12 trials each in painful and warm conditions. Study 2 included a mean (±SD) of 24±13 trials for pain and 36±9 trials for warmth, depending on the ratings. Study 3 included 8 trials each in painful and warm conditions. Study 4 included 3 trials for pain and 3 for warmth in the before-drug-treatment condition and in the condition with peak drug concentration. CI denotes confidence interval.
The tradeoff between sensitivity and specificity at different thresholds was assessed by means of receiver-operating-characteristic (ROC) plots; the signature-response threshold that minimized overall classification errors is reported here.
For the area under the ROC curve, chance is 0.5. Discriminability is a measure of effect size under a gaussian model. Performance varied across studies, according to the number of trials averaged to form the condition maps.
For the two-choice (forced-choice) discrimination test, the classification threshold for the difference between paired observations is 0. The sensitivity, specificity, and positive predictive value are the same and are equal to the decision accuracy.
Painful conditions were defined as temperatures greater than 44.5°C and as ratings of more than an average of 5.80 points on a visual-analogue scale (VAS), and warm conditions as temperatures of less than 44.5°C and ratings of less than 3.34 points on the VAS.
Study 2 was conducted with the use of a scanner with a different field strength (3 T), so the threshold was reestimated.
Participants made judgments of painful versus nonpainful conditions for each trial.
Participants rated pain or warmth intensity on a continuous VAS, with scores ranging from 0 to 99 points for warmth and from 100 to 200 points for pain. Pain was defined as a score of more than 125 points, near the pain threshold as a score of 75 to 125 points, high warmth as a score of 50 to 100 points, and low warmth as a score of O to 50 points.
The threshold derived from study 1 was applied.
In forced-choice discrimination, two activation maps from the same participant were compared, and the image with the higher overall signature response (i.e., the stronger expression of the signature pattern) was classified as associated with more pain. Forced-choice tests are particularly suitable for fMRI because they do not compare the signature response with a threshold that is fixed across persons. Therefore, they do not require people to use the pain-reporting scale in the same way, and they do not require the scale of fMRI activity to be the same across scanners (see the Supplementary Appendix). Sensitivity, specificity, positive predictive value, and decision accuracy are all equivalent in the forced-choice test. The MATLAB code for implementing all analyses is available at http://wagerlab.colorado.edu/.
RESULTS
CROSS-VALIDATED PREDICTION OF PAIN
In study 1, the neurologic signature included significant positive weights in regions including the bilateral dorsal posterior insula, the secondary so-matosensory cortex, the anterior insula, the ventro lateral and medial thalamus, the hypothalamus, and the dorsal anterior cingulate cortex (q < 0.05, corrected for the false discovery rate) (Fig. 1A, and Table S1 in the Supplementary Appendix), which is consistent with the view of pain as a distributed process.15,16 In a leave-one-participant-out cross-validation test, the neurologic signature accurately predicted continuous pain ratings, with a mean (±SD) error of 0.96±0.33 points on the 9-point VAS and a prediction-outcome correlation coefficient of 0.74 (Fig. 1B).
The signature response increased nonlinearly with increasing stimulus intensity during thermal stimulation, but as expected, it was uniformly low for the pain-anticipation and pain-recall periods (Fig. 1C). To test the discrimination of painful from nonpainful warmth, we compared painful conditions (> 45°C, a temperature level that activates specific nociceptors17 and that was above the median temperature associated with reported pain) with warm conditions (< 45°C, which was below the median temperature associated with reported pain). Both sensitivity and specificity in the discrimination of pain from no pain were 94% or more for comparisons of pain versus nonpainful warmth, pain versus anticipation, and pain versus pain recall (Fig. 1D and Table 1).
Forced-choice tests showed 100% sensitivity and specificity for all three comparisons (Table 1), indicating that the signature response was always higher for painful stimulation than for anticipation or recall within an individual participant. In addition, the signature discriminated between relative differences in pain, with sensitivity and specificity of 93% or more when pain ratings differed by 2 or more points on the 9-point VAS (see the Supplementary Appendix). Thus, the neurologic signature was sensitive and specific to pain, with improved performance in the forced-choice test.
