Abstract
Background
Chronic heart failure is an important cause for morbidity and mortality in adults with congenital heart disease (ACHD). While NT-proBNP is an established biomarker for heart failure of non-congenital origin, its application in ACHD has limitations. The angiogenic factors Angiopoietin-1 and -2 (Ang-1, Ang-2), vascular endothelial growth factor (VEGF), and soluble receptor tyrosine kinase of the Tie family (sTie2) correlate with disease severity in heart failure of non-congenital origin. Their role in ACHD has not been studied.
Methods
In 91 patients Ang-2 and NT-proBNP were measured and related to New York Heart Association class, systemic ventricular function and parameters of cardiopulmonary exercise testing. Ang-1, VEGF, and sTie2 were also measured.
Results
Ang-2 correlates with NYHA class and ventricular dysfunction comparable to NT-proBNP. Further, Ang-2 showed a good correlation with parameters of cardiopulmonary exercise testing. Both, Ang-2 and NT-proBNP identified patients with severely limited cardiopulmonary exercise capacity. Additionally, Ang-2 is elevated in patients with a single ventricle physiology in contrast to NT-proBNP. VEGF, Ang-1, and sTie2 were not correlated with any clinical parameter.
Conclusion
The performance of Ang-2 as a biomarker for heart failure in ACHD is comparable to NT-proBNP. Its significant elevation in patients with single ventricle physiology indicates potential in this patient group and warrants further studies.
Introduction
Chronic heart failure (CHF) is an important cause for morbidity and mortality in adults with congenital heart disease (ACHD) [1]. Heart failure symptoms may not always correlate with objective measures like systemic ventricular function or parameters of cardiopulmonary exercise testing [2], [3]. The rarity of individual malformations and the complex anatomy and physiology make assessing the cardiac function difficult [4]. Therefore, the prevalence of heart failure in these patients is underappreciated [5], [6]. A simple investigation like a blood test to detect early stages of heart failure and predict those at risk of deterioration would be valuable [4]. B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP) are established biomarkers for diagnosis and management of heart failure due to acquired heart disease [7]. Unfortunately, the clinical use of those markers in adults with congenital heart disease is limited [8], [9]. Therefore diagnosis and treatment monitoring is frequently based on cardiopulmonary exercise testing [4], [6], [10], [11], which is time consuming and not feasible in special patient groups.
Endothelial dysfunction is one of the hallmarks in patients with chronic heart failure of non-congenital origin, and has also already been described in some forms of congenital heart disease [12],[13]. Recently we provided evidence for the promising role of circulating levels of asymmetrical dimethylarginine (ADMA), the most potent endogenous nitrix oxid synthase (NOS) inhibitor, for the diagnosis of heart failure in patients with ACHD [14]. Angiopoietins as growth factors of angiogenesis also play a role in endothelial dysfunction. Angiopoietin-1 (Ang-1) and Angiopoietin-2 (Ang-2) are antagonistic ligands of the Tie2 receptor, which is the second vascular specific receptor tyrosine kinase (the first being the vascular endothelial growth factor (VEGF)/VEGF receptor). The Ang/Tie2 ligand receptor system is a non-redundant gatekeeper of endothelial activation and controls the endothelial phenotype during angiogenesis and inflammation [15],[16]. Ang-1 is continuously produced and released by pericytes. Binding of Ang-1 to Tie2 enhances vascular integrity, prevents vascular leakage and suppresses inflammatory gene expression [17],[18]. In contrast, Ang-2 competitively inhibits binding of Ang-1 to Tie2 and thereby disrupts protective Ang-1 signalling leading to loss of vessel integrity, vascular leakage and expression of inflammatory genes [16],[19]. Ang-2 is stored in the endothelium and rapidly released upon different activators e.g. hypoxemia [20]. Recently published studies report a significant increase of the soluble Tie2 receptor, Ang-2 and VEGF in patients with CHF due to acquired heart disease when compared with healthy controls [21],[22]. Serum Ang-2 correlates with an impaired exercise capacity and reduced ventilatory capacity in CHF patients [22]. The role of these circulating endothelial factors in ACHD has not been studied before. Therefore, the aim of this study was to elucidate the potential diagnostic value of Ang-1, Ang-2, soluble Tie2 and VEGF for heart failure in adults with congenital heart disease.
Materials and Methods
Ethics Statement
The study was approved by the local Ethics Committee of Hannover Medical School, Germany. All patients gave written informed consent.
The patients were recruited during a routine outpatient visit at the Adult Congenital Heart Disease Clinic of the Hannover Medical School. All patients in whom a venous blood sampling was feasible were eligible for this study.
