Abstract
Bacterial type IV pili are essential for adhesion to surfaces, motility, microcolony formation, and horizontal gene transfer in many bacterial species. These polymers are strong molecular motors that can retract at two different speeds. In the human pathogen Neisseria gonorrhoeae speed switching of single pili from 2 µm/s to 1 µm/s can be triggered by oxygen depletion. Here, we address the question how proton motive force (PMF) influences motor speed. Using pHluorin expression in combination with dyes that are sensitive to transmembrane ΔpH gradient or transmembrane potential ΔΨ, we measured both components of the PMF at varying external pH. Depletion of PMF using uncouplers reversibly triggered switching into the low speed mode. Reduction of the PMF by ≈ 35 mV was enough to trigger speed switching. Reducing ATP levels by inhibition of the ATP synthase did not induce speed switching. Furthermore, we showed that the strictly aerobic Myxococcus xanthus failed to move upon depletion of PMF or oxygen, indicating that although the mechanical properties of the motor are conserved, its regulatory inputs have evolved differently. We conclude that depletion of PMF triggers speed switching of gonococcal pili. Although ATP is required for gonococcal pilus retraction, our data indicate that PMF is an independent additional energy source driving the high speed mode.
Introduction
Bacterial type IV pili (T4P, pili) are extracellular polymers that are generated by various bacterial species [1]. They are involved in adhesion to surfaces, motility, microcolony formation and biofilm architecture, and in transformation.
The type IV pilus mainly consists of pilin subunits that assemble to form helical polymer with a width of 6 nm and an average length of 1 µm [2]. The length of T4P is dynamic, i.e. pili elongate by polymerization and retract by depolymerization [3,4]. The ATPase PilF is essential for polymerization of pili [5] and the ATPase PilT is essential for pilus retraction in Neisseria gonorrhoeae (N. gonorrhoeae, gonococcus) [6]. Both ATPases form hexameric rings and structural data suggests coordinated ATPase cycles of the individual motors in the ring [7]. Cycles of pilus elongation, adhesion at surfaces, and retraction power bacterial surface motility, also called twitching motility. Multiple T4P cooperate for generating surface motility (Figure 1a) [8]. During retraction, single pili can generate considerable force exceeding 100 pN [9]. Potential functions of high force generation include the rearrangement of the host cytoskeleton [10–12] and force-induced change of epitope exposure on the T4P [13].
Figure 1. Oxygen depletion triggers speed switching of T4P retraction.
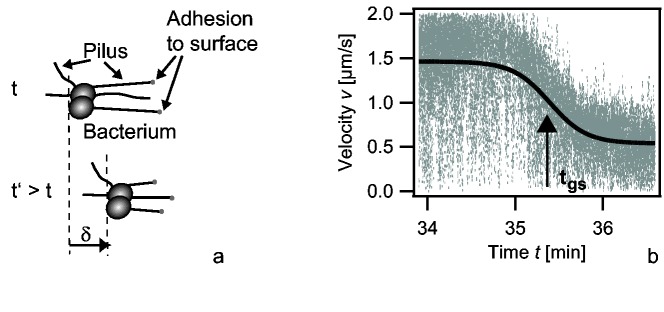
a) Scheme of T4P driven surface motility. Multiple pili adhere to the surface and when they retract, they pull the cell towards the point of attachment. b) Overlay of the speed of twitching motility of multiple bacteria during global speed switching. Full line: fit to eq. 1.
The physical parameters of T4P retraction can be fine-tuned [14]. At the genetic level, PilT2 enhances the speed of T4P retraction [15]. We have recently shown that type IV pili of N. gonorrhoeae can switch between different velocities, namely retraction at two different speed modes and elongation [16–18]. Speed switching is conserved in Myxococcus xanthus [19]. For N. gonorrhoeae we found that oxygen depletion triggers the switch from the high speed mode of single pilus retraction at vH ≈ 2 µm/s to the low speed mode at vL ≈ 1 µm/s [20]. Switching occurred at the level of individual pili, was reversible, and independent of protein expression. Twitching motility of gonococci exhibits a global switch from a high speed mode of surface motility v = 1.5 µm/s to a low speed mode v = 0.5 µm/s upon oxygen depletion [20] (Figure 1b). As multiple pili interact for generating bacterial motility, a two-state model for describing the time course of speed evolution was derived:
| , | (1) |
where t gs is the time point of global switching, and k is the rate at which the free energy difference between the states changes. The time point of global switching t gs decreases inversely with the oxygen consumption rate (or concentration of cells) when the bacteria are the only consumers of oxygen in the sample [20]. We hypothesize that this multistate-system enables bacteria to tune T4P dynamics for rapidly responding to environmental conditions. However, the mechanism of oxygen-sensing is unclear.
Here, we investigated the influence of proton motive force (PMF), ATP-depletion, and nitrite on the speed of bacterial motility. Our data indicate that ATP is required for pilus retraction, but gonococci boost pilus retraction speed by a factor of two by using PMF as an additional energy source.
Results
Oxygen is the final electron acceptor of the respiratory chain that helps maintaining the proton gradient between the cell’s interior and its exterior space. It was therefore conceivable that oxygen depletion correlated with depletion of the proton motive force. To our knowledge, the proton motive force (PMF) has not been characterized systematically in N. gonorrhoeae so far and thus we determined the PMF as a function of the external pH before addressing the question whether depletion of PMF triggered speed switching of gonococcal pili.
Proton motive force ofNeisseria gonorrhoeae at varying external pH
The proton motive force PMF = −61 · ΔpH + ΔΨ has two contributions, namely an entropic component (ΔpH) and an electrostatic component (ΔΨ). We determined both components using fluorescence microscopy at 37°C. For measuring the transmembrane pH difference, gonococci were loaded with the ratiometric pH-sensitive dye 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (cFDA-SE). Calibration was performed as explained in the Methods S1 and Figure S1 in File S1. These experiments were conducted inside a flow cell during twitching motility assays. In all experiments availability of oxygen was verified by eye-inspection of twitching motility in high speed mode. The intracellular pHin increased with increasing external pH (pHex) (Figure 2a).
Figure 2. Components of the proton motive force of N. gonorrhoeae.
