Abstract
Lipid rafts are specialized plasma membrane microdomains that serve as platforms for integrating cellular signal transductions. We have recently reported that autoantibodies against cardiac membrane proteins are present in patients with postural orthostatic tachycardia syndrome (POTS). In this study, we examined the presence of autoimmunoreactive IgGs against lipid raft proteins in these patients. IgGs were purified from the sera of 10 patients and 7 normal controls. Cardiac lipid raft preparations were isolated from normal human heart tissue. The lipid raft-associated proteins were resolved by 2DE and immunoblotted against IgGs from each subject. Protein spots that reacted specifically with patient IgGs were identified by nanoLC-MS/MS. Thirty-four such protein spots, and 72 unique proteins were identified. The targets of autoimmunoreactive IgGs include proteins associated with caveolae structure, adrenergic signaling, calcium signaling, cytostructures, chaperone and energy metabolism. Multiple pathways were involved including those that regulate caveolae-mediated signaling, oxidative phosphorylation, fatty acid metabolism, protein ubiquitination, and cardiac β-adrenergic signaling. Our results suggest that cardiac lipid raft-associated proteins are targets of autoimmunoreactive IgGs from patients with POTS. Autoimmunity may play a role in the pathogenesis of POTS.
Postural orthostatic tachycardia syndrome (POTS) is a common form of orthostatic intolerance, and the etiology of the syndrome is complex and unexplained in most patients. Various causes and mechanisms have been proposed for the disease including autoimmunity and ganglionic acetylcholine receptor (AChR) antibodies1. We have recently reported the presence of autoantibodies against cardiac membranes in POTS patients2. In this study, we examined whether cardiac proteins in lipid raft microdomains constitute targets of autoantibodies in POTS patients.
Subcellular fractionation can reduce sample complexity for proteomic analysis and is most efficient when combined with 2-dimensional gel electrophoresis (2DE) and mass spectrometry (MS) studies3. Numerous studies have shown the importance of lipid raft/caveolae microdomains in the regulation of cellular signal transduction, mechanosensing, lipid metabolism, cholesterol homeostasis, and ion channel activities4–13. Many critical signaling proteins and their effectors are compartmentalized in lipid rafts and if they become autoimmunoreactive, normal cellular functions may be disturbed. These microdomains are richly and tightly packed with cholesterol, sphingolipids, and glycosyl-phosphatidylinositol (GPI) anchored proteins, and thus have a low buoyant density in sucrose gradient fractionations. Caveolae are particularly abundant in terminally differentiated cells such as adipocytes, squamous epithelial cells, and muscle cells14.
METHODS
Study subjects and IgG isolation
This study was approved by the Mayo Clinic Institutional Review Board and all participants provided written informed consent. Patients were excluded if they had a history of confirmed autoimmune diseases. Seven control subjects (6 females and 1 male, average age 36.1 years) and 10 patients with the diagnosis of POTS (7 females and 3 males, average age 35.1 years) provided 30 ml of venous blood. The detailed clinical and laboratory profiles of the subjects and controls are outlined in Table 1. POTS was diagnosed in the POTS Clinic at the Mayo Clinic under the supervision of Dr. Phillip Low and they satisfied the criteria of such syndrome as previously described15. IgGs were purified from each serum sample using the Melon gel IgG isolation kit (Pierce Biotechnology). Normal human heart tissue was obtained from the National Disease Research Interchange (NDRI).
Table 1.
Clinical profiles and laboratory findings of controls and POTS patients
| Age | Sex | Allergies | PMH | Medications | Symptoms & Study Findings | ECG HR BPM | Holter HR BPM Avg (range) | Tilt Study Baseline HR (BP) | Tilt Study 10 min HR (BP) | ECHO (LVEF %) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | |||||||||||
| 1 | 43 | F | Codeine, procaine | Benign thyroid nodule | Levapro | ||||||
| 2 | 23 | F | |||||||||
| 3 | 45 | F | Lipids, LBP, Herpes zoster | ||||||||
| 4 | 37 | F | Azithromycin, Pseudoephed rine | Lipids | |||||||
| 5 | 44 | F | PCN, shellfish | Sinus rhinitis | Advil, fish oil, Imitrex | ||||||
| 6 | 24 | F | Asthma | Alavert | |||||||
| 7 | 37 | M | HBP, GERD | Lisinopril, Prilosec | |||||||
| POTS Subjects | |||||||||||
| 1 | 44 | F | Sulfur, Levofloxacin | Melanoma, meningitis, lipids, fibromyalgia, eczema | RetinA cream | ortho intolerance | 126 | 101 (70–169) | 99 (122/80) | 122 (110/84) | Normal (60–65%) |
