Abstract
Oral Δ9-tetrahydrocannabinol (Δ9-THC) has been evaluated as a medication for cannabis dependence, but repeated administration of acute oral doses up to 40 mg has not been effective at reducing drug-taking behavior. Larger doses might be necessary to affect cannabis use. The purpose of the present study was therefore to determine the physiological and behavioral effects of oral Δ9-THC at acute doses higher than those tested previously. The pharmacokinetic and pharmacodynamic profile of oral Δ9-THC, administered in ascending order in 15 mg increments across separate sessions, up to a maximum of 90 mg, was determined in seven cannabis users. Five subjects received all doses and two experienced untoward side effects at lower doses. Δ9-THC produced a constellation of effects consistent with previous clinical studies. Low cannabinoid concentrations were associated with significant effects on drug- sensitive measures, although progressively greater levels did not lead to proportionately larger drug effects. Considerable variability in Cmax and tmax was observed. Doses of oral Δ9-THC larger than those tested previously can be administered to individuals with a history of cannabis use, although given the pharmacokinetic variability of oral Δ9-THC and individual differences in sensitivity, individualized dose adjustment is needed to avoid side effects and maximize therapeutic response.
Keywords: marijuana, cannabis, subjective effects, repeated acquisition task, digit-symbol-substitution task
Introduction
Oral Δ9-tetrahydrocannabinol (i.e., Δ9-THC, dronabinol), a primary active constituent of cannabis (Cannabis sativa, Cannabis indica), is approved in the US and other countries to control nausea/vomiting and stimulate appetite, and has been considered for various other therapeutic indications.1 Non-medical use of oral preparations of cannabis is also common, albeit to a lesser degree than via the smoked route. Though differing in time course, oral Δ9-THC and smoked cannabis produce comparable behavioral and physiological responses.2,3 Consequently, oral Δ9-THC is also used in clinical research as a proxy to study the pharmacodynamic effects of cannabis and has been tested for initial efficacy in human laboratory studies as a substitution treatment for cannabis-use disorders.4
Because of the likely tolerance to oral Δ9-THC stemming from prior cannabis experience, supratherapeutic doses have been used in clinical research with cannabis users. For reference, the recommended initial acute doses of oral Δ9-THC for appetite stimulation in the treatment of AIDS-related anorexia and chemotherapy-induced emesis is between 2.5–10 mg.5 In contrast, recent research with cannabis users has tested acute doses up to 40 mg.6–8 and some early chronic dosing studies included doses of 60 mg, although limited information is available about the acute pharmacological response to this higher dose.9 Studies that have characterized the acute effects of oral Δ9-THC have demonstrated that the abovementioned doses produced increased heart rate, a moderate subjective response, and somewhat less consistently, performance impairment.10 Further, human laboratory research evaluating the potential of oral Δ9-THC as a pharmacotherapy for cannabis-use disorders in non-treatment-seeking subjects suggested that 20–40 mg of oral Δ9-THC administered 3–4x/day (i.e., up to 80–120 mg/day) suppressed withdrawal signs and symptoms and modestly attenuated some of the subject-rated effects of smoked cannabis.8,11–13 However, these doses failed to block the subjective response to cannabis in other studies, and have not been effective at reducing cannabis self-administration under controlled laboratory conditions.8,13,14
Consistent with the laboratory findings, in a recently published clinical trial testing therapeutic efficacy in 156 treatment-seeking, cannabis-dependent subjects, 20 mg oral Δ9-THC administered twice daily improved study retention and reduced withdrawal symptoms relative to placebo, but did not increase the proportion of subjects abstinent for a 2-week period.15 The authors of that report suggested that higher doses might be necessary to suppress continued cannabis use. Increasing daily intake during outpatient treatment could be accomplished by decreasing the dosing interval, as done in controlled laboratory studies (i.e., 3–4x/day), but the use of fewer, larger acute doses rather than numerous smaller doses each day would be predicted to improve medication compliance.16 Concerns over cardiovascular side effects (i.e., tachycardia and orthostatic hypotension) and excessive intoxication, including psychomotor impairment, have previously deterred the evaluation of larger doses of oral Δ9-THC. However, given the inability of maintenance on these previously characterized doses to consistently attenuate the subjective and reinforcing effects of smoked cannabis, research on further doses is warranted. The purpose of the present study was therefore to determine the physiological and behavioral effects of oral Δ9-THC at acute doses higher than those tested previously in humans to inform dose choice in future clinical studies.
Methods
Subjects
Adult men and women reporting regular cannabis use were recruited from the local community. Potential subjects completed demographic, drug-use and medical history questionnaires, as well as medical screens. A cannabis use history questionnaire was used to determine current patterns of use, years of use, and total lifetime uses. With respect to the last of these measures, a chart that provided examples for the number of times cannabis was used in the past year based on daily and weekly use (e.g., using 3 times per day, 5 days per week would result in 720 times used in the past year), as well as a calculator, was provided to facilitate more accurate reporting. Individuals with current or past histories of Axis I disorder according to DSM-IV criteria,17 including substance dependence other than tobacco, were excluded from participating. The Institutional Review Board of the University of Kentucky Medical Center approved the study and the informed consent document.
Seven subjects (4 white males, 3 white females) completed the experiment. Three additional subjects were enrolled and completed at least one session in which a drug dose was administered, but discontinued their participation and were unable to be contacted to determine the reason. Completers ranged in age from 18 to 25 years (median = 20 years), in education from 12 to 16 years (median = 12), and in weight from 52 to 101 kg (median = 76 kg). All subjects reported current cannabis use ranging from 2 to 7 days/week (mean = 4.8). Lifetime cannabis use ranged from 100 to 7500 times (mean = 2442.9 uses) across 2 to 10 years (mean = 4.6). See Table 1 for individual subject cannabis use characteristics. At the time of screening, subjects reported consuming 1 to 18 standard alcohol-containing beverages per week (mean = 8.0). One subject reported daily tobacco use (3 cigarettes/day), four subjects reported weekly use of tobacco (cigarettes in all subjects, smokeless tobacco in one subject; 2–6 uses per week); another subject reported using one cigarette per month. Other lifetime non-medical drug use included benzodiazepines (3 subjects, one reporting use in the month prior to screening), hallucinogens (three subjects, none in the month prior to screening), opioids (two subjects, none in the month prior to screening) and stimulants (cocaine: two subjects, methylenedioxymethamphetamine: one subject, and mixed-salt amphetamines: six subjects, with amphetamines being used by two subjects in the month prior to screening). At the time of screening (and prior to each experimental session) subjects provided a urine sample that was screened via qualitative tests of drug use (Integrated E-Z Split Cut, Acon Laboratories, San Diego, CA) and pregnancy (hCG Assay, Rapid Detect, Inc., Poteau, OK). All subjects provided a urine sample positive for cannabinoids but negative for other substances prior to study initiation.