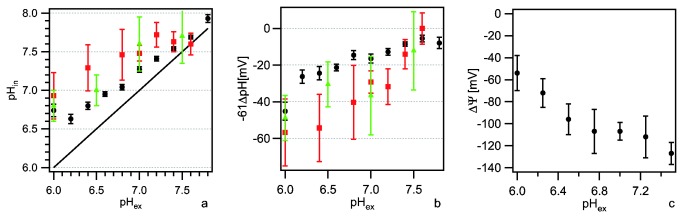
(a) Intracellular pH (pHin) and (b) ΔpH-component of the PMF versus extracellular pH (pHex). black: cFDA-SE (N = 100-1000 cells per data point), red: single cell analysis of pHluorin expressing cells (N = 240-530 cells per data point), green spectroscopic analysis of of pHluorin expressing cells (3 independent experiments). Full line in (a): pHin = pHex. (c) Transmembrane potential ΔΨ as a function of pHex (N = 100-200 cells per data point).
Since our results showed that homeostasis in N. gonorrhoeae is remarkably poor as compared to experiments with other bacterial species [21], we used a different method for determining pHin. We generated a gonococcal strain that expressed ratiometric pHluorin, a pH-sensitive GFP derivative [21] [22] (Figure S2 in File S1). Using ratiometric single cell fluorescence microscopy of the pHluorin expressing bacteria, we found slightly higher internal pHin as a function of external pHex as compared to cFDA-SE (Figure 2 a, b). Since the calibration of the dye sensitively depends on full depletion of ΔpH, we compared our data with spectroscopic data where cells have been lysed for calibration and found good agreement (Figure 2 a, b). We conclude that although the absolute value measured for the internal pHin is somewhat sensitive to the method used, pH homeostasis in the range of pHex 6 to 7.5 is not as pronounced as in other bacterial species such as Escherichia coli.
For determination of the transmembrane potential ΔΨ the cationic dye tetramethylrhodamine methyl ester (TMRM) was used. TMRM partitions between the cytoplasm and the exterior of the cell according to Boltzmann’s distribution and can be used to determine ΔΨ (Materials and Methods, Figure S3 in File S1). In agreement with the assumption that the ΔpH-component and the ΔΨ -component of the PMF can compensate each other, ΔΨ increased with decreasing ΔpH (Figure 2c and Table 1). These results demonstrate that the ΔΨ -component is highly dominant in retraction assay medium (RAM, pH 6.8) with a ΔpH-component −61 · ΔpH ≈ -30 mV and a membrane potential ΔΨ ≈ −110 mV.
Table 1. Bacterial strains.
| Strain | Parental strain | Genotype | Source of strain |
|---|---|---|---|
| MS11 | wt | ||
| N400 | MS11 | P lac recA (tetM) | [52] |
| pHluorin | N400 | iga::P pilE pHluorin (erm) | This study |
| DK1622 | wt |
Depletion of proton motive force triggers speed switching
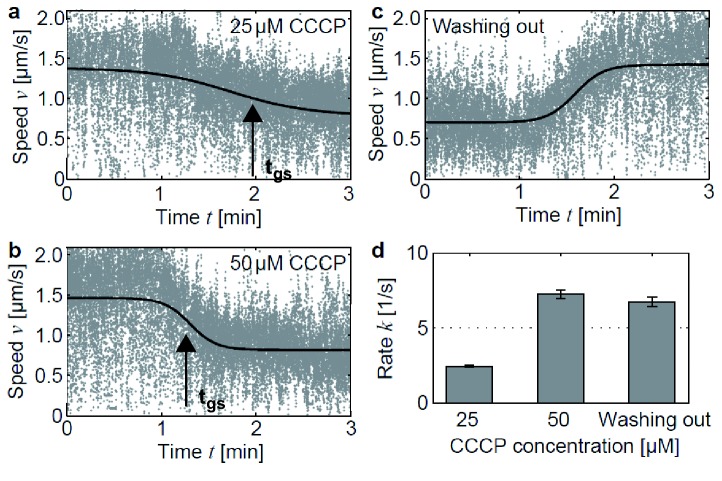
To understand the role of bioenergetics in oxygen-dependent speed switching, we investigated twitching motility in response to PMF depletion by application of carbonyl cyanide m-chlorophenyl hydrazone (CCCP). The protonophore CCCP is a potent uncoupler which depletes PMF on a time scale below 1 min at the concentrations we used [23] [24]. We conducted twitching motility assays inside a flow cell and injected CCCP at two different concentrations: 25 µM and 50 µM. In both cases, injection was followed by rapid speed switching, demonstrating that global switching can be induced by depletion of PMF (Figure 3a, b). The transition rate increased with increasing CCCP concentration. In addition, we proved that this process is fully reversible. After washing out CCCP, all bacteria switched back to the high speed mode within a time window of less than 60s (Figure 3c). The transition can be described using the two-state model eq. 1 (Figure 3d). This behavior is comparable to re-energizing cells after oxygen depletion with fresh oxygen-rich medium. The kinetics of switching from the high speed state to the low speed state is reminiscent of switching triggered by oxygen depletion [20]. This experiment is in agreement with the assumption that depletion of PMF is the underlying reason for speed switching in response to oxygen depletion and suggests that direct oxygen sensing is not involved.
Figure 3. Depletion of proton motive force induces global switching and is fully reversible.
(a) Global switching during injection of 25 µM CCCP. Overlay of speeds of 48 bacterial tracks versus time. Solid line: fit to eq. 1. (b) Global switching during injection of 50 µM CCCP. Overlay of speeds of 40 bacterial tracks. (c) Washing out CCCP is accompanied by switching back to high speed mode. Overlay of speeds of 35 bacterial tracks. (d) Transition rate as obtained by fit to eq. 1.
Inhibition of ATP synthase does not trigger speed switching of twitching motility
Synthesis of ATP by the ATP synthase relies on the proton motive force. Therefore, we characterized the kinetics of decrease in ATP levels after treatment with 50 µM CCCP using a luciferase/luciferin assay (Figure 4a). The ATP concentration decreased considerably within minutes with a characteristic decay time of ≈ 5 min.
Figure 4. Effect of ATP depletion on twitching dynamics by direct inhibition of the ATP synthase.
