Abstract
PTEN inhibitors administered prior to or immediately after experimental stroke confer acute neuroprotection. However, it remains unclear if delayed treatment with a PTEN inhibitord improves long-term functional recovery after stroke. We addressed the issue in this study. Adult male mice were subjected to 1 hour of middle cerebral artery occlusion (MCAO) followed by treatment with a well-established PTEN inhibitor bpv or saline daily for 14 days, starting at 24 hours after MCAO. Functional recovery was assessed with behavioral tests and acute infarct volumes were analyzed histologically. Delayed bpv treatment did not reduce infarction during the acute phase, but significantly improved long-term functional recovery after MCAO. Since PTEN is a critical intrinsic inhibitory factor in axonal regeneration, we further examined bpv effects on axonal densities following MCAO using bielschowsky silver staining and immunohistochemistry with antibodies against myelin basic protein. Delayed bpv treatment significantly increased axon densities in the ischemic brain at 14 days after MCAO. Moreover, PTEN expression persistently remained high in the ischemic brain over 14 days after MCAO, and bpv treatment increased post-ischemic activation of Akt and mTOR in the ischemic brain. Akt and mTOR activation are the well-established cascades downstream to PTEN inhibition and have been shown to contribute to post-injury axonal regrowth in response to PTEN inhibition. Consistently, in an in vitro neuronal ischemia model, bpv enhanced axonal outgrowth of primary cortical neurons after oxygen-glucose deprivation and the enhancing effects were abolished by Akt/mTOR inhibition. In conclusion, delayed bpv treatment improved functional recovery from experimental stroke possibly via enhancing axonal growth and Akt/mTOR activation contributed to bpv-enhanced post-stroke axon growth.
Keywords: PTEN inhibitor, bpv, functional recovery, axonal densities, stroke
Introduction
Stroke is a major cause of death and disabilities globally, yet therapeutic approaches are rather limited. For instance, the only approved drug for ischemic stroke is tissue plasminogen activator, which must be administered within 4.5 h of stroke onset. Thus, there is an unmet need for stroke therapies that could be commenced beyond this therapeutic window (Moskowitz et al, 2010). Several strategies have been suggested for extending the therapeutic windows of stroke therapies. The one that is drawing growing interest is to develop the therapies that aim at enhancing post-stroke functional recovery.
PTEN (phosphatase and tensin homolog deleted on chromosome 10) is a dual specificity phosphatase that dephosphorylates both lipids and phosphoproteins. By activating Akt (Li et al., 2009) or preserving -aminobutyric acid subtype A receptors (Liu et al, 2010a), PTEN inhibitors administered prior to or immediately after experimental stroke confer acute neuroprotection following cerebral ischemia Interestingly, PTEN may also serve as a restorative target since emerging data show that PTEN deletion induces axonal regrowth following both CNS and peripheral nerve injuries (Park et al., 2008, Christie et al., 2010, Liu et al, 2010b). However, it is currently not clear whether PTEN inhibitors improve long-term functional recovery after stroke, nor is it clear whether the therapeutic window of PTEN inhibitors could be beyond 4.5 hours following ischemic stroke.
Thus, we investigated if delayed treatment with a well-established PTEN inhibitor bpv (Schmid et al, 2004, Li et al, 2009, Christie et al, 2010, Liu et al, 2010a) improves long-term functional recovery following cerebral ischemia To explore the possible mechanisms underlying bpv restorative effects, we also investigated if delayed bpv treatment increases post-ischemic axonal densities in the ischemic boundary zone (IBZ) where neural repair is thought to occur following cerebral ischemia.
Material and Methods
Transient Middle Cerebral Artery Occlusion (MCAO) and drug administration
All animal experiments were approved by Ethical Review Panels of Changhai Hospital and Soochow University and subject to the Experimental Animal Act, 1988. To determine the restorative effects of bpv, adult male CD-1 mice weighing 30 ± 2g received 1 hour intraluminal MCAO according to previous publications (Chen et al, 2001b, Gibson and Murphy, 2004, Ren et al, 2011). In brief, mice were anesthetized and body temperature was maintained by warming pads. A lysine-coated nylon monofilament with a heat-blunted tip (diameter 0.22 ± 0.02 mm) was inserted into the right internal carotid artery via the external carotid artery. The filament was secured and the surgical site was closed when the tip of the filament reached the origin of the middle cerebral artery. After 60 minutes of occlusion, the filament was withdrawn to allow for reperfusion. Vascular occlusion (< 30% of baseline) and reperfusion (> 75% of baseline) were verified with laser Doppler flowmetry (PeriFlux System 5000, Perimed Inc, Stockholm, Sweden) by affixing a laser probe to the mouse skull to monitor cortical perfusion. Sham-operated mice received identical surgery with the exception of filament insertion to produce occlusion.
At 24 hours after reperfusion, neurological deficit were assessed using modified neurological severity score (mNSS). Mice showing neurological deficits were randomly divided into two groups to receive: 1) intraperitoneal (IP) injection of the PTEN inhibitor [bpv (phen)] (EMD Chemicals, Inc, Gibbstown, NJ, United States) at a dose of 0.2 mg / kg / day for 14 days, starting at 24 hours after MCAO; or 2) an equal volume of saline. IP injection of bpv at this concentration has been shown to inhibit cerebral PTEN and confer neuroprotection following experimental stroke (Li et al, 2009, Shi et al, 2011). Over 14 days after MCAO, the mortalities of bpv- and saline- treated groups were: 12 out of 42 and 22 out of 42 mice, respectively Gross examination revealed that, regardless of treatments, most mice died from lung infection after MCAO.
Behavioral testing
Modified neurological severity scores (mNSS) were examined in bpv-treated mice (n = 12) and saline-treated mice (n = 12) before and at 1, 3, 5, 7, 9, 11 and 14 days after MCAO in a blinded manner. mNSS is a comprehensive test for evaluating motor, sensory, reflex and balance abilities. Neurological deficits were graded on a scale of 0 to 18. Table 1 describes the set of mNSS in detail (Chen et al., 2001a, Zhang et al., 2010). According to table 1, score points were awarded when mice were unable to perform the tests or lacked tested reflexes. Thus, the higher the scores are, the more severe the injury is.
Table 1.