A clinical workup including medical history, physical examination, 12-lead electrocardiogram, transthoracic echocardiography, and cardiopulmonary exercise testing was performed.
The severity of the congenital heart defect was graded according to complexity as proposed by guidelines [23]. The patients were further classified according to their symptoms of heart failure using the New York Heart Association (NYHA) functional classification which is based on patient’s symptoms and the limitations to normal physical activities [7].
Laboratory Methods
Blood samples for measurement of plasma Ang-1, Ang-2, VEGF, sTie2 and NT-proBNP, and routine biochemistry were drawn. Blood samples were immediately cooled on ice, centrifuged at 1,500 g and 4°C for 10 min. Supernatants were stored in 1 ml aliquots at –80°C until further use. Plasma concentrations of Ang-1 and Ang-2 were measured by an in-house immunoluminometric assay as was previously reported in detail [24]. In brief the assays had detection limits of 0.12 ng/mL (Ang-1) and 0.2 ng/mL (Ang-2). Inter- and intra-assay imprecision was ≤8.8 and 3.7% for Ang-1 and was ≤4.6 and 5.2% for Ang-2, respectively. Plasma VEGF (biologically active VEGF-A121 and VEGF-A165) and sTie2 were measured using commercially available sandwich ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. All other measurements were done with routine laboratory tests using certified assay methods.
Echocardiography
A standard 2D-Doppler transthoracic echocardiogram was performed according to the recommendations for the assessment of ventricular function and valvular heart disease issued by the American Society of Echocardiography [25]. Systemic ventricular systolic function was assessed qualitatively (i.e. normal, moderately or severely impaired).
Cardiopulmonary Exercise Studies
Cardiopulmonary exercise studies were performed on a bicycle in sitting position, starting with 25 W, increasing further 25 W every 2 min. All patients exercised to the end of their tolerance. A 12-lead ECG was recorded throughout the exercise test to determine heart rate and heart rate response. Systolic blood pressure and blood pressure response, as well as work rate (W/kg) were measured. Ventilation, oxygen uptake (VO2), and carbon dioxide production (VCO2), were measured continuously by a breath-by-breath method. Subjects breathed through a fitted mask and a hot-wire anemometer (Oxycon Delta, Jäger, Hoechberg, Germany) measuring inspired and expired flow continuously.
Statistical Analysis
Continuous data are presented as mean ± standard deviation. Categorical data are presented as counts and proportions. Between group comparisons were examined using Student’s t test for continuous and Mann-Whitney-U test for categorical variables. One-way ANOVA was used if more than two groups were compared. The least significant difference (LSD) method was used as a post hoc-test.
For the parameters of cardiopulmonary exercise testing cut off values representing patients with limitations of their cardiopulmonary exercise capacity were defined as previously described [14]: peak oxygen uptake (peak VO2) <20 ml/min/kg, ventilatory equivalent for carbon dioxide (EQCO2) >34, ventilatory equivalent for oxygen (EQO2) >34, oxygen pulse if female <9 ml/heartbeat, if male <12 ml/heartbeat. A group of patients that was severely affected was defined by a peakVO2<20 ml/min/kg or an EQCO2>34 or a combination of both. The cut-offs were chosen according to published data and clinical experience [2],[3],[26].
Receiver-operating characteristic curves (ROC curve) for these parameters were drawn and the areas under the curves calculated. All tests were two-sided and significance was accepted at p<0.05. Data analysis was performed using SPSS (SPSS Inc., Chicago, IL, USA). Figures were prepared using GraphPad Prism (GraphPad Prism Software Inc., San Diego, CA, USA).
Results
Ninety-one patients were included in this cross-sectional study. Ang-1, VEGF and Tie2 could only be analyzed in 80 patients. Table 1 and Table 2 show the clinical characteristics of the study population. Cardiopulmonary exercise testing was performed in 70 patients. Five of these performed a submaximal exercise test (respiratory exchange ratio <1.05) and were excluded from analysis. Nine patients were cyanotic. Out of these three had an exercise test. The values for EQCO2 were not used in these patients since EQCO2 is not a marker of prognosis in cyanotic patients.