PAINFUL VERSUS NONPAINFUL HEAT
In study 2, the signature response increased mono-tonically across the six temperatures (Fig. 2A), with an expected nonlinear increase with temperature, and it correlated with both the reported level of pain (r = 0.73) and the stimulus temperature (r = 0.65). Signature responses increased with subjective intensity on a continuum across painful and nonpainful events (Fig. 2B), a finding that is consistent with contributions by colocal-ized wide-dynamic-range neurons and nociceptive-specific neurons.17-19 However, mixed-effects regression analyses showed that the signature response increased more strongly with ratings of pain intensity than with ratings of warmth intensity (β = 0.66, t = 2.58, P = 0.02) (Fig. 2B).
Figure 2. Application of the Neurologic Signature in Study 2.
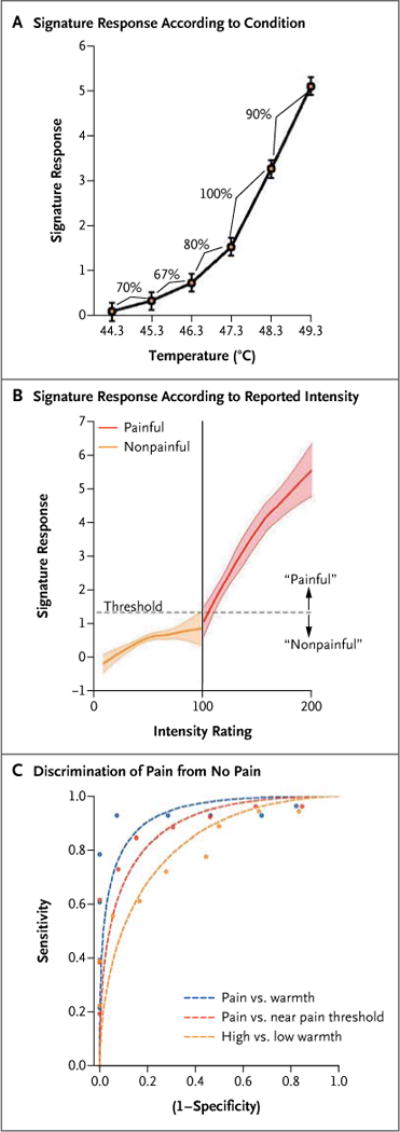
Panel A shows the signature response across the temperatures used in study 2. The signature response was defined as the dot product of the signature-pattern weights from study 1 and the activation maps for each temperature within each individual participant. I bars show the standard error for the within-participant data. The signature response increased with increasing temperature, as did the level of reported pain. Percentages indicate the sensitivity and specificity for adjacent temperatures in the forced-choice classification. Sensitivity and specificity are equivalent for the forced-choice test and reflect the proportion of participants for whom the prediction based on the signature response was correct. Panel B shows the signature response as a function of reported intensity, for conditions rated as warm (nonpainful; orange) and those rated as painful (red). Loess smoothing was used to visualize the relationship; shaded areas show bootstrapped standard errors. The vertical line (at 100) divides conditions explicitly rated as painful from those rated as nonpainful, and the dashed horizontal line (at 1.32) is the classification threshold that maximizes the classification accuracy for painful versus nonpainful conditions. Panel C shows the discrimination performance for comparisons of pain and no pain. Performance (circles) was generally better than predicted by the gaussian model (dashed lines), suggesting a super-gaussian distribution of the signature response. Discrimination in the forced-choice test showed 100% sensitivity and specificity in all comparisons (data not shown).
In trials involving painful heat, the neurologic signature strongly predicted pain intensity (β = 0.20, t = 6.84, P < 0.001), even when we controlled for linear and nonlinear effects of temperature (β = 0.13, t = 4.51, P < 0.001). In trials involving nonpainful heat, the neurologic signature weakly predicted warmth intensity (β = 0.06, t = 2.04, P = 0.08) and did not predict warmth intensity after adjustment for temperature (β = 0.05, t = 1.30, P = 0.22). These results suggest that the signature is related principally to the subjective sensation of pain but also reflects the overall intensity of somatic stimulation to some degree.