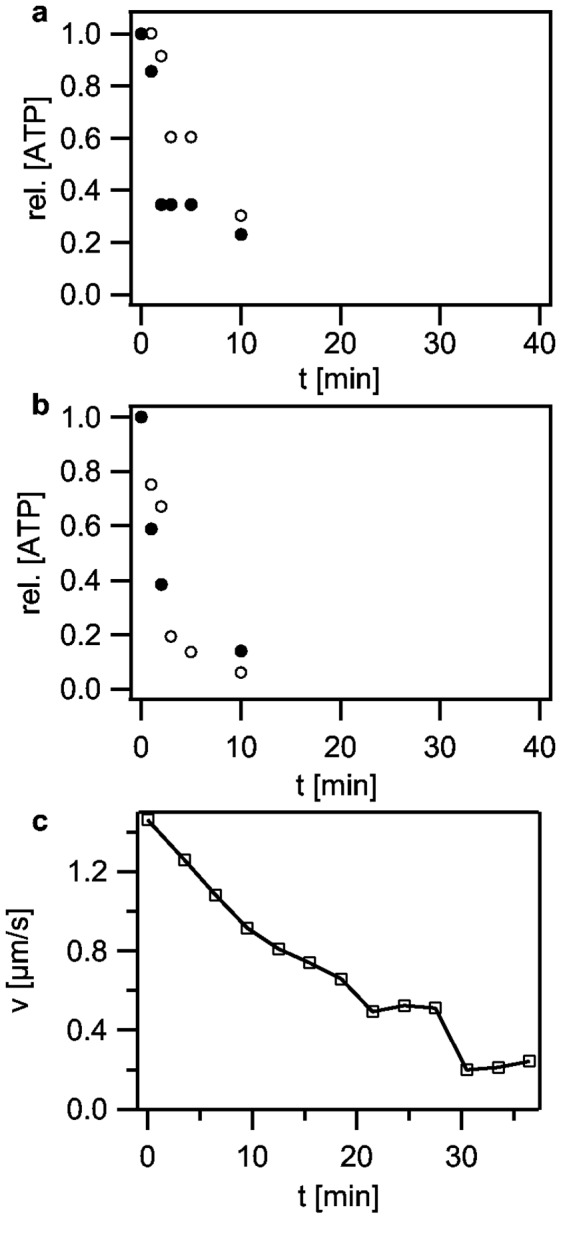
(a) Kinetics of ATP depletion upon treatment with 50 µM CCCP. Open and closed grey circles depict independent determinations of the relative ATP concentration, respectively. (b) Kinetics of ATP depletion upon treatment with 200 µM DCCD. Open and closed grey circles depict independent determinations of the relative ATP concentration, respectively. (c) Twitching speed averaged over 3 min versus incubation time in 200 µM DCCD.
It was conceivable that the rapid decrease of ATP levels triggered speed switching. Thus, we applied the ATP synthase inhibitor N,N’-dicyclohexylcarbodiimide (DCCD) at 200 µM. The ATP decay kinetics was in agreement with an exponential function and the characteristic decay time was ≈ 2 min (Figure 4b). Next, we monitored twitching motility during treatment with DCCD. Again, these experiments were conducted inside a flow cell and twitching motility was directly monitored after DCCD injection. At 200 µM DCCD the twitching speed decreased very slowly (Figure 4c). The effect of DCCD on viability was very heterogeneous determined by twitching and cell appearance. The number of motile bacteria decreased drastically with increasing incubation time. However, fast speed switching, as induced by depletion of oxygen or PMF was not observed, even though ATP depletion occurred more rapidly as in the case of treatment with CCCP (Figure 3a,b). Below 100 µM DCCD, we observed no effect on twitching motility (Figure S4 in File S1). At 300 µM DCCD twitching speed decreased continuously until all bacteria stopped movement after ≈ 12 min of incubation (Figure S4 in File S1). We conclude that speed switching was not triggered by depletion of ATP.
Depletion of ΔpH triggers speed switching and speed switching upon oxygen depletion is accompanied by reduction of ΔpH
Nigericin is a H+–K+-antiporter and exclusively depletes ΔpH while maintaining ΔΨ. To monitor twitching motility during nigericin injection and to determine the membrane potential before and after drug treatment, we used a flow cell and loaded cells with TMRM. These experiments were conducted in RAM (pH 6.8) in which the ΔΨ -component of the PMF is dominant. Interestingly, application of 5 µM nigericin induced rapid speed switching (Figure 5a). If a single component of the PMF is depleted, e.g. by application of an ionophore, bacteria can rapidly upregulate the other component within several seconds up to a few minutes to maintain the PMF [23] [25]. We found that the membrane potential remained constant (Figure 5b). Thus assuming that the ΔpH was fully depleted, the reduction of PMF is only from PMF ≈ −140 mV before global switching to PMF ≈ −105 mV after global switching.
Figure 5. Global switching correlates with reduction of transmembrane ΔpH.
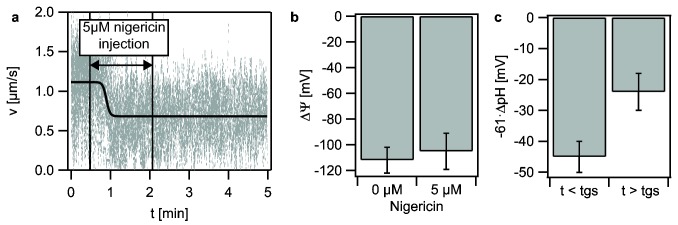
(a) Addition of 5 µM nigericin induces global switching (overlay of 31 bacterial tracks). (b) Transmembrane potential ΔΨ before and after nigericin treatment. (c) −61·ΔpH before and after global switching induced by oxygen scavenger treatment at pHex = 6.0.
Next, we determined the ΔpH before and after global switching in response to oxygen depletion. Again, twitching motility assays inside a flow cell were performed and in this case cells were loaded with the pH-sensitive dye cFDA-SE. Because ΔpH was highest at pHex 6.0, we adjusted the medium to pHex 6.0 to obtain a significant effect. Global switching was induced by application of an oxygen scavenger system based on 2.5 mM protocatechuic acid (PCA) and 50 nM protocatechuate 3,4-dioxygenase (PCD) as described elsewhere [20]. Since global switching is reversible, these experiments could be repeated in the same sample. We found an average ΔpH = 0.74 ± 0.08 in the high speed mode and a ΔpH = 0.40 ± 0.11 in the low speed mode (Figure 5c). Although significant, again the reduction in ΔpH was not very high.
To confirm that the important component for speed switching was the pH difference over the cell membrane and not the internal pH, we assessed whether we were able to see speed switching upon oxygen depletion at varying extracellular pHex which correlates with varying intracellular pHin (Figure 2). We found that speed switching upon oxygen depletion occurred between pHex 6.0 and pHex 7.8. We conclude therefore, that changes of internal pH cannot trigger global switching.
Taken together, we demonstrated that depletion of ΔpH induces speed switching and that oxygen depletion and reduction of ΔpH are strongly correlated.