Table 1. Clinical characteristics of study population.
| Age (yrs) | 30.4±10.7 |
| Sex | |
| female | 37 (40.7) |
| male | 54 (59.3) |
| BMI (kg/m2) | 23.5±4.2 |
| Complexity of congenital heart disease | |
| simple | 18 (19.8) |
| moderate | 36 (39.6) |
| severe | 37 (40.7) |
| Systemic ventricle | |
| left | 65 (71.4) |
| right | 12 (13.2) |
| single ventricle | 14 (15.4) |
| Systemic ventricular function | |
| normal | 52 (57.1) |
| moderately impaired | 32 (35.2) |
| severely impaired | 7 (7.7) |
| NYHA class | |
| NYHA I | 55 (60.4) |
| NYHA II | 21 (23.1) |
| NYHA III | 15 (16.5) |
Data are expressed as mean±SD or as counts (percentage).
Table 2. Type of congenital heart defect grouped according to ventricular function.
| Ventricular function | ||||
| Congenital heart defect | All | Normal | Moderately impaired | Severely impaired |
| TGA and CCTGA | 11 (12.1) | 2 (18.2) | 5 (45.5) | 4 (36.4) |
| Tetralogy of Fallot | 12 (13.2) | 6 (50) | 6 (50) | 0 |
| Coarctation of the aorta | 10 (11.0) | 9 (90) | 1 (10) | 0 |
| ASD or VSD | 11 (12.1) | 9 (81.8) | 2 (18.2) | 0 |
| AVSD | 5 (5.5) | 5 (100) | 0 | 0 |
| Marfan syndrome | 7 (7.7) | 3 (42.9) | 4 (57.1) | 0 |
| Congenital AS or PS | 11 (12.1) | 9 (81.8) | 2 (18.2) | 0 |
| Single ventricle physiology | 13 (14.3) | 4 (30.8) | 6 (46.2) | 3 (23.1) |
| Miscellaneous | 11 (12.1) | 5 (45.5) | 6 (54.5) | 0 |
Data are expressed as counts (percentage); TGA = transposition of the great arteries; CCTGA = congenital corrected transposition of the great arteries; ASD = atrial septal defect; VSD = ventricular septal defect; AVSD = atrioventricular septal defect; AS = aortic valve stenosis; PS = pulmonary valve stenosis; miscellaneous: Ebstein’s anomaly, subaortic stenosis, supravalvular aortic stenosis, pulmonary atresia, Eisenmenger.
Ventricular Function
Ang-1, VEGF and Tie2 were not statistically different in patients with normal ventricular function compared to patients with moderate or severe ventricular dysfunction. ( Table 3 ). Ang-2 did reach a statistically significant difference in patients with normal ventricular function compared to patients with severe ventricular dysfunction (3.53±4.19 ng/ml vs. 7.48±7.57 ng/ml, p<0.05), but not to those with moderate ventricular dysfunction (5.3±5.18, p = 0.11). There was also no significant difference between the last two. ( Table 3 and Figure 1A ).
Table 3. Ang-1, Ang-2, VEGF, Tie2 and NT-proBNP levels according to NYHA class, ventricular function and systemic ventricle physiology.
| Ang-1 (ng/ml) | Ang-2 (ng/ml | VEGF (pg/ml) | Tie2 (ng/ml) | NT-proBNP (pg/ml) | |
| NYHA Class | |||||
| I | 5.8±3.8 | 2.5±1.6 | 111.4±62.5 | 19.9±3.3 | 128±200 |
| II | 6.5±5.7 | 6.8±6.6a | 119.2±114.4 | 23.1±5.7 | 432±517a |
| III | 4.5±3.2 | 8.2±6.9b | 156.7±142.7 | 21.0±3.9 | 796±1081b,c |
| Ventricular function | |||||
| normal | 5.1±3.2 | 3.5±4.2 | 117.7±84.8 | 20.5±4.2 | 142±177 |
| moderately impaired | 6.7±5.5 | 5.3±5.1 | 128.6±111.1 | 21.0±4.1 | 394±477d |
| severely impaired | 5.7±3.8 | 7.5±7.6e | 122.2±110.8 | 23.2±4.4 | 1156±1540f |
| Systemic ventricle | |||||
| right | 7.5±7.3 | 4.9±5.2 | 117±85.5 | 21.6±4.2 | 642±758 |
| left | 5.6±3.4 | 2.9±2.8 | 116.9±81.6 | 20.1±3.7 | 180±250g |
| single | 4.6±3.0 | 11.2±6.9h | 144.5±145.9 | 23.1±5.4 | 617±1091i |
Values are expressed as mean ± SD;
p<0.01 to NYHA I - Ang-2 and p<0.05 for NT-proBNP;
p<0.001 to NYHA I - Ang-2 and NT-proBNP;
p<0.05 for NT-proBNP and NYHA II;
p<0.01 vs. normal ventricular function;
p<0.05 vs. normal ventricular function;
p<0.01 vs. moderate ventricular dysfunction and p<0.001 vs. normal ventricular function;
p<0.01 vs. systemic right ventricle;
p<0.001 vs. systemic left and right ventricle;
p<0.01 vs. systemic left ventricle.
