Abstract
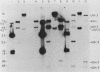
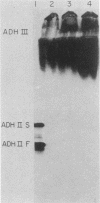
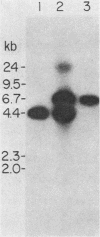
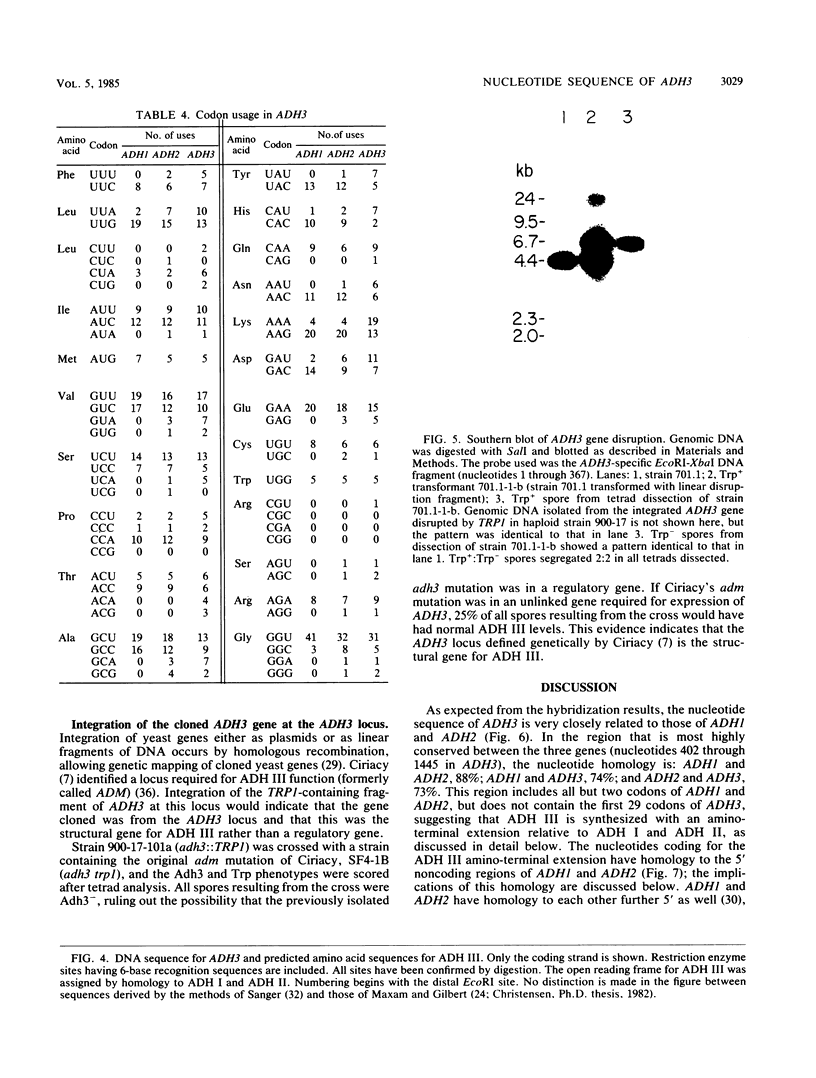
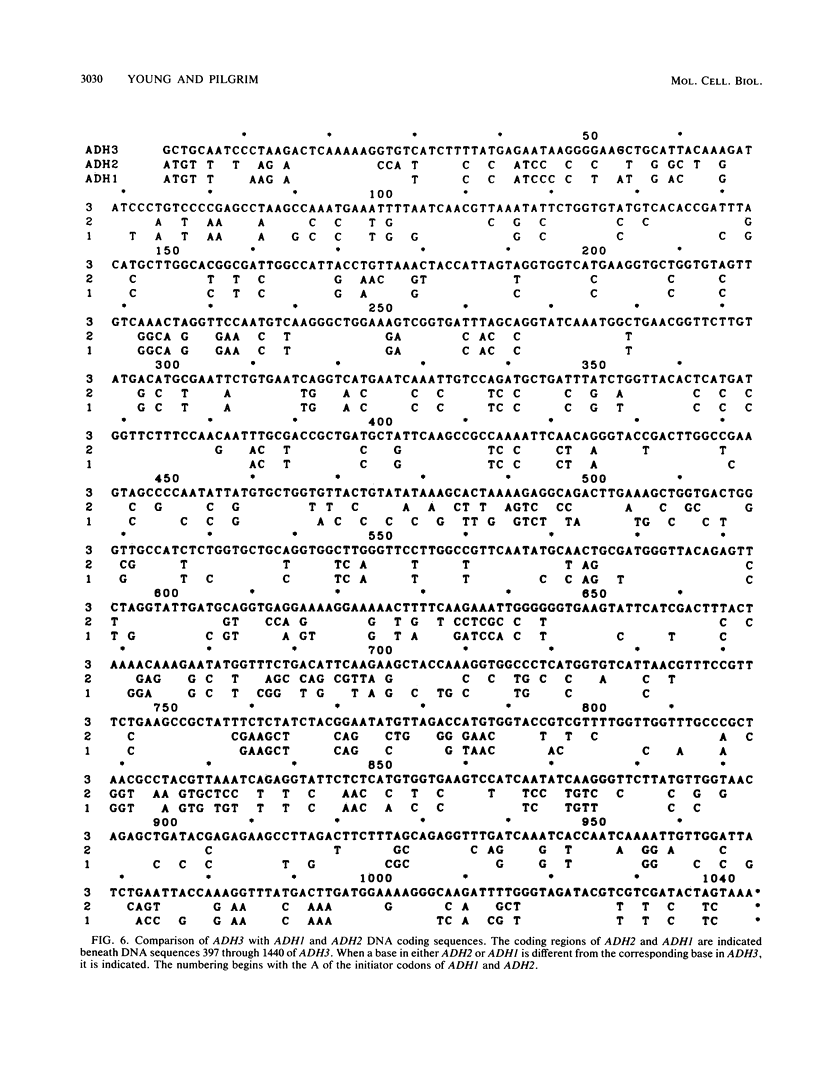
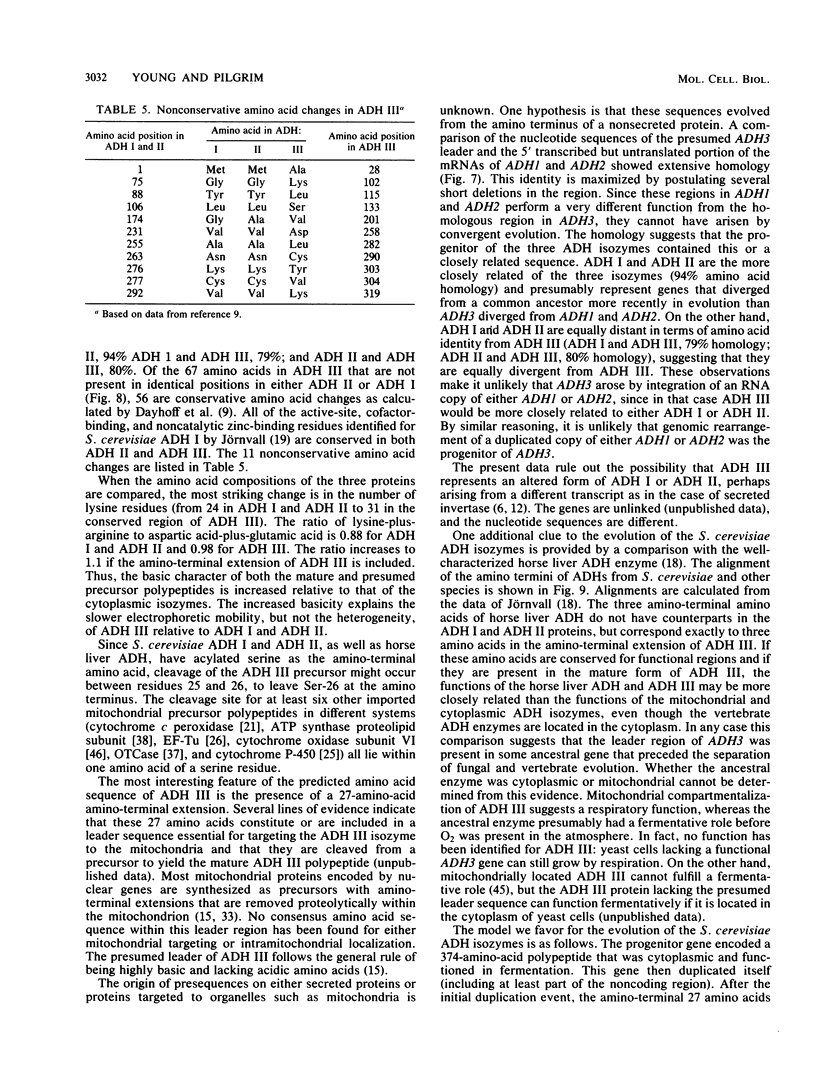
The Saccharomyces cerevisiae nuclear gene, ADH3, that encodes the mitochondrial alcohol dehydrogenase isozyme ADH III was cloned by virtue of its nucleotide homology to ADH1 and ADH2. Both chromosomal and plasmid-encoded ADH III isozymes were repressed by glucose and migrated heterogeneously on nondenaturing gels. Nucleotide sequence analysis indicated 73 and 74% identity for ADH3 with ADH1 and ADH2, respectively. The amino acid identity between the predicted ADH III polypeptide and ADH I and ADH II was 79 and 80%, respectively. The open reading frame encoding ADH III has a highly basic 27-amino-acid amino-terminal extension relative to ADH I and ADH II. The nucleotide sequence of the presumed leader peptide has a high degree of identity with the untranslated leader regions of ADH1 and ADH2 mRNAs. A strain containing a null allele of ADH3 did not have a detectably altered phenotype. The cloned gene integrated at the ADH3 locus, indicating that this is the structural gene for ADH III.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammerer G. Expression of genes in yeast using the ADCI promoter. Methods Enzymol. 1983;101:192–201. doi: 10.1016/0076-6879(83)01014-9. [DOI] [PubMed] [Google Scholar]
- Beier D. R., Young E. T. Characterization of a regulatory region upstream of the ADR2 locus of S. cerevisiae. Nature. 1982 Dec 23;300(5894):724–728. doi: 10.1038/300724a0. [DOI] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. The primary structure of the Saccharomyces cerevisiae gene for alcohol dehydrogenase. J Biol Chem. 1982 Mar 25;257(6):3018–3025. [PubMed] [Google Scholar]
- Bennetzen J. L., Swanson J., Taylor W. C., Freeling M. DNA insertion in the first intron of maize Adh1 affects message levels: cloning of progenitor and mutant Adh1 alleles. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4125–4128. doi: 10.1073/pnas.81.13.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Ciriacy M. Genetics of alcohol dehydrogenase in Saccharomyces cerevisiae. II. Two loci controlling synthesis of the glucose-repressible ADH II. Mol Gen Genet. 1975;138(2):157–164. doi: 10.1007/BF02428119. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Denis C. L., Ciriacy M., Young E. T. A positive regulatory gene is required for accumulation of the functional messenger RNA for the glucose-repressible alcohol dehydrogenase from Saccharomyces cerevisiae. J Mol Biol. 1981 Jun 5;148(4):355–368. doi: 10.1016/0022-2836(81)90181-9. [DOI] [PubMed] [Google Scholar]