Nitrite and global switching
In the absence of oxygen, nitrite is an alternative electron acceptor for the respiratory chain in N. gonorrhoeae [26]. The ability of gonococci to reduce nitrite is regulated by the master regulator FNR whose expression is upregulated in the presence of nitrite after oxygen depletion. We tested whether addition of nitrite to RAM medium caused the cells to switch back to the high speed mode.
Following Falsetta et al. who found enhanced biofilm formation in the presence of 100 µM NaNO2 [27] [28], we conducted twitching motility assays inside enclosed chambers at the same concentration of nitrite in the absence of ascorbic acid. Higher concentrations of nitrite (10mM to 20mM) were also tested but showed a toxic effect determined by a strongly increasing number of non-motile cells with increasing incubation time.
The time point of global switching after oxygen depletion did not depend on the presence of nitrite. This was expected because denitrification must be induced by the oxygen-sensing transcription factor FNR [26] [29]. Although a few minutes after global switching a very small fraction of bacteria (< 5%) showed a twitching speed almost comparable to the high speed mode under aerobic conditions, addition of nitrite did not induce a back-switch of the population to the high speed mode within 1h after oxygen depletion.
Mode switching is triggered by different inputs in Myxococcus xanthus
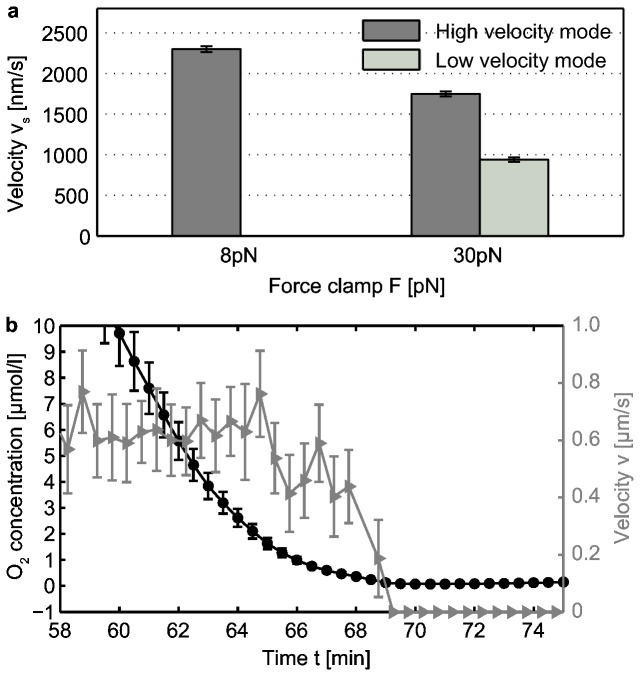
At the level of single pilus retraction, we have previously found that bimodality in speed occurred both in N. gonorrhoeae and in M. xanthus [19]. Here, we investigated pilus retractions of M. xanthus at a very low load of 8 pN, a force regime that we had not addressed in previous laser tweezers experiments. Interestingly, we found a dominant high velocity mode leading to a monomodal Gaussian distribution after averaging all 62 events with an average speed of vs = (2300 ± 37) nm/s (Figure 6a). By increasing the clamp force to 30 pN, we observed frequent switching between the high and the low speed mode during single retraction events. This observation was independent of the time post sealing, i.e. of the oxygen concentration in the sample. In contrast, for N. gonorrhoeae we did not observe speed switching under aerobic conditions at force up to 60pN [20]. At very high force of 120pN, however, we detected speed switching even at high oxygen concentration (Kurre & Maier, unpublished).
Figure 6. Oxygen-dependent mode switching is not conserved in M. xanthus.
(a) Average speeds v of single pilus retraction at 8 pN (62 events) and 30 pN (56 events). (b) Oxygen concentration (black circles) and average speed v of moving bacteria (grey triangles, 5 bacteria per time point) as a function of time.
Next, we applied the oxygen scavenger system to M. xanthus. We did not observe pilus retraction after incubation with the oxygen scavenger. To confirm that oxygen depletion affected motility in M. xanthus, we simultaneously measured the speed of surface motility and of oxygen concentration as demonstrated previously (details in [20]). We added a 50 µl cell suspension of M. xanthus to an oxygen sensor and sealed the sample. After attachment to the PDMS surface of the oxygen sensor, bacteria performed surface motility with an average speed v = (0.67 ± 0.01) µm/s (Figure 6b). At the time point of full oxygen depletion, motility was not detectable any more. Furthermore, we depleted PMF via 50 µM CCCP prior to sealing the sample. Again, pilus retraction was not detected.
Together, we have shown that although both speed modes exist in M. xanthus, switching between both modes is not triggered by oxygen depletion or depletion of PMF.
Discussion
Putative mechanisms of proton motive force induced speed switching
All of the evidence that we have obtained indicates that speed switching of gonococcal T4P retraction is triggered by depletion of PMF but not by ATP depletion. Our data is consistent with a picture in which the T4P system is in the high speed mode when the PMF is high and switches to the low speed mode upon reduction of PMF. We propose therefore, that the gonococcal T4P motor uses two sources of energy, namely ATP which supports the low speed mode and PMF for increasing the speed.
How does PMF couple to T4P retraction? One putative molecular mechanism would be that in addition to the retraction ATPase PilT another protein supports T4P retraction in a PMF dependent fashion. Furthermore, binding of regulatory proteins to the T4P complex may generate different states of pilus retraction. The PMF may be translated into motor speed through tuning the functionality of the motor by a regulating protein, reminiscent of the molecular brake in E. coli or the molecular clutch in B. subtilis interacting with flagellar rotation [30–33]. Another mechanism would involve PilT itself. PilT has a PAS-like N-terminal domain [7] that may be act as a sensor domain. PAS domains play a crucial role in sensing diverse physicochemical stimuli [34–36].
One possible mechanism for changing the molecular composition or conformation of the pilus motor would be a change of pHin. However, oxygen-dependent speed switching occurred at pHex ranging from 6.0 to 7.8, and changing pHex only influenced the oxygen depletion rate without affecting the absolute speed of the high and low speed mode. Since pHin increased with increasing pHex, a change of pHin is unlikely to trigger mode switching.
Contribution of the components of PMF
As it was expected from experiments in B. subtilis, Escherichia coli, or Helicbacter pylori [37–39], we have shown that pHin, ΔpH as well as ΔΨ are functions of pHex in N. gonorrhoeae. Consequently, ΔpH decreased with increasing pHex, whereas ΔΨ increased to stabilize the PMF at changing pHex. We conclude therefore, that our qualitative behavior for ΔpH as well as ΔΨ agree well with the literature values, even though pH homeostasis appears to be relatively poor. Please note that comparison of absolute bioenergetic parameters between different studies is quite challenging, since they strongly depend on experimental conditions such as surface charge [40].