| 2 | 51 | F | Bipolar, HBP | Catapres, Metoprolol, Lipitor, Clonidine, Provera | ortho Intolerance, mild auto neuropathy | 117 | 114 (89–165 | 92 (148/92) | 134 (125/76) | Normal (62%) | |
| 3 | 48 | M | Fibromyalgia, chronic fatigue, PTSD | Vit D, Lyrica, Imitrex, Inderal, Florinef | ortho intolerance, mild postgang sudomoter impair, mild multifocal anhidrosis | 63 | 73 (50–145) | 74 (122/78) | 125 (110/70) | Normal (60%) | |
| 4 | 56 | M | GI dysmotility | Florinef, Neurontin, K, Midodrine, Mestinon | severe autonomic neuropathy, severe cardiovagal and adrenergic failure, distal anhidrosis, ortho hypotension | 67 | 64 (164/94) | 64 (50/−) (1.5 min) | |||
| 5 | 35 | F | Asthma, lipids, depression | Klonopin, Midodrine, Nadolol, BCP, inhaler | mild vascular adrenergic impairment, minor hypohidrosis | 88 | 93 (64–152) | 88 (122/74) | 165 (104/82) | Normal (63%) | |
| 6 | 18 | F | Hydrocodone | Irritable bowel, hypovitaminosis D | Metoprolol, Citalopram, vit D | ortho tachycardia | 88 | 76 (108/64) | 120 (100/80) | ||
| 7 | 20 | F | Amoxacillin | Allegra, Microgestin | ↓ortho tolerance, hyperadrenergic state | 92 | 82 (110/56) | 131 (100/70) | Normal (61%) | ||
| 8 | 21 | F | Tramadol, Sprintec | Mononucleosis, irritable bowel | Florinef, Neurontin, Lexapro, Metoprolol, Prilosec, Mestinon | focal sudomotor failure, ortho intolerance | 67 | 90 (64–137) | 72 (92/64) | 101 (86/64) | |
| 9 | 26 | F | Amoxacillin | Eczema. GERD, sinus, periurethral cyst | Klonopin, Microgestin, Zyrtec, Norco, Cymbalta | ortho intolerance | 82 | 84 (67–137) | 83 (114/70) | 137 (108/84) | |
| 10 | 32 | M | HBP, lipids, arthritis, asthma. GI dysmotility | Flexeril, Florinef, Neurontin, Klonopin, Mestinon, Propranolol | ortho intolerance, mild adrenergic vasoconstr failure | 71 | 87 (54–136) | 80 (110/76) | 131 (112/78) |
Purification of lipid raft fractions from human heart tissue
Lipid raft fractions were prepared by the non-detergent method as we have described16. Briefly, frozen heart tissue from all four chambers was homogenized and sonicated with 2 ml of 500 mM sodium carbonate (pH11) with protease inhibitors (Roche Diagnostics, Germany). The homogenate was centrifuged at 2,500 × g for 10 min and the supernatant containing 3 mg of proteins was adjusted to 40% sucrose by mixing with 2 ml of 80% sucrose prepared in MBS (0.15 M NaCl, 25 mM 2-[N-Morpholono] ethanesulfonic acid, pH6.5) and placed at the bottom of an ultracentrifuge tube. Discontinuous sucrose gradients of 5% and 30% were layered on top (4 ml of 5% sucrose/4 ml of 30% sucrose, both in MBS containing 250 mM sodium carbonate, pH11). Samples were then centrifuged at 260,000 × g for 20 hours at 4°C. Twelve fractions of 1 ml each were collected sequentially from the top. The lipid raft fractions were identified by immunoblotting against anti-caveolin-3 antibodies (1:1000, BD Transduction labs), dialyzed against ammonium bicarbonate (50 mM, pH7.8) and lyophilized to reduce sample volume.
2DE and immunoblotting
The experiments were performed as we have described2. Proteins from the human cardiac lipid raft fractions prepared above were resolved by 2DE and transferred electrophoretically onto a PVDF membrane. IgGs isolated from each control and patient were used to develop a separate immunoblot. Antibodies against Desmin (1:1000, Cell Signaling), HSP70 (1:1000, Enzo Life Sciences) and cavin-1 (1:2000, Bethyl Laboratory, Inc) were used for 2DE gel immunoblotting to identify their spot locations in 2DE gels.
Protein identification and data analysis
Protein spots specifically reacting against patient IgGs were selected and processed for protein identification by in-gel trypsin digestion and nano-LC-electrospray tandem mass spectrometry as previously described2.
Protein interaction network analysis
Ingenuity Pathway Analysis (IPA, Ingenuity® Systems) was used to identify signaling pathways and networks as previously described2. The composite was laid out graphically using the network visualization algorithm Cytoscape 2.8.217.
RESULTS
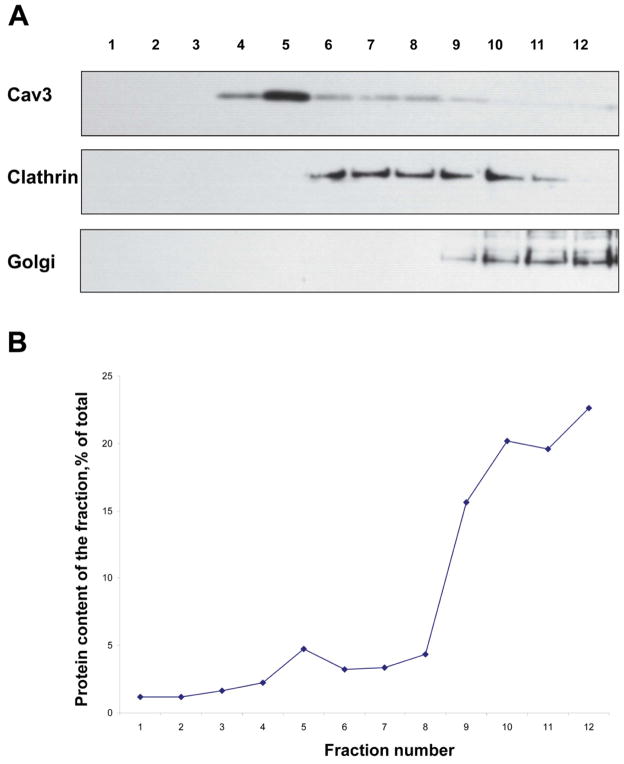
We first evaluated the effectiveness of lipid raft/caveolae-rich fraction isolation. Caveolin-3, the marker of cardiomyocyte caveolae, was enriched in the low buoyant density fractions (Figure 1). In contrast, the non-lipid raft membrane marker, clathrin, and the subcellular organelle marker Golgi 58, were differentially distributed (Figure 1). Clathrin appeared predominantly in the heavier fractions of the gradient, suggesting the exclusion of clathrin-coated pits as well as clathrin-associated membranes from our caveolae-rich lipid raft fractions. The Golgi apparatus was also distributed in the heavy density fractions. These results suggest that the lipid raft fraction was relatively free of contamination by intracellular organelles.