Modified Neurological Severity Score
| Points | |
|---|---|
| Motor tests | |
| Raising mice by the tail | |
| Forelimb flextion | 1 |
| Hindlimb flextion | 1 |
| Head moving more than 10 degrees to vertical axis within 30 s | 1 |
| Placing mice on the floor (Normal = 0; Maximum = 3) | |
| Unable to walk straight | 1 |
| Circling toward paretic sides | 2 |
| Falling down to paretic sides | 3 |
| Beam balancing tests (Normal = 0; Maximum = 6) | |
| Grasp of beam side | 1 |
| Hugging the beam plus one limb falling down from the beam | 2 |
| Hugging the beam plus two limbs falling down from the beam (> 1 min) | 3 |
| Attempting to keep balance on the beam but falling down (> 40 s) | 4 |
| Attempting to keep balance on the beam but falling down (> 20 s) | 5 |
| Falling down without attempt to keep balance or hang on to the beam | 6 |
| Sensory tests | |
| Visual and tactile tests | 1 |
| Deep sensory (Proprioceptive tests) | 1 |
| Absence of the reflexes | |
| Head shaking when the auditory meatus is touched | 1 |
| Eye blinking when the cornea is touched lightly with a cotton tip | 1 |
| Motor reflexes in responses to a brief noise from paper snapping | 1 |
| Abnormal movements | |
| Myoclonus, myodystony and seizures | 1 |
| Maximum points | 18 |
Limb placing, a test initially used for assessing lateralized sensorimotor dysfunction of rats after experimental stroke, has been translated to the mouse MCAO model recently (Jin et al, 2010). We performed the forelimb placement test in bpv-treated mice (n = 12) and saline-treated mice (n = 12) before and at 1, 3, 5, 7, 9, 11 and 14 days after MCAO, as previously described for MCAO-treated mice (Wang et al, 2009, Jin et al, 2010). Briefly, mice were brought laterally towards the benchtop, allowing for spontaneous placement of the forelimbs. Mice were then gently pulled down, forcing the limbs away from the bench top edge. Forelimb retrieval and placement were observed and graded as follows: 0 = immediate and complete placement; 1 = delayed or incomplete placement (> 2 seconds); and 2 = no placement.
Elevated body swing test was performed in bpv-treated (n = 13) and saline-treated mice (n = 11) before and at 3, 9 and 13 days after MCAO to evaluate asymmetrical motor behavior (Wang et al., 2009). Mice were held by the tail, the direction of the body swing, defined as an upper body turn of > 10 degrees to either side, was recorded for 30 trials each time. The numbers of left and right turns were counted, and final results were presented as the percentages of turns to the ischemia-impaired side (left side) to 30 (total trials).
Infarct volume assessment
Infarction analysis was performed in bpv- (n = 7) and saline-treated mice (n = 7) at 4 days after MCAO. Briefly, mice were subjected to MCAO followed by daily injection of bpv or saline, starting at 24 hours after MCAO. At 4 days after MCAO, mice were decapitated, and brains were harvested and cut into 2-mm-thick coronal sections. Slices were stained in a 1.2% solution of 2, 3, 5-triphenyltetrazolium chloride (TTC, Sigma, St Louis, MO) and then photographed. Unstained (infarcted) and stained (uninfarcted) areas were analyzed with digital image analysis software (SigmaScan Pro, Jandel, San Rafael, CA) and integrated across all slices, as previously described (Kitano et al., 2007).
Bielschowsky silver staining and MBP immunohistochemistry
Bielschowsky silver is a marker for axons (Pluchino et al, 2003, Karnezis et al, 2004). Enhanced bielschowsky silver staining has been correlated with axonal regrowth in rodent brains following MCAO (Chen et al, 2010, Cui et al., 2010). Thus, we performed bielschowsky silver staining to visualize myelinated axons as described with modifications (Cui et al, 2010). Mice treated with bpv or saline (n = 8 for each group) were euthanized at 14 days after MCAO. Transcardial perfusion with saline followed by 4% paraformaldehyde was performed to fix the brains. After overnight immersion in 4% paraformaldehyde, the brains were embedded in paraffin. Paraffin blocks were obtained from the lesion center (bregma −1 to +1 mm) and serially cut into 8 μm-thick slices. Slices were placed in 20% silver nitrate at 37°C in the dark for 25 minutes followed by rinsing with distilled water. Ammoniacal silver solution was then added dropwise to the slices. After rinsing with water, slices were immersed serially in 10% formaldehyde, distilled water and 5% sodium thiosulfate.
MBP, a marker of myelination, is critical for axon functions and exhibits irreversible decrease after cerebral ischemia (Irving et al, 2001, Moxon-Emre and Schlichter, 2010, Chu et al, 2012). Accordingly, MPB levels have been used as markers of post-ischemic white matter injury and repair (Irving et al, 2001, Iwai et al, 2010). For immunohistochemistrical assessment of the expression of MBP, mice treated with bpv or saline (n = 8 for each group) were euthanized at 14 days after MCAO. A series of 6-μm coronal sections were cut from the block that surrounded the lesion center (bregma −1 to +1 mm). Sections were then deparafinized, sequentially incubated with MBP monoclonal antibodies derived from rats (1:200, Abcam, Cambridge, UK) and HRP-labeled secondary antibodies.
Quantification of bielschowsky and MBP immunostaining was performed in a blinded manner. As described in detail in the publication (Chen et al, 2010), every 10th coronal section for a total 5 sections was collected for quantification, with each section containing pre-assigned eight fields in the striatal IBZs, which were adjacent to the ischemic core. Images were digitized under a 20X objective (Olympus BX40) using a CCD-color video system interfaced with the imaging analysis system (Image pro-plus). Positive areas of bielschowsky silver and MBP were quantified in these eight regions of white matter bundles located in the striatal IBZs. Data were expressed as the percentages of bielschowsky silver and MBP positive areas.