Table 1.
| Subject | Sex | Cannabis Use (years) | Lifetime Cannabis Uses | Cannabis Use Frequency (x/week) | Max Δ9-THC Dose (mg) |
|---|---|---|---|---|---|
|
| |||||
| S0456 | M | 3 | 300 | 2 | 90 |
| S0473 | F | 2.5 | 1900 | 7 | 60 |
| S0529 | F | 2 | 300 | 4 | 90 |
| S0539 | M | 4 | 100 | 4 | 30 |
| S0549 | F | 5 | 750 | 5 | 90 |
| S0554 | M | 10 | 7500 | 4.5 | 90 |
| S0561 | M | 6 | 6250 | 7 | 90 |
General Procedures
Experimental sessions were conducted at a fixed time, Monday through Thursday. Subjects initially completed one drug-free practice session on an outpatient basis at the University of Kentucky Clinical Services Core (CSC) to become familiarized with session procedures and behavioral tasks. Subjects were then admitted as 23-h inpatients on separate days for up to seven experimental sessions. Experimental activities lasted approximately 7 h; subjects then remained in the hospital overnight and were released the following morning. Sessions were separated by at least 48 h; the median number of days between sessions across subjects was 7.
Subjects were informed that they would receive placebo and Δ9-THC, but were blind to the dose and order of administration. They were asked to abstain from illicit drugs other than cannabis for the duration of the experiment, and any drug use on the day of experimental sessions. They were also asked to avoid any over-the-counter medication without investigator approval, with the exception of non-steroidal anti-inflammatory analgesics. Subjects were asked to refrain from food intake for 4 h prior to each experimental session; at intake, prior to dosing, they consumed a low-fat snack, and 3 h after capsule administration they were provided with a hospital meal consisting of items they pre-selected from the available menu. Subjects were also asked to abstain from caffeine for 4 h prior to the session, and alcohol for 12 h prior to and following each experimental session. The subject who reported daily tobacco cigarette use was also asked to abstain from smoking for four hours prior to the session, but was allowed to smoke a single tobacco cigarette upon arrival to the laboratory to avoid testing under conditions of nicotine withdrawal. This subject was not allowed to smoke again until the session had ended.
Urine samples collected at the beginning of each session were negative for substances other than cannabis metabolites (i.e., 11-nor-9-carboxy-Δ9-THC) and hCG throughout the study, with one exception. The qualitative urine-drug screen conducted at the beginning of one of the experimental sessions indicated recent amphetamine use in a single subject; a quantitative confirmation test was not conducted. That session was rescheduled at a later date.
At session intake, subjects also completed a modified version of the U.S. Department of Transportation Drug Evaluation and Classification Screening18 (walk and turn, timed one-leg balance or Romberg balance, time interval reproduction and the finger-to-nose tests) and were observed by the research staff for signs of cannabis intoxication (e.g., bloodshot, glassy eyes); no cannabis intoxication was detected during intake throughout the study. Subjects were reassessed the following morning for possible intoxication and/or impairment using these procedures prior to release. In addition, subjects were required to report no further drug effects. If necessary, subjects were retained beyond the scheduled session time until residual drug effects dissipated.
Subjects received placebo and six doses of oral Δ9-THC, in 15 mg increments, up to a maximum of 90 mg in ascending order. This dose run-up design, based on published methods19 was used to test acute oral doses of Δ9-THC higher than those tested previously, while avoiding adverse events that might result from individual differences in sensitivity to Δ9-THC. Criteria were established to exclude subjects from further participation, which included: 1) severe functional impairment, defined by an inability to complete the experimental tasks; 2) clinically significant cardiovascular or psychiatric symptoms, as determined by the on call psychiatrist; and 3) withdrawal of consent for further participation by the subject due to undesirable drug effects. Five subjects received all possible doses; two subjects experienced nausea and vomited following submaximal doses and were unable to complete the session activities, excluding them from testing with higher doses (see Table 1). Other than the gastrointestinal issues, no other side effects beyond those captured by the pharmacodynamic assessments were noted.
Measures
Data were collected in fixed order, immediately prior to drug administration, and 1, 2, 3, 4, 5 and 6 h after Δ9-THC administration, with the exceptions noted below. Behavioral data were collected on an Apple Macintosh computer. Subjects remained in a semi-reclined position in a standard hospital bed for the duration of the data collection.
Pharmacokinetics
In an effort to more fully characterize the pharmacological profile of high dose oral Δ9-THC, Δ9-THC and 11-OH-Δ9-THC levels were measured prior to capsule administration, hourly for 6 hours, and then 12 h after dosing. During the intake procedures at the beginning of each session, a heparin-locked catheter was placed in the brachial vein of subjects’ non-dominant arm. Blood was collected through the catheter at the scheduled times and placed into a chilled vacutainer tube containing dipotassium ethylenediaminetetraacetic acid (EDTA). Immediately following sample collection, the catheter was flushed with 3 mL heparin. Blood samples were placed into a refrigerated centrifuge and spun at 3000 rpm for 15 min. Plasma was then transferred to siliconized tubes for storage at −80 °C freezer for subsequent analysis.
Plasma concentrations of Δ9-THC and 11-OH-Δ9-THC were determined using liquid chromatography/mass spectrometry (LC/MS) using a modified previously published method.20
Extraction
Fifty microliters of the plasma sample was placed into a siliconized microcentrifuge tube and extracted with 500 μL of acetonitrile: ethyl acetate (1:1, v/v). The mixture was vortexed for 1 min and centrifuged at 10,000 × g for 20 min. The supernatant was pipetted into a silanized 3 mL glass test tube and evaporated at 37°C under nitrogen. The residue was reconstituted with 100 μL of acetonitrile, vortexed for 1 min and sonicated for 5 min. The sample was transferred into autosampler vials containing silanized low volume inserts and 25 μL was injected onto the HPLC column.
