Abstract
Adiponectin is an anti-inflammatory, anti-atherogenic adipokine elevated in heart failure (HF) that may protect against endothelial dysfunction by influencing underlying nitric oxide bioavailablity. In this study, we examine the relationship between plasma adiponectin levels and measures of nitric oxide bioavailability and myocardial performance in patients with chronic systolic HF. In 139 ambulatory patients with stable, chronic systolic HF (left ventricular [LV] ejection fraction ≤40%, New York Heart Association [NYHA] class I to IV), we measured plasma levels of adiponectin, asymmetric dimethylarginine (ADMA) and global arginine bioavailability (GABR), and performed comprehensive echocardiography with assessment of cardiac structure and performance. Adverse events (all-cause mortality or cardiac transplantation) were prospectively tracked for a median of 39 months. Plasma adiponectin levels directly correlated with plasma ADMA levels (Spearman’s r=0.41, p<0.001) and NT-proBNP levels (r=0.55, p<0.001), inversely correlated with GABR (r= −0.39, p<0.001), and were not associated with hsCRP (p=0.81) or MPO (p=0.07). Interestingly, increased plasma adiponectin levels remained positively correlated with plasma ADMA levels only in patients with elevated NT-proBNP levels (r= 0.33, p=0.009). Higher plasma adiponectin levels were associated with worse LV diastolic dysfunction (rank sums p=0.002), RV systolic dysfunction (rank sums p=0.002), and RV diastolic dysfunction (rank sums p=0.011), but not after adjustment for plasma ADMA and NT-proBNP levels. Plasma adiponectin levels predicted increased risk of adverse clinical events (HR [95% CI]: 1.45 [1.02–2.07], p=0.038) but not after adjustment for plasma ADMA and NT-proBNP levels, or echocardiographic indices of diastolic or RV systolic dysfunction. In patients with chronic systolic HF, adiponectin production is more closely linked with nitric oxide bioavailability than inflammation, and appears to be more robust in the setting of cardiac dysfunction or elevated natriuretic peptide levels.
Keywords: Congestive heart failure, adiponectin, ADMA, natriuretic peptides, diastolic dysfunction
INTRODUCTION
Adiponectin is an adipocyte-derived hormone (or “adipokine”) that plays a fundamental role in regulation of inflammation and energy homeostasis. Previous studies have identified adiponectin as a potent anti-inflammatory, anti-atherogenic, anti-oxidant and insulin-sensitizing cytokine reduced in the setting of cardiovascular disease, obesity, and diabetes mellitus.1,2 Increasing mechanistic evidence supports a direct role for adiponectin as a protective factor against endothelial dysfunction. Low adiponectin levels have been linked to impaired endothelium-dependent vasodilation,3 and adiponectin has been found to stimulate NO production via AMP-kinase-mediated endothelial nitric oxide synthase (NOS) phosphorylation.4,5 Recent data have also reported that adiponectin inhibits endothelial cell accumulation of the endogenous NOS inhibitor, asymmetrical dimethylarginine (ADMA), through increased activity of the ADMA degrading enzyme dimethylarginine dimethylaminohydrolase.6 In renal transplant recipients, low gene expression of adiponectin in visceral fat tissue was directly linked to increased plasma ADMA levels and development of endothelial dysfunction.7 Recent reports have also linked low 25-hydroxyvitamin D3 levels to elevated ADMA levels and endothelial dysfunction8, while parallel reports have described low 25-hydroxyvitamin D3 levels to be linked to low plasma adiponectin levels and postulate 1,25-dihydroxyvitamin D3 positively regulates adiponectin gene expression 9,10.
We have previously reported that evidence of systemic inflammation and endothelial dysfunction were both associated with LV diastolic dysfunction, elevated natriuretic peptide levels, and adverse long-term clinical outcomes in patients with chronic systolic heart failure.11,12 Recent data have revealed that natriuretic peptides directly enhance adipocyte adiponectin production13–15 and that adiponectin may be synthesized by and released from the failing heart itself as a compensatory response in proportion to the extent of cardiac dysfunction.16,17 We therefore hypothesize that in patients with chronic systolic heart failure, production of adiponectin may be closely associated with determinants of natriuretic peptide release (i.e. degree of left ventricular (LV) diastolic dysfunction or right ventricular (RV) systolic dysfunction). We aim to further explore the determinants of counter-regulatory responses mediated by adiponectin by examining the potential contributions of systemic inflammation (measured by C-reactive protein) and endothelial dysfunction (measured by ADMA) on adiponectin production in the setting of cardiac insufficiency.