Figure 1. Immunoblot analysis of human cardiac membrane sucrose density gradient fractions.
(A) An equal volume of each sucrose density gradient fraction was loaded onto each lane. Enrichment of caveolin-3 in the low buoyant density fractions indicated the successful separation of caveolae/lipid raft fraction (top panel). The middle and lower panels show clathrin and Golgi 58, the non-lipid raft plasma membrane and intracellular organelle marker proteins, respectively, are predominantly detected in the heavy fractions, but not in the caveolae-rich fraction. (B) The distribution of protein content across the gradient fractions was expressed as a percentage of the total amount of protein recovered in the gradient.
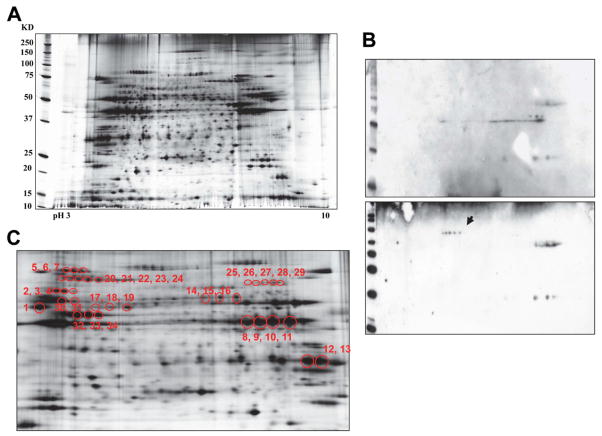
After 2DE separation and silver staining, 1159 protein spots were visualized (analyzed by PDQuest, Bio-Rad Laboratories, Inc.) (Figure 2A). 2DE gel immunoblotting analysis demonstrated that 34 spots reacted specifically with patient IgGs (Figure 2B& C, Table 2). These spots were further analyzed by nanoLC-MS/MS and the results identified 72 unique proteins (Table 3). A variety of cardiac lipid-raft associated proteins were targeted by the IgGs from POTS patients. These include proteins associated with caveolae structure (cavin), adrenergic signaling (protein kinase A), calcium signaling (sarcalumenin and S100), cytostructures (desmin, desmoplakin, desmoglein, vimentin, and plakoglobin), chaperone (heat shock 70), and energy metabolism.
Figure 2. 2DE gel immunoblotting of human cardiac lipid raft/caveolae fractions.
(A) Protein maps resolved by 2DE with isoelectric focusing, SDS-PAGE and silver staining. (B) Protein spots in representative 2DE immunoblots reactive against purified IgGs from a healthy control (upper panel) and a patient with POTS (lower panel). Arrow shows patient-specific immunoreactive protein spots. (C) Patient-specific immunoreactive spots sampled from silver-stained 2DE gel for protein identification, spots 1 to 34.
Table 2.
POTS patient specific immunoreactive spots
| Patient # | Patient specific spots* |
|---|---|
| 009 | 2, 3, 4 |
| 010 | 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 |
| 011 | 5, 6, 7 |
| 012 | 14, 15, 16, 30, 31, 32, 33, 34 |
| 013 | 1 |
| 014 | 17, 18, 19, 30, 31, 2, 3, 4 |
| 015 | 20, 21, 22, 23, 24 |
| 016 | |
| 017 | |
| 018 | 3, 4, 30, 31, 25, 26, 27, 28, 29, 12, 13 |
Spot code refer to Figure 2C
Table 3.