To assess discrimination performance, we averaged the neurologic signature response for painful conditions (rating, ≥ 100; mean rating, 138 points) and nonpainful conditions (rating, < 100; mean rating, 60 points) for each participant. Because the field strengths of the scanners used in studies 1 and 2 differed (1.5 T vs. 3.0 T), we reestimated the signature-response threshold for painful versus nonpainful events, which was estimated to be 1.32 in study 2, as compared with 1.40 in study 1. The average signature response across trials accurately discriminated painful from nonpainful conditions with 93% sensitivity and specificity in the test of pain versus no pain (95% confidence interval [CI], 84 to 100 for both comparisons), and with 100% sensitivity and specificity (95% CI, 100 to 100) in the forced-choice test (Table 1).
The signature response also discriminated between clearly painful conditions and conditions near the pain threshold (mean score, 150 vs. 98 points) with 88% sensitivity (95% CI, 77 to 97) and 85% specificity (95% CI, 72 to 95) in the test of pain versus no pain and with 100% sensitivity and specificity in the forced-choice test. However, the signature response also discriminated between intense nonpainful warmth and mild nonpainful warmth (Table 1), suggesting that hyperalgesia or allodynia would be indicated by positive results of both the test of pain versus no pain and the forced-choice test.
Finally, tests of forced-choice discrimination across painful temperatures showed good performance, and tests across nonpainful temperatures showed poor performance, supporting the use of for a temperature of 48.3°C versus 47.3°C, with 15 trials performed for each condition. However, performance dropped to near-chance levels when low temperatures were used (Fig. 2A, and the Supplementary Appendix).the signature to assess nociceptive responses. Sensitivity and specificity were 90% (95% CI, 81 to 97) for a temperature of 49.3°C versus 48.3°C, with only 4 trials performed at 49.3°C, and 100%
SPECIFICITY OF NEUROLOGIC SIGNATURE FOR PHYSICAL PAIN
In study 3, comparisons of rejecter versus friend and pain versus warmth yielded similar levels of self-reported negative affect, and overlapping portions of many regions related to pain intensity were activated, including the bilateral anterior in-sula, medial thalamus, secondary somatosensory cortex, and dorsal posterior insula.6 These findings provided a good basis for a test of specificity.
The neurologic signature response was substantially stronger for physical pain than for any of the other conditions (warmth, rejecter, or friend) (Fig. 3A) and predicted pain ratings (r = 0.68, P < 0.001, with a mean prediction error of 0.84 points). As in study 1, the signature response predicted intensity ratings for noxious stimuli (r = 0.44, P < 0.01) but not innocuous stimuli (r = 0.02, P > 0.90). With the use of the threshold derived from study 1, the response for the discrimination between pain and no pain had 85% sensitivity (95% CI, 76 to 94) and 78% specificity (95% CI, 67 to 89) for pain versus warmth and 93% sensitivity and specificity (95% CI, 86 to 98) for forced-choice discrimination, with similar performance for the comparison of pain and rejecter conditions (P < 0.001 for all comparisons) (Table 1). Discrimination between the rejecter and friend conditions was no better than would be expected by chance (Table 1).
Figure 3. Application of the Neurologic Signature to Physical and Social Pain Stimuli in Study 3.
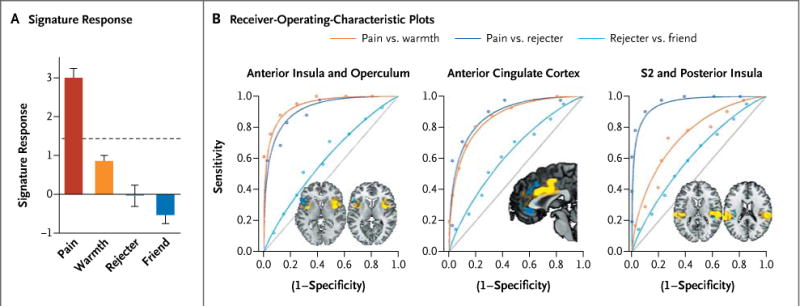
Panel A shows the signature response in each condition. The dashed horizontal line shows the threshold derived from the classification of pain versus warmth in study 1. I bars indicate standard errors. Panel B shows the receiver-operating-characteristic plots for the forced-choice test, assessed only from the pattern within a single region of interest. A physical-pain signature would ideally show high sensitivity and specificity for pain versus warmth (orange line) and pain versus rejecter (dark blue line) but chance performance for rejecter versus friend (light blue line). The brain images (insets) show the positive (yellow) and negative (blue) signature weights in each region of interest, with the magnitude of the weights represented by the intensity of the color.