Western blotting
For analysis of PTEN expression following MCAO, cortical and striatal tissues were harvested from IBZs, as described in our previous publication (Cheng et al, 2007), at 1, 3, 7, 14 days after MCAO (n = 4 for each timepoint). To examine bpv inhibitory effects on PTEN cascade following MCAO, western blot assessment of total and phosphorylated PTEN, Akt and mTOR was performed with striatal tissues harvested from IBZs of bpv- (n = 3) and saline-treated mice (n = 3) at 4 days after MCAO. Whole protein extracts were obtained by homogenizing tissue samples in RIPA lysis buffer. Denatured protein extracts (30 μg) were electrophoresed on 10% SDS-PAGE gels and then transferred to PVDF membranes. Membranes were blocked with 5% non-fat milk and probed with following primary antibodies purchased from Cell Signaling (Beverly, MA, USA): PTEN (1:1000), phosphorylated PTEN (p-PTEN, 1:1000), Akt (1:1000), phosphorylated-Akt (p-Akt, 1:1000), S6 protein (1:1000) and phosphorylated-S6 (p-S6, 1:1000). After washing three times, membranes were probed with appropriate secondary antibodies (Cell Signaling, Beverly, MA, USA): anti-rabbit IgG, HRP-linked antibody (1:2000) or anti-mouse IgG, HRP-linked antibody (1:2000) for 1 hour at room temperature. Protein bands were visualized with a Kodak imaging system using a SuperSignal West Pico chemiluminescence kit (Pierce, Rockford, IL, USA). β-actin was used as a loading control. The optical densities of protein bands were semi-quantified with Image J software, and final results were expressed as the ratios of phosphorylated proteins to total proteins.
Measurement of neurite outgrowth of primary cortical neurons (PCN) following oxygen-glucose deprivation (OGD)
The in vitro OGD model has been extensively used to investigate the mechanisms underlying post-ischemic axon regeneration (Chen et al, 2010, Cui et al., 2010, Yan et al, 2012). We performed this model as described by these previous publications. Briefly, PCNs were obtained from 16-days mouse embryos and cultured in neurobasal media supplemented with B27. For assessment of neurite outgrowth following ischemic injury, PCNs were subjected to 1 hour OGD. PCNs were then removed from the anaerobic chamber, re-fed with neurobasal media containing: 1) bpv (100 nM); 2) bpv (100 nM) + Akt inhibitor LY294002 (10 μM); 3) bpv (100 nM) + mTOR inhibitor rapamycin (100 nM); or 4) vehicle. PCNs were returned to a normal incubator. At 24 hours after reoxygenation, immunostaining was performed using antibodies against TUJ1 to assess dendrite length, as described previously (Chen et al, 2010). For each condition, the experiments were repeated six times and a total of 20 neurons were imaged in a well per experiment. The total dendrite length of 20 neurons was averaged and results were presented as averaged length per neuron.
Statistical analysis
Data were presented as mean ± SEM and evaluated using student t-tests. Data involving different time points (behavioral) were analyzed using repeated measurements ANOVA, followed by Tukey-Kramer post-hoc tests. For all analyses, the null-hypothesis was rejected at the 0.05 level.
Results
Delayed bpv treatment improved functional recovery
We administered bpv at 24 hours after MCAO and monitored functional recovery over 14 days following MCAO with mNSS, forelimb placement and elevated body swing tests. As indicated by the mNSS scores, the neurological deficits were equivalent between bpv- and saline- groups at the time when bpv treatment started at 24 hours after MCAO (Figure 1A). At 11 and 14 days after MCAO, bpv-treated MCAO mice displayed significantly lower mNSS scores than saline-treated MCAO mice. Bpv-treated mice also performed better in the forelimb placement test at 11 and 14 days after MCAO (Figure 1B). The results from elevated body swing test also showed that delayed bpv treatment, commenced at 24 hours after MCAO, significantly improved asymmetrical motor deficits at 13 days after MCAO (Figure 1C).
Figure 1.
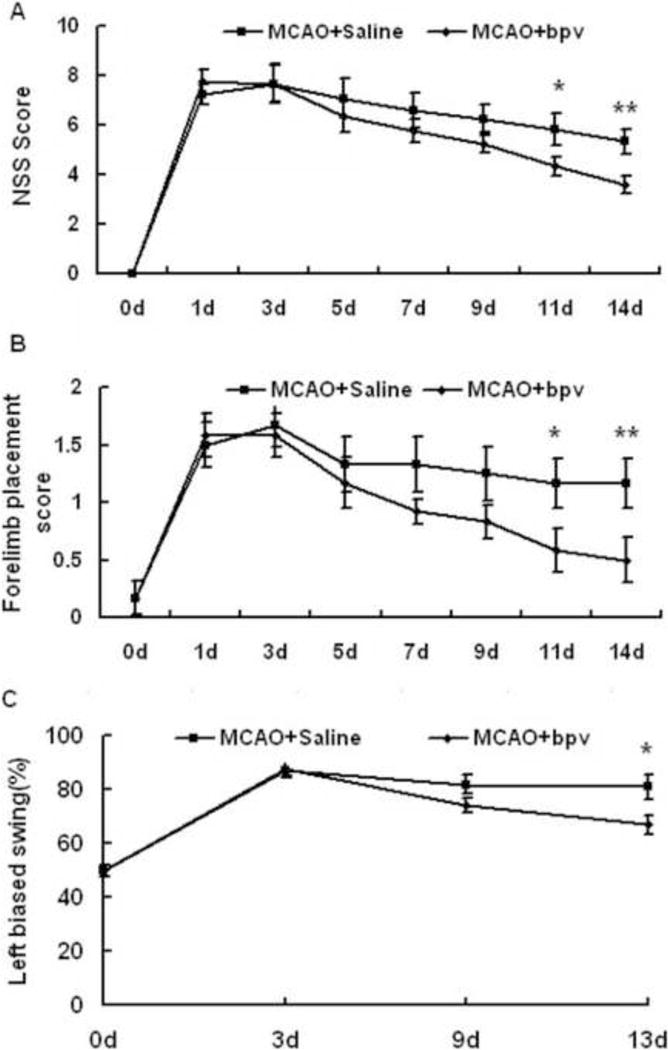
Delayed bpv treatment improved functional recovery from MCAO in mice. A: Modified neurological severity scores (mNSS) were significantly lower in bpv-treated vs. saline-treated mice from 11 days following MCAO (n = 12 per group). B: bpv-treated mice performed better in the forelimb placement test from 11 days after MCAO (n = 12 per group). C: Compared to saline-treated mice (n = 11), bpv-treated mice (n = 13) exhibited improved asymmetrical motor deficits at 13 days after MCAO in the elevated body swing test. * p < 0.05, and ** p < 0.01 compared to the saline-treated group.