Analysis
Chromatography was performed on a Waters Symmetry® C18 (2.1 × 150 mm, 5 μm) column at 35°C with a mobile phase consisting of ammonium acetate (2 mM):acetonitrile (20:80, v/v) at a flow-rate of 0.25 mL/min. A Waters Symmetry® C18 (2.1 × 10 mm, 3.5 μm) guard column was used. The LC/MS system consisted of a Waters Alliance 2695 HPLC Separations Module and a Waters Micromass® ZQ™ detector (Waters, Milford, MA) using electrospray ionization (ESI) for ion production. Selected ion monitoring (SIM) was performed in negative mode for ions m/z 313 [THC-H]-(dwell time 30 s) and m/z 329 [THC-OH-H]-(dwell time 30 s). Capillary voltage was 3.5 kV and cone voltage was 30V. The source block and desolvation temperatures were 120 and 250°C, respectively. Nitrogen was used as a nebulization and drying gas at flow rates of 50 and 450 L/h, respectively. The retention times for Δ9-THC and 11-OH-Δ9-THC were 7.20 ± 0.10 and 3.25 ± 0.05 min, respectively. Calibration graphs were constructed using a linear regression of the drug peak-area of the product ions versus nominal drug concentrations.
Pharmacodynamics
Physiological Indices
Heart Rate and Blood Pressure
Heart rate and blood pressure were recorded prior to, and every 15 min after, capsule administration using an automated monitor (DINAMAP, Johnson and Johnson, Alexandria, TX).
Temperature
An infrared thermographic scanner (Derma-Temp, Exergen Corporation, Watertown, MA) was used to measure skin temperature on the tip of the index finger. An electronic thermometer was used to measure oral temperature.
Performance Tasks
These tasks were chosen because prior research has found them to be sensitive to the impairing effects of oral Δ9-THC and smoked cannabis.21–26 Subjects did not receive compensation based on task performance.
Digit-Symbol-Substitution Task (DSST)
A modified version of the computerized DSST was used.27 Briefly, subjects used a numeric keypad to enter the geometric pattern associated with one of nine patterns identified on a given trial for 90 s. The dependent measures were the number of patterns the subject entered correctly (i.e., trials correct; accuracy) and the total number of patterns entered (i.e., trials completed; response rate).
Repeated Acquisition of Response Sequences Task (RA task)
During the initial acquisition component, subjects pressed 4 keys (1, 3, 7 and 9) on a numeric keypad to learn a new, randomly-determined 10-response sequence (a “chain”) for 180 s. When a correct key in the sequence was pressed, a “position” counter on the screen increased by 1. When the tenth and final key in the sequence was pressed, a “points” counter increased by one, and the position counter reset. A 60-s performance component of this task, in which the 10-response sequence remained the same across trials, followed the acquisition component. The primary dependent measures for this task were the number of chains completed (i.e., accuracy) and the total number of responses emitted (i.e., response rate).
Time Reproduction Task
Two time periods, 30 and 60 s were presented. Subjects responded to start a timer, and held down the response key until they believed that the interval had elapsed.
Subject-Rated Questionnaires
Visual Analog Scale (VAS) Subject-Rated Drug-Effect Questionnaire. Subjects rated 20 items (I feel: any drug effect, anxious, a bad drug effect, discomfort, dizzy or light headed, forgetful, a good drug effect, high, hungry, nauseated, tired or sedated, shaky or jittery, stoned, suspicious, thirsty; I am seeing or hearing unusual things; I am confused or having difficulty concentrating; I like the drug effect; I would take this drug again; I would pay for this drug) presented individually on the computer by marking a 100-unit line anchored on the extremes by “Not At All” and “Extremely”.
Multiple-Choice Procedure
This task provided an assessment of the monetary value of each dose condition.28 Subjects made a series of nine discrete choices between the drug dose received during that session and ascending amounts of money that doubled across the choices ($0.25, 0.50, 1.00, 2.00, 4.00, 8.00, 16.00, 32.00 and 64.00). The dependent measure on the Multiple-Choice Procedure was the maximum dollar value at which subjects chose drug over money (i.e., “crossover point”). There were no contingencies associated with subject choices on this task. This task was only completed at the end of the 6-h assessment.
Drug Administration
Doses of Δ9-THC were prepared by encapsulating commercially available capsules of Marinol (Δ9-THC in sesame oil, Solvay Pharmaceuticals, Marietta, GA) in five opaque green size 00 capsules. Cornstarch was used to fill the remainder of all capsules. Placebo capsules contained only cornstarch.
Data Analyses
Data from all seven subjects were included in the analyses. Summary statistics were calculated for peak plasma concentrations (Cmax) of Δ9-THC and 11-OH-Δ9-THC as well as time to peak plasma concentrations (tmax). Data from individual pharmacodynamic outcomes were fit to a linear mixed model (SAS Institute Inc., v9.3, Cary, NC), expressing the mean level of the outcome as a function of dose, which was treated as a categorical variable, and included a subject-specific random effect. To account for differences in baseline mood, and for learning effects on performance tasks, behavioral data were analyzed as change scores from the baseline assessment conducted prior to dose administration during each session. Crossover point data from the Multiple-Choice Procedure were subjected to a square-root transformation to reduce non-normality. Physiological data were analyzed as raw scores. In order to 1) incorporate data from all time points, 2) more closely correspond with the analytical strategy and 3) facilitate interpretation of the figures (i.e., provide values that approximate raw data), area-under-the-curve (AUC) values were calculated and then divided by the number of post-drug data points used to calculate AUC for graphical representation.29
Results for overall effects of a predictor in a given model were judged by a Type III F test of fixed effects. Statistical significance was defined by p ≤0.05. When a significant overall effect of dose was identified, post-hoc tests were used to compare placebo to each active dose.