Proteins detected from patient specific spots
| Symbols | Protein Name | Acc. numbers | Function |
|---|---|---|---|
| S100A8 | Protein S100-A8 | S10A8_HUMAN | apoptosis |
| PPP2R1A | Serine/threonine-protein phosphatase 2A | 2AAA_HUMAN | apoptosis, chromosome segregation |
| HSPA9 | Stress-70 protein | GRP75_BOVIN | Chaperone, cell proliferation and aging |
| PDIA6 | Protein disulfide-isomerase A6 | PDIA6_HUMAN | Chaperone, isomerase |
| HSPD1 | 60 kDa heat shock protein | CH60_HUMAN | Chaperone, protein folding |
| HSPA1A | Heat shock 70 kDa protein 1A/1B | HSP71_HUMAN | Chaperone, protein folding |
| HSPA8 | Heat shock cognate 71 kDa protein | HSP7C_HUMAN | Chaperone, protein folding |
| LMNB2 | Lamin-B2 | LMNB2_HUMAN | structural molecule activity, lipodystrophy |
| TUBA | Tubulin alpha chain | TBA_XENTR | microtubule, structural molecule activity |
| TUBB2C | Tubulin beta-2C chain | TBB2C_HUMAN | microtubule, structural molecule activity |
| TUBB5 | Tubulin beta-5 chain | TBB5_HUMAN | microtubule, structural molecule activity |
| ACT | Actin, alpha sarcomeric/cardiac | ACT2_XENLA | cytoskeleton, cell motility |
| VIM | Vimentin | VIME_HUMAN | cytoskeleton, intermediate filaments |
| DES | Desmin | DESM_HUMAN | intermediate filament, cardiomyopathy |
| MYH7 | Myosin-7 | MYH7_HUMAN | contractile protein, hypertrophic CM |
| DSP | Desmoplakin | DESP_HUMAN | desmosomal protein, cardiomyopathy |
| PSMC5 | 26S protease regulatory subunit 8 | PRS8_HUMAN | degradation of ubiquitinated proteins |
| FHL1 | Four and a half LIM domains protein 1 | FHL1_HUMAN | muscle development, hypertrophy |
| FLG2 | Filaggrin-2 | FILA2_HUMAN | cell differentiation |
| DSG1 | Desmoglein-1 | DSG1_HUMAN | mediating cell-cell adhesion |
| MFGE8 | Lactadherin | MFGM_HUMAN | cell adhesion, angiogenesis |
| JUP | Junction plakoglobin | PLAK_HUMAN | Cell adhesion, cardiomyopathy, arrhythmia |
| CS | Citrate synthase | CISY_HUMAN | Carbohydrate metabolism, Tricarboxylic acid cycle |
| ACAA2 | 3-ketoacyl-CoA thiolase | THIM_HUMAN | apoptosis and mitochondrial damage |
| CKM | Creatine kinase M-type | KCRM_HUMAN | energy transduction |
| CKMT2 | Creatine kinase S-type | KCRS_HUMAN | energy transduction |
| ACOT1 | Acyl-coenzyme A thioesterase 1 | ACOT1_HUMAN | hydrolysis of acyl-CoAs |
| SLC25A4 | ADP/ATP translocase 1 | ADT1_HUMAN | exchange of ADP and ATP |
| ACAT1 | Acetyl-CoA acetyltransferase | THIL_HUMAN | ketone body metabolism |
| HADH | Hydroxyacyl-coenzyme A dehydrogenase | HCDH_HUMAN | Fatty acid metabolism |
| FH | Fumarate hydratase | FUMH_HUMAN | fumarate metabolic process |
| MAOB | Amine oxidase | AOFB_HUMAN | metabolism of neuroactive and vasoactive amines |
| MT-CO2 | Cytochrome c oxidase subunit 2 | COX2_HUMAN | electron transport |
| UQCRC1 | Cytochrome b-c1 complex subunit 1 | QCR1_HUMAN | electron transport, ATP |
| UQCRC2 | Cytochrome b-c1 complex subunit 2 | QCR2_HUMAN | electron transport, ATP |
| SAMM50 | Sorting and assembly machinery component 50 homolog | SAM50_HUMAN | mitochondrial function |
| NDUFS1 | NADH-ubiquinone oxidoreductase | NDUS1_HUMAN | NADH oxidation |
| PDHX | Pyruvate dehydrogenase protein X component | ODPX_HUMAN | pyruvate metabolic, acyltransferase activity |
| TUFM | Elongation factor Tu | EFTU_HUMAN | Elongation factor |
| ATP5A1 | ATP synthase subunit alpha | ATPA_HUMAN | oxidative phosphorylation |
| ATP5B | ATP synthase subunit beta | ATPB_HUMAN | oxidative phosphorylation |
| ATP5C1 | ATP synthase subunit gamma | ATPG_HUMAN | oxidative phosphorylation |
| DECR1 | 2,4-dienoyl-CoA reductase | DECR_HUMAN | Oxidoreductase |
| ALDH2 | Aldehyde dehydrogenase | ALDH2_HUMAN | Oxidoreductase |
| DLD | Dihydrolipoyl dehydrogenase | DLDH_HUMAN | Oxidoreductase |
| DLAT | Dihydrolipoyllysine-residue acetyltransferase- pyruvate dehydrogenase complex | ODP2_HUMAN | Oxidoreductase |