Delayed bpv administration did not confer acute neuroprotection, but increased axon densities in the ischemic brain following MCAO
Bpv, when administered prior to cerebral ischemia, decreases infarct sizes during the acute phase of experimental stroke (Shi et al, 2011). To investigate if acute neuroprotection contributes to the beneficial effects of delayed bpv treatment on recovery from MCAO, we compared acute infarct damage between bpv- and saline-treated mice. Bpv was administered daily starting at 24 hours after MCAO. At 4 days after MCAO, infarct volumes were not different in bpv- vs. saline-treated mice (Figure 2A, p = 0.92, n = 7 per group).
Figure 2.
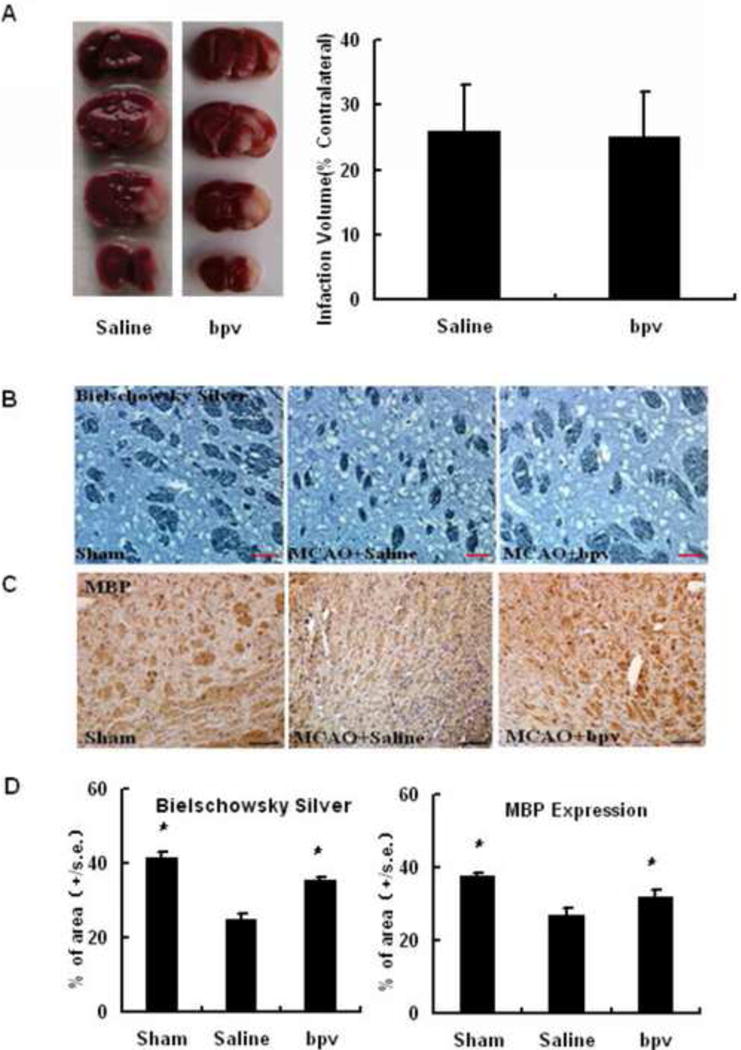
Delayed bpv treatment did not reduce acute infarction at 4 days after MCAO but increased axonal densities at 14 day after MCAO. Acute infarct damage was analyzed in bpv- and saline-treated mice using TTC histology. Bielschowsky silver and MBP expression were assessed in sham-, saline-treated and bpv-treated MCAO mice. A: Representative images of TTC histology and the bar graph. B: Bielschowsky silver staining (scale bar: 50 μm); B, MBP immunostaining (scale bar: 50 μm); C quantitative data of bielschowsky silver staining; D: quantitative data of MBP immunostaining * p < 0.05 compared to saline-treated mice (n = 8 per group).
To examine if delayed bpv treatment increases axonal densities following MCAO, we used bielschowsky silver staining and MBP immunohistochemistry to assess the post-ischemic densities of myelinated axons in the striatal IBZs, where neural repair is thought to occur after MCAO (Chen et al., 2010, Cui et al., 2010). Compared to sham controls, MCAO mice displayed significantly decreased bielschowsky silver staining in the striatal IBZs at 14 days after MCAO. Bpv treatment restored bielschowsky silver staining to the levels comparable to those seen in sham controls (Figure 2B and the left panel of 2C). However, bpv did not enhance axon densities in the corresponding regions of the contralateral sides as assessed by bielschowsky staining (Data not shown). Similarly, we observed that MBP immunoreactivity in the ipsilateral striatal IBZs was decreased at 14 days after MCAO, which was remarkably restored by delayed bpv treatment (Figure 2B and the right panel of 2C). Bpv had no effect on MBP immunohistochemistry in the corresponding regions of the contralateral sides either (Data not shown).
High levels of PTEN were maintained in the ischemic brain
Previous studies show that baseline expression of PTEN is high in intact adult neurons, which persists after injury and intrinsically inhibits axon regeneration (Park et al, 2008, Christie et al, 2010, Liu et al, 2010b). Thus, we examined if PTEN expression persists after MCAO in cortical and striatal IBZs, where neural repair occurs following cerebral ischemia The total amount and phosphorylated PTEN protein in the cortical and striatal IBZs did not change compared to those seen in the same regions of naïve adult mice, and high levels of PTEN were maintained in these regions over 14 days after MCAO (Figure 3). Thus, persistently expressed PTEN may serve as an intrinsic inhibitory factor of axon regrowth following cerebral ischemia.
Figure 3.