The role of the denitrification pathway in speed switching
It has been shown that N. gonorrhoeae can grow under anaerobic conditions in the presence of nitrite, using a truncated denitrification pathway. Anaerobiosis plays an important physiological role, since biofilm formation is accompanied by a transition to anaerobic conditions followed by anaerobic growth [28,41,42]. Therefore we addressed the question whether denitrification in the presence of nitrite can affect oxygen-dependent speed switching. Twitching motility assays revealed that the time point of global switching was unchanged. This was expected because denitrification must be induced by the oxygen-sensing transcription factor FNR [26] [29]. We did not observe a global switching back to the high speed mode even 60 min after the first global switching event, indicating that the denitrification pathway does not show a major influence on oxygen-dependent speed switching. Interestingly, both reductases of the truncated denitrification pathway, AniA and NorB, do not support PMF generation [43,44], indicating that PMF is significantly lower under exclusive nitrite respiration. Therefore, we hypothesize that the high speed mode, which is very likely coupled to the PMF by a so far unknown mechanism, cannot be driven by denitrification exclusively. We would like to point out, however, that we did not monitor the expression of aniA and norB, and therefore we cannot exclude that one or both genes may not have been induced under the conditions used in our experiments.
Although the mechanical properties of the T4P motors are conserved, the trigger for speed switching is not
Interestingly, M. xanthus shows two retraction modes comparable in speed to N. gonorrhoeae but it shows a different response to oxygen depletion. Pilus retraction switches readily between both speed modes when forces exceed 8 pN, demonstrating that they are in a bistable regime under aerobic conditions. Similar bistability was found for N. gonorrhoeae only at forces exceeding 100 pN. Upon oxygen depletion, however, M. xanthus switched within minutes to a non-motile state, reminiscent of the oxygen response of E. coli [45]. Furthermore, pilus retractions were not detected after depletion of PMF via CCCP, indicating that depletion of PMF induces the switch to the non-motile state. Thus, speed switching in M. xanthus may be triggered by a so far undiscovered environmental input.
If reduction of cellular ATP levels and hence the occupation of the hexameric retraction ATPase PilT with ATPs would be exclusively responsible for speed switching, we expect that M. xanthus and N. gonorrhoeae responded in a similar way to cellular energy depletion. Recently, Sun et al. have shown that treatment of M. xanthus with nigericin does not affect T4P-dependent motility [46] in contrast to our results with N. gonorrhoeae. We conclude, therefore, that although the mechanical characteristics of T4P dynamics are well-conserved between bacterial species with different lifestyles, they have evolved to respond to different regulatory inputs.
In contrast to M. xanthus, which is an obligate aerobe, oxygen-dependent speed switching may have evolved in N. gonorrhoeae due to its oxygen-limited habitat in the human urogenital mucosa. In this scenario, N. gonorrhoeae switches to the low speed mode, which serves as a “power saving mode”, to save ATP for processes of vital importance when PMF is reduced and ATP is limited. This behavior could prevent energy depletion without needing to switch off T4P dynamics completely.
Conclusion
Our experiments clearly demonstrate that reduction of proton motive force triggers speed switching of type IV pilus retraction to the low speed mode. Reduction of ATP levels does not trigger speed switching. We therefore suggest that the low speed mode of the pilus motor is exclusively driven by ATP-hydrolysis, whereas the high speed mode is caused by an additional coupling to the pH gradient/proton flux.
Materials and Methods
Bacterial strains, growth conditions, and media
N . gonorrrhoeae (Table 1) was grown overnight at 37°C and 5% CO2 on agar plates containing gonococcal base agar (10 g/l BactoTM agar (BD Biosciences, Bedford, MA, USA), 5 g/l NaCl (Roth, Darmstadt, Germany), 4 g/l K 2HPO4 (Roth), 1 g/l KH2PO4 (Roth), 15 g/l BactoTM Proteose Peptone No. 3 (BD), 0.5g/l soluble starch (Sigma-Aldrich, St. Louis, MO, USA)) and the following supplements: 1g/l D-Glucose (Roth), 0.1 g/l L-glutamine (Roth), 0.289 g/l L-cysteine-HCL×H20 (Roth), 1 mg/l thiamine pyrophosphate (Sigma-Aldrich), 0.2 mg/l Fe(NO3)3 (Sigma-Aldrich), 0.03 mg/l thiamine HCl (Roth), 0.13 mg/l 4-aminobenzoic acid (Sigma-Aldrich), 2.5mg/l β-nicotinamide adenine dinucleotide (Roth) and 0.1 mg/l vitamin B12 (Sigma-Aldrich). Before each experiment gonococcal colonies were resuspended in retraction assay medium (RAM) consisting of phenol red-free Dulbecco’s Modified Eagle Medium (GIBCO, Grand Island, NY, USA), 4.5 g/l Glucose (GIBCO), 2 mM L-glutamine (Roth), 8 mM sodium pyruvate (GIBCO), 5 mM ascorbic acid (Roth) and 30 mM HEPES (Roth). RAM had a final pH of 6.8. To adjust pH, RAM was titrated with HCl or sodium hydroxide.
To generate anaerobic conditions, an oxygen scavenger system based on 2.5 mM protocatechuic acid (Sigma-Aldrich, St. Louis, MO) and 50 nM protecatechuate-3,4-dioxygenase (Sigma-Aldrich) was added to the media (Aitken, 2008)
For M. xanthus wild type DK1622 [47] was used. Cells were grown in liquid medium or on 1.5% agar containing 1% CTT as described [48]. For experiments bacteria were grown to an optical density at 600nm of 0.3 at 32°C and 230 rpm and then directly transferred either to an oxygen sensor for twitching assays [20] or to a polystyrene coated glass slide for pilus retraction assays.
Construction of pHluorin expressing strains
The pHluorin gene under the control of the pilE promoter was inserted into the iga locus of N400. The pHluorin sequence was amplified from the plasmid pGex, using the primers NK45 and NK52-SacI (Table S1 in File S1). The pilE promoter region was amplified from gDNA using the primers 83-SacI and NK44. The PCR fragments were fused and then cloned into the p2/16/1 plasmid using the SacI restriction site. Plasmid p2/16/1 is a derivative of pUP6 with a 4 kb fragment containing the gonococcal IgA1 protease gene (iga) and an erm R cassette. The plasmid with pHluorin gene was transformed into DH5α E. coli on 50µg/ml kanamycin LB plates and purified from E. coli.