| DLST | Dihydrolipoyllysine-residue succinyltransferase-2-oxoglutarate dehydrogenase complex | ODO2_HUMAN | Oxidoreductase |
| ECSIT | Evolutionarily conserved signaling intermediate in Toll pathway | ECSIT_HUMAN | Oxidoreductase |
| HSDL2 | Hydroxysteroid dehydrogenase-like protein 2 | HSDL2_HUMAN | Oxidoreductase |
| IDH2 | Isocitrate dehydrogenase | IDHP_HUMAN | Oxidoreductase |
| LDHA | L-lactate dehydrogenase A chain | LDHA_HUMAN | Oxidoreductase |
| MDH2 | Malate dehydrogenase | MDHM_HUMAN | Oxidoreductase |
| ACADM | Medium-chain specific acyl-CoA dehydrogenase | ACADM_HUMAN | Oxidoreductase |
| LDHD | Probable D-lactate dehydrogenase | LDHD_HUMAN | Oxidoreductase |
| AIFM1 | Apoptosis-inducing factor 1 | AIFM1_HUMAN | oxidoreductase, apoptosis |
| VDAC1 | Voltage-dependent anion-selective channel protein 1 | VDAC1_HUMAN | Oxidoreductase, apoptosis |
| ACADVL | Very long-chain specific acyl-CoA dehydrogenase | ACADV_HUMAN | Oxidoreductase, cardiomyopathy |
| PRKAR1A | cAMP-dependent protein kinase type I-alpha regulatory subunit | KAP0_HUMAN | Post-translational modification |
| PRKAR2A | cAMP-dependent protein kinase type II-alpha regulatory subunit | KAP2_HUMAN | Post-translational modification |
| LDB3 | LIM domain-binding protein 3 | LDB3_HUMAN | PKC-mediated signaling |
| PGK1 | Phosphoglycerate kinase 1 | PGK1_HUMAN | Kinase, transferase |
| PKM2 | Pyruvate kinase isozymes M1/M2 | KPYM_HUMAN | pyruvate, apoptosis, calcium signaling |
| PTRF | Polymerase I and transcript release factor | PTRF_HUMAN | caveolae formation, cell membrane repair |
| APOD | Apolipoprotein D | APOD_HUMAN | ligands transport |
| PACSIN3 | Protein kinase C and casein kinase substrate in neurons protein 3 | PACN3_HUMAN | vesicle formation and transport |
| SRL | Sarcalumenin | SRCA_HUMAN | regulation of calcium transport |
| FGB | Fibrinogen beta chain | FIBB_HUMAN | platelet aggregation |
| FGG | Fibrinogen gamma chain | FIBG_HUMAN | platelet aggregation |
| ANXA2 | Annexin A2 | ANXA2_HUMAN | stress response, exocytosis |
| ANXA6 | Annexin A6 | ANXA6_HUMAN | regulate intracellular Ca2+ release |
| ANXA11 | Annexin A11 | ANX11_HUMAN | cytokinesis |
| ANXA7 | Annexin A7 | ANXA7_HUMAN | membrane fusion, exocytosis |
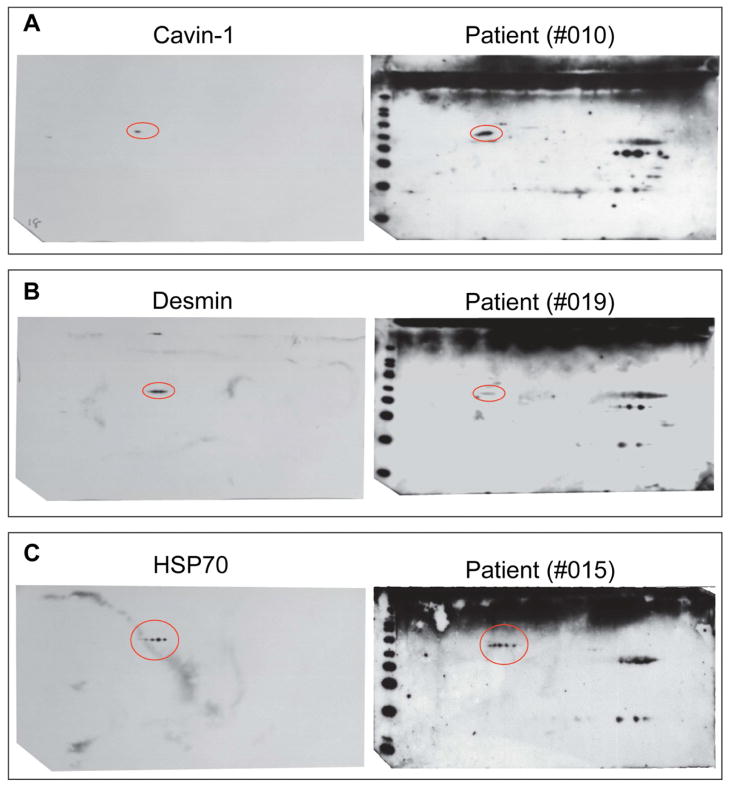
To validate the results from nanoLC-MS/MS, specific antibodies against desmin, cavin-1 and HSP70 were used against 2DE blots of cardiac lipid raft/caveolae fractions (Figure 3). The results are in agreement with those from proteomic analysis, confirming that the protein identification results were correct.
Figure 3. 2DE gel immunoblot of human heart lipid raft/caveolae fractions against anti-cavin-1, -desmin and -HSP70 antibodies.
Immunoblot analyses of 2DE of lipid raft/caveolae fractions against commercially available antibodies (left panel) and against patient serum IgGs (right panel). The results show agreement for anti-cavin-1 (A), anti-desmin (B), and anti-HSP70 (C).
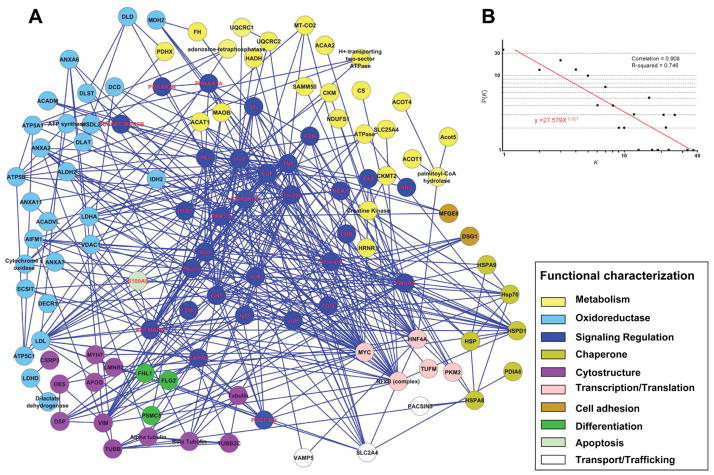
To help understand the physiological and pathophysiological relevance of the immunoreactive lipid raft proteins, we performed network and canonical pathway analysis on the data from Table 3 using the IPA. Seventy-two identified proteins were integrated into a composite neighborhood comprised of 113 nodes linked by 389 interactions or edges (Figure 4). Network topology was assessed as an undirected network and the results demonstrated that the network topology was not random. A nonstochastic property was confirmed by examination of the interrelationship between node degree (k) and degree distribution (P[k]) (Figure 4). The organized assemblage followed a power law distribution falling within the predicted confidence range of biological networks18.