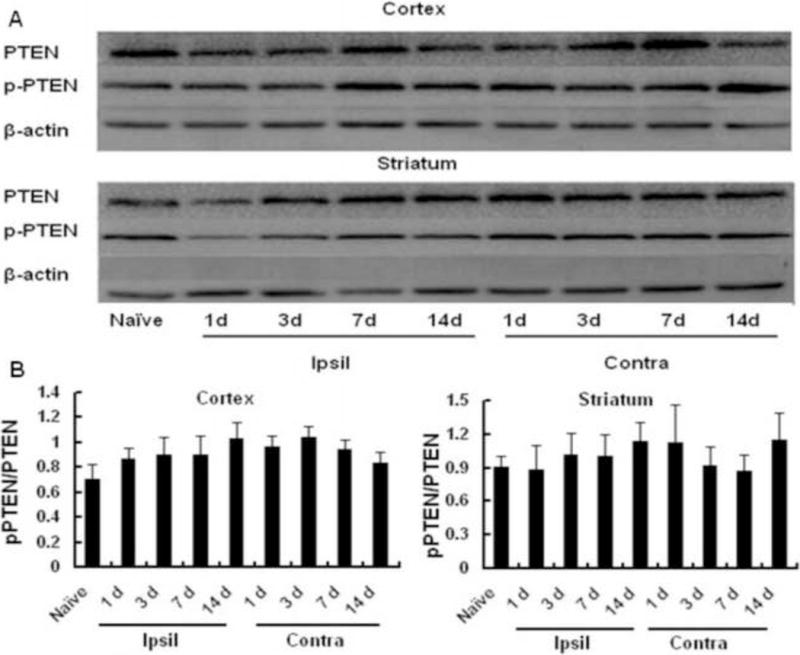
PTEN expression persistently remained high levels in the ischemic boundary zones (IBZs) over 14 days after MCAO. A: representative images of western blot analysis of total PTEN and phosphorylated (inactive) PTEN in the IBZs of the cortex and striatum. B: Quantitative data of ratios of phosphorylated/total PTEN in the ischemic cortex (n = 4 per group, left panel) and striatum (n = 4 per group, right panel). Ipsil: ipsilateral sides, Contra: contralateral sides.
Delayed bpv treatment inhibited PTEN and activated Akt/mTOR in the ischemic brain
Akt and mTOR activation are well-established cascades downstream to PTEN inhibition, which contributes to axonal regrowth induced by PTEN deletion following neural injuries (Christie et al, 2010, Liu et al, 2010b, Park et al., 2010). Furthermore, Akt activation is a specific marker for PTEN inhibition and has been used to indicate bpv (or PTEN siRNA) inhibitory preference and potency toward PTEN (Ning et al, 2004, Schmid et al, 2004, Walker et al., 2012) since PTEN exhibits a high phosphatase specificity towards 3-phosphorylated phosphoinositides that activate Akt. Thus, we investigated if bpv, as a potent PTEN inhibitor, activated Akt and downstream mTOR in the ischemic brain. Phosphorylated Akt was used as a marker for Akt activation and phosphorylated S6 as a marker for mTOR activation (Park et al., 2008). Western blotting was performed to examine activation of Akt and mTOR in the striatal IBZ, where post-MCAO axonal densities were enhanced by delayed bpv treatment (Figure 2). We found that IP injection of bpv daily, starting at 24 hours after MCAO, significantly increased phosphorylation of Akt and S6 in the striatal IBZ at 4 days after MCAO compared to saline injection (Figure 4A, 4B and 4C). However, IP injection of bpv did not increase Akt and S6 phosphorylation in the contralateral sides (Figure 4A, 4B and 4C). Finally, we examined if delayed bpv treatment affected the cerebral levels of phosphorylated (inactive) PTEN and total PTEN following MCAO. Compared to vehicle, delayed treatment with bpv decreased PTEN expression in the striatal IBZ at 4 days after MCAO (Figure 4D). The results were consistent with a recent publication showing that bpv inhibits PTEN protein expression (Pi et al, 2012) and provides direct evidence that PTEN was inhibited by delayed bpv treatment following MCAO Collectively, these results indicated that intraperitoneally injected bpv crossed the blood-brain barrier to induce PTEN inhibition in the ischemic sides of the brain.
Figure 4.
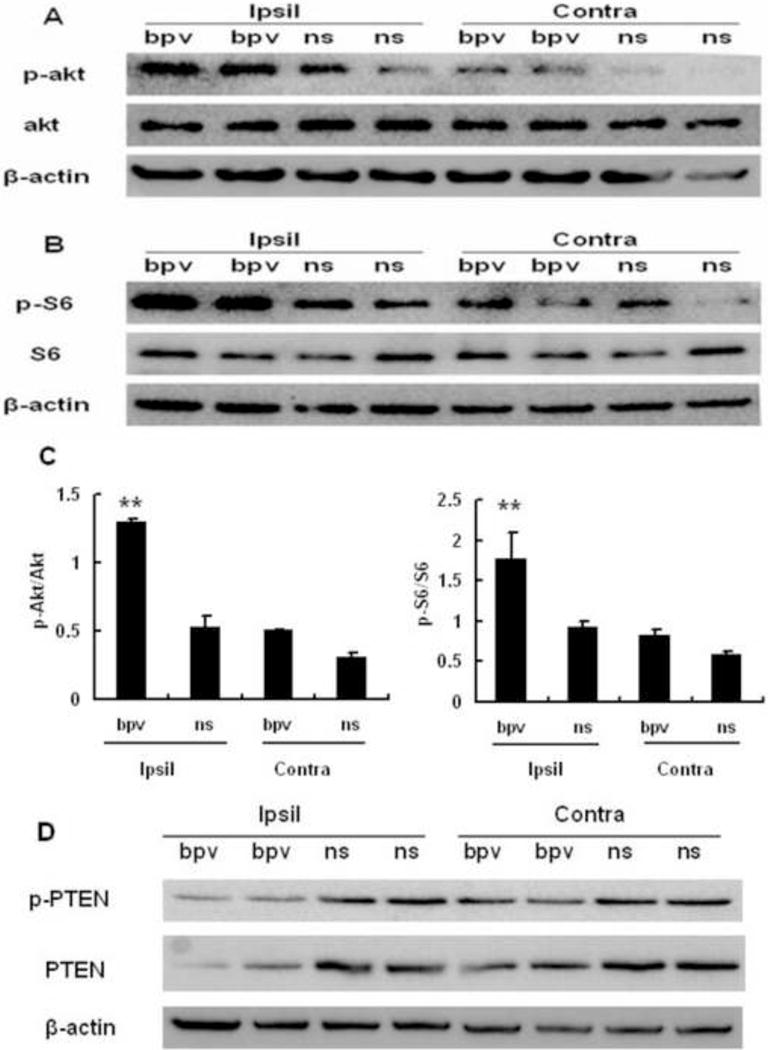
The PTEN inhibitor bpv inhibited cerebral PTEN and activated cerebral Akt and mTOR following MCAO at 4 days after MCAO. Compared to saline-injected mice (ns), bpv-treated mice (bpv) displayed elevated activation of AKT (phosphorylation, panel A) and elevated activation of and mTOR (S6 phosphorylation, panel B) in the IBZs of the ipsilateral (Ipsil) striatum. β-actin was used as a loading control. C: quantitative data of ratios of phosphorylated/total Akt (left panel) and phosphorylated/total S6 (right panel). D: Compared to saline, bpv inhibited PTEN expression in the IBZs of the ipsilateral (Ipsil) striatum. **p < 0.01 compared to other groups, n = 3 per group. Ipsil: ipsilateral sides, Contra: contralateral sides.