- Denis C. L., Young E. T. Isolation and characterization of the positive regulatory gene ADR1 from Saccharomyces cerevisiae. Mol Cell Biol. 1983 Mar;3(3):360–370. doi: 10.1128/mcb.3.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Schauer I., Hansen W., Esmon P., Schekman R. Invertase beta-galactosidase hybrid proteins fail to be transported from the endoplasmic reticulum in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Nov;4(11):2347–2355. doi: 10.1128/mcb.4.11.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler P. W., Ball A. J., Griffiths D. E. The control of alcohol dehydrogenase isozyme synthesis in Saccharomyces cerevisiae. Can J Biochem. 1972 Jan;50(1):35–43. doi: 10.1139/o72-007. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of yeast transfer RNAs and the occurrence of the respective codons in protein genes. Differences in synonymous codon choice patterns of yeast and Escherichia coli with reference to the abundance of isoaccepting transfer RNAs. J Mol Biol. 1982 Jul 15;158(4):573–597. doi: 10.1016/0022-2836(82)90250-9. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H. Differences between alcohol dehydrogenases. Structural properties and evolutionary aspects. Eur J Biochem. 1977 Feb;72(3):443–452. doi: 10.1111/j.1432-1033.1977.tb11268.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Eklund H., Brändén C. I. Subunit conformation of yeast alcohol dehydrogenase. J Biol Chem. 1978 Dec 10;253(23):8414–8419. [PubMed] [Google Scholar]
- Jörnvall H. The primary structure of yeast alcohol dehydrogenase. Eur J Biochem. 1977 Feb;72(3):425–442. doi: 10.1111/j.1432-1033.1977.tb11267.x. [DOI] [PubMed] [Google Scholar]
- Kaput J., Goltz S., Blobel G. Nucleotide sequence of the yeast nuclear gene for cytochrome c peroxidase precursor. Functional implications of the pre sequence for protein transport into mitochondria. J Biol Chem. 1982 Dec 25;257(24):15054–15058. [PubMed] [Google Scholar]
- Kreitman M. Nucleotide polymorphism at the alcohol dehydrogenase locus of Drosophila melanogaster. Nature. 1983 Aug 4;304(5925):412–417. doi: 10.1038/304412a0. [DOI] [PubMed] [Google Scholar]