To determine whether cannabis use history (i.e., current weekly use, years of use and estimated total number of uses) impacted the response to Δ9-THC and should be controlled for in the analyses, each of these factors was individually added to the linear mixed model for each outcome. None of the cannabis use variables significantly improved model fit and were therefore not considered further.
Lastly, because 11-OH-Δ9-THC is an active metabolite, and there is a larger ratio of 11-OHΔ9-THC to Δ9-THC following oral administration of Δ9-THC,30–32 versions of the model were also fit using Δ9-THC and 11-OH-Δ9-THC as predictors of the outcomes rather than the administered dose. Because these competing models were not nested, the Akaike Information Criterion (AIC) was used to identify the best predictor of drug response.
Results
Pharmacokinetics
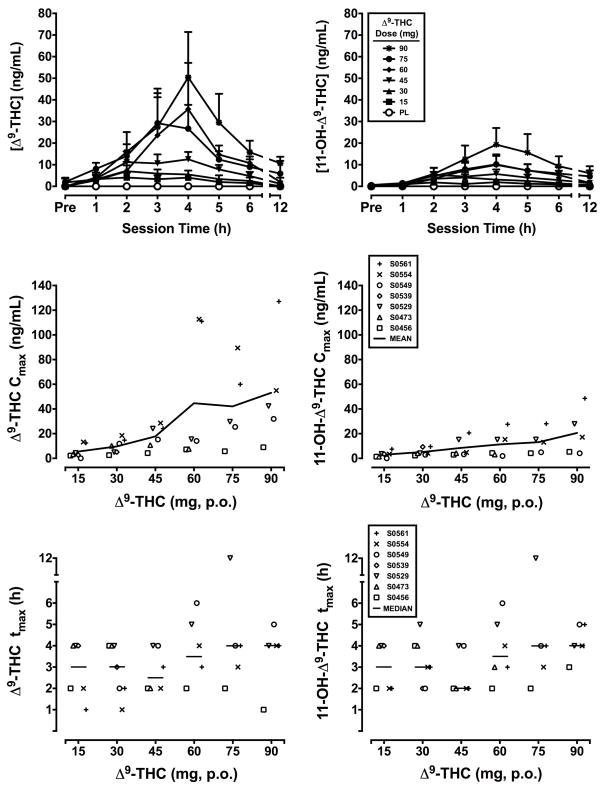
The top panels of Figure 1 present the time course for plasma concentrations of Δ9-THC (left panels) and 11-OH-Δ9-THC (right panels) as a function of oral Δ9-THC dose. The middle and bottoms panels show Cmax (top panels) and tmax values (bottom panels), respectively, for individual subjects (symbols), as well as means (Cmax) and medians (tmax) as solid lines. As illustrated in the figure, Cmax generally increased as a function of dose, but varied considerably across subjects, particularly at higher doses. Substantial variability was also found for tmax, both within and between subjects, ranging across all post-drug sampling time points, with an overall median of 3.3 h for both Δ9-THC and 11-OH-Δ9-THC.
Figure 1.
Time course (top panels), Cmax (middle panels) and tmax values (bottom panels) for plasma Δ9-THC (left panels) and 11-OH-Δ9-THC (right panels) concentrations following administration of escalating doses of oral Δ9-THC. Data points in the top panels represent means of 5–7 subjects. Uni-directional brackets indicate 1 SEM. Symbols in the bottom four panels represent individual subject data; solid lines represent means (Cmax) or medians (tmax). The x-axis in the top panels shows the time after dose in h. Pre indicates the pre-dose measurement. The x-axis for remaining panels represents Δ9-THC dose in mg. PL denotes placebo.
Pharmacodynamics
Heart Rate, Blood Pressure and Temperature
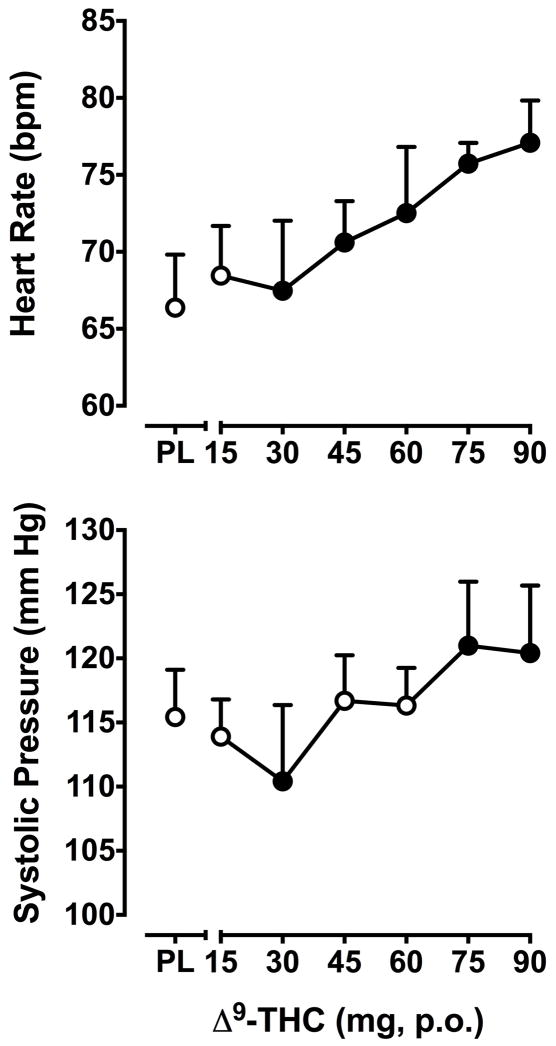
Δ9-THC dose-dependently elevated heart rate (F25,150 = 1.5, p < 0.001). Heart rate was significantly increased relative to placebo at all but the lowest Δ9-THC dose (15 mg; Figure 2).
Figure 2.
Effects of Δ9-THC on heart rate (top panel) and systolic blood pressure (bottom panel). Data are presented as AUC values divided by the number of post-drug data points used to calculate AUC. Filled symbols indicate values that are significantly different from placebo. Data points show means of 5–7 subjects. Uni-directional brackets indicate 1 SEM. Other details are as in Figure 1.