Bpv-enhancing effects on neurite outgrowth of primary neurons following OGD were abolished by Akt or mTOR inhibition
The in vitro OGD model has been extensively used to investigate the mechanisms underlying post-ischemic axon regeneration (Chen et al., 2010, Cui et al., 2010, Yan et al, 2012). Our in vivo data showed that bpv promoted post-MCAO functional recovery (Figure 1) and enhanced axonal densities and Akt/mTOR activation in the ischemic brain (Figure 2 and 4). Thus, we used the in vitro neuronal OGD model to investigate if bpv enhances post-ischemic axonal outgrowth of primary cortical neurons (PCNs) and if Akt and mTOR activation contribute to axonal outgrowth in response to bpv treatment. Compared to saline, bpv significantly increased post-OGD neurite outgrowth of PCNs (Figure 5). The PI3K/Akt inhibitor LY294002 or mTOR inhibitor rapamycin significantly attenuated bpv-enhanced neurite outgrowth in post-OGD neurons, suggesting that activated Akt and mTOR at least in part contribute to post-ischemic neurite outgrowth enhanced by bpv.
Figure 5.
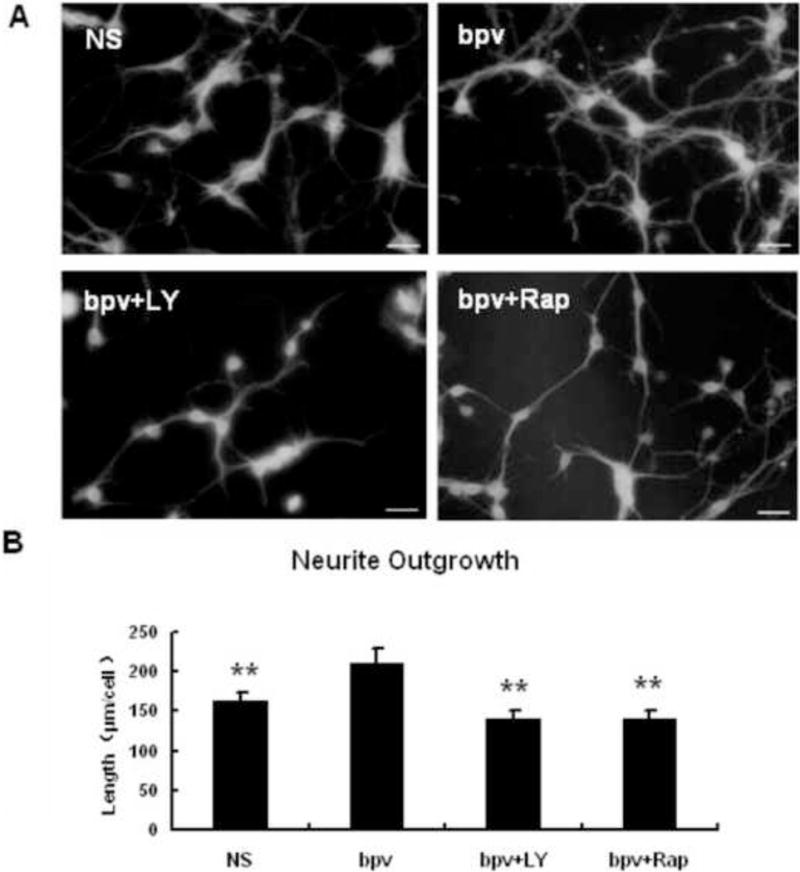
The PTEN inhibitor bpv enhanced neurite outgrowth of primary cortical neurons following OGD, which was abolished by Akt or mTOR inhibition. A: TUJ1 immunostaining in post-OGD neurons treated with vehicle, bpv, bpv + ly (bpv plus the Akt inhibitor LY294002) and bpv + Rap (bpv plus the mTOR inhibitor rapamycin). Scale bar: 20 μm. B: Quantitative data of neurite outgrowth. ** p < 0.01 compared with the bpv-treated neurons (n = 6 per group).
Discussion
The major finding of this study is that delayed administration of the potent PTEN inhibitor bpv did not confer acute neuroprotection but proved long-term functional recovery following MCAO. Furthermore, we found that bpv increased post-ischemic axonal densities, which likely contributed to bpv restorative effects. Finally, bpv activated cerebral Akt and mTOR in vivo and Akt or mTOR inhibition abolished the bpv-enhanced neurite outgrowth in vitro, suggesting that Akt and mTOR activation contributed to bpv-enhancing effects on post-ischemic axonal densities.
Treatment with PTEN inhibitors before or immediately after experimental stroke confers acute neuroprotection in experimental stroke (Li et al, 2009, Liu et al, 2010a, Shi et al., 2011). Here we investigated if delayed treatment of a PTEN inhibitor improved long-term functional recovery following cerebral ischemia Although further histological and studies are needed to examine the restorative effects of PTEN inhibition commenced at 3 days after MCAO, our results showed that bpv, given at 24 hours after MCAO, accelerated long-term recovery. The results suggested that the therapeutic window of PTEN inhibitors could be beyond 4.5 hours after ischemic stroke. Of note, delayed bpv treatment only improved recovery from 11 days after MCAO, and did not improve functions during the acute phase. The time-course of bpv-accelerated recovery suggested that delayed bpv treatment might act through restorative rather than acute neuroprotective cascades to promote functional recovery.