- Lutstorf U., Megnet R. Multiple forms of alcohol dehydrogenase in Saccharomyces cerevisiae. I. Physiological control of ADH-2 and properties of ADH-2 and ADH-4. Arch Biochem Biophys. 1968 Sep 10;126(3):933–944. doi: 10.1016/0003-9861(68)90487-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight G. L., Kato H., Upshall A., Parker M. D., Saari G., O'Hara P. J. Identification and molecular analysis of a third Aspergillus nidulans alcohol dehydrogenase gene. EMBO J. 1985 Aug;4(8):2093–2099. doi: 10.1002/j.1460-2075.1985.tb03897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K., Fujii-Kuriyama Y., Okada Y., Sogawa K., Hirose T., Inayama S., Omura T. Molecular cloning and nucleotide sequence of cDNA for mRNA of mitochondrial cytochrome P-450(SCC) of bovine adrenal cortex. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4647–4651. doi: 10.1073/pnas.81.15.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Tsunetsugu-Yokota Y., Naito A., Kaziro Y. Molecular cloning and sequence determination of the nuclear gene coding for mitochondrial elongation factor Tu of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6192–6196. doi: 10.1073/pnas.80.20.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Chen H. Y., Brinster R. L. Differential regulation of metallothionein-thymidine kinase fusion genes in transgenic mice and their offspring. Cell. 1982 Jun;29(2):701–710. doi: 10.1016/0092-8674(82)90186-6. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Smith M., Williamson V. M., Young E. T. Nucleotide sequence of the yeast alcohol dehydrogenase II gene. J Biol Chem. 1983 Feb 25;258(4):2674–2682. [PubMed] [Google Scholar]
- Russell P. R., Hall B. D. The primary structure of the alcohol dehydrogenase gene from the fission yeast Schizosaccharomyces pombe. J Biol Chem. 1983 Jan 10;258(1):143–149. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G., Butow R. A. How are proteins imported into mitochondria? Cell. 1983 Feb;32(2):316–318. doi: 10.1016/0092-8674(83)90450-6. [DOI] [PubMed] [Google Scholar]
- Sugar J., Schimpfessel L., Rozen E., Crokaert R. The mitochondrial alcohol dehydrogenase of the yeast "Saccharomyces cerevisiae". Arch Int Physiol Biochim. 1970 Dec;78(5):1009–1010. [PubMed] [Google Scholar]