Figure 4. Network analysis of the proteins identified by MS from patient-specific protein spots.
(A) Seventy-two unique proteins identified by MS submitted to Ingenuity Pathways Analysis as focus nodes were integrated into a composite neighborhood comprised of 113 protein interaction network linked by 389 interactions or edges. Nodes are listed by Swiss-Prot gene designations and the color corresponds to ontological function. (B) Network degree distribution, (P[k]) versus degree (k), followed a power law distribution indicating nonstochastic scale-free network architecture a typical biological networks.
Canonical pathway analysis demonstrated that many of the proteins targeted by patient IgGs are interrelated in pathways based on the IPA library (Table 4). Many pathways are involved including those that regulate caveolae-mediated signaling, oxidative phosphorylation, fatty acid metabolism, protein ubiquitination, and cardiac β-adrenergic signaling (Table 4).
Table 4.
Canonical pathways involved in proteins targeted by autoantibodies
| Ingenuity Canonical Pathways | Molecules (Symbols) |
|---|---|
| Mitochondrial Dysfunction | ATP5C1, NDUFS1, ATP5B, UQCRC2, MT-CO2, UQCRC1, AIFM1 |
| Oxidative Phosphorylation | ATP5C1, NDUFS1, ATP5B, UQCRC2, MT-CO2, UQCRC1, ATP5F1 |
| Pyruvate Metabolism | PKM2, LDHD, DLAT, PDHX, ACAT1 |
| Valine, Leucine and Isoleucine Degradation | ACADVL, ACAT1, ACAA2, HADH |
| Citrate Cycle | CS, DLST, IDH2 |
| Purine Metabolism | PKM2, ATP5C1, ATP5B, MYH7, HSPD1, ATP5F1 |
| Fatty Acid Metabolism | ACADVL, ACAT1, ACAA2, HADH |
| Lysine Degradation | DLST, ACAT1, HADH |
| Extrinsic Prothrombin Activation Pathway | FGB, FGG |
| Fatty Acid Elongation in Mitochondria | ACAA2, HADH |
| Glycolysis/Gluconeogenesis | PKM2, DLAT, PDHX |
| Intrinsic Prothrombin Activation Pathway | FGB, FGG |
| Alanine and Aspartate Metabolism | DLAT, PDHX |
| Acute Phase Response Signaling | ECSIT, FGB, FGG |
| Butanoate Metabolism | ACAT1, HADH |
| Propanoate Metabolism | ACADVL, ACAT1 |
| IL-1 Signaling | ECSIT, PRKAR2A |
| Synthesis and Degradation of Ketone Bodies | ACAT1 |
| Cardiomyocyte Differentiation via BMP Receptors | MYH7 |
| AMPK Signaling | CKM, PRKAR2A |
| Tryptophan Metabolism | ACAT1, HADH |
| Tight Junction Signaling | PRKAR2A, MYH7 |
| Calcium Signaling | PRKAR2A, MYH7 |
| ILK Signaling | MYH7, DSP |
| Huntington’s Disease Signaling | ATP5B, HSPA9 |
| Protein Ubiquitination Pathway | HSPA9, HSPD1 |
| Glucocorticoid Receptor Signaling | HSPA9, FGG |
| Caveolar-mediated Signaling | PTRF |
| Nitric Oxide Signaling in the Cardiovascular System | PRKAR2A |
| Arginine and Proline Metabolism | CKM |
| Melanocyte Development and Pigmentation Signaling | PRKAR2A |
| α-Adrenergic Signaling | PRKAR2A |
| Cardiac β-adrenergic Signaling | PRKAR2A |
| Insulin Receptor Signaling | PRKAR2A |
| Role of NFAT in Cardiac Hypertrophy | PRKAR2A |
| cAMP-mediated signaling | PRKAR2A |
| Cardiac Hypertrophy Signaling | PRKAR2A |
DISCUSSION
In this pilot study, we have made the following observations. First, autoantibodies against cardiac lipid raft microdomain proteins are present in patients with POTS. Second, multiple lipid raft/caveolae proteins are potential targets of the autoantibodies in POTS. Third, the presence of autoantibodies may affect the regulation of multiple cellular processes in patients with POTS.
Proteomic technologies have provided ideal tools in the search of antigens and antibodies in autoimmune diseases. The approach combining 2DE gel immunoblotting and MS has been effective in the identification of new antigens/antibodies in autoimmune and other diseases19. While the analytical power of MS-based technologies has become greatly improved in recent years, sample preparation remains a limitation for optimal sensitivity of proteomic analysis. In extremely complex protein preparations, the presence of high abundance proteins reduces the ability to detect low abundance proteins. To reduce sample complexity and augment low abundance proteins, we prepared lipid raft/caveolae fractions which are abundant in cardiomyocytes. Caveolae are specialized lipid rafts enriched with cholesterol, caveolins, and signaling molecules and they provide platforms for integrating specific cellular signal transduction processes13. Interaction between autoantibodies and lipid raft/caveolae proteins may trigger alterations in signaling pathways to produce the cardiovascular abnormalities seen in patients with POTS.