This observed specificity may be driven by finegrained differences in activity patterns in regions activated by both physical and social pain, an explanation that is consistent with the notion that different groups of neurons code for different affective events, or by differential activation of sensory-system–specific regions (e.g., the secondary somatosensory cortex for heat vs. the occipital cortex for images). If the first explanation holds, the pattern of activation, rather than the overall level of activation of a region, is the critical agent of discrimination.
To test these alternatives, we assessed the neurologic signature response derived from patterns within the dorsal anterior cingulate cortex, anterior insula and operculum, and secondary somato-sensory cortex and dorsal posterior insula individually (Fig. 3B, 3C, and 3D). Each region was activated by social pain (rejecter vs. friend) overall. However, in each region, the signature response reliably discriminated pain from the warm condition and pain from the rejecter condition (mean sensitivity and specificity in the forced-choice test, 78%) (Table S2 in the Supplementary Appendix) and performed at chance levels for the rejecter versus friend condition (mean sensitivity and specificity, 58%), suggesting that the pattern within these regions is critical for predicting pain.
REMIFENTANIL TREATMENT RESPONSE
Before drug infusion, in study 4, the signature response was greater for painful stimuli than for warm stimuli in both the open-infusion trials and the hidden-infusion trials (t = 5.21 and t = 4.84, respectively; P < 0.001 for both comparisons) (Fig. S6 in the Supplementary Appendix). During infusion, the signature response was reduced in parallel with increases in the drug effect-site concentration (t = −2.78 for trials with open infusion, and t = −2.77 for trials with hidden infusion; P = 0.01 for both comparisons).
At the maximum drug concentration, remifen tanil was associated with a reduction of 53% in the signature response, with no differences across the open and hidden infusions (P = 0.94). The sensitivity and specificity for the discrimination between painful and warm stimuli in the forced-choice test were both 90% (95% CI, 79 to 100), with 95% sensitivity (95% CI, 86 to 100) and 62% specificity (95% CI, 43 to 79) in the test of pain versus no pain (P < 0.001) (Table 1). Lower accuracy was expected because preinfusion signature responses in each condition were estimated from only three trials.
DISCUSSION
We identified an fMRI-based neurologic signature associated with thermal pain, discriminates physical pain from several other salient, aversive events, and is sensitive to the analgesic effects of opi-oids. This signature consisted of interpretable, stable patterns across regions known to show increased activity in association with experimentally induced pain, hyperalgesic or allodynic states,20,21 experimentally induced acute pain in patients,22 and experimentally induced tonic pain (pain caused by a stimulus of extended duration) in healthy persons.23
The signature is distinguished from a general salience signal by its inclusion of somatic-specific regions, such as the ventrolateral thalamus,24 the secondary somatosensory cortex,6 and the dorsal posterior insula,6 and by the identification of patterns of activity that are specific to physical pain within regions that are activated across many psychological processes (e.g., the anterior insula and the anterior cingulate cortex11). Specificity to pain at the pattern level is consistent with findings that the anterior cingulate cortex and other association regions contain nociceptive-specific neurons as well as neurons with other properties25 and that machine learning can identify fMRI patterns with specific functional properties.26
The neurologic signature is predominantly bilateral but shows evidence of contralateral specificity in the primary and secondary somatosensory cortexes, an observation that is consistent with previous work.15,27 These results build on previous studies16,28–31 by showing a signature that has more than 90% sensitivity and specificity for pain at the level of the individual person and that is consistently accurate across studies and scanners. The forced-choice classification test we used could be translated into a test of hyperalgesia or allodynia in clinical studies, although the signature has not been validated for clinical pain and cannot currently be used in clinical tests.
If our findings are extended to clinical populations, brain-based signatures could be useful in confirming pain in situations in which patients are unable to communicate pain effectively or when self-reports are otherwise suspect. Such signatures could also help identify functional neuropathologic disorders32 that may underlie or confer a predisposition to chronic pain, even in the absence of overt structural lesions.33 More broadly, brain-based signatures could accelerate the identification of neurophysiological subtypes of pain and intermediate markers for treatment discovery.34 Such signatures could not, however, rule out the presence of pain with a nonnormative neurophysiological basis.