Figure 1. Levels of biomarkers in comparison between normal, moderate and severe impairment of ventricular function.
Ang-2 (A) and NT-proBNP (B) (*p<0.05, **p<0.001, ***p<0.0001).
NT-proBNP reached a statistically significant difference in patients with normal ventricular function compared to those with severe ventricular dysfunction (142±177 pg/ml vs. 1156±1540 pg/ml, p<0.0001) and between patients with normal ventricular function and moderate ventricular dysfunction (142±177 pg/ml vs. 394±477 pg/ml, p<0.05) as well as between patients with moderate and severe ventricular dysfunction (394±477 pg/ml vs. 1156±1540 pg/ml, p<0.001). ( Table 3 and Figure 1B ).
NYHA Class
Ang-2 differentiated between patients in NYHA class I (2.52±1.6 ng/ml) and NYHA class II (6.83±6.56 ng/ml, p<0.0001), as well as those in NYHA class III (8.23±6.88 ng/ml, p<0.0001), but not between patients in NYHA class II and III (p = 0.34). ( Table 3 and Figure 2A ) The results for Ang-2 as a function of NYHA class in each ventricular function subset can be found in Table 4 . The highest values were found in the subgroup of patients in NYHA class III and severe ventricular function, while patients in NYHA class I and normal ventricular function had the lowest values. It seems that Ang-2 could mark NYHA class within a given ventricular function better than marking ventricular function within a given NYHA class, but the sample size in each group is too small to provide conclusive answers.
Figure 2. Levels of biomarkers in comparison between NYHA classes.
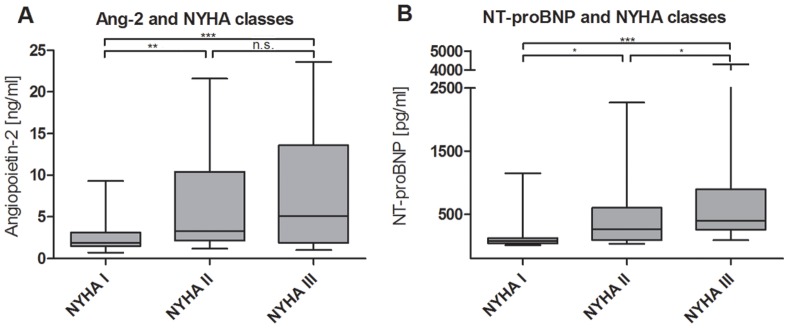
Ang-2 (A) and NT-proBNP (B) (*p<0.05, **p<0.001, ***p<0.0001).
Table 4. Ang-2 as a function of NYHA class in each ventricular function subset.
| NYHA I | NYHA II | NYHA III | ||||||
| Ventricular function | n | Ang-2 | n | Ang-2 | n | Ang-2 | ||
| normal | 40 | 2.2±1.4 | 7 | 8.1±7.7a | 5 | 7.6±6.3b | ||
| moderately impaired | 15 | 3.3±1.8c | 11 | 6.6±6.9a | 6 | 7.8±6.3a,d | ||
| severely impaired | 0 | n/a | 3 | 4.7±1.9 | 4 | 9.6±9.9a,d | ||
Values are expressed as mean ± SD;
p<0.01 to NYHA I – normal;
p<0.05 to NYHA I – normal;
p<0.05 to NYHA II – normal;
p<0.05 to NYHA I – moderate. All other comparisons were not significant.
NT-proBNP was significantly different in patients in NYHA class I (128±202 pg/ml) compared to patients in NYHA class III (796±1081 pg/ml, p<0.0001) and to patients in NYHA class II (432±517 pg/ml, p<0.05) as well as between the last two (p<0.05). ( Table 3 and Figure 2B ). Ang-1, VEGF and Tie2 were not different between NYHA classes. ( Table 3 ).
Cardiopulmonary Exercise Testing
Peak VO2 was significantly higher in patients in NYHA class I (28.6±7.4 ml/min/kg) vs. patients in NYHA class II (23.3±4.5 ml/min/kg, p = 0.01) and patients in NYHA class III (14.1±5.2 ml/min/kg, p<0.001) and also in comparison between NYHA class II and III (p = 0.001). Patients with a normal ventricular function had a significantly higher peak VO2 (28.5±7.6 ml/min/kg) compared to patients with a moderate (20.6±7.2 ml/min/kg, p<0.001) or severe ventricular dysfunction (21.0±8.4 ml/min/kg, p = 0.018). There was no significant difference between the last two (p = 0.917).