METHODS
Study design and population
This analysis is part of the neurohormonal sub-study of Assessment of Doppler Echocardiography in Prognosis and Therapy (ADEPT) study, a single-center, prospective cohort study.18 This study was approved by the Cleveland Clinic Institutional Review Board, and informed consent was obtained from all subjects. Briefly, 139 ambulatory subjects with stable, chronic systolic heart failure (LV ejection fraction ≤40%, NYHA functional class I-IV) underwent echocardiographic evaluation of systolic and diastolic performance with plasma sample collection. Glomerular filtration rate was calculated using the standard 4-variable Modification of Diet in Renal Disease equation.19 Plasma NT-proBNP levels were assayed using a commercially available assay (Roche Elecsys® proBNP assay, Roche Diagnostics, Indianapolis IN). Plasma high-sensitivity C-reactive protein (hsCRP) was determined by the particle-enhanced immunonephelometry assay (Siemens Healthcare Diagnostics, Deerfield IL). Plasma myeloperoxidase (MPO) was determined by enzyme-linked immunosorbent assay (CardioMPO, Cleveland Heart Labs, Cleveland OH). The composite endpoint of clinical events (all-cause mortality or cardiac transplantation) was prospectively tracked for a median of 39 months by scheduled telephone calls as previously described.18
Transthoracic echocardiography
Comprehensive transthoracic echocardiography was performed using commercially available HDI 5000 (Phillips Medical Systems, N.A., Bothell, Washington) and Acuson Sequoia (Siemens Medical Solutions USA Inc., Malvern, Pennsylvania) machines. Two-dimensional and color Doppler imaging was performed in standard parasternal and apical views. Diastolic indices (including pulse-wave Doppler, color M-mode [CMM], and tissue Doppler imaging) were acquired over ten consecutive beats using sweep speeds of 50 and 100 cm/s using previously described techniques.18,20 Classification of diastolic stage was determined as follows: (1) Stage I (impaired relaxation) consists of mitral E/A <1, deceleration time (DT) >220 ms, pulmonary vein S/D >1, color M-mode propagation velocity (Vp) <45 cm/s; (2) Stage II (pseudonormal) shows mitral E/A 1–2, pulmonary vein S/D <1, DT <220 ms, Vp <45 cm/s; (3) Stage III (restrictive) gives mitral E/A >2, pulmonary vein S/D <1, DT <150 ms, Vp <45 cm/s. The LV ejection fraction and cardiac volumes were measured using Simpson’s biplane method. Right ventricular (RV) systolic dysfunction was qualitatively determined by visual assessment (on a scale of 0 to 4+). All ventricular volume measurements were indexed to body surface area. Measurements were averaged over three cycles (five cycles for atrial fibrillation), and measured by two experienced individuals prior to analyses of the neurohormonal data.
Plasma adiponectin and arginine metabolomic assays
All samples were collected using EDTA-plasma vacuum collecting tubes, processed and immediately frozen in aliquots at −80°C until analyzed. Plasma adiponectin levels were determined by the adiponectin sandwich enzyme-linked immunosorbent assay (Quantikine, R&D Systems). This assay demonstrated a minimum detection limit (as calculated by interpolation of the mean plus two standard deviations) of 0.025 μg/mL, with a within-run variation of 3.5%. Normal control values for plasma adiponectin have been reported to be 1.9–17 μg/mL.21 Arginine metabolomic profiles were measured as previously described.11 In brief, each sample solution was injected onto a HPLC column and ADMA, L-arginine, L-ornithine, and L-citrulline levels were quantified by LC/ESI/MS/MS analysis using an ABI 365 triple quadrupole mass spectrometer (Applied Biosystems Inc., Foster City, CA, USA) with Ionics EP 10+ redesigned source (Concord, Ontario, Canada) and electrospray ionization (ESI) needle connected to an Aria LX4 series multiplexed HPLC system with Flux pumps (Cohesive Technologies, Franklin, MA). Global arginine bioavailability ratio (GABR) was calculated as the quotient of L-arginine levels and the sum of L-ornithine plus L-citrulline levels. The imprecision of measurement for ADMA was 8.1%. The accuracy of measurement of ADMA was 99.2%.
Statistical analysis
Continuous variables were summarized as mean ± standard deviation if normally distributed, and as median and interquartile range [IQR] if non-normally distributed. Normality was assessed by the Shapiro-Wilk W test. Spearman’s rank correlation method was used as a nonparametric measure of association for correlations between plasma adiponectin levels and clinical and echocardiographic indices. For multiple regression analysis, a stepwise variable selection procedure was used to select variables significantly related with plasma adiponectin levels with a probability of 0.1 to enter or leave. Natural logarithmic transformations were applied to non-normally distributed variables. The Wilcoxon or Kruskal-Wallis (rank sums) tests were used to compare differences in plasma adiponectin levels across categorical variables. The Cox proportional hazards model was used to assess the clinical risk of all-cause mortality or cardiac transplantation associated with increasing continuous standardized increments of natural logarithm-transformed plasma adiponectin levels. The proportional hazards assumption was verified with log(time) vs. long[−log(survival)] plots. All-cause mortality and cardiac transplantation were followed for a median of 39 months. All p-values reported are from two-sided tests and a p-value <0.05 was considered statistically significant. For multiple hypothesis testing correction, we recommend considering Bonferroni correction with a significance level of α/n, but note the increased risk for type II error. Statistical analyses were performed using JMP 9.0.0 (SAS Institute, Cary, NC).