Before fMRI-based signatures for pain can be tested in medical decision-making settings, the generalizability of our findings must be assessed. Pain classification may be less accurate in patients than in healthy persons. Clinical use would require calibration across persons, scanning protocols, and research sites. Of the tests studied, the test of pain versus no pain is likely to be clinically useful in the broadest range of situations, but it is less strongly predictive than the forced-choice test. Finally, pain-associated fMRI patterns may differ according to body site,35,36 type of pain (visceral vs. cutaneous),37 and clinical cause, potentially necessitating the development of multiple pain signatures. Nonetheless, our findings represent a step toward developing neurologic signatures for multiple types of pain and other cognitive and affective processes.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute on Drug Abuse (1RC1DA028608 and R01DA027794), the National Institute of Mental Health (R01MH076136), and the National Science Foundation (0631637, to Dr. Wager).
We thank Drs. Ed Smith, John Jonides, Basil Margolis, Doug Noll, Robert Welsh, and Daphna Shohamy for helpful discussion and comments on an earlier version of the manuscript; Dr. Robert Whittington for participation in the drug administration and data collection in study 4; Damon Abraham and Kate Dahl for help with data collection; Dr. Tal Yarkoni for help with meta-analytic maps; Dr. Jason Buhle for assistance in planning study 2; Dr. Steven Shafer for assistance with pharmokinetic modeling; and Dr. Lew Harvey for consultation on binary classification analyses.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.Flor H, Turk D. Chronic pain: an integrated biobehavioral approach. Seattle: IASP Press; 2011. [Google Scholar]
- 3.Wager TD, Fields H. Placebo analgesia. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s textbook of pain. Oxford, United Kingdom: Churchill Livingstone; (in press) [Google Scholar]
- 4.Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning: data mining, inference, and prediction. 2. New York: Springer; 2009. [Google Scholar]
- 5.Mitchell T. Does machine learning really work? AI Magazine. 1997;18:11. [Google Scholar]
- 6.Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci USA. 2011;108:6270–5. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. Dissociable influences of opiates and expectations on pain. J Neurosci. 2012;32:8053–64. doi: 10.1523/JNEUROSCI.0383-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macdonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychol Bull. 2005;131:202–23. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- 9.Minto CF, Schnider TW, Egan TD, et al. Influence of age and gender on the pharmacokinetics and pharmacodynam-ics of remifentanil. I. Model development. Anesthesiology. 1997;86:10–23. doi: 10.1097/00000542-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci. 2011;31:439–52. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–70. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baliki MN, Geha PY, Apkarian AV. Parsing pain perception between nocicep-tive representation and magnitude estimation. J Neurophysiol. 2009;101:875–87. doi: 10.1152/jn.91100.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindquist MA, Meng Loh J, Atlas L, Wager TD. Modeling the hemodynamic response function in fMRI: efficiency, bias and mis-modeling. Neuroimage. 2009;45(Suppl):S187–S198. doi: 10.1016/j.neuroimage.2008.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 15.Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–43. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 16.Brodersen KH, Wiech K, Lomakina EI, et al. Decoding the perception of pain from fMRI using multivariate pattern analysis. Neuroimage. 2012;63:1162–70. doi: 10.1016/j.neuroimage.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craig AD, Krout K, Andrew D. Quantitative response characteristics of ther-moreceptive and nociceptive lamina I spinothalamic neurons in the cat. J Neuro-physiol. 2001;86:1459–80. doi: 10.1152/jn.2001.86.3.1459. [DOI] [PubMed] [Google Scholar]
- 18.Dong WK, Salonen LD, Kawakami Y, Shiwaku T, Kaukoranta EM, Martin RF. Nociceptive responses of trigeminal neurons in SII-7b cortex of awake monkeys. Brain Res. 1989;484:314–24. doi: 10.1016/0006-8993(89)90375-2. [DOI] [PubMed] [Google Scholar]