Our results showed that proteins regulating multiple cellular processes, such as signaling, metabolism, oxidoreductases, chaperones, catabolic, cytostructure and transcription, could be the targets of autoantibodies in patients with POTS. Many of the proteins have previously been implicated in cardiac dysfunction or cardiac disease, such as S100A820, HSP7021, desmin22, and desmoplakin23. However, involvement of these proteins in the pathogenesis of POTS is unknown. Further studies are needed to substantiate the role of these proteins in POTS. Although it is difficult to predict the prevalence of autoantibodies in the POTS population from the limited number of patients studied, our results indicate that a multitude of cardiac lipid raft-associated proteins could be targeted by autoimmunoreactive IgGs in these patients (Table 2). As shown in Table 2, the putative autoantigen profile of each individual patient was variable. Analyses of large population-based data sets are needed to provide further information on these issues.
Validation of the nanoLC-MS/MS results using affinity purified antibodies of desmin, cavin-1 and HSP70 was performed (Figure 3). The results are consistent with those from MS analyses indicating that this technology is a valuable approach in identifying potential autoimmune targets. Desmin, HSP70 and cavin-1 have been shown to be associated with caveolae24–26 and cardiovascular diseases22. Desmin is a critical component of cardiac myocyte architecture, structure and signaling. Mice without desmin have impaired mitochondrial function with abnormalities in cardiac and skeletal muscles27. These animals are weak and easily fatigable, and the lack of desmin renders myofibers more susceptible to damage during contraction28. Increased plasma levels of anti-HSP70 were found to be a cardiac risk factor associated with a significant incidence of ECG abnormalities characteristic of chronic myocardial ischemia, conduction abnormality, or heart displacement29. Previous study also suggested that higher serum HSP70 antibody level may be a marker for subsequent development of postoperative atrial fibrillation30. Our findings suggest that the presence of autoantibodies against HSP70 could be a risk factor for the development of cardiac dysfunction in POTS.
Interestingly, a newly described protein, cavin-1, also known as polymerase I and transcript release factor (PTRF), is one of the targets of the autoantibodies in POTS. Cavin-1 is required for the formation of caveolae in all mammalian and plays a critical role in regulating caveolae function in endothelial cells26. Cavin-1 also facilitates repair of damaged muscle cells31. Cavin-1 mutations in human caused a secondary deficiency of caveolins resulting in muscular dystrophy, as well as arrhythmias and atrial fibrillation32. Therefore, further studies are warranted to investigate the effect of immune reactions against cavin-1.
Cellular proteins are connected through extensive networks of interacting pathways. Assembly of biologically relevant interactomes may help better understand the effects produced by the binding of autoimmunoreactive IgGs to lipid raft/caveolae proteins. The unbiased gene ontology-based network interrogation further indicates that autoimmune reactions to cardiac proteins are biologically relevant with nonrandom effects on cardiac functions (Figure 4). Some of the targeted proteins are important signaling proteins and they encompass many signaling pathways (Table 4). The potential involvement of the β-adrenergic signaling pathway in POTS is particularly intriguing as a putative mechanism that underlies the clinical manifestation in POTS is an imbalance between sympathetic/parasympathetic regulations. Hence, further studies on the functional relevance of autoantibodies and their potential cardiac membrane protein targets may provide important insights into the pathogenesis of POTS. We hope that this study may serve as an impetus for further investigations in this area.
Acknowledgments
The authors thank Dr. Kent Arrell (Mayo Clinic, Rochester) for his assistance on network analysis, and Mayo Proteomics Core for protein identification.
This work was supported by grants from the National Institutes of Health HL74180 and HL080118 (to H.L.), and the Mayo Clinic Foundation (to W.K.S).
Abbreviations
- POTS
postural orthostatic tachycardia syndrome
- IPA
Ingenuity Pathway Analysis
- 2DE
2-dimensional gel electrophoresis
- MS
mass spectrometry
Footnotes
All authors have declared no potential conflict of interest, and have read the policy on disclosure of potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847–855. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- 2.Wang XL, Chai Q, Charlesworth MC, Figueroa JJ, Low P, Shen WK, Lee HC. Autoimmunoreactive iggs from patients with postural orthostatic tachycardia syndrome. Proteomics Clin Appl. 2012;6:615–625. doi: 10.1002/prca.201200049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huber LA, Pfaller K, Vietor I. Organelle proteomics: Implications for subcellular fractionation in proteomics. Circ Res. 2003;92:962–968. doi: 10.1161/01.RES.0000071748.48338.25. [DOI] [PubMed] [Google Scholar]
- 4.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 5.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nature medicine. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. The Journal of clinical investigation. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy MA, Li SL, Sahar S, Kim YS, Xu ZG, Lanting L, Natarajan R. Key role of src kinase in s100b-induced activation of the receptor for advanced glycation end products in vascular smooth muscle cells. The Journal of biological chemistry. 2006;281:13685–13693. doi: 10.1074/jbc.M511425200. [DOI] [PubMed] [Google Scholar]