RESULTS
Plasma Adiponectin Levels in Study Population
In our study cohort, the mean and median plasma adiponectin levels were 14 ± 13 μg/mL and 10.4 [5.6, 18.1] μg/mL respectively, in similar ranges as previously reported.22,23 Mean and median plasma ADMA levels were 0.47 ± 0.15 μM and 0.45 [0.36, 0.56] μM, respectively (Table 1). In our overall study cohort, plasma adiponectin levels were inversely correlated with BMI (Spearman’s r = −0.32, p<0.001) and eGFR (r = −0.25, p=0.004), and directly associated with increasing age (r = 0.29, p<0.001) and plasma NT-proBNP levels (r = 0.55, p<0.0001). Plasma adiponectin levels were not associated with gender (p=0.34). Plasma adiponectin levels were significantly higher in patients with ischemic versus non-ischemic heart failure etiology (12.5 [7.6, 20.4] versus 9.2 [4.4, 16.8] μg/mL, p=0.034). Plasma adiponectin levels were also significantly lower in patients on beta-blocker therapy (8.2 [5.3, 14.6] versus 13.6 [8.0, 24.1] μg/mL, p=0.006), but this association was not significant after adjustment for NT-proBNP levels or echocardiographic indices of cardiac performance (p>0.10).
Table 1.
Subject Characteristics (n=139)
| Variable | Value |
|---|---|
| Age (years) | 58 ± 13 |
| Male gender (%) | 107 (77%) |
| Heart failure history: | |
| NYHA class III or IV (%) | 45 (33%) |
| Ischemic etiology (%) | 57 (42%) |
| Co-morbid Conditions: | |
| Hypertension (%) | 77 (57%) |
| Diabetes mellitus (%) | 41 (30%) |
| Body mass index (kg/m2) | 28 ± 5 |
| Medications: | |
| ACE inhibitors/ARB (%) | 127 (94%) |
| Beta-blockers (%) | 81 (60%) |
| Spironolactone (%) | 36 (28%) |
| Loop diuretics (%) | 106 (78%) |
| Digoxin (%) | 77 (60%) |
| Echocardiographic indices: | |
| LV ejection fraction (%) | 26 ± 6 |
| LVED volume index (mL/m2) | 112 ± 35 |
| LV Diastolic stage II or III (%) | 76 (62%) |
| Mitral annular septal E/Ea | 16.6 [12.0, 24.0] |
| eGFR (mL/min/1.73 m2) | 71 ± 24 |
| Laboratory Data | |
| NT-proBNP (pg/mL) | 1,238 [517, 3318] |
| Adiponectin (μg/mL) | 10.4 [5.6, 18.1] |
| ADMA (μM) | 0.45 [0.36, 0.56] |
| hsCRP (mg/L) | 3.23 [1.42, 7.17] |
Abbreviations: NYHA, New York Heart Association; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; LV, left ventricular; LVED, left ventricular end-diastolic; eGFR, estimated glomerular filtration rate; NT-proBNP, amino-terminal pro-B-type natriuretic peptide; ADMA, asymmetric dimethylarginine; hsCRP, high-sensitivity C-reactive protein.
Plasma Adiponectin Levels in Myocardial Structure and Performance
Table 2 presents the relationship between plasma adiponectin levels and echocardiographic indices of cardiac structure and function. There were no statistically significant correlations between plasma adiponectin levels and cardiac structure and LV ejection fraction. In contrast, plasma adiponectin levels were directly correlated with indices of left ventricular diastolic dysfunction, including mitral inflow E/A ratio (r = 0.19, p = 0.027), mitral E/septal Ea ratio (r = 0.27, p = 0.002), and large left atrial volume index (r = 0.27, p=0.006) (Table 2). Higher plasma adiponectin levels were also directly associated with overall increasing LV diastolic dysfunction stage (rank sums p=0.002). Increasing plasma adiponectin levels were also associated with echocardiographic indices of right-sided dysfunction, including high RV systolic dysfunction stage (rank sums p=0.002), high RV diastolic dysfunction stage (rank sums p=0.011), high pulmonary artery systolic pressure (r = 0.22, p=0.031), large right atrial volume index (r = 0.42, p<0.0001), large tricuspid regurgitant jet area (r = 0.33, p <0.001), tricuspid E/A ratio (r = 0.19, p=0.032), tricuspid DT (r = −0.20, p=0.028), and low hepatic vein S/D ratio (r = −0.35, p<0.001).
Table 2.
Univariable and multivariable correlations between plasma adiponectin levels and clinical and echocardiographic indices.