The transformations in Neisseria gonorrhoeae were carried out with 500 ng of DNA and 500 µl of gonococcal cells (~ 5x108 cells/ml) in GC broth plus supplements (see above) with 7 mM MgCl2, 1mM IPTG at 37 °C and 5% CO2. After 30 min of incubation cells were diluted 1:10 and further incubated for 3 h under constant shaking with 250 rpm before plating at different dilutions onto 2.5 µg/ml erythromycin agar plates. Using the twitching motility assay, we verified that pHluorin expressing cells were motile.
Depletion and quantification of proton motive force
The PMF was depleted by adding carbonyl cyanide m-chlorophenyl hydrazone (CCCP, Sigma-Aldrich). CCCP stock solution (10 mM in DMSO) was stored at -20°C. To break down the transmembrane gradient, the K+–H+ antiporter nigericin (Invitrogen, Carlsbad, CA, USA) was used. Nigericin stock solutions (10mM in DMSO) were stored at -20°C. We verified, that DMSO added at the final concentrations used for the experiments, did not alter twitching motility.
The internal pH of N. gonorrhoeae was determined by the pH-sensitive flourophore 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (cFDA-SE, Sigma-Aldrich) [25] [49]. Stock solutions (1 mM in DMSO) were stored at -20°C. Cells were loaded with 5µM cFDA-SE for 10 min in loading buffer (pH 8.0) containing 1x PBS (Roth), 30 mM HEPES (Roth) and 1 mM EDTA (pH 7.4, Roth). After uptake, cFDA-SE is hydrolysed by cellular esterases to 5(6)-carboxyfluorecein succinimidyl ester (cFSE) and subsequently conjugated to aliphatic amine functions [49]. A detailed protocol of the calibration can be found in Methods S1 in File S1. Unbound cFDA-SE was flushed out by washing with RAM.
Transmembrane potential Δψ of N. gonorrhoeae was measured by 0.1 µM tetramethylrhodamine methylester (TMRM, Sigma-Aldrich) [50]. Stock solutions (100 µM in DMSO) were stored at 4°C. Cells were pre-treated with 10 mM EDTA (pH 7.4, Roth) for 10 min before loading to increase membrane permeability for TMRM. A detailed protocol of the calibration can be found in Methods S1 in File S1.
Fluorescence microscopy
Fluorescence measurements were conducted in epifluorescence mode at an inverted microscope (Eclipse TE2000, Nikon) equipped with a 120 W metal halogenide fluorescence lamp (X-Cite 120, EXFO), an EMCCD camera (Roper Cascade II:512, Photometrics), a computer-controlled motorized microscope stage (H117 ProScan, Prior Scientific) and a perfect focus system (T-PFB, Nikon). Two different 100 x oil immersion objectives were used: a CFI Plan Fluor objective (NA 1.3, Nikon) for PtTFPP (oxygen) and cFDA-SE (∆pH) and a CFI Apochromat TIRF series objective (NA 1.49, Nikon) for TMRM (Δψ). To equilibrate samples at 32°C (M. xanthus) or 37°C (N. gonorrhoeae) the microscope stage was incorporated into a heated thermal insulation box. Fully automated acquisition and device control was achieved by imaging software (NIS-Elements AR, Nikon instruments Inc.). More detailed methods may be found in the Supplementary information.
Twitching motility assays
Twitching motility assays of N. gonorrhoeae were performed on bovine serum albumin (BSA, Sigma-Aldrich) coated glass in a commercial microscope equipped with a heated thermal insulation box equilibrated at 37 °C. Bacterial motility was monitored via standard video microscopy (10 frames per second) and subsequent cell tracking in MATLAB R2009b (MathWorks Inc.). Tracking is based on the algorithms of J.C. Crocker and D. Grier originally written in IDL and transferred to MATLAB code by D. Blair and E. Dufresne [51]. (Download: http://physics.georgetown.edu/matlab/.) Applying this tracking algorithm to long rod-shaped M. xanthus cells was not successful. In this case, cells were manually tracked within the image processing software ImageJ.
Single pilus retraction assays
Single pilus retraction events were measured with an optical tweezers in force clamp mode [16]. Bacteria were fixed to polystyrene spin-coated glass slides. Carboxylated polystyrene microspheres (Molecular Probes, Eugene, Oregon, USA) with 2 µm in diameter were added to cell suspension before sealing the chamber.
Supporting Information
Methods S1. Table S1, Primers used in this study. Figure S1, Measurement of ΔpH using cFDA-SE. Figure S2, Calibration of pHluorin expressing cells. Figure S3, Correction for point spread function for determination of ΔΨ. Figure S4, Effect of different DCCD concentration on twitching dynamics.
(PDF)
Acknowledgments
We thank Heike Gangel, Claudia Meel, Michael Koomey, and Michael Hippler for helpful discussions, and Ingo Flügge and Kirsten Jung for donation of plasmids.