Bpv, given at 24 hours after MCAO, increased Akt activation (Figure 4), a well-established neuronal survival signal in response to ischemia However, delayed bpv treatment did not reduce acute infarction. Indeed, bpv has been shown to exert acute neuroprotection only when it is administered prior to or immediately after experimental stroke (Li et al, 2009, Liu et al, 2010a, Shi et al, 2011). Consistently, PTEN activation exacerbates ischemic neuronal death only when PTEN is activated before or immediately after experimental stroke (Kilic et al., 2010). Thus, these results support that the restorative effects of delayed bpv treatment we observed in this study do not relate to bpv acute neuroprotection.
In this study, the mortality of bpv-treated group was remarkably lower than that of saline-treated group over 14 days after MCAO (mortalities: 12/42 vs. 22/42), thus raising a possibility that bpv given at 24 hours after MCAO exerts delayed neuroprotection, which contribute to better recovery of bpv-treated mice. However, regardless of treatments, most mice died during 3 to 7 days after MCAO (11 out of 12 dead mice in bpv-treated group and 19 out of 22 dead mice in saline-treated group). It is reported that the major reason for mouse death during 3 to 7 days after MCAO is lung infection (Prass et al, 2003). Thus, the reduced mortality of bpv-treated mice likely resulted from bpv inhibition on post-stroke peripheral infection. Indeed, we already obtained data showing that delayed bpv treatment reduced bacterial loads in the lung and protected mice from developing spontaneous pneumonia after MCAO (manuscript in preparation). Consistently, a recent publication reports that PTEN-deficiency mice display less severe signs of pneumonia and prolonged survival after streptococcus pneumoniae challenge (Schabbauer et al, 2010). Thus, we think that bpv protective effects in terms of reducing mortality over 14 days after MCAO are attributed to bpv inhibition on post-stroke peripheral infection and unlikely confound our interpretation of bpv enhancing effects on functional recovery following MCAO.
Axon regeneration contributes to post-ischemic functional recovery. PTEN is a key intrinsic inhibitory factor in post-injury axonal regeneration (Park et al, 2010). PTEN gene deletion alone successfully induces axonal regeneration following both central and peripheral nerve injuries (Christie et al, 2010, Liu et al, 2010b, Saver, 2010). Thus, we examined if bpv enhanced axonal densities following MCAO. By using bielschowsky silver staining and MBP immunohistochemistry, we found that bpv enhanced axonal densities in the striatal penumbral areas, where neural repair is thought to occur in MCAO-treated rodents. Furthermore, in the in vitro neuronal ischemia model, bpv directly promoted neurite outgrowth of primary neurons following OGD. Together, these results suggest that the enhancement of axonal regrowth is a possible mechanism underlying restorative effects of bpv following cerebral ischemia Finally, we investigated if bpv-enhancing effects on post-ischemic axonal densities are derived from its action on PTEN inhibition and downstream cascades. First, we showed that PTEN remained high expression levels over 14 days after MCAO in the regions where brain repair is thought to occur after stroke, suggesting that PTEN is persistently present in the ischemic brain to inhibit post-ischemic axonal regrowth. Then, we showed that, in the ischemic brain, bpv decreased PTEN expression and increased activation of Akt/mTOR, the well-established cascades downstream to PTEN inhibition, which have been shown to contribute to post-injury axonal regrowth in response to PTEN inhibition. Collectively, our results suggested that bpv did cross the brain blood barrier to induce cerebral PTEN inhibition following cerebral ischemia. To further demonstrate that Akt/mTOR activation contributes to post-ischemic axonal regrowth in response to bpv, we used the in vitro neuronal OGD model and found that bpv enhancing effects on post-OGD neurite outgrowth were abolished by Akt/mTOR inhibition. Togather, these data suggest that bpv enhanced post-ischemic axonal densities likely via activating Akt and mTOR. However, whether other cascades downstream to PTEN inhibition also contributes to bpv beneficial effects following cerebral ischemia needs further investigation.
In conclusion, our results suggested that delayed treatment with the PTEN inhibitor bpv improved functional recovery from experimental stroke and that Akt/mTOR activation contributed to bpv-enhanced post-stroke axon growth.
Highlights
Delayed bpv treatment improved long-term functional recovery following MCAO.
Delayed bpv treatment did not reduce acute infarction following MCAO.
Delayed bpv treatment enhanced axon densities in the ischemic brain.
Bpv activated Akt/mTOR in the ischemic brain following MCAO.
Akt/mTOR inhibition abolished bpv-enhanced axonal outgrowth following OGD in vitro.
Acknowledgments
Source of funding
The project is supported by the grants from National Science Foundation of China (81041094, 81171246, 81100918, and 81070959), Shanghai Natural Science Funding (11ZR1432200), National Basic Research Plan (2011CB5C4403), Shanghai Research Foundation for Nanometer (1052NM01300) and the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
Abbreviations
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- MCAO
middle cerebral artery occlusion
- OGD
oxygen-glucose deprivation
- MBP
myelin basic protein
- IBZ
ischemic boundary zone
- IP
intraperitoneal
- PCN
primary cortical neuron
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
A patent application on PTEN restorative effects in neurodegenerative diseases has been filed to China Intellectual Property Office (201210044967.0).