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
|
| ||||
| Variable | Spearman’s r | p-value | Standardized β | p-value |
| Age (years) | 0.29 | <0.001 | -- | --- |
| Body mass index (kg/m2) | −0.32 | <0.001 | −0.23 | 0.004 |
| Echocardiographic indices: | ||||
| LV ejection fraction (%) | −0.14 | 0.108 | -- | --- |
| LVED volume index (mL/m2) | −0.02 | 0.834 | -- | --- |
| Mitral E/A ratio | 0.19 | 0.027 | -- | --- |
| Mitral DT (ms) | −0.17 | 0.062 | -- | --- |
| PV S/D ratio | −0.15 | 0.088 | -- | --- |
| E/Septal Ea ratio | 0.27 | 0.002 | -- | --- |
| Tricuspid regurgitant jet area (cm2) | 0.33 | <0.001 | -- | --- |
| PA systolic pressure (mmHg) | 0.22 | 0.031 | -- | --- |
| LAVi (ml/m2) | 0.27 | 0.006 | -- | --- |
| RAVi (ml/m2) | 0.42 | <0.0001 | -- | --- |
| eGFR (mL/min/1.73 m2) | −0.25 | 0.004 | -- | --- |
| NT-proBNP (pg/mL) | 0.55 | <0.0001 | 0.51 | <0.0001 |
| ADMA (μM) | 0.41 | <0.0001 | -- | --- |
| GABR | −0.39 | <0.0001 | -- | --- |
| hsCRP (mg/L) | −0.02 | 0.812 | -- | -- |
Abbreviations: LV, left ventricular; LVED, left ventricular end-diastolic; DT, deceleration time; PV, pulmonary vein; PA, pulmonary artery; LAVi, left atrial volume index; RAVi, right atrial volume index; eGFR, estimated glomerular filtration rate; NT-proBNP, amino-terminal pro-B-type natriuretic peptide; ADMA, asymmetric dimethylarginine; hsCRP, high-sensitivity C-reactive protein.
Inflammation and Arginine Bioavailability as Determinants of Plasma Adiponectin Levels
Overall, plasma adiponectin levels were positively correlated with plasma ADMA levels (Spearman’s r = 0.41, p<0.0001) and inversely correlated with GABR (Spearman’s r = −0.39, p<0.0001), including after adjustment for age, BMI, gender, eGFR, diabetic status, and heart failure etiology (Standardized [Std] β = 0.37, p<0.0001, and Std β = −0.36, p<0.001, respectively). After adjustment for plasma NT-proBNP, plasma adiponectin levels were not associated with plasma ADMA (Std β = 0.09, p=0.280) and only modestly associated with GABR (Std β = −0.17, p=0.050). In stepwise multiple regression analysis, plasma NT-proBNP and BMI were independently associated with plasma adiponectin levels (p<0.0001 and p=0.004, respectively; Table 2).
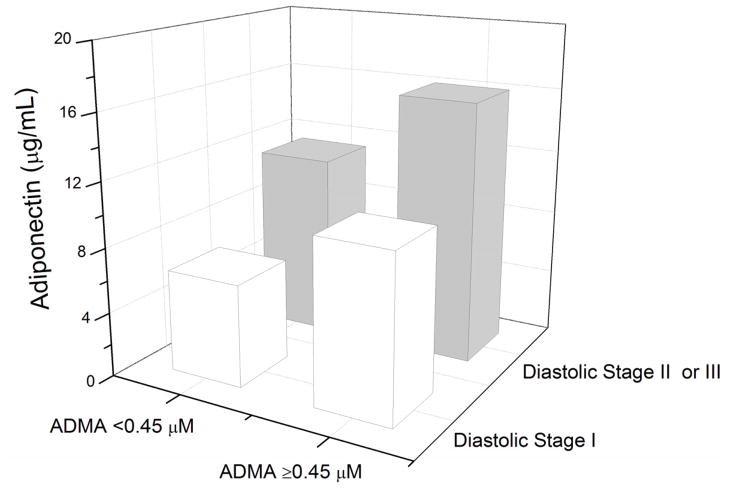
Figure 1 presents the relationship between plasma adiponectin and ADMA levels in our overall study cohort and subgroups stratified by median NT-proBNP. Plasma adiponectin levels remained significantly associated with plasma ADMA levels in patients with high (above median levels of) plasma NT-proBNP (r = 0.33, p=0.009) but not low plasma NT-proBNP (r=0.21, p=0.096). Plasma levels of adiponectin remained associated with ADMA in patients with preserved renal function (eGFR≥60 ml/min/1.73 m2); (r = 0.047, p<0.001). This was similar when stratified according to diastolic dysfunction (Figure 2). In contrast, overall plasma adiponectin levels did not demonstrate any clear relationships with hsCRP (r = −0.02, p = 0.81) or MPO (r= 0.16, p=0.07).
Figure 1.