Systolic blood pressure was also altered by Δ9-THC (F25,150 = 1.3, p < 0.01). Following administration of the 30 mg dose, systolic blood pressure was significantly lower compared to the placebo, whereas the two highest doses, 75 and 90 mg, significantly increased systolic blood pressure (Figure 2). Diastolic blood pressure did not significantly change as a function of dose (data not shown).
Finger temperature was decreased by Δ9-THC (F6,36 = 2.6, p ≤0.05), but not in a dose-dependent manner. Compared to placebo, the 30 and 45 mg doses significantly reduced finger temperature. Oral temperature was not significantly influenced by Δ9-THC (data not shown).
Performance
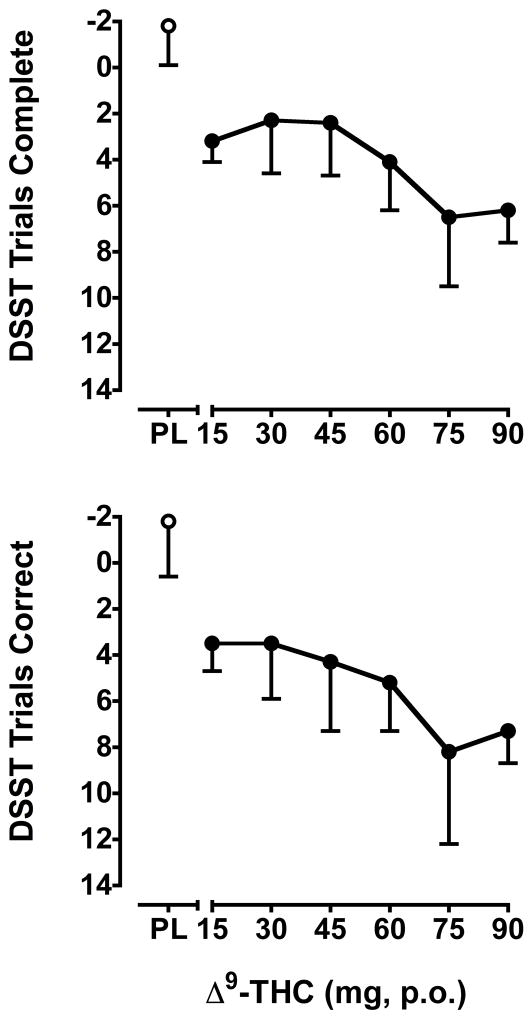
Δ9-THC reduced both rate (F6,36 = 3.0, p < 0.001) and accuracy (F6,36 = 3.0, p < 0.001) on the DSST (change from baseline; Figure 3). Relative to placebo, all active doses reduced DSST performance.
Figure 3.
Effects of Δ9-THC on the rate (top panel) and accuracy (bottom panel) on the DSST. Data are change from baseline values. Note that the Y-axis is inverted to visually represent a reduction in performance. Other details are as in Figure 2.
Δ9-THC also reduced the number of chains completed (F6,36 = 2.7, p < 0.001) and total number of responses emitted (F6,36 = 2.6, p < 0.001) on the acquisition component of the RA task. Relative to placebo, all active doses, except for 45 mg, significantly impaired rate and accuracy on this task component. Δ9-THC also reduced the number of chains completed (F6,36 = 3.6, p < 0.001) and total number of responses emitted (F6,36 = 3.8, p < 0.001) on the performance component of the RA task. Relative to placebo, all but the lowest Δ9-THC dose (15 mg) significantly impaired rate and accuracy on this task component.
Δ9-THC did not significantly affect the reproduction of time (data not shown).
Subject Ratings
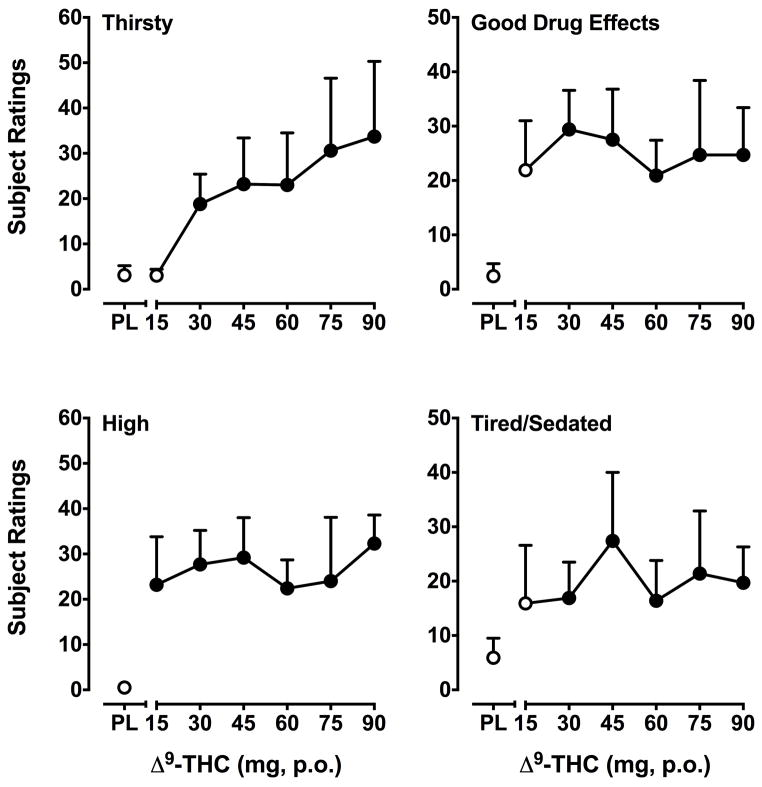
Δ9-THC significantly (F’s6,36 = 1.5–3.6, p’s ≤ 0.05) increased change scores on 10 out of 20 VAS items: Any Drug Effect, Bad Drug Effects, Good Drug Effects, High, Tired/Sedated, Pay for Drug, Thirsty, Stoned, Forgetful and Confused/Difficulty Concentrating. The subjective response to Δ9-THC varied by item. For Any Drug Effect and Thirsty*, ratings increased as a function of dose. Ratings for the item Pay for Drug peaked at the 30 mg dose and then declined. Significantly higher ratings of Bad Drug Effects only emerged at the highest dose (90 mg). For the remaining items (i.e., Good Drug Effects*, High*, Tired/Sedated*, Stoned, Forgetful and Confused/Difficulty Concentrating), doses larger than 30 mg were not consistently associated with higher ratings, with a relatively flat overall dose-response. Items marked with an asterisk (*) are presented in Figure 4.