- Taguchi A. K., Ciriacy M., Young E. T. Carbon source dependence of transposable element-associated gene activation in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Jan;4(1):61–68. doi: 10.1128/mcb.4.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi M., Miura S., Mori M., Tatibana M., Nagata S., Kaziro Y. Molecular cloning and nucleotide sequence of cDNA for rat ornithine carbamoyltransferase precursor. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7412–7416. doi: 10.1073/pnas.81.23.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viebrock A., Perz A., Sebald W. The imported preprotein of the proteolipid subunit of the mitochondrial ATP synthase from Neurospora crassa. Molecular cloning and sequencing of the mRNA. EMBO J. 1982;1(5):565–571. doi: 10.1002/j.1460-2075.1982.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger J. I., Bernofsky C. Mitochondrial alcohol dehydrogenase from Saccharomyces cerevisiae. Biochim Biophys Acta. 1971 Mar 10;227(3):479–490. doi: 10.1016/0005-2744(71)90001-5. [DOI] [PubMed] [Google Scholar]
- Williamson V. M., Bennetzen J., Young E. T., Nasmyth K., Hall B. D. Isolation of the structural gene for alcohol dehydrogenase by genetic complementation in yeast. Nature. 1980 Jan 10;283(5743):214–216. doi: 10.1038/283214a0. [DOI] [PubMed] [Google Scholar]
- Williamson V. M., Cox D., Young E. T., Russell D. W., Smith M. Characterization of transposable element-associated mutations that alter yeast alcohol dehydrogenase II expression. Mol Cell Biol. 1983 Jan;3(1):20–31. doi: 10.1128/mcb.3.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson V. M., Young E. T., Ciriacy M. Transposable elements associated with constitutive expression of yeast alcohol dehydrogenase II. Cell. 1981 Feb;23(2):605–614. doi: 10.1016/0092-8674(81)90156-2. [DOI] [PubMed] [Google Scholar]
- Wills C., Jörnvall H. The two major isozymes of yeast alcohol dehydrogenase. Eur J Biochem. 1979 Sep;99(2):323–331. doi: 10.1111/j.1432-1033.1979.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Wills C., Phelps J. A technique for the isolation of yeast alcohol dehydrogenase mutants with altered substrate specificity. Arch Biochem Biophys. 1975 Apr;167(2):627–637. doi: 10.1016/0003-9861(75)90506-8. [DOI] [PubMed] [Google Scholar]
- Wright R. M., Ko C., Cumsky M. G., Poyton R. O. Isolation and sequence of the structural gene for cytochrome c oxidase subunit VI from Saccharomyces cerevisiae. J Biol Chem. 1984 Dec 25;259(24):15401–15407. [PubMed] [Google Scholar]
- Yaffe M. P., Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]