References
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001a;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001b;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Cui X, Shehadah A, Jiang H, Roberts C, Lu M, Chopp M. Treatment of stroke with a synthetic liver X receptor agonist, TO901317, promotes synaptic plasticity and axonal regeneration in mice. J Cereb Blood Flow Metab. 2010;30:102–109. doi: 10.1038/jcbfm.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Alkayed NJ, Hurn PD. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab. 2007;27:1553–1562. doi: 10.1038/sj.jcbfm.9600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie KJ, Webber CA, Martinez JA, Singh B, Zochodne DW. PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J Neurosci. 2010;30:9306–9315. doi: 10.1523/JNEUROSCI.6271-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M, Hu X, Lu S, Gan Y, Li P, Guo Y, Zhang J, Chen J, Gao Y. Focal cerebral ischemia activates neurovascular restorative dynamics in mouse brain. Front Biosci (Elite Ed) 2012;4:1926–1936. doi: 10.2741/513. [DOI] [PubMed] [Google Scholar]
- Cui X, Chopp M, Zacharek A, Roberts C, Buller B, Ion M, Chen J. Niacin treatment of stroke increases synaptic plasticity and axon growth in rats. Stroke. 2010;41:2044–2049. doi: 10.1161/STROKEAHA.110.589333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab. 2004;24:805–813. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- Irving EA, Bentley DL, Parsons AA. Assessment of white matter injury following prolonged focal cerebral ischaemia in the rat. Acta Neuropathol. 2001;102:627–635. doi: 10.1007/s004010100416. [DOI] [PubMed] [Google Scholar]
- Iwai M, Stetler RA, Xing J, Hu X, Gao Y, Zhang W, Chen J, Cao G. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke. 2010;41:1032–1037. doi: 10.1161/STROKEAHA.109.570325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Greenberg DA. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl Acad Sci U S A. 2010;107:7993–7998. doi: 10.1073/pnas.1000154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnezis T, Mandemakers W, McQualter JL, Zheng B, Ho PP, Jordan KA, Murray BM, Barres B, Tessier-Lavigne M, Bernard CC. The neurite outgrowth inhibitor Nogo A is involved in autoimmune-mediated demyelination. Nat Neurosci. 2004;7:736–744. doi: 10.1038/nn1261. [DOI] [PubMed] [Google Scholar]
- Kilic E, ElAli A, Kilic U, Guo Z, Ugur M, Uslu U, Bassetti CL, Schwab ME, Hermann DM. Role of Nogo-A in neuronal survival in the reperfused ischemic brain. J Cereb Blood Flow Metab. 2010;30:969–984. doi: 10.1038/jcbfm.2009.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano H, Young JM, Cheng J, Wang L, Hurn PD, Murphy SJ. Gender-specific response to isoflurane preconditioning in focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:1377–1386. doi: 10.1038/sj.jcbfm.9600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Qu Y, Mao M, Zhang X, Li J, Ferriero D, Mu D. Involvement of the PTEN-AKT-FOXO3a pathway in neuronal apoptosis in developing rat brain after hypoxia-ischemia. J Cereb Blood Flow Metab. 2009;29:1903–1913. doi: 10.1038/jcbfm.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Li L, Zhang Q, Chang N, Wang D, Shan Y, Wang H, Feng H, Zhang L, Brann DW, Wan Q. Preservation of GABAA receptor function by PTEN inhibition protects against neuronal death in ischemic stroke. Stroke. 2010a;41:1018–1026. doi: 10.1161/STROKEAHA.110.579011. [DOI] [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010b;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon-Emre I, Schlichter LC. Evolution of inflammation and white matter injury in a model of transient focal ischemia. J Neuropathol Exp Neurol. 2010;69:1–15. doi: 10.1097/NEN.0b013e3181c3ce6c. [DOI] [PubMed] [Google Scholar]
- Ning K, Pei L, Liao M, Liu B, Zhang Y, Jiang W, Mielke JG, Li L, Chen Y, El-Hayek YH, Fehlings MG, Zhang X, Liu F, Eubanks J, Wan Q. Dual neuroprotective signaling mediated by downregulating two distinct phosphatase activities of PTEN. J Neurosci. 2004;24:4052–4060. doi: 10.1523/JNEUROSCI.5449-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Kanter JL, He Z. PTEN/mTOR and axon regeneration. Exp Neurol. 2010;223:45–50. doi: 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi W, Guo X, Su L, Xu W. BMP-2 up-regulates PTEN expression and induces apoptosis of pulmonary artery smooth muscle cells under hypoxia. PLoS One. 2012;7:e35283. doi: 10.1371/journal.pone.0035283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, Offner H. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci. 2011;31:8556–8563. doi: 10.1523/JNEUROSCI.1623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver JL. Targeting the brain: neuroprotection and neurorestoration in ischemic stroke. Pharmacotherapy. 2010;30:62S–69S. doi: 10.1592/phco.30.pt2.62S. [DOI] [PubMed] [Google Scholar]
- Schabbauer G, Matt U, Gunzl P, Warszawska J, Furtner T, Hainzl E, Elbau I, Mesteri I, Doninger B, Binder BR, Knapp S. Myeloid PTEN promotes inflammation but impairs bactericidal activities during murine pneumococcal pneumonia. J Immunol. 2010;185:468–476. doi: 10.4049/jimmunol.0902221. [DOI] [PubMed] [Google Scholar]
- Schmid AC, Byrne RD, Vilar R, Woscholski R. Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 2004;566:35–38. doi: 10.1016/j.febslet.2004.03.102. [DOI] [PubMed] [Google Scholar]
- Shi GD, OuYang YP, Shi JG, Liu Y, Yuan W, Jia LS. PTEN deletion prevents ischemic brain injury by activating the mTOR signaling pathway. Biochem Biophys Res Commun. 2011;404:941–945. doi: 10.1016/j.bbrc.2010.12.085. [DOI] [PubMed] [Google Scholar]
- Walker CL, Walker MJ, Liu NK, Risberg EC, Gao X, Chen J, Xu XM. Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS One. 2012;7:e30012. doi: 10.1371/journal.pone.0030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, van Hoecke M, Tang XN, Lee H, Zheng Z, Swanson RA, Yenari MA. Pyruvate protects against experimental stroke via an anti-inflammatory mechanism. Neurobiol Dis. 2009;36:223–231. doi: 10.1016/j.nbd.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Chopp M, Ye X, Liu Z, Zacharek A, Cui Y, Roberts C, Buller B, Chen J. Niaspan increases axonal remodeling after stroke in type 1 diabetes rats. Neurobiol Dis. 2012;46:157–164. doi: 10.1016/j.nbd.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chopp M, Zhang RL, Wang L, Zhang J, Wang Y, Toh Y, Santra M, Lu M, Zhang ZG. Erythropoietin amplifies stroke-induced oligodendrogenesis in the rat. PLoS One. 2010;5:e11016. doi: 10.1371/journal.pone.0011016. [DOI] [PMC free article] [PubMed] [Google Scholar]