Ang-2 was elevated in patients with limited cardiopulmonary exercise compared to their peers. Significant differences were observed for EQO2 (p = 0.04), and oxygen pulse (p = 0.03), but not for peak VO2 (p = 0.23) and EQCO2 (p = 0.11). NT-proBNP was not elevated in patients with limited cardiopulmonary exercise capacity. There was no significant difference for peak VO2 (p = 0.16), EQCO2 (p = 0.30), EQO2 (p = 0.12), and oxygen pulse (p = 0.05). ( Table 5 ).
Table 5. Association of endothelial factors and NT-proBNP with parameters of cardiopulmonary exercise testing.
| peak VO2 in ml/min/kg | EQCO2 | EQO2 | Oxygen pulse in ml/beat | |||||||||
| < cut off | > cut off | p | < cut off | > cut off | p | < cut off | > cut off | p | < cut off | > cut off | p | |
| Ang-2 (ng/ml) | 6.6±7.3 | 4.0±4.4 | 0.23 | 3.7±3.9 | 17.7±8.6 | 0.11 | 3.4±3.5 | 7.5±7.5 | 0.04 | 7.2±7.5 | 3.2±2.9 | 0.03 |
| NT-proBNP (pg/ml) | 670±1159 | 203±371 | 0.16 | 229±412 | 1903±2098 | 0.30 | 186±315 | 612±1079 | 0.12 | 608±1020 | 151±209 | 0.05 |
| Ang-1 (ng/ml) | 5.1±2.9 | 6.2±4.6 | 0.41 | 5.8±3.7 | 11.1±12.3 | 0.53 | 5.7±3.3 | 6.9±6.5 | 0.47 | 7.0±5.0 | 5.5±3.9 | 0.21 |
| Tie2 (ng/ml) | 21.9±5.4 | 20.8±4.1 | 0.43 | 20.7±3.9 | 24.6±10.4 | 0.58 | 20.5±4.0 | 22.5±5.1 | 0.10 | 22.5±5.3 | 20.2±3.7 | 0.08 |
| VEGF (pg/ml) | 91±54 | 122±84 | 0.21 | 115±81 | 161±69 | 0.34 | 110±87 | 133±52 | 0.32 | 106±51 | 123±91 | 0.36 |
Severely Limited Exercise Capacity
There were 16 patients with severely limited exercise capacity in our cohort of 65 patients with cardiopulmonary exercise testing (defined as peakVO2<20 ml/min/kg or an EQCO2>34 or a combination of both). The areas under the receiver-operating characteristic (ROC) curves for identifying patients with severely limited cardiopulmonary exercise capacity were 0.784 for NT-proBNP and 0.656 for Ang-2.
Ventricular Physiology
Ang-2 was significantly elevated in patients with a single ventricle physiology (11.21±6.94 ng/ml) in comparison with patients with a systemic left (2.93±2.75 ng/ml, p<0.0001) or right ventricle (4.86±5.22 ng/ml, p<0.0001), but not between the last two. ( Table 3 and Figure 3A ) NT-proBNP was elevated in patients with a single ventricle (617±1091 pg/ml) in comparison with a systemic left ventricle (180±250 pg/ml, p<0.001) but not with a systemic right ventricle (642±758 pg/ml). ( Table 3 and Figure 3B ).
Figure 3. Levels of biomarkers according to ventricular physiology.
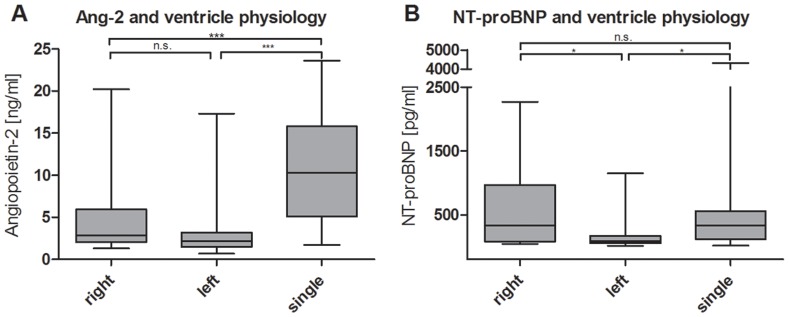
Ang-2 (A) and NT-proBNP (B) (*p<0.05, **p<0.001, ***p<0.0001).