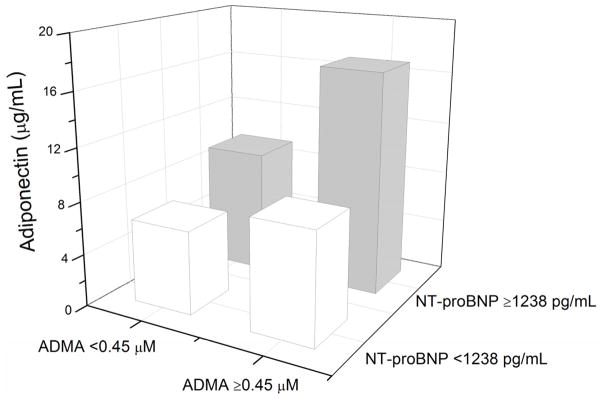
Median plasma adiponectin levels stratified by median plasma ADMA (0.45 μM) and median plasma NT-proBNP (1,238 pg/mL) levels.
Figure 2.
Median plasma adiponectin levels stratified by median plasma ADMA level (0.45 μM) and diastolic dysfunction stages.
Plasma Adiponectin Levels and Prognosis
In Cox proportional hazard analysis, natural logarithm-transformed adiponectin demonstrated increased risk for adverse clinical events (unadjusted HR 1.45, 95% CI: 1.02 – 2.07, p=0.038). In multivariate Cox proportional hazard analysis, natural logarithm-transformed plasma adiponectin predicted risk independent of age, BMI and diabetic status (HR 1.55, 95% CI: 1.08 – 2.23, p = 0.038). However, plasma adiponectin levels did not demonstrate independent prognostic value when adjusted for echocardiographic indices of LV diastolic function, indices of RV systolic or diastolic function, plasma NT-proBNP levels, or plasma ADMA levels (Table 3).
Table 3.
Multivariable Cox proportional hazard analyses of adverse clinical events.
| Variable | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Model 1 (32 events) | ||
| Ln Adiponectin (μg/mL) * | 1.45 (1.02 – 2.07) | 0.038 |
| Model 2 (28 events) | ||
| Ln Adiponectin (μg/mL) * | 1.55 (1.08 – 2.23) | 0.019 |
| Age (years) * | 0.87 (0.60 – 1.28) | 0.461 |
| Body Mass Index (kg/m2) * | 0.98 (0.63 – 1.49) | 0.927 |
| Diabetes mellitus | 2.27 (1.04 – 4.93) | 0.040 |
| Model 3 (30 events) | ||
| Ln Adiponectin (μg/mL) * | 1.26 (0.85 – 1.89) | 0.250 |
| Ln E/Septal Ea ratio * | 1.69 (1.13 – 2.56) | 0.010 |
| Model 4 (32 events) | ||
| Ln Adiponectin (μg/mL) * | 1.25 (0.85 – 1.83) | 0.255 |
| RV systolic dysfunction class† | 1.44 (1.10 – 1.89) | 0.007 |
| Model 5 (30 events) | ||
| Ln Adiponectin (μg/mL) * | 1.13 (0.73 – 1.71) | 0.577 |
| Ln NT-proBNP (pg/mL) * | 1.73 (1.17 – 2.52) | 0.006 |
| Model 6 (32 events) | ||
| Ln Adiponectin (μg/mL) * | 1.26 (0.85 – 1.85) | 0.248 |
| Ln ADMA (μM) * | 1.53 (1.04 – 2.27) | 0.030 |
Hazard ratios per 1 standard deviation (SD) increments (1 SD for Ln Adiponectin = 0.81 μg/mL; 1 SD for Age = 13.5 years; 1 SD for BMI = 4.87 kg/m2; 1 SD for Ln ADMA = 0.30 μM; 1 SD for Ln LV ejection fraction = 0.25 (%); 1 SD for Ln E/Septal Ea ratio = 0.56; 1 SD for Ln NT-proBNP = 1.34 pg/mL).
Hazard ratios per +1 increments
Abbreviations: Ln, natural log; RV, right ventricular; NT-proBNP, amino-terminal pro-B-type natriuretic peptide; ADMA, asymmetric dimethylarginine; SD = standard deviation.
DISCUSSION
Over the past decade, there has been a growing understanding of the role of metabolic factors, in particular adipokines, in the pathophysiology of heart failure. In this study, we report a direct association between higher plasma levels of adiponectin and greater impairment of diastolic dysfunction and right ventricular systolic dysfunction in patients with chronic systolic heart failure. Furthermore, we observed a link between circulating adiponectin levels and the degree of underlying nitric oxide bioavailability (either in the form of substrate-product balance as measured by GABR, or the presence of endogenous NO synthase inhibitor as measured by ADMA) rather than with measures of systemic inflammation (as measured by MPO or hsCRP) in the setting of cardiac insufficiency. Remarkably, these correlations were stronger with increasing disease severity as indicated by higher NT-proBNP levels. These findings support the potential role of adiponectin as a counter-regulatory response to cardiac insufficiency rather than a metabolic mediator of disease progression.