- 19.Kenshalo DR, Iwata K, Sholas M, Thomas DA. Response properties and organization of nociceptive neurons in area 1 of monkey primary somatosensory cortex. J Neurophysiol. 2000;84:719–29. doi: 10.1152/jn.2000.84.2.719. [DOI] [PubMed] [Google Scholar]
- 20.Meier ML, Brügger M, Ettlin DA, et al. Brain activation induced by dentine hy-persensitivity pain — an fMRI study. J Clin Periodontol. 2012;39:441–7. doi: 10.1111/j.1600-051X.2012.01863.x. [DOI] [PubMed] [Google Scholar]
- 21.Maihöfner C, Handwerker HO, Birklein F. Functional imaging of allo-dynia in complex regional pain syndrome. Neurology. 2006;66:711–7. doi: 10.1212/01.wnl.0000200961.49114.39. [DOI] [PubMed] [Google Scholar]
- 22.Derbyshire SW, Jones AK, Creed F, et al. Cerebral responses to noxious thermal stimulation in chronic low back pain patients and normal controls. Neuroimage. 2002;16:158–68. doi: 10.1006/nimg.2002.1066. [DOI] [PubMed] [Google Scholar]
- 23.Schreckenberger M, Siessmeier T, Viertmann A, et al. The unpleasantness of tonic pain is encoded by the insular cortex. Neurology. 2005;64:1175–83. doi: 10.1212/01.WNL.0000156353.17305.52. [DOI] [PubMed] [Google Scholar]
- 24.DaSilva AF, Becerra L, Makris N, et al. Somatotopic activation in the human tri-geminal pain pathway. J Neurosci. 2002;22:8183–92. doi: 10.1523/JNEUROSCI.22-18-08183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikes RW, Vogt BA. Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophysiol. 1992;68:1720–32. doi: 10.1152/jn.1992.68.5.1720. [DOI] [PubMed] [Google Scholar]
- 26.Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nat Neurosci. 2005;8:679–85. doi: 10.1038/nn1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bingel U, Quante M, Knab R, Bromm B, Weiller C, Büchel C. Single trial fMRI reveals significant contralateral bias in responses to laser pain within thalamus and somatosensory cortices. Neuroimage. 2003;18:740–8. doi: 10.1016/s1053-8119(02)00033-2. [DOI] [PubMed] [Google Scholar]
- 28.Schulz E, Zherdin A, Tiemann L, Plant C, Ploner M. Decoding an individual’s sensitivity to pain from the multivariate analysis of EEG data. Cereb Cortex. 2012;22:1118–23. doi: 10.1093/cercor/bhr186. [DOI] [PubMed] [Google Scholar]
- 29.Brown JE, Chatterjee N, Younger J, Mackey S. Towards a physiology-based measure of pain: patterns of human brain activity distinguish painful from non-painful thermal stimulation. PLoS One. 2011;6(9):e24124. doi: 10.1371/journal.pone.0024124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marquand A, Howard M, Brammer M, Chu C, Coen S, Mourão-Miranda J. Quantitative prediction of subjective pain intensity from whole-brain fMRI data using Gaussian processes. Neuroimage. 2010;49:2178–89. doi: 10.1016/j.neuroimage.2009.10.072. [DOI] [PubMed] [Google Scholar]
- 31.Rish I, Cecchi G, Baliki MN, Apkarian V. Sparse regression models of pain perception; Proceedings of the International Conference on Brain Informatics; Toronto. August 28–30, 2010. [Google Scholar]
- 32.Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15:1117–9. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen SP, White RL, Kurihara C, et al. Epidural steroids, etanercept, or saline in subacute sciatica: a multicenter, randomized trial. Ann Intern Med. 2012;156:551–9. doi: 10.7326/0003-4819-156-8-201204170-00397. [DOI] [PubMed] [Google Scholar]
- 34.Borsook D, Becerra L, Hargreaves R. A role for fMRI in optimizing CNS drug development. Nat Rev Drug Discov. 2006;5:411–24. doi: 10.1038/nrd2027. [DOI] [PubMed] [Google Scholar]
- 35.Brooks JCW, Zambreanu L, Godinez A, Craig ADB, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27:201–9. doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 36.Hua LH, Strigo IA, Baxter LC, Johnson SC, Craig ADB. Anteroposterior so-matotopy of innocuous cooling activation focus in human dorsal posterior insular cortex. Am J Physiol Regul Integr Comp Physiol. 2005;289:R319–R325. doi: 10.1152/ajpregu.00123.2005. [DOI] [PubMed] [Google Scholar]
- 37.Strigo IA, Duncan GH, Boivin M, Bushnell MC. Differentiation of visceral and cutaneous pain in the human brain. J Neurophysiol. 2003;89:3294–303. doi: 10.1152/jn.01048.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