Discussion
This is the first study to evaluate the role of circulating endothelial factors in adults with congenital heart disease. Ang-2 correlated with parameters of heart failure like NYHA classes and ventricular function. Furthermore, there was a good correlation between Ang-2 and parameters of cardiopulmonary exercise testing. Interestingly, elevated Ang-2 levels were found in patients with a single ventricle physiology.
Two recent studies elucidated the role of circulating endothelial factors in CHF due to acquired heart disease. Both showed an increase of circulating Ang-2 in patients with CHF [21],[22]. Furthermore, Eleuteri et al. reported a stepwise increase of Ang-2 in CHF with increasing NYHA class. We also found elevated Ang-2 levels with increasing NYHA class in our patients. This is of importance, since Norozi et al. showed that the risk of heart failure increases with NYHA class in ACHD [3]. They found an odds ratio for patients in NYHA II compared to patients in NYHA I of 3.4 and for patients in NYHA III of 11.6 [3]. Therefore, Ang-2 might act as a surrogate marker for heart failure in ACHD.
In our study population there was no difference in Ang-1 levels according to NYHA class or ventricular function. This is in accordance with recent studies that reported stable Ang-1 levels in NYHA class I-IV with a trend to lower values in NYHA class III in CHF patients [21],[22]. ( Table 3 ).
We observed an increase of Ang-2 levels with worsening ventricular function. There was also a good correlation between ventricular function and NT-proBNP concentrations. This is in contrast to the finding of Larsson and colleagues [4]. In their study, the association of BNP/NT-proBNP with ventricular function was weak and only statistically significant when BNP and NT-proBNP data were combined [4]. It appeared that BNP/NT-proBNP had especially poor value in differentiating between patients with no or mild ventricular impairment, which suggested a limited ability of BNP/NT-proBNP to diagnose heart failure at the initial stages [4].
In patients with CHF an association of elevated circulating Ang-2 and parameters of impaired exercise capacity was recently reported. In 87 patients with heart failure of non-congenital origin circulating Ang-2 levels were associated with lower peak oxygen consumption (peak VO2), increased VE/VCO2 slope and shorter exercise duration [22]. This is in accordance with our results that a limited cardiopulmonary exercise capacity in ACHD patients is associated with elevated Ang-2 levels. Further, Ang-2 was able to distinguish patients with an especially limited exercise capacity demonstrated by lower peak VO2 and increased EQCO2. It has been shown that poor exercise capacity identifies ACHD at risk for hospitalization or death [2]. Peak VO2 predicted hospitalization or death and was related to the frequency and duration of hospitalization in a large cohort of ACHD [2].
Furthermore, Ang-2 levels were elevated in patients with a single ventricle physiology. This is an interesting finding, because NT-proBNP might have its limitations in this subgroup. It was not elevated in patients with a single ventricle physiology in our study and also showed mixed results regarding its association with a variety of different parameters of heart failure in this population in a recent systematic review [9]. NT-proBNP and BNP are released from cardiomyocytes in response to increased myocardial wall stress due to volume- or pressure-overload states [27]. But in adults with congenital heart disease increased myocardial wall stress due to volume- or pressure-overload states is often not the main pathophysiologic mechanism of heart failure. This is especially true for patients with a single ventricle after the Fontan palliation in whom the leading problem is a limitation of preload [28]. This might explain the restrictions of NT-proBNP as a biomarker for heart failure in this patient group. Further, in patients who underwent the Fontan palliation elevated pulmonary vascular resistance can be found [28]. It was already demonstrated that Ang-2 is elevated and a promising biomarker in patients with elevated pulmonary vascular resistance in the context of idiopathic pulmonary arterial hypertension [29]. Therefore, one possible explanation for the elevated Ang-2 levels in patients with a Fontan circulation could be that elevated pulmonary vascular resistance leads to elevated Ang-2 levels in this subgroup. Interestingly, one study showed that BNP is only elevated in patients with a single ventricle physiology when the systemic ventricle fails but not when there is a failure of the Fontan connection [30]. Thus, Ang-2 may be a valuable biomarker for failure of the Fontan connection in this patient group.