The relationship between adiponectin levels and cardiac dysfunction is complex. Adiponectin has been found to be present in damaged cardiomyocytes,16 and is thought to be upregulated in response to signals of local inflammatory and oxidative stress24 as well as sensors of energy imbalance.25,26 Recent reports have also revealed that adiponectin may be synthesized by and released from the failing heart itself in proportion to the extent of LV dysfunction16,17 and that natriuretic peptides enhance adipocyte adiponectin synthesis.13–15
In our overall study cohort, plasma adiponectin also was directly associated with increasing overall LV diastolic dysfunction stage and with individual echocardiographic indices of diastolic dysfunction, as has been previously reported. These findings confirm previous reports of a direct association between plasma adiponectin and NT-proBNP levels, and corroborate recent findings of a direct association between increased plasma adiponectin levels and echocardiographic indices of LV diastolic dysfunction in patients with hypertrophic cardiomyopathy.27 In addition, we demonstrate for the first time new associations between increased plasma adiponectin levels and echocardiographic estimates of right ventricular systolic and diastolic dysfunction, including RAVi, hepatic vein S/D ratio, tricuspid E/A ratio, and tricuspid DT. RAVi, as a measure of the chronicity of RV diastolic function over time,28 was associated with plasma adiponectin levels independent of plasma NT-proBNP levels. Hepatic congestion secondary to right-sided failure may increase plasma adiponectin levels independent of plasma natriuretic peptide levels as the liver is the primary site of clearance of adiponectin.29 In addition, plasma adiponectin levels may be independently raised in response to metabolic derangements. Tricuspid regurgitation and increased estimated pulmonary arterial systolic pressure are known to correlate with hepatic congestion and malnutrition.30 In fact, tricuspid regurgitation itself may be an accentuating and accelerating risk factor for cardiac cachexia on account of a greater hypoalbuminemia and hyponatremia, which presumably results from the associated protein-losing enteropathy.31
In direct contrast to previous reports of an inverse correlation between plasma adiponectin and ADMA levels in the non-heart failure setting, our data indicate a direct correlation between plasma adiponectin and ADMA levels. Since increased adiponectin levels have been reported to attenuate the TNFα-mediated decrease in the activity of the ADMA degrading enzyme, dimethylarginine dimethylaminohydrolase,6,7 our data imply that natriuretic peptide enhanced upregulation of adipocyte adiponectin production is a counter-regulatory effect in response to underlying endothelial dysfunction that is likely contributing to or associated with cardiac insufficiency. In support of this possible mechanistic link, the positive association we observed between plasma adiponectin and ADMA levels in this study population was not independent of plasma NT-proBNP levels, and in subgroup analysis, was preserved only in patients with above-median NT-proBNP levels alone. Furthermore, plasma adiponectin levels were not predictive of long-term clinical outcomes independent of plasma NT-proBNP levels, plasma ADMA levels, or echocardiographic indices of diastolic dysfunction. In contrast, no such relationships were observed between plasma adiponectin levels and C-reactive protein, a marker of systemic inflammation shown to provide independent prognostic value in heart failure.12
Study Limitations
Different isoforms of adiponectin, including low-, middle-, and high-molecular weight forms were not separately determined. Alternate methods of assessing endothelial function, including assessment of flow-mediated dilation by strain-gauge venous occlusion plethysmography, were not performed. Estimates of diastolic dysfunction were derived from non-invasive echocardiographic assessment as opposed to direct invasive measurements of intracardiac pressures. Due to the cross-sectional nature of this study, any causal relationship between adiponectin levels and plasma ADMA, GABR and echocardiographic indices will require further investigation. Nevertheless, our data report for the first time a positive association between plasma adiponectin and ADMA levels (a finding opposite to that observed in non-heart failure populations) as well as a negative association between plasma adiponectin and GABR, especially in patients with chronic systolic heart failure. In addition, we confirm recent reports of a direct association between increased plasma adiponectin levels and echocardiographic indices of LV diastolic dysfunction, and demonstrate for the first time novel associations between plasma adiponectin levels and RV systolic and diastolic dysfunction. Further studies are needed to determine in which heart failure subgroups increased plasma adiponectin levels are protective, and, in subgroups where increased adiponectin levels mark worse prognosis, whether adiponectin is simply a marker of a failed counter-regulatory response or an active contributor to worsening heart failure progression. Finally, the potential risks and benefit of therapeutic strategies that directly raise plasma adiponectin levels need to be examined carefully in the setting of chronic systolic heart failure.
CONCLUSIONS
In our cohort of symptomatic patients with chronic systolic heart failure, higher adiponectin levels were associated with lower nitric oxide bioavailability as well as more impaired diastolic performance and right (but not left) ventricular systolic dysfunction. Our observations also support one of the notions that adiponectin is more likely generated in response to endothelial dysfunction than to systemic inflammation.
Brief Commentary.