Funding Statement
This project was funded by the Deutsche Forschungsgemeinschaft through MA3898 and SFB 680. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pelicic V (2008) Type IV pili: e pluribus unum? Mol Microbiol 68: 827-837. doi:10.1111/j.1365-2958.2008.06197.x. PubMed: 18399938. [DOI] [PubMed] [Google Scholar]
- 2. Craig L, Volkmann N, Arvai AS, Pique ME, Yeager M et al. (2006) Type IV pilus structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. Mol Cell 23: 651-662. doi:10.1016/j.molcel.2006.07.004. PubMed: 16949362. [DOI] [PubMed] [Google Scholar]
- 3. Skerker JM, Berg HC (2001) Direct observation of extension and retraction of type IV pili. Proc Natl Acad Sci U S A 98: 6901-6904. doi:10.1073/pnas.121171698. PubMed: 11381130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merz AJ, So M, Sheetz MP (2000) Pilus retraction powers bacterial twitching motility. Nature 407: 98-102. doi:10.1038/35024105. PubMed: 10993081. [DOI] [PubMed] [Google Scholar]
- 5. Freitag NE, Seifert HS, Koomey M (1995) Characterization of the pilF-pilD pilus-assembly locus of Neisseria gonorrhoeae. Mol Microbiol 16: 575-586. doi:10.1111/j.1365-2958.1995.tb02420.x. PubMed: 7565116. [DOI] [PubMed] [Google Scholar]
- 6. Wolfgang M, Lauer P, Park HS, Brossay L, Hébert J et al. (1998) PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol Microbiol 29: 321-330. doi:10.1046/j.1365-2958.1998.00935.x. PubMed: 9701824. [DOI] [PubMed] [Google Scholar]
- 7. Satyshur KA, Worzalla GA, Meyer LS, Heiniger EK, Aukema KG et al. (2007) Crystal structures of the pilus retraction motor PilT suggest large domain movements and subunit cooperation drive motility. Structure 15: 363-376. doi:10.1016/j.str.2007.01.018. PubMed: 17355871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holz C, Opitz D, Greune L, Kurre R, Koomey M et al. (2010) Multiple pilus motors cooperate for persistent bacterial movement in two dimensions. Phys Rev Lett 104: 178104. doi:10.1103/PhysRevLett.104.178104. PubMed: 20482147. [DOI] [PubMed] [Google Scholar]
- 9. Maier B, Potter L, So M, Long CD, Seifert HS et al. (2002) Single pilus motor forces exceed 100 pN. Proc Natl Acad Sci U S A 99: 16012-16017. doi:10.1073/pnas.242523299. PubMed: 12446837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Howie HL, Glogauer M, So M (2005) The N. gonorrhoeae type IV pilus stimulates mechanosensitive pathways and cytoprotection through a pilT-dependent mechanism. PLOS Biol 3: e100. doi:10.1371/journal.pbio.0030100. PubMed: 15769184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Opitz D, Clausen M, Maier B (2009) Dynamics of gonococcal type IV pili during infection. ChemPhysChem 10: 1614-1618. doi:10.1002/cphc.200800654. PubMed: 19266528. [DOI] [PubMed] [Google Scholar]
- 12. Opitz D, Maier B (2011) Rapid cytoskeletal response of epithelial cells to force generation by type IV pili. PLOS ONE 6: e17088. doi:10.1371/journal.pone.0017088. PubMed: 21340023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biais N, Higashi DL, Brujic J, So M, Sheetz MP (2010) Force-dependent polymorphism in type IV pili reveals hidden epitopes. Proc Natl Acad Sci U S A 107: 11358-11363. doi:10.1073/pnas.0911328107. PubMed: 20534431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maier B (2013) The type IV pilus system - a tunable molecular motor. Soft Matter 9: 5667-5671. doi:10.1039/c3sm50546d. [Google Scholar]
- 15. Kurre R, Höne A, Clausen M, Meel C, Maier B (2012) PilT2 enhances the speed of gonococcal type IV pilus retraction and of twitching motility. Mol Microbiol 86: 857-865. PubMed: 23035839. [DOI] [PubMed] [Google Scholar]
- 16. Clausen M, Koomey M, Maier B (2009) Dynamics of type IV pili is controlled by switching between multiple states. Biophys J 96: 1169-1177. doi:10.1016/j.bpj.2008.10.017. PubMed: 19186152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maier B, Koomey M, Sheetz MP (2004) A force-dependent switch reverses type IV pilus retraction. Proc Natl Acad Sci U S A 101: 10961-10966. doi:10.1073/pnas.0402305101. PubMed: 15256598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allemand JF, Maier B (2009) Bacterial translocation motors investigated by single molecule techniques. FEMS Microbiol Rev 33: 593-610. doi:10.1111/j.1574-6976.2009.00166.x. PubMed: 19243443. [DOI] [PubMed] [Google Scholar]
- 19. Clausen M, Jakovljevic V, Søgaard-Andersen L, Maier B (2009) High-force generation is a conserved property of type IV pilus systems. J Bacteriol 191: 4633-4638. doi:10.1128/JB.00396-09. PubMed: 19429611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kurre R, Maier B (2012) Oxygen depletion triggers switching between discrete speed modes of gonococcal type IV pili. Biophys J 102: 2556-2563. doi:10.1016/j.bpj.2012.04.020. PubMed: 22713571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martinez KA 2nd, Kitko RD, Mershon JP, Adcox HE, Malek KA et al. (2012) Cytoplasmic pH response to acid stress in individual cells of Escherichia coli and Bacillus subtilis observed by fluorescence ratio imaging microscopy. Appl Environ Microbiol 78: 3706-3714. doi:10.1128/AEM.00354-12. PubMed: 22427503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miesenböck G, De Angelis DA, Rothman JE (1998) Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394: 192-195. doi:10.1038/28190. PubMed: 9671304. [DOI] [PubMed] [Google Scholar]
- 23. Repaske DR, Adler J (1981) Change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellents. J Bacteriol 145: 1196-1208. PubMed: 7009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shioi J, Taylor BL (1984) Oxygen taxis and proton motive force in Salmonella typhimurium. J Biol Chem 259: 10983-10988. PubMed: 6381491. [PubMed] [Google Scholar]
- 25. Breeuwer P, Drocourt J, Rombouts FM, Abee T (1996) A Novel Method for Continuous Determination of the Intracellular pH in Bacteria with the Internally Conjugated Fluorescent Probe 5 (and 6-)-Carboxyfluorescein Succinimidyl Ester. Appl Environ Microbiol 62: 178-183. PubMed: 16535209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lissenden S, Mohan S, Overton T, Regan T, Crooke H et al. (2000) Identification of transcription activators that regulate gonococcal adaptation from aerobic to anaerobic or oxygen-limited growth. Mol Microbiol 37: 839-855. doi:10.1046/j.1365-2958.2000.02050.x. PubMed: 10972806. [DOI] [PubMed] [Google Scholar]