The reason of elevated VEGF and Ang-2 levels in CHF and ACHD remain unclear, because higher levels of these factors have not been translated into clinically significant new vessel formation, as in cancer growth for example [31]. An increase of these angiogenic factors is amongst others possibly triggered by tissue hypoxia [21]. It is hypothesised that elevation of Ang-2 and VEGF in heart failure promotes endothelial repair mechanisms but does not lead to angiogenesis [31],[32]. Although there is a proangiogenic milieu with elevated Ang-2 and VEGF additional endothelial nitric oxidase synthase (eNOS) is needed for the formation of new blood vessels [31]. ENOS is the downstream mediator for VEGF and is lacking in CHF. Current data of our group shows significantly elevated levels of ADMA, the most potent inhibitor of eNOS, in patients with ACHD and heart failure [14]. This could explain why despite elevated angiogenic factors their presence may not necessarily translate into angiogenesis. Therefore, these abnormal levels of angiogenic factors in ACHD may simply play a role in repair and maintenance of a dysfunctional or damaged endothelium [31]. However endothelial repair mechanisms involved remain to be determined.
A limitation of our study is its cross sectional design. A longitudinal design would be needed to elucidate the predictive value of circulating angiogenic factors in patients with ACHD. A long-term follow up study of the patients that participated in this study is planned. This would be of great interest since previous studies suggest that the predictive value of NT-proBNP may be limited in patients with heart failure in ACHD and its true prognostic value is unclear [4],[9] Further, the number of patients enrolled especially those with a single ventricle was small. Therefore, our results have a hypothesis-generating character.
In conclusion, Ang2 could have potential as a biomarker of heart failure in ACHD. It shows especially promising results for patients with a Fontan circulation. A prospective study with a larger patient size is warranted to evaluate the diagnostic and prognostic potential of Ang-2 in this patient group.
Funding Statement
The article processing charge was funded by means of the DFG-Project “Open Access Publishing“ by the German Research Council. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Shaddy RE, Webb G (2008) Applying heart failure guidelines to adult congenital heart disease patients. Expert Rev Cardiovasc Ther 6: 165–174. [DOI] [PubMed] [Google Scholar]
- 2. Diller GP, Dimopoulos K, Okonko D, Li W, Babu-Narayan SV, et al. (2005) Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation 112: 828–835. [DOI] [PubMed] [Google Scholar]
- 3. Norozi K, Wessel A, Buchhorn R, Alpers V, Arnhold JO, et al. (2007) Is the Ability index superior to the NYHA classification for assessing heart failure?: comparison of two classification scales in adolescents and adults with operated congenital heart defects. Clin Res Cardiol 96: 542–547. [DOI] [PubMed] [Google Scholar]
- 4. Larsson DA, Meurling CJ, Holmqvist F, Waktare JE, Thilen UJ (2007) The diagnostic and prognostic value of brain natriuretic peptides in adults with a systemic morphologically right ventricle or Fontan-type circulation. Int J Cardiol 114: 345–351. [DOI] [PubMed] [Google Scholar]
- 5. Bolger AP, Coats AJ, Gatzoulis MA (2003) Congenital heart disease: the original heart failure syndrome. Eur Heart J 24: 970–976. [DOI] [PubMed] [Google Scholar]
- 6. Bolger AP, Sharma R, Li W, Leenarts M, Kalra PR, et al. (2002) Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation 106: 92–99. [DOI] [PubMed] [Google Scholar]
- 7. Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, et al. (2008) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 29: 2388–2442. [DOI] [PubMed] [Google Scholar]
- 8.Eindhoven JA, van den Bosch AE, Boersma E, Roos-Hesselink JW (2012) The usefulness of brain natriuretic peptide in simple congenital heart disease - a systematic review. Cardiol Young: 1–10. [DOI] [PubMed]
- 9. Eindhoven JA, van den Bosch AE, Jansen PR, Boersma E, Roos-Hesselink JW (2012) The usefulness of brain natriuretic Peptide in complex congenital heart disease: a systematic review. J Am Coll Cardiol 60: 2140–2149. [DOI] [PubMed] [Google Scholar]
- 10. Garg R, Raman SV, Hoffman TM, Hayes J, Daniels CJ (2008) Serum markers of systemic right ventricular function and exercise performance. Pediatr Cardiol 29: 641–648. [DOI] [PubMed] [Google Scholar]