Figure 4.
Effects of Δ9-THC on the self-report questionnaire items Thirsty, Good Drug Effects, High and Tired/Sedated. Data are change from baseline values. Other details are as in Figure 2.
Multiple-Choice Procedure
Crossover point was significantly increased by Δ9-THC (F6,36 = 2.9, p < 0.05). As with subject ratings of Pay for Drug, crossover point was highest for the 30 mg dose, and declined at higher doses. The crossover point for all but the 75 mg dose was significantly greater than for placebo (data not shown).
Model Fit for Dose vs. Cannabinoid Metabolites
The AIC was used to compare the goodness-of-fit of models incorporating dose of Δ9-THC, or cannabinoid plasma concentrations (i.e., Δ9-THC or 11-OH-Δ9-THC). For almost all outcomes, models containing plasma cannabinoid concentrations revealed stronger associations compared to models containing Δ9-THC dose. Exceptions were outcomes for the performance component of the RA task and crossover point on a Multiple Choice Procedure. Further, statistical significance emerged for additional outcomes when plasma cannabinoid concentrations replaced dose in the model (i.e., diastolic blood pressure, subject ratings of Like Drug and Take Again, and reproduction of the 60-s time interval). Overall, Δ9-THC concentration was a stronger predictor than its active metabolite 11-OH-Δ9-THC (13 vs. 7 outcomes), suggesting that Δ9-THC is mainly responsible for the effects of oral dosing, although its primary active metabolite also significantly contributes to the response.
Discussion
The present study determined the pharmacokinetic and pharmacodynamic profile of an escalating range of doses of oral Δ9-THC that, to our knowledge, included some of the largest doses administered on an acute basis to human subjects under controlled laboratory conditions. These doses produced a constellation of physiological (e.g., elevated heart rate) and behavioral (e.g. subject ratings of Thirsty and Stoned, psychomotor task performance impairment) effects consistent with previous clinical studies in which Δ9-THC and/or cannabis was administered. Interestingly, relatively low Cmax values were associated with significant effects on drug-sensitive measures (e.g., 9.7 ng/mL at 30 mg), and progressively greater Δ9-THC and 11-OH-Δ9-THC levels did not lead to proportionately larger effects on many study outcomes. Considerable variability in Cmax and tmax was observed, particularly at higher doses (e.g., Cmax range: 9.0–127.1 ng/mL at the 90 mg dose; tmax range: 1–12 h across the 75–90 mg doses).
The subjects enrolled in this study were current cannabis users with varying use patterns and histories (current use of 2–7 days per week, 100–7500 lifetime uses, across 2–10 years). Five of the seven subjects enrolled received the full range of doses, and two subjects experienced side effects (i.e., nausea and vomiting) that precluded testing with the highest doses. Perhaps surprisingly, cannabis use history was not a predictor of Δ9-THC effects or the maximum tolerated dose. For example, a subject reporting daily use and 1900 lifetime uses over 2.5 years experienced untoward side effects after receiving the 60 mg dose, whereas another subject who received all doses reported use only 2 times per week, with 300 lifetime uses over 3 years. Because Δ9-THC is a highly lipophilic molecule, fat content in the diet also could have influenced absorption and the emergence of gastrointestinal side effects; however, examination of menu choices by the subjects did not reveal an orderly pattern that might have resulted in differential response (data not shown). Determination of the key variables that impact the onset and magnitude of Δ9-THC effects after oral dosing will require further investigation.
Elevated heart rate is considered one of the most reliable biomarkers of cannabinoid effects in humans,33 and of possible concern with the administration of large Δ9-THC doses. AUC data presentation reflected a significant and dose-related increase in heart rate following administration of oral Δ9-THC. However, the peak response in the 5 subjects who were tested with all doses occurred across the 15–75 mg doses (peak mean = 117, range = 108–135 bpm; baseline mean = 69, range = 60–76 bpm). Worth noting is that the time at which peak heart rates occurred coincided with the highest concentration of plasma cannabinoids detected during the session in which those doses were administered, but not with the highest cannabinoid concentrations measured across the entire study for a given subject. Systolic blood pressure was also elevated at the two highest doses. Like heart rate, peak systolic pressures were not dose dependent in these 5 subjects, and also occurred across the 15–75 mg doses (peak mean = 146, range = 128–167 mmHg; baseline mean = 114, range = 104–141 mmHg). None of the subjects exceeded the predetermined cardiovascular safety parameters or experienced any concurrent side effects that might have been indicative of a more serious cardiac condition (e.g., sweating, blurry vision). Overall, the cardiovascular response to these doses of Δ9-THC was transient and not of clinical concern for an acute drug effect.
Behavioral side effects consisted of impaired performance on the DSST and the repeated acquisition task, and increased ratings on some “negative” subject-rated VAS items (i.e., Bad Drug Effects, Confused/Difficulty Concentrating, Forgetful). Worth noting is that these self-reported side effects did not occur in all subjects, suggesting that tailored dosing might help to avoid negative mood effects of oral Δ9-THC. With respect to psychomotor performance impairment, DSST accuracy, for example, was reduced approximately 20% by the highest doses of oral Δ9-THC. For comparison, this is consistent with disruptions in DSST performance observed following recommended clinical doses of oral triazolam in cannabis users),34 indicating that even large doses of Δ9-THC impact performance to a degree considered acceptable for an approved medication.
Maintenance on oral Δ9-THC that included administration of acute doses up to 40 mg (120 mg/day) has not been effective at reducing cannabis self-administration under controlled laboratory conditions or in the natural environment.8,13–15 Incorporating tailored Δ9-THC dosing and using higher doses to maximize the response has been suggested as a strategy to adjust for individual differences in sensitivity to Δ9-THC.8 Worth noting is that although such an approach has been adopted for community-based treatment, its incorporation into research designs to evaluate putative pharmacotherapy effectiveness for substance-use disorders is rare.35 The present results support this strategy by demonstrating that doses of oral Δ9-THC higher than those used previously can be administered to individuals with a history of cannabis use, although dose escalation and careful monitoring are needed to balance therapeutic response with side effects on an individual basis. Future medications development research would benefit from this type of design. These results also highlight the possibility that the pharmacokinetic variability of oral Δ9-THC might have negatively influenced the results of the prior studies in that incomplete or delayed absorption might have reduced treatment effectiveness on cannabis self-administration or abstinence. Related, flexible-dosing strategies should also include provisions for the individual to self-regulate the amount of Δ9-THC used to account for inconsistent absorption that might occur.