Background
Adiponectin is an anti-inflammatory, anti-atherogenic adipokine elevated in heart failure and previously associated with poor prognosis. Recent mechanistic data report natriuretic peptides enhance adiponectin production, and suggest adiponectin may increase catabolism of asymmetric dimethylarginine (ADMA).
Translational Significance
We observe that plasma adiponectin levels, as previously reported to have prognostic value in heart failure, were not predictive of long-term clinical outcomes independent of plasma natriuretic peptide or ADMA levels, or diastolic dysfunction. Hence, natriuretic peptide-enhanced upregulation of adiponectin may constitute a compensatory (and potentially favorable) response to endothelial dysfunction, and rather than deleterious effects associated with systemic inflammation.
Acknowledgments
This research was supported by National Institutes of Health grants P01HL076491, P01HL103453, P01HL098055, R01HL103866, R01HL103931, P20HL113452 and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439-06).
Footnotes
All authors have read the journal’s policy on conflicts of interest.
DISCLOSURE
Dr. Tang has previously received research grant support from Abbott Laboratories, Inc, and has served as consultant for Medtronic Inc and St Jude Medical Inc. Dr. Hazen reports being listed as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Hazen reports having been paid as a consultant or speaker for the following companies: Abbott Diagnostics, Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., Merck & Co., Inc., and Pfizer Inc. Dr. Hazen reports receiving research funds from Abbott, Cleveland Heart Lab, Liposcience Inc., and Pfizer Inc. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Abbott Laboratories, Inc., Cleveland Heart Lab., Esperion, Frantz Biomarkers, LLC, Liposcience Inc., and Siemens.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond) 2006;110:267–78. doi: 10.1042/CS20050182. [DOI] [PubMed] [Google Scholar]
- 2.Duncan BB, Schmidt MI, Pankow JS, Bang H, Couper D, Ballantyne CM, Hoogeveen RC, Heiss G. Adiponectin and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2004;53:2473–8. doi: 10.2337/diabetes.53.9.2473. [DOI] [PubMed] [Google Scholar]
- 3.Tan KC, Xu A, Chow WS, Lam MC, Ai VH, Tam SC, Lam KS. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab. 2004;89:765–9. doi: 10.1210/jc.2003-031012. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–6. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 5.Hattori Y, Suzuki M, Hattori S, Kasai K. Globular adiponectin upregulates nitric oxide production in vascular endothelial cells. Diabetologia. 2003;46:1543–9. doi: 10.1007/s00125-003-1224-3. [DOI] [PubMed] [Google Scholar]
- 6.Eid HM, Lyberg T, Arnesen H, Seljeflot I. Insulin and adiponectin inhibit the TNFalpha-induced ADMA accumulation in human endothelial cells: the role of DDAH. Atherosclerosis. 2007;194:e1–8. doi: 10.1016/j.atherosclerosis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Teplan V, Schuck O, Racek J, Lecian D, Haluzik M, Kudla M, Vitko S. Asymmetric dimethylarginine in obesity after renal transplantation. J Ren Nutr. 2008;18:513–20. doi: 10.1053/j.jrn.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Ngo DT, Sverdlov AL, McNeil JJ, Horowitz JD. Does vitamin d modulate asymmetric dimethylarginine and C-reactive protein concentrations? Am J Med. 123:335–41. doi: 10.1016/j.amjmed.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Liu E, Meigs JB, Pittas AG, McKeown NM, Economos CD, Booth SL, Jacques PF. Plasma 25-hydroxyvitamin d is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutr. 2009;139:329–34. doi: 10.3945/jn.108.093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nimitphong H, Chanprasertyothin S, Jongjaroenprasert W, Ongphiphadhanakul B. The association between vitamin D status and circulating adiponectin independent of adiposity in subjects with abnormal glucose tolerance. Endocrine. 2009;36:205–10. doi: 10.1007/s12020-009-9216-9. [DOI] [PubMed] [Google Scholar]
- 11.Tang WH, Tong W, Shrestha K, Wang Z, Levison BS, Delfraino B, Hu B, Troughton RW, Klein AL, Hazen SL. Differential effects of arginine methylation on diastolic dysfunction and disease progression in patients with chronic systolic heart failure. Eur Heart J. 2008;29:2506–13. doi: 10.1093/eurheartj/ehn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang WH, Shrestha K, Van Lente F, Troughton RW, Martin MG, Borowski AG, Jasper S, Klein AL. Usefulness of C-reactive protein and left ventricular diastolic performance for prognosis in patients with left ventricular systolic heart failure. Am J Cardiol. 2008;101:370–3. doi: 10.1016/j.amjcard.