- 11. Giannakoulas G, Dimopoulos K, Bolger AP, Tay EL, Inuzuka R, et al. (2010) Usefulness of natriuretic Peptide levels to predict mortality in adults with congenital heart disease. Am J Cardiol 105: 869–873. [DOI] [PubMed] [Google Scholar]
- 12. Tousoulis D, Charakida M, Stefanadis C (2005) Inflammation and endothelial dysfunction as therapeutic targets in patients with heart failure. Int J Cardiol 100: 347–353. [DOI] [PubMed] [Google Scholar]
- 13. Diller GP, van Eijl S, Okonko DO, Howard LS, Ali O, et al. (2008) Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation 117: 3020–3030. [DOI] [PubMed] [Google Scholar]
- 14. Tutarel O, Denecke A, Bode-Boger SM, Martens-Lobenhoffer J, Lovric S, et al. (2012) Asymmetrical dimethylarginine–more sensitive than NT-proBNP to diagnose heart failure in adults with congenital heart disease. PLoS One 7: e33795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brindle NP, Saharinen P, Alitalo K (2006) Signaling and functions of angiopoietin-1 in vascular protection. Circ Res 98: 1014–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fiedler U, Augustin HG (2006) Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol 27: 552–558. [DOI] [PubMed] [Google Scholar]
- 17. Kim I, Kim HG, So JN, Kim JH, Kwak HJ, et al. (2000) Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circ Res 86: 24–29. [DOI] [PubMed] [Google Scholar]
- 18. Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O’Connor DS, et al. (2000) Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem 275: 9102–9105. [DOI] [PubMed] [Google Scholar]
- 19. Fiedler U, Krissl T, Koidl S, Weiss C, Koblizek T, et al. (2003) Angiopoietin-1 and angiopoietin-2 share the same binding domains in the Tie-2 receptor involving the first Ig-like loop and the epidermal growth factor-like repeats. J Biol Chem 278: 1721–1727. [DOI] [PubMed] [Google Scholar]
- 20. Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, et al. (2004) The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103: 4150–4156. [DOI] [PubMed] [Google Scholar]
- 21. Chong AY, Caine GJ, Freestone B, Blann AD, Lip GY (2004) Plasma angiopoietin-1, angiopoietin-2, and angiopoietin receptor tie-2 levels in congestive heart failure. J Am Coll Cardiol 43: 423–428. [DOI] [PubMed] [Google Scholar]
- 22. Eleuteri E, Di Stefano A, Tarro Genta F, Vicari C, Gnemmi I, et al. (2011) Stepwise increase of angiopoietin-2 serum levels is related to haemodynamic and functional impairment in stable chronic heart failure. Eur J Cardiovasc Prev Rehabil 18: 607–614. [DOI] [PubMed] [Google Scholar]
- 23. Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, et al. (2001) Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol 37: 1170–1175. [DOI] [PubMed] [Google Scholar]
- 24. Lukasz A, Hellpap J, Horn R, Kielstein JT, David S, et al. (2008) Circulating angiopoietin-1 and angiopoietin-2 in critically ill patients: development and clinical application of two new immunoassays. Crit Care 12: R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, et al. (2003) Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 16: 777–802. [DOI] [PubMed] [Google Scholar]
- 26. Dimopoulos K, Okonko DO, Diller GP, Broberg CS, Salukhe TV, et al. (2006) Abnormal ventilatory response to exercise in adults with congenital heart disease relates to cyanosis and predicts survival. Circulation 113: 2796–2802. [DOI] [PubMed] [Google Scholar]
- 27. Kim HN, Januzzi JL Jr (2011) Natriuretic peptide testing in heart failure. Circulation 123: 2015–2019. [DOI] [PubMed] [Google Scholar]
- 28. Gewillig M, Brown SC, Eyskens B, Heying R, Ganame J, et al. (2010) The Fontan circulation: who controls cardiac output? Interact Cardiovasc Thorac Surg 10: 428–433. [DOI] [PubMed] [Google Scholar]
- 29. Kumpers P, Nickel N, Lukasz A, Golpon H, Westerkamp V, et al. (2010) Circulating angiopoietins in idiopathic pulmonary arterial hypertension. Eur Heart J 31: 2291–2300. [DOI] [PubMed] [Google Scholar]
- 30. Law YM, Ettedgui J, Beerman L, Maisel A, Tofovic S (2006) Comparison of plasma B-type natriuretic peptide levels in single ventricle patients with systemic ventricle heart failure versus isolated cavopulmonary failure. Am J Cardiol 98: 520–524. [DOI] [PubMed] [Google Scholar]
- 31. Chong AY, Caine GJ, Lip GY (2004) Angiopoietin/tie-2 as mediators of angiogenesis: a role in congestive heart failure? Eur J Clin Invest 34: 9–13. [DOI] [PubMed] [Google Scholar]
- 32. Elsasser A, Schlepper M, Klovekorn WP, Cai WJ, Zimmermann R, et al. (1997) Hibernating myocardium: an incomplete adaptation to ischemia. Circulation 96: 2920–2931. [DOI] [PubMed] [Google Scholar]