One caveat to the potential use of oral Δ9-THC as a treatment for cannabis-use disorders is that higher doses might not be more effective at reducing use. Δ9-THC functions as a partial agonist under preclinical in vitro experimental conditions36 and in some in vivo models.37,38 One characteristic of a partial agonist is a “flat” dose effect curve; that is, higher doses do not produce correspondingly larger responses. Such a profile was demonstrated here on several measures, most notably the “positive” self-reported effects (e.g., Good Drug Effects and High), consistent with modeled concentration-effect relationships of Δ9-THC.39 On the one hand, this profile is promising for a putative pharmacotherapy because it limits the abuse potential of higher doses. On the other hand, however, higher doses might be associated with additional side effects in the absence of further therapeutic value. Further research is needed to determine the ability of larger, patient-adjusted doses of oral Δ9-THC to reduce cannabis use.
Limitations of the present study include the small number of subjects and the use of an ascending dose order. However, the former limitation is offset in large part by the within-subjects design, and also by the complimentary pharmacokinetic data. That said, a larger sample might have yielded a more clear relationship between cannabis user characteristics and the response to oral Δ9-THC. The ascending dose order was necessary given the administration of previously untested doses. In addition, this limitation was addressed by using change scores for the behavioral data to account for learning effects on the tasks and systematic changes in mood across participation.
In conclusion, the present study contributes to the literature by demonstrating that, with dose escalation and cautious side effect monitoring, large doses of oral Δ9-THC can be administered to humans with a history of cannabis use, which might be useful for managing cannabis-use disorders. A secondary, but informative, contribution is the documentation of the pharmacokinetic variability of oral Δ9-THC within individual subjects. These findings are likely not surprising to professionals with experience administering oral Δ9-THC to clinical research subjects or patients receiving treatment, but published reports from controlled studies illustrating this variability are scarce. Related, these results support the therapeutic use of other orally available cannabinoids such as nabilone, or alternative oral formulations of Δ9-THC with more favorable pharmacokinetic profiles.40 Lastly, the use of such an extensive range of doses permitted the demonstration of a partial agonist profile for Δ9-THC in humans.
Acknowledgments
Funding: This research and the preparation of this manuscript were supported by National Institutes of Health grants awarded to Dr. Lile (K01 DA018772, K02 DA031766, P50 DA005312 pilot project), Dr. Kelly (P20 RR015592 Project 5) and the University of Kentucky Center for Clinical and Translational Science (UL1TR000117).
We appreciate the pharmacy services of Dr. Steve Sitzlar of the University of Kentucky Investigational Drug Service. We also appreciate the nursing services of the University of Kentucky Clinical Services Core Staff. We thank David Hudson, Elizabeth Forester and Matt Neltner for medical oversight. We also thank David Bardach, Desiree Nangle, David Pinsky and Lisa Purdy for protocol execution, and Dana Hammell and Leta Barnes for plasma sample management and analysis.
Footnotes
Declaration of Conflicting Interests: None
References
- 1.Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009;156(3):397–411. doi: 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral Δ9-THC in humans. Psychopharmacology. 2002;164(4):407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- 3.Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology. 2002;161(4):331–9. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- 4.Vandrey R, Haney M. Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs. 2009;23(7):543–53. doi: 10.2165/00023210-200923070-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marinol [package insert] Highpoint, NC: Solvay Pharmaceuticals Inc; 2004. [Google Scholar]
- 6.Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32(6):1391–403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- 7.Lile JA, Kelly TH, Pinsky DJ, Hays LR. Substitution profile of Δ9-THC, triazolam, hydromorphone and methylphenidate in humans discriminating Δ9-THC. Psychopharmacology. 2009;203(2):241–50. doi: 10.1007/s00213-008-1393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandrey R, Stitzer ML, Mintzer MZ, Huestis MA, Murray JA, Lee D. The dose effects of short- term dronabinol (oral THC) maintenance in daily cannabis users. Drug Alcohol Depend. 2012;128(1–2):64–70. doi: 10.1016/j.drugalcdep.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones RT, Benowitz N, Bachman J. Clinical studies of cannabis tolerance and dependence. Ann NY Acad Sci. 1976;282(1):221–239. doi: 10.1111/j.1749-6632.1976.tb49901.x. [DOI] [PubMed] [Google Scholar]
- 10.Cooper ZD, Haney M. Actions of delta-9-tetrahydrocannabinol in cannabis: relation to use, abuse, and dependence. Int Rev Psychiatr. 2009;21(2):104–112. doi: 10.1080/09540260902782752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86(1):22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Haney M, Hart CL, Vosburg SK, et al. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29(1):158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- 13.Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2008;197(1):157–168. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart CL, Haney M, Ward AS, Fischman WM, Foltin RW. Effects of oral THC maintenance on smoked marijuana self-administration. Drug Alcohol Depend. 2002;67(3):301–309. doi: 10.1016/s0376-8716(02)00084-4. [DOI] [PubMed] [Google Scholar]
- 15.Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116(1):142–50. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15(6):22–33. [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text revision. [Google Scholar]