- 8.Frank PG, Pavlides S, Cheung MW, Daumer K, Lisanti MP. Role of caveolin-1 in the regulation of lipoprotein metabolism. American journal of physiology. 2008;295:C242–248. doi: 10.1152/ajpcell.00185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank PG, Cheung MW, Pavlides S, Llaverias G, Park DS, Lisanti MP. Caveolin-1 and regulation of cellular cholesterol homeostasis. Am J Physiol Heart Circ Physiol. 2006;291:H677–686. doi: 10.1152/ajpheart.01092.2005. [DOI] [PubMed] [Google Scholar]
- 10.Davies LM, Purves GI, Barrett-Jolley R, Dart C. Interaction with caveolin-1 modulates vascular atp-sensitive potassium (katp) channel activity. J Physiol. 2010;588:3255–3266. doi: 10.1113/jphysiol.2010.194779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang XL, Ye D, Peterson TE, Cao S, Shah VH, Katusic ZS, Sieck GC, Lee HC. Caveolae targeting and regulation of large conductance ca(2+)-activated k+ channels in vascular endothelial cells. J Biol Chem. 2005;280:11656–11664. doi: 10.1074/jbc.M410987200. [DOI] [PubMed] [Google Scholar]
- 12.Davies LM, Purves GI, Barrett-Jolley R, Dart C. Interaction with caveolin-1 modulates vascular atp-sensitive potassium (katp) channel activity. J Physiol. 2010;588:3255–3266. doi: 10.1113/jphysiol.2010.194779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razani B, Woodman SE, Lisanti MP. Caveolae: From cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 15.Low PA, Sandroni P, Joyner M, Shen WK. Postural tachycardia syndrome (pots) J Cardiovasc Electrophysiol. 2009;20:352–358. doi: 10.1111/j.1540-8167.2008.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu T, Zhang DM, Wang XL, He T, Wang RX, Chai Q, Katusic ZS, Lee HC. Regulation of coronary arterial bk channels by caveolae-mediated angiotensin ii signaling in diabetes mellitus. Circ Res. 2010;106:1164–1173. doi: 10.1161/CIRCRESAHA.109.209767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barabasi AL, Oltvai ZN. Network biology: Understanding the cell’s functional organization. Nat Rev Genet. 2004;5:101–113. doi: 10.1038/nrg1272. [DOI] [PubMed] [Google Scholar]
- 19.Katsumata Y, Kawaguchi Y, Baba S, Hattori S, Tahara K, Ito K, Iwasaki T, Yamaguchi N, Oyama M, Kozuka-Hata H, Hattori H, Nagata K, Yamanaka H, Hara M. Identification of three new autoantibodies associated with systemic lupus erythematosus using two proteomic approaches. Mol Cell Proteomics. 2011;10:M110 005330. doi: 10.1074/mcp.M110.005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyd JH, Kan B, Roberts H, Wang Y, Walley KR. S100a8 and s100a9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ Res. 2008;102:1239–1246. doi: 10.1161/CIRCRESAHA.107.167544. [DOI] [PubMed] [Google Scholar]
- 21.Genth-Zotz S, Bolger AP, Kalra PR, von Haehling S, Doehner W, Coats AJ, Volk HD, Anker SD. Heat shock protein 70 in patients with chronic heart failure: Relation to disease severity and survival. Int J Cardiol. 2004;96:397–401. doi: 10.1016/j.ijcard.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 22.McLendon PM, Robbins J. Desmin-related cardiomyopathy: An unfolding story. Am J Physiol Heart Circ Physiol. 2011;301:H1220–228. doi: 10.1152/ajpheart.00601.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abreu-Velez AM, Howard MS, Jiao Z, Gao W, Yi H, Grossniklaus HE, Duque-Ramirez M, Dudley SC., Jr Cardiac autoantibodies from patients affected by a new variant of endemic pemphigus foliaceus in colombia, south america. J Clin Immunol. 2011;31:985–997. doi: 10.1007/s10875-011-9574-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mermelstein CS, Martins ER, Portilho DM, Costa ML. Association between the muscle-specific proteins desmin and caveolin-3 in muscle cells. Cell & Tissue Research. 2007;327:343–351. doi: 10.1007/s00441-006-0296-z. [DOI] [PubMed] [Google Scholar]
- 25.Black AT, Hayden PJ, Casillas RP, Heck DE, Gerecke DR, Sinko PJ, Laskin DL, Laskin JD. Regulation of hsp27 and hsp70 expression in human and mouse skin construct models by caveolae following exposure to the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide. Toxicol Appl Pharmacol. 2011;253:112–120. doi: 10.1016/j.taap.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, Ravid K, Pilch PF. Deletion of cavin/ptrf causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 2008;8:310–317. doi: 10.1016/j.cmet.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linden M, Li Z, Paulin D, Gotow T, Leterrier JF. Effects of desmin gene knockout on mice heart mitochondria. J Bioenerg Biomembr. 2001;33:333–341. doi: 10.1023/a:1010611408007. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Mericskay M, Agbulut O, Butler-Browne G, Carlsson L, Thornell LE, Babinet C, Paulin D. Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J Cell Biol. 1997;139:129–144. doi: 10.1083/jcb.139.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan J, Yang M, Yao H, Zheng J, Yang Q, Chen S, Wei Q, Tanguay RM, Wu T. Plasma antibodies to heat shock protein 60 and heat shock protein 70 are associated with increased risk of electrocardiograph abnormalities in automobile workers exposed to noise. Cell Stress Chaperones. 2005;10:126–135. doi: 10.1379/CSC-95R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oc M, Ucar HI, Pinar A, Akbulut B, Oc B, Akyon Y, Kanbak M, Dogan R. Heat shock protein70: A new marker for subsequent atrial fibrillation development? Artif Organs. 2008;32:846–850. doi: 10.1111/j.1525-1594.2008.00640.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhu H, Lin P, De G, Choi KH, Takeshima H, Weisleder N, Ma J. Polymerase transcriptase release factor (ptrf) anchors mg53 protein to cell injury site for initiation of membrane repair. J Biol Chem. 2011;286:12820–12824. doi: 10.1074/jbc.C111.221440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi YK, Matsuda C, Ogawa M, Goto K, Tominaga K, Mitsuhashi S, Park YE, Nonaka I, Hino-Fukuyo N, Haginoya K, Sugano H, Nishino I. Human ptrf mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest. 2009;119:2623–2633. doi: 10.1172/JCI38660. [DOI] [PMC free article] [PubMed] [Google Scholar]