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 13.Tsukamoto O, Fujita M, Kato M, Yamazaki S, Asano Y, Ogai A, Okazaki H, Asai M, Nagamachi Y, Maeda N, Shintani Y, Minamino T, Asakura M, Kishimoto I, Funahashi T, Tomoike H, Kitakaze M. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol. 2009;53:2070–7. doi: 10.1016/j.jacc.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T, Tsutamoto T, Sakai H, Nishiyama K, Fujii M, Yamamoto T, Horie M. Effect of atrial natriuretic peptide on adiponectin in patients with heart failure. Eur J Heart Fail. 2008;10:360–6. doi: 10.1016/j.ejheart.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Yamaji M, Tsutamoto T, Tanaka T, Kawahara C, Nishiyama K, Yamamoto T, Fujii M, Horie M. Effect of carperitide on plasma adiponectin levels in acute decompensated heart failure patients with diabetes mellitus. Circ J. 2009;73:2264–9. doi: 10.1253/circj.cj-09-0371. [DOI] [PubMed] [Google Scholar]
- 16.Pineiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, Dieguez C, Gualillo O, Gonzalez-Juanatey JR, Lago F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005;579:5163–9. doi: 10.1016/j.febslet.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 17.Takano H, Obata JE, Kodama Y, Kitta Y, Nakamura T, Mende A, Kawabata K, Saito Y, Fujioka D, Kobayashi T, Yano T, Sano K, Kugiyama K. Adiponectin is released from the heart in patients with heart failure. Int J Cardiol. 2009;132:221–6. doi: 10.1016/j.ijcard.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 18.Troughton RW, Prior DL, Frampton CM, Nash PJ, Pereira JJ, Martin M, Fogarty A, Morehead AJ, Starling RC, Young JB, Thomas JD, Lauer MS, Klein AL. Usefulness of tissue doppler and color M-mode indexes of left ventricular diastolic function in predicting outcomes in systolic left ventricular heart failure (from the ADEPT study) Am J Cardiol. 2005;96:257–62. doi: 10.1016/j.amjcard.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 20.Troughton RW, Prior DL, Pereira JJ, Martin M, Fogarty A, Morehead A, Yandle TG, Richards AM, Starling RC, Young JB, Thomas JD, Klein AL. Plasma B-type natriuretic peptide levels in systolic heart failure: importance of left ventricular diastolic function and right ventricular systolic function. J Am Coll Cardiol. 2004;43:416–22. doi: 10.1016/j.jacc.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 21.Tsao TS, Lodish HF, Fruebis J. ACRP30, a new hormone controlling fat and glucose metabolism. Eur J Pharmacol. 2002;440:213–21. doi: 10.1016/s0014-2999(02)01430-9. [DOI] [PubMed] [Google Scholar]
- 22.George J, Patal S, Wexler D, Sharabi Y, Peleg E, Kamari Y, Grossman E, Sheps D, Keren G, Roth A. Circulating adiponectin concentrations in patients with congestive heart failure. Heart. 2006;92:1420–4. doi: 10.1136/hrt.2005.083345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, Hildebrandt P. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–62. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 24.Delaigle AM, Senou M, Guiot Y, Many MC, Brichard SM. Induction of adiponectin in skeletal muscle of type 2 diabetic mice: in vivo and in vitro studies. Diabetologia. 2006;49:1311–23. doi: 10.1007/s00125-006-0210-y. [DOI] [PubMed] [Google Scholar]
- 25.Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95:568–78. doi: 10.1161/01.RES.0000141774.29937.e3. [DOI] [PubMed] [Google Scholar]
- 26.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–22. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unno K, Shibata R, Izawa H, Hirashiki A, Murase Y, Yamada T, Kobayashi M, Noda A, Nagata K, Ouchi N, Murohara T, Murohara T. Adiponectin acts as a positive indicator of left ventricular diastolic dysfunction in patients with hypertrophic cardiomyopathy. Heart. 2009 doi: 10.1136/hrt.2009.172320. [DOI] [PubMed] [Google Scholar]
- 28.Sallach JA, Tang WH, Borowski AG, Tong W, Porter T, Martin MG, Jasper SE, Shrestha K, Troughton RW, Klein AL. Right atrial volume index in chronic systolic heart failure and prognosis. JACC Cardiovasc Imaging. 2009;2:527–34. doi: 10.1016/j.jcmg.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Halberg N, Schraw TD, Wang ZV, Kim JY, Yi J, Hamilton MP, Luby-Phelps K, Scherer PE. Systemic Fate of the Adipocyte-Derived Factor Adiponectin. Diabetes. 2009 doi: 10.2337/db08-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carr JG, Stevenson LW, Walden JA, Heber D. Prevalence and hemodynamic correlates of malnutrition in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1989;63:709–13. doi: 10.1016/0002-9149(89)90256-7. [DOI] [PubMed] [Google Scholar]
- 31.Ajayi AA, Adigun AQ, Ojofeitimi EO, Yusuph H, Ajayi OE. Anthropometric evaluation of cachexia in chronic congestive heart failure: the role of tricuspid regurgitation. Int J Cardiol. 1999;71:79–84. doi: 10.1016/s0167-5273(99)00117-5. [DOI] [PubMed] [Google Scholar]