- 18.Toland SL, Green W. DRE field testing of drug impaired drivers. In: Watts V, editor. The Effects of Drugs on Human Performance and Behavior: Drugs and Driving/Drugs in the Workplace. Colorado: American Academy of Forensic Sciences; 1991. [Google Scholar]
- 19.Carter LP, Richards BD, Mintzer MZ, Griffiths RR. Relative abuse liability of GHB in humans: A comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital, and GHB. Neuropsychopharmacology. 2006;31(11):2537–2551. doi: 10.1038/sj.npp.1301146. [DOI] [PubMed] [Google Scholar]
- 20.Valiveti S, Stinchcomb AL. Liquid chromatographic-mass spectrometric quantitation of Delta9-tetrahydrocannabinol and two metabolites in pharmacokinetic study plasma samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;803(2):243–8. doi: 10.1016/j.jchromb.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Hart CL, Haney M, Vosburg SK, Comer SD, Foltin RW. Reinforcing effects of oral Delta9-THC in male marijuana smokers in a laboratory choice procedure. Psychopharmacology. 2005;181(2):237–243. doi: 10.1007/s00213-005-2234-2. [DOI] [PubMed] [Google Scholar]
- 22.Kamien JB, Bickel WK, Higgins ST, Hughes JR. The effects of Δ9-THC on repeated acquisition and performance of response sequences and on self-reports in humans. Behav Pharmacol. 1994;5(1):71–78. doi: 10.1097/00008877-199402000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Heishman SJ, Stitzer ML, Yingling JE. Effects of THC content on marijuana smoking behavior, subjective reports and performance. Pharmacol Biochem Behav. 1989;34(1):173–179. doi: 10.1016/0091-3057(89)90369-9. [DOI] [PubMed] [Google Scholar]
- 24.Kelly TH, Foltin RW, Fischman MW. Multidimensional behavioral effects of marijuana. Prog Neuropsychopharmacolo Biol Psychiatr. 1990;14(6):885–902. doi: 10.1016/0278-5846(90)90075-r. [DOI] [PubMed] [Google Scholar]
- 25.Kelly TH, Foltin RW, Emurian CS, Fischman MW. Performance-based testing for drugs of abuse: dose and time profiles of marijuana, amphetamine, alcohol and diazepam. J Anal Toxicol. 1993;17(5):264–272. doi: 10.1093/jat/17.5.264. [DOI] [PubMed] [Google Scholar]
- 26.Wilson WH, Ellinwood EH, Mathew RJ, Johnson K. Effects of marijuana on performance of a computerized cognitive-neuromotor test battery. Psychiatry Res. 1994;51:115–25. doi: 10.1016/0165-1781(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 27.McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Method. 1982;14(5):463–466. [Google Scholar]
- 28.Griffiths RR, Troisi JR, Silverman K, Mumford GK. Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behav Pharmacol. 1993;4(1):3–13. [PubMed] [Google Scholar]
- 29.Lile JA, Stoops WW, Glaser PE, Hays LR, Rush CR. Physiological and subjective effects of acute intranasal methamphetamine during extended-release alprazolam maintenance. Drug Alcohol Depend. 2011;119(3):187–193. doi: 10.1016/j.drugalcdep.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodwin RS, Gustafson RA, Barnes A, Nebro W, Moolchan ET, Huestis MA. Delta(9)- tetrahydrocannabinol, 11-hydroxy-delta(9)-tetrahydrocannabinol and 11-nor-9-carboxy- delta(9)-tetrahydrocannabinol in human plasma after controlled oral administration of cannabinoids. Ther Drug Monit. 2006;28(4):545–551. doi: 10.1097/00007691-200608000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. II. Models for the prediction of time of marijuana exposure from plasma concentrations of delta 9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-delta 9-tetrahydrocannabinol (THCCOOH) J Anal Toxicol. 1992;16(5):283–290. doi: 10.1093/jat/16.5.283. [DOI] [PubMed] [Google Scholar]
- 32.Lemberger L, Crabtree RE, Rowe HM. 11-hydroxy-9-tetrahydrocannabinol: pharmacology, disposition, and metabolism of a major metabolite of marihuana in man. Science. 1972;177(4043):62–64. doi: 10.1126/science.177.4043.62. [DOI] [PubMed] [Google Scholar]
- 33.Zuurman L, Ippel AE, Moin E, van Gerven JM. Biomarkers for the effects of cannabis and THC in healthy volunteers. Br J Clin Pharmacol. 2009;67(1):5–21. doi: 10.1111/j.1365-2125.2008.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lile JA, Kelly TH, Hays LR. The reinforcing, self-reported, performance and physiological effects of Δ9-THC, triazolam, hydromorphone and methylphenidate in cannabis users. Behav Pharmacol. 2010;21(1):29–38. doi: 10.1097/FBP.0b013e32833470d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennedy AP, Karran AP, Epstein DH, Reamer DA, Schmittner J, Preston KL. A randomized investigation of methadone doses at or over 100 mg/day, combined with contingency management. Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.10.025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burkey TH, Quock RM, Consroe P, Roeske WR, Yamamura HI. delta 9- Tetrahydrocannabinol is a partial agonist of cannabinoid receptors in mouse brain. Eur J Pharmacol. 1997;323(2–3):R3–4. doi: 10.1016/s0014-2999(97)00146-5. [DOI] [PubMed] [Google Scholar]
- 37.Hruba L, Ginsburg BC, McMahon LR. Apparent inverse relationship between cannabinoid agonist efficacy and tolerance/cross-tolerance produced by Δ-tetrahydrocannabinol treatment in rhesus monkeys. J Pharmacol Exp Ther. 2012;342(3):843–849. doi: 10.1124/jpet.112.196444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paronis CA, Nikas SP, Shukla VG, Makriyannis A. 3(9)-Tetrahydrocannabinol acts as a partial agonist/antagonist in mice. Behav Pharmacol. 2012;23(8):802–805. doi: 10.1097/FBP.0b013e32835a7c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strougo A, Zuurman L, Roy C, et al. Modelling of the concentration-effect relationship of THC on central nervous system parameters and heart rate-insight into its mechanisms of action and a tool for clinical research and development of cannabinoids. J Psychopharmacol. 2008;22(7):717–726. doi: 10.1177/0269881108089870. [DOI] [PubMed] [Google Scholar]
- 40.Klumpers LE, Beumer TL, van Hasselt JG, et al. Novel Δ (9)-tetrahydrocannabinol formulation Namisol® has beneficial pharmacokinetics and promising pharmacodynamics effects. Br J Clin Pharmacol. 2012 doi: 10.1111/j.1365-2125.2012.04164.x. http://www.ncbi.nlm.nih.gov/pubmed/23278647. [DOI] [PMC free article] [PubMed]