- 27. Falsetta ML, Bair TB, Ku SC, Vanden Hoven RN, Steichen CT et al. (2009) Transcriptional profiling identifies the metabolic phenotype of gonococcal biofilms. Infect Immun 77: 3522-3532. doi:10.1128/IAI.00036-09. PubMed: 19528210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Falsetta ML, McEwan AG, Jennings MP, Apicella MA (2010) Anaerobic metabolism occurs in the substratum of gonococcal biofilms and may be sustained in part by nitric oxide. Infect Immun 78: 2320-2328. doi:10.1128/IAI.01312-09. PubMed: 20231417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whitehead RN, Overton TW, Snyder LA, McGowan SJ, Smith H et al. (2007) The small FNR regulon of Neisseria gonorrhoeae: comparison with the larger Escherichia coli FNR regulon and interaction with the NarQ-NarP regulon. BMC Genomics 8: 35. doi:10.1186/1471-2164-8-35. PubMed: 17261178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boehm A, Kaiser M, Li H, Spangler C, Kasper CA et al. (2010) Second messenger-mediated adjustment of bacterial swimming velocity. Cell 141: 107-116. doi:10.1016/j.cell.2010.01.018. PubMed: 20303158. [DOI] [PubMed] [Google Scholar]
- 31. Paul K, Nieto V, Carlquist WC, Blair DF, Harshey RM (2010) The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a "backstop brake" mechanism. Mol Cell 38: 128-139. doi:10.1016/j.molcel.2010.03.001. PubMed: 20346719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blair KM, Turner L, Winkelman JT, Berg HC, Kearns DB (2008) A molecular clutch disables flagella in the Bacillus subtilis biofilm. Science 320: 1636-1638. doi:10.1126/science.1157877. PubMed: 18566286. [DOI] [PubMed] [Google Scholar]
- 33. Guttenplan SB, Blair KM, Kearns DB (2010) The EpsE flagellar clutch is bifunctional and synergizes with EPS biosynthesis to promote Bacillus subtilis biofilm formation. PLOS Genet 6: e1001243 PubMed: 21170308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taylor BL, Zhulin IB, Johnson MS (1999) Aerotaxis and other energy-sensing behavior in bacteria. Annu Rev Microbiol 53: 103-128. doi:10.1146/annurev.micro.53.1.103. PubMed: 10547687. [DOI] [PubMed] [Google Scholar]
- 35. Möglich A, Ayers RA, Moffat K (2009) Structure and signaling mechanism of Per-ARNT-Sim domains. Structure 17: 1282-1294. doi:10.1016/j.str.2009.08.011. PubMed: 19836329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Philip AF, Kumauchi M, Hoff WD (2010) Robustness and evolvability in the functional anatomy of a PER-ARNT-SIM (PAS) domain. Proc Natl Acad Sci U S A 107: 17986-17991. doi:10.1073/pnas.1004823107. PubMed: 20889915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shioi JI, Matsuura S, Imae Y (1980) Quantitative measurements of proton motive force and motility in Bacillus subtilis. J Bacteriol 144: 891-897. PubMed: 6254950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Setty OH, Hendler RW, Shrager RI (1983) Simultaneous measurements of proton motive force, delta pH, membrane potential, and H+/O ratios in intact Escherichia coli. Biophys J 43: 371-381. doi:10.1016/S0006-3495(83)84360-4. PubMed: 6354293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meyer-Rosberg K, Scott DR, Rex D, Melchers K, Sachs G (1996) The effect of environmental pH on the proton motive force of Helicobacter pylori. Gastroenterology 111: 886-900. doi:10.1016/S0016-5085(96)70056-2. PubMed: 8831583. [DOI] [PubMed] [Google Scholar]
- 40. Hong Y, Brown DG (2009) Variation in bacterial ATP level and proton motive force due to adhesion to a solid surface. Appl Environ Microbiol 75: 2346-2353. doi:10.1128/AEM.02671-08. PubMed: 19218409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Steichen CT, Shao JQ, Ketterer MR, Apicella MA (2008) Gonococcal cervicitis: a role for biofilm in pathogenesis. J Infect Dis 198: 1856-1861. doi:10.1086/593336. PubMed: 18973432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Falsetta ML, Steichen CT, McEwan AG, Cho C, Ketterer M et al. (2011) The Composition and Metabolic Phenotype of Neisseria gonorrhoeae Biofilms. Front Microbiol 2: 75 PubMed: 21833322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wasser IM, de Vries S, Moënne-Loccoz P, Schröder I, Karlin KD (2002) Nitric oxide in biological denitrification: Fe/Cu metalloenzyme and metal complex NO(x) redox chemistry. Chem Rev 102: 1201-1234. doi:10.1021/cr0006627. PubMed: 11942794. [DOI] [PubMed] [Google Scholar]
- 44. Clark VL, Isabella V, Barth K, Overton C, editors (2010) Regulation and Function of the Neisserial Denitrification Pathway. Caister Academic Press. [Google Scholar]
- 45. Douarche C, Buguin A, Salman H, Libchaber A (2009) E. Coli and oxygen: a motility transition. Phys Rev Lett 102: 198101. doi:10.1103/PhysRevLett.102.198101. PubMed: 19518998. [DOI] [PubMed] [Google Scholar]
- 46. Sun M, Wartel M, Cascales E, Shaevitz JW, Mignot T (2011) Motor-driven intracellular transport powers bacterial gliding motility. Proc Natl Acad Sci U S A 108: 7559-7564. doi:10.1073/pnas.1101101108. PubMed: 21482768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaiser D (1979) Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci U S A 76: 5952-5956. doi:10.1073/pnas.76.11.5952. PubMed: 42906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Søgaard-Andersen L, Kaiser D (1996) C factor, a cell-surface-associated intercellular signaling protein, stimulates the cytoplasmic Frz signal transduction system in Myxococcus xanthus. Proc Natl Acad Sci U S A 93: 2675-2679. doi:10.1073/pnas.93.7.2675. PubMed: 8610100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Riondet C, Alcaraz G, Divi C (1997) Measurement of the intracellular pH in Escherichia coli with the internally conjugated fluorescent probe 5- (and 6-) carboxyfluorescein succinimidyl ester. Biotechnol Tech: 735-738. [Google Scholar]
- 50. Lo CJ, Leake MC, Pilizota T, Berry RM (2007) Nonequivalence of membrane voltage and ion-gradient as driving forces for the bacterial flagellar motor at low load. Biophys J 93: 294-302. doi:10.1529/biophysj.106.095265. PubMed: 17416615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Crocker JC, Grier DG (1996) When Like Charges Attract: The Effects of Geometrical Confinement on Long-Range Colloidal Interactions. Phys Rev Lett 77: 1897-1900. doi:10.1103/PhysRevLett.77.1897. PubMed: 10063199. [DOI] [PubMed] [Google Scholar]
- 52. Tønjum T, Freitag NE, Namork E, Koomey M (1995) Identification and characterization of pilG, a highly conserved pilus-assembly gene in pathogenic Neisseria. Mol Microbiol 16: 451-464. doi:10.1111/j.1365-2958.1995.tb02410.x. PubMed: 7565106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods S1. Table S1, Primers used in this study. Figure S1, Measurement of ΔpH using cFDA-SE. Figure S2, Calibration of pHluorin expressing cells. Figure S3, Correction for point spread function for determination of ΔΨ. Figure S4, Effect of different DCCD concentration on twitching dynamics.
